Possible Effects and Mechanisms of Dietary Natural Products and Nutrients on Depression and Anxiety: A Narrative Review
Abstract
:1. Introduction
2. Depression
2.1. Epidemiological Studies
2.2. Experimental Studies
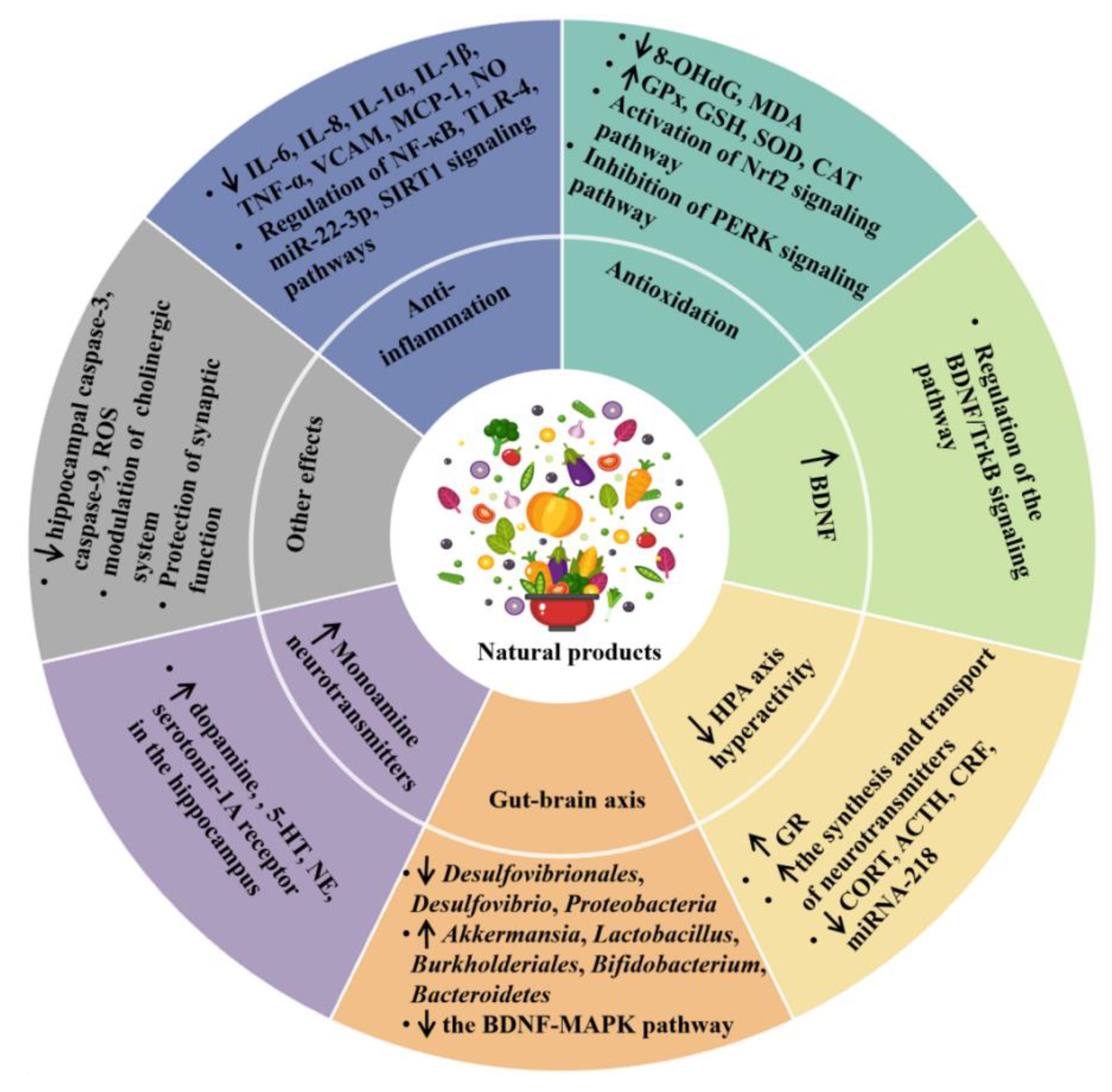
2.2.1. Anti-Inflammatory Effect
2.2.2. Antioxidant Effect
2.2.3. Modulating the Production of Monoamine Neurotransmitters
2.2.4. Promoting the Production of Neurotrophins
2.2.5. Inhibition of the HPA Axis Hyperactivity
2.2.6. Modulation of Microbiota–Gut–Brain Axis
2.2.7. Other Mechanisms
| Name | Study Type | Model | Dose | Effects and Mechanisms | Ref. |
|---|---|---|---|---|---|
| Animal Foods | |||||
| Fish oil | In Vivo | Lupus-prone MRL/lpr mice | 728 mg/kg | Increased BDNF and synaptic protein Enhanced Nrf2-mediated antioxidant defenses; | [52] |
| Plant Foods | |||||
| Geum japonicum | In Vivo | ICR mice | 30, 100, 300 mg/kg | Exerted neuroprotective effects Upregulated expression of BDNF in hippocampus; | [60] |
| In Vitro | SH-SY5Y cells | 0, 50, 100 μg/mL | Decreased CORT-induced neurotoxicity; | ||
| Royal jelly | In Vivo | CUMS mice | 4.5 g/kg | Attenuated CUMS-induced depression Inhibited the biosynthesis of CORT; | [68] |
| Purple cauliflower | In Vivo | CUMS mice | 50, 100, 200 mg/kg | Improved depressive symptoms Increased content of monoamine neurotransmitter Suppressed activity of MAO Upregulated BDNF, TrkB; | [19] |
| Semen sojae praeparatum | In Vivo | CUMS rats | 0.97 g/kg | Exerted antidepressant effect Upregulated Ruminococcaceae_UCG-008 Regulated the 5-HT, NE, GABA, BDNF content | [89] |
| Deoiled sunflower seeds | In Vivo | CUMS mice | NA | Ameliorated depression-like behaviors Upregulated dopamine, 5-HT, acetylcholine, NE, BDNF; | [54] |
| Bifidobacteria-fermented red ginseng | In Vivo | C57BL/6 mice | 10, 25, 50 mg/kg | Exerted protective effects against Escherichia coli-induced depression Enhanced the abundance of Bacteroidetes Reduced the abundance of Proteobacteria Upregulated expression of BDNF; | [84] |
| Navel orange essential oil | In Vivo | Kunming mice | 0.5, 1, 2% | Exerted anti-depressive effects Increased serotonin and dopamine levels in brain; | [56] |
| Garlic essential oil | In Vivo | CUMS rats | 25, 50 mg/kg | Exerted anti-depressive effect Upregulated hippocampal BDNF, CREB, protein kinase B; | [59] |
| Beverages | |||||
| Grewia asiatica berry juice | In Vivo | SD male rats | Free access to 5%, 10%, 20%, 30% dilutions | Decreased oxidative damage Increased SOD and GPx levels Modulated the cholinergic system Decreased acetylcholinesterase and MDA levels; | [50] |
| Maqui berry | In Vivo | Male balb/c mice | 25, 50, 100 mg/kg | Ameliorated post-stroke depression Upregulated expression level of GSH Enhanced activities of SOD, CAT; | [51] |
| Adzuki bean sprout fermented milk | In Vivo | C57BL/6 mice | 0.1, 0.2, 0.4 mL/mouse | decreased depressive symptoms Upregulated the expression levels of 5-HT, NE, dopamine; | [57] |
| Probiotics | |||||
| Bifidobacterium E41 and M2CF22M7 | In Vivo | C57BL/6J mice | 1 × 109 CFU/mouse | Suppressed depression-like behaviors Improved the gut microbial dysbiosis Enhanced 5-HT and BDNF; | [79] |
| Prevotella histicola | In Vivo | C57 BL/6 mice | 1 × 109 CFU/mouse | Protective effect against estrogen deficiency-induced depression Downregulated VCAM, MCP-1, IL-6, IL-8, TNF-α Increased the abundance of Akkermansia and Lactobacillus; | [44] |
| Lacticaseibacillus paracasei NK112 | In Vivo | C57 BL/6J mice | NA | Exerted protective effects against Escherichia coli-induced depression Decreased IL-1α, IL-6, TNF-α Inhibited the activity of NF-κB; | [45] |
| Lactobacillus casei | In Vivo | CUMS rats | NA | Mitigated depressive symptoms Reversed the structure change of gut microbiota Regulated BDNF/TrkB signaling; | [78] |
| Lactobacillus casei | In Vivo | Pregnant rats | 8 × 108 CFU/kg | Exerted anti-depressive effects Changed the composition of gut microbiota Increased expression level of BDNF Suppressed BDNF–MAPK pathway | [77] |
| Lactobacillus gasseri NK109 | IN VIVO | Mice | 1 × 108, 1 × 109 CFU/mouse | Improved Escherichia coli K1-induced depression Decreased the expression level of IL-1α Regulated gut microbiota; | [81] |
| Lactobacillus kefiranofaciens ZW3 | In Vivo | CUMS mice | 1 × 107, 1 × 108, 1 × 109 CFU/mouse | Improved the symptoms of depression Regulated the gut microbiota composition Ameliorated constipation; | [80] |
| Lactobacillus helveticus strain MCC1848 | In Vivo | C57BL/6J mice | 1 × 1011 CFU/mouse | Ameliorated depressive symptoms Improved the alteration of gene expression in nervous system development and signal transduction; | [90] |
| Nutrients | |||||
| SCFAs | In Vivo | High fructose-fed mice | NA | Decreased depression-like behaviors Inhibited microglia activation Reduced blood–brain barrier damage; | [94] |
| n-3 PUFA | In Vivo | Wistar rats | 0 en% of n-3 PUFA, 1 en% of n-3 PUFA | Ameliorated post-menopausal depression Increased brainstem serotonin level Increased serotonin-1A receptor, BDNF, CREB, miRNA-155, GR Downregulated IL-6, IL-1β, TNF-α, prostaglandin E-2, CORT, ACTH, CRF, miRNA-218; | [42] |
| n-3 PUFA | In Vivo | Wistar rats | 0 en% of n-3 PUFA, 1 en% of n-3 PUFA | Regulated the HPA axis Reduced circulating levels of ACTH and CORT Downregulated expression of CRF; | [67] |
| EGCG | In Vivo | CUMS rats | 50 mg/kg | Exerted anti-depressive effects Reduced IL-6 and NO Decreased caspase-3 and caspase-9; | [46] |
| EGCG | In Vivo | CUMS rats | 50 mg/kg | Improved depression-like behaviors Reduced serotonin in the colon Increased serotonin in the hippocampus; | [55] |
| EPA-PL | In Vivo | ICR mice | NA | Ameliorated depressive symptoms Suppressed HPA axis hyperactivity; | [65] |
| Sesamin | In Vivo | CUMS mice | 50 mg/kg | Improved depressive symptoms Increased BDNF, NT-3; | [61] |
| Resveratrol | In Vivo | Wistar-Kyoto male rats | 40 mg/kg | alleviated depression-like behaviors increased BDNF, NT3; | [63] |
| Dietary fiber | In Vivo | C57BL/6J mice | NA | Increased the expression levels of NE, 5-HT Inhibited neuroinflammation Promoted formation of short-chain fatty acids and reconstruction of gut microbiome; | [82] |
| Dietary pectins | In Vivo | BALB/c mice | 50 mg/kg | Suppressed depression-like behaviors Increased the levels of IL-6, IFN-γ Downregulated the protein level of STAT3; | [95] |
| Coniferyl ferulate | In Vivo | C57BL/6 SPF mice | 50 mg/kg | Exerted protective effect Improved the reconstruction of gut microbiome Downregulated the expression levels of IL-6, IL-1β, TNF-α; | [88] |
| Coniferyl ferulate | In Vitro | PC12 cells | 0.2, 2, 20 μmol/L | Exerted anti-depressive effect Decreased the production of ROS Suppressed mitochondrial apoptotic pathways; | [96] |
| Nicotinamide riboside | In Vivo | C57BL/6J mice | 400 mg/kg | Changed the composition of gut microbiota Decreased inflammation-related cytokines Increased BDNF levels; | [86] |
| Soy isoflavones | In Vivo | CUMS rats | 40, 80, 160 mg/kg | Improved depressive symptoms Enhanced the diversity of gut microbiota Upregulated monoamine neurotransmitter levels; | [87] |
| Curcumin | In Vivo | Wistar male rats | 40 mg/kg | Attenuated depression-like behaviors Suppressed excessive synaptic loss Improved synaptic function; | [92] |
| Lycopene | In Vitro | SH-SY5Y cells | 1~10 μM | Alleviated oxidative damage Reduced 8-OHdG, MDA, and protein carbonyls expressions Inhibited PERK signaling pathway; | [48] |
| Chlorogenic acid | In Vivo | Wistar rats | 500 mg/kg | Had anti-depressive effects Increased Burkholderiales, Bifidobacterium Reduced Desulfovibrionales, Desulfovibrio; | [83] |
| Apple phenolic | In Vivo | Kunming mice | 200 ppm in normal saline | Improved lead acetate-induced depression-like behaviors Attenuated neuroinflammation and neuronal apoptosis Regulated miR-22-3p/SIRT1 signaling pathway; | [39] |
| Alkaloids | In Vivo | C57BL/6N mice | 200 mg/kg | Mitigated LPS-induced depressive symptoms Inhibited neuroinflammation Repressed BDNF-mediated endoplasmic reticulum stress Increased autophagy; | [91] |
| In Vitro | BV2 cells | 50 μg/mL | Inhibited pro-inflammatory mediators and NO production; | ||
| Aponin compounds | In Vivo | CMS rats | 240 mg/kg | Exerted anti-depressive effect Inhibited the hyperactivation of HPA axis Improved the synthesis and transport processes of neurotransmitters; | [66] |
| Saccharina japonica ethanol extract | In Vivo | C57BL/6 mice | 1, 2, 4 g/kg | Decreased the depression-like behaviors Increased activity of superoxide dismutase Increased anti-inflammatory cytokines Downregulated NF-κB, NOD-like receptor 3, TLR-4; | [43] |
2.3. Clinical Trials
3. Anxiety
3.1. Epidemiological Studies
3.2. Experimental Studies
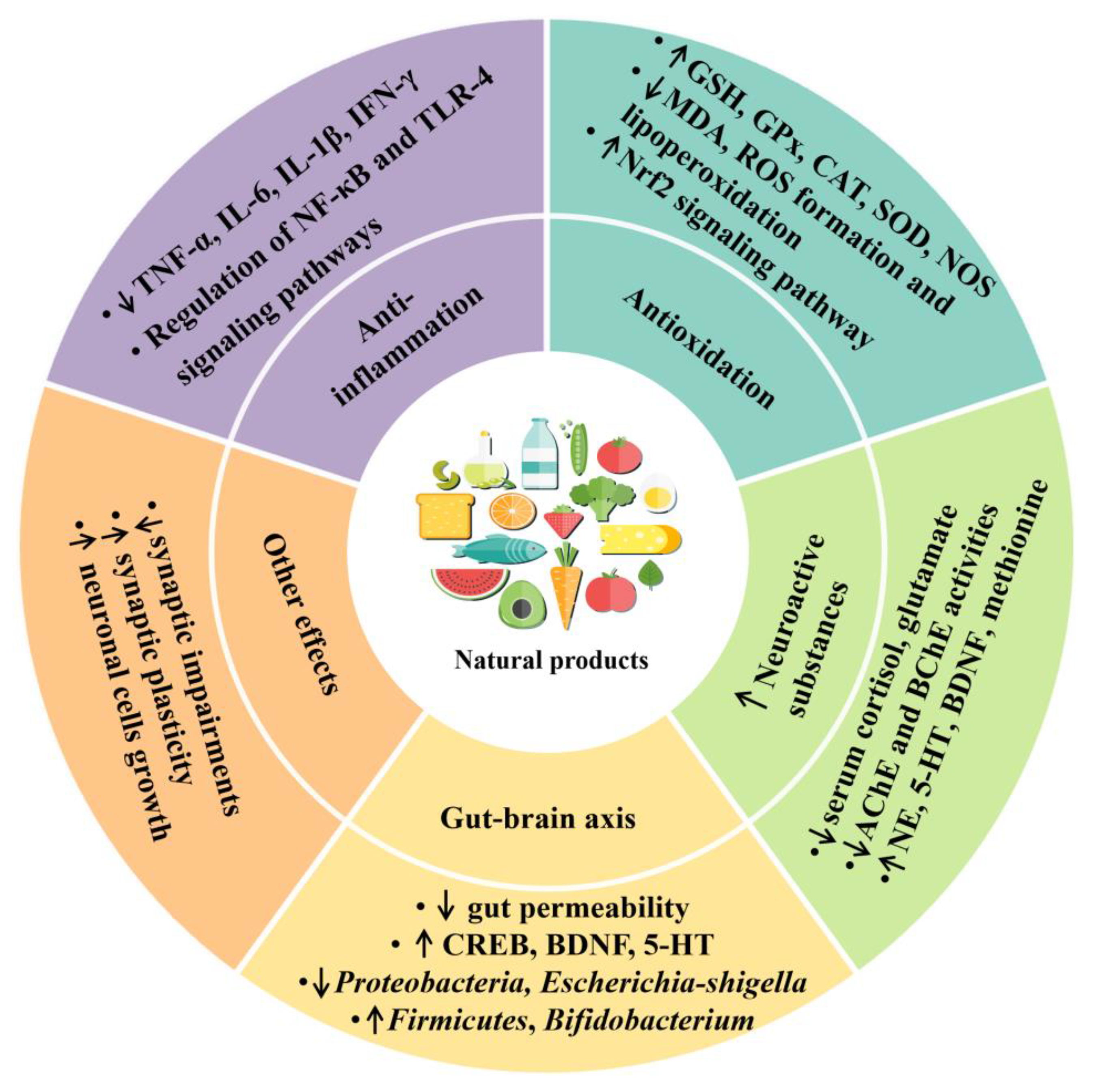
3.2.1. Anti-Inflammatory Effect
3.2.2. Antioxidant Effect
3.2.3. Modulation of Gut Microbiota
3.2.4. Regulation of Production of Neuroactive Substances
3.2.5. Other Mechanisms
| Name | Study Type | Model | Dose | Effects and Mechanisms | Ref. |
|---|---|---|---|---|---|
| Animal foods | |||||
| Honey | In Vivo | Wistar rats | 0.26, 0.31, 0.36 g/kg | Had protective effects against anxiety Decreased TNF-α, IL-6; | [20] |
| Plant foods | |||||
| Saffron | In Vivo | Rats | 30, 60 mg/kg | Exerted anxiolytic effect Downregulated serum cortisol level Upregulated BDNF in hippocampal; | [128] |
| Bergamot essential oil | In Vivo | SD rats | 200 mg/kg | Improved anxiety Decreased IL-1β, IL-6, TNF-α Enhanced the activity of GPx, CAT, SOD; | [111] |
| Red pomegranate fruit extract-based formula | In Vivo | C57BL/6J mice | 2.0, 1.5, 1.0 mg/g | Exerted anxiolytic effect Increased 5-HT in hippocampus Suppressed indoleamine-2,3-dioxygenase Improved tryptophan hydroxylase Reduced NF-κ B, TNF-α, IL-6, IL-1β, IFN-γ, MDA Promoted the activities of NOS, SOD and CAT; | [113] |
| Beverages | |||||
| Low-dose alcohol | In Vivo | C57BL/6 mice | 0.8 g/kg | Decreased anxiety-like behaviors Upregulated adiponectin Activated Nrf2 signaling pathway; | [117] |
| Breadfruit pulp | In Vivo | Zebrafish | NA | Exerted anxiolytic effect Regulated the serotoninergic system | [126] |
| Probiotics | |||||
| Lactobacillus sakei | In Vivo | Mice | OK67: 2 × 108, 1 × 109, 2 × 109 CFU/mouse PK16: 1 × 109, 5 × 109 CFU/mouse | Mitigated anxiety-like behaviors Inhibited the population of Proteobacteria Decreased fecal lipopolysaccharide levels Inhibited NF-κB Increased AMPK; | [121] |
| Lactococcus lactis WHH2078 | In Vivo | CUMS mice | 1 × 109 CFU/mouse | Decreased serum CORT Increased 5-HT, BDNF Restored abundances of Firmicutes and Bacteroidetes; | [120] |
| Escherichia coli Nissle 1917 | In Vivo | C57BL/6 mice | 0.5 × 1010, 1 × 1010 CFU/mouse | Attenuated anxiety-like behaviors Inhibited the pathologic gut–brain circuit; | [122] |
| Weissella paramesenteroides WpK4 | In Vivo | C57BL/6 mice | 1 × 108 CFU/mouse | Mitigated anxiety-related behaviors Decreased gut permeability Regulation of gut–brain axis; | [124] |
| Pediococcus acidilactici CCFM6432 | In Vivo | C57BL/6 mice | 5 × 109 CFU/mouse | Attenuated anxiety-like behaviors Improved the gut microbial composition Inhibited hyperactivity of HPA axis Upregulated phosphorylated CREB; | [123] |
| Nutrients | |||||
| Caffeine and caffeic acid | In Vivo | Swiss albino mice | Caffeine: 15 mg/kg Caffeine + caffeic acid: 10 mg/kg + 5 mg/kg | Attenuated anxiety-like behaviors Decreased inflammatory markers levels; | [110] |
| Sesamol | In Vivo | C57BL/6J mice | 100 mg/kg | Reduced anxiety-like behaviors Decreased neuroinflammatory responses Inhibition of TLR-4/NF-κB pathway Stimulated Nrf2 signaling pathway Increased BDNF, NE, 5-HT Repaired synaptic impairments Regulated BDNF/TrkB/CREB signaling pathway; | [109] |
| Curcumin | In Vivo | Rats | 0.1, 0.5% | Low doses: exerted anxiolytic effect, improved the synaptic plasticity, enhanced neural circuits High doses: reversed anxiolytic effect, induced neuroinflammation; | [131] |
| Curcumin | In Vivo | Swiss albino mice | 20, 40, 80, 160 mg/kg | Ameliorated anxiety-like behaviors Promoted the production of neuronal cells Suppressed neuroinflammation; | [135] |
| n-3 PUFA | In Vivo | Wistar rats | 500 mg/kg | Attenuated obesity-induced anxiety Exerted anti-inflammatory effect Decreased IL-6, TNF-α; | [112] |
| Blumea lacera leaf methanol extract | In Vivo | Swiss albino mice | 200, 400 mg/kg | Mitigated anxiety-like behaviors Suppressed ROS formation; | [116] |
| Chamomile decoction | In Vivo | Wistar rats | 100 mg/kg | Ameliorated high fat diet-induced anxiety Suppressed lipoperoxidation Promoted antioxidant enzymes activities Inhibited AChE, BChE; | [118] |
| Goat milk fat | In Vivo | Wistar rats | NA | Palliated anxiety-like behaviors Decreased MDA Increased GSH; | [114] |
| Tangeretin | In Vivo | Rats | 100, 200 mg/kg | Decreased anxiety-like behaviors Activated Nrf2; | [115] |
| Panaxynol | In Vivo | CUMS mice | 1.0 mg/kg | Improved CUMS-induced anxiety symptoms Suppressed the HPA axis hyperfunction Increased the release of 5-HT Promoted synaptic plasticity; | [127] |
| L-theanine | In Vivo | WKY rats | 0.4 mg/kg | Attenuated anxiety-related behaviors Decreased glutamate levels Increased methionine levels Improved hippocampal activity; | [129] |
| Queen bee acid | In Vivo | SD rats | 12, 24 mg/kg | Decreased anxiety-like behaviors Promoted the growth of neurons; | [130] |
3.3. Clinical Trials
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abautret-Daly, A.; Dempsey, E.; Parra-Blanco, A.; Medina, C.; Harkin, A. Gut-brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease. Acta Neuropsychiatr. 2018, 30, 275–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, A.J.; Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Ashbaugh, C.; Erskine, H.E.; Charlson, F.J.; Degenhardt, L.; Scott, J.G.; McGrath, J.J.; et al. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- Friedrich, M.J. Depression is the leading cause of disability around the world. JAMA 2017, 317, 1517. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Dolcetti, E.; Rizzo, F.R.; Fresegna, D.; Musella, A.; Gentile, A.; De Vito, F.; Caioli, S.; Guadalupi, L.; Bullitta, S.; et al. Inflammation-associated synaptic alterations as shared threads in depression and multiple sclerosis. Front. Cell. Neurosci. 2020, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, L.; O’Donnell, S.; McRae, L.; Grenier, J. The burden of generalized anxiety disorder in Canada. Health Promot. Chronic Dis. Prev. Can.-Res. Policy Pract. 2017, 37, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Rothenberg, D.O.N.; Zhang, L. Mechanisms underlying the anti-depressive effects of regular tea consumption. Nutrients 2019, 11, 1361. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Gan, R.-Y.; Li, B.-Y.; Mao, Q.-Q.; Shang, A.; Xu, X.-Y.; Li, H.-Y.; Li, H.-B. Effects and mechanisms of tea on parkinson’s disease, alzheimer’s disease and depression. Food Rev. Int. 2021, in press. [CrossRef]
- Methiwala, H.N.; Vaidya, B.; Addanki, V.K.; Bishnoi, M.; Sharma, S.S.; Kondepudi, K.K. Gut microbiota in mental health and depression: Role of pre/pro/synbiotics in their modulation. Food Funct. 2021, 12, 4284–4314. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Murphy, A.B.; Cryan, J.F.; Ross, P.R.; Dinan, T.G.; Stanton, C. Microbiome in brain function and mental health. Trends Food Sci. Technol. 2016, 57, 289–301. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Z.F.; Cheng, L.; Zhang, X.; Yang, H.N. The role of the intestinal microbiota in the pathogenesis of host depression and mechanism of TPs relieving depression. Food Funct. 2021, 12, 7651–7663. [Google Scholar] [CrossRef]
- Lee, A.R.Y.B.; Tariq, A.; Lau, G.; Tok, N.W.K.; Tam, W.W.S.; Ho, C.S.H. Vitamin E, alpha-tocopherol, and its effects on depression and anxiety: A systematic review and meta-analysis. Nutrients 2022, 14, 656. [Google Scholar] [CrossRef] [PubMed]
- Stroehle, A.; Gensichen, J.; Domschke, K. The diagnosis and treatment of anxiety disorders. Dtsch. Arztebl. Int. 2018, 115, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Hou, Y.; Wang, D.; Zhao, X. Flavonoids for depression and anxiety: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, in press. [CrossRef]
- Wang, S.-J.; Chen, M.-Y. The effects of sunlight exposure therapy on the improvement of depression and quality of life in post-stroke patients: A RCT study. Heliyon 2020, 6, e04379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-L.; Jiang, W.-T.; Wang, X.; Cai, Z.-D.; Liu, Z.-H.; Liu, G.-R. Exercise, brain plasticity, and depression. CNS Neurosci. Ther. 2020, 26, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Gong, Q.; Huang, C.; Guo, F.; Li, W.; Wang, Y.; Cheng, X. The relationship between sunlight exposure duration and depressive symptoms: A cross-sectional study on elderly Chinese women. PLoS ONE 2021, 16, e0254856. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Luo, M.; Huang, S.-Y.; Saimaiti, A.; Shang, A.; Gan, R.-Y.; Li, H.-B. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxidative Med. Cell. Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef]
- Shang, A.; Li, J.; Zhou, D.-D.; Gan, R.-Y.; Li, H.-B. Molecular mechanisms underlying health benefits of tea compounds. Free Radic. Biol. Med. 2021, 172, 181–200. [Google Scholar] [CrossRef]
- Fang, J.L.; Luo, Y.; Jin, S.H.; Yuan, K.; Guo, Y. Ameliorative effect of anthocyanin on depression mice by increasing monoamine neurotransmitter and up-regulating BDNF expression. J. Funct. Foods 2020, 66, 103757. [Google Scholar] [CrossRef]
- Adeniyi, I.A.; Babalola, K.T.; Adekoya, V.A.; Oyebanjo, O.; Ajayi, A.M.; Onasanwo, S.A. Neuropharmacological effects of honey in lipopolysaccharide-induced neuroinflammation, cognitive impairment, anxiety and motor impairment. Nutr. Neurosci. 2022, in press. [CrossRef]
- Milaneschi, Y.; Lamers, F.; Berk, M.; Penninx, B.W.J.H. Depression heterogeneity and its biological underpinnings: Toward immunometabolic depression. Biol. Psychiatry 2020, 88, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Upton, N. Developing our understanding of nutrition in depression. Br. J. Nutr. 2022, 127, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Businaro, R.; Vauzour, D.; Sarris, J.; Munch, G.; Gyengesi, E.; Brogelli, L.; Zuzarte, P. Therapeutic opportunities for food supplements in neurodegenerative disease and depression. Front. Nutr. 2021, 8, 669846. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.P.; Gao, Q.; Gwee, X.; Chua, D.Q.L. Tea consumption and depression from follow up in the singapore longitudinal ageing study. J. Nutr. Health Aging 2021, 25, 295–301. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J. Green tea, coffee, and caffeine consumption are inversely associated with self-report lifetime depression in the korean population. Nutrients 2018, 10, 1201. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Y.; Ju, Y.; Cui, L.L.; Liu, T.; Hou, Y.Y.; Wu, Q.; Ojo, O.; Du, X.J.; Wang, X.H. Association between dietary fiber intake and incidence of depression and anxiety in patients with essential hypertension. Nutrients 2021, 13, 4159. [Google Scholar] [CrossRef]
- de Almeida, T.L.F.; Petarli, G.B.; Cattafesta, M.; Zandonade, E.; Bezerra, O.; Tristao, K.G.; Salaroli, L.B. Association of selenium intake and development of depression in Brazilian farmers. Front. Nutr. 2021, 8, 671377. [Google Scholar] [CrossRef]
- Saghafian, F.; Malmir, H.; Saneei, P.; Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Fruit and vegetable consumption and risk of depression: Accumulative evidence from an updated systematic review and meta-analysis of epidemiological studies. Br. J. Nutr. 2018, 119, 1087–1101. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.S.; Byeon, S.; Shin, D.M. Sources of dietary fiber are differently associated with prevalence of depression. Nutrients 2020, 12, 2813. [Google Scholar] [CrossRef]
- Arab, L.; Guo, R.; Elashoff, D. Lower depression scores among walnut consumers in nhanes. Nutrients 2019, 11, 275. [Google Scholar] [CrossRef]
- Yang, Y.J.; Je, Y.J. Fish consumption and depression in Korean adults: The Korea National Health and Nutrition Examination Survey, 2013–2015. Eur. J. Clin. Nutr. 2018, 72, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Villegas, A.; Alvarez-Perez, J.; Toledo, E.; Salas-Salvado, J.; Ortega-Azorin, C.; Zomeno, M.D.; Vioque, J.; Martinez, J.A.; Romaguera, D.; Perez-Lopez, J.; et al. Seafood consumption, omega-3 fatty acids intake, and life-time prevalence of depression in the PREDIMED-plus trial. Nutrients 2018, 10, 2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, A.M.; Abasheva, D.; Martinez-Gonzalez, M.A.; Ruiz-Estigarribia, L.; Martin-Calvo, N.; Sanchez-Villegas, A.; Toledo, E. Coffee consumption and the risk of depression in a middle-aged cohort: The SUN project. Nutrients 2018, 10, 1333. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Zhang, M.; Dong, S.-S.; Wang, J.-H.; Zhang, K.; Guo, J.; Guo, Y.; Yang, T.-L. Bidirectional two-sample Mendelian randomization analysis identifies causal associations between relative carbohydrate intake and depression. Nat. Hum. Behav. 2022, in press. [CrossRef] [PubMed]
- Anjom-Shoae, J.; Sadeghi, O.; Keshteli, A.H.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Legume and nut consumption in relation to depression, anxiety and psychological distress in Iranian adults. Eur. J. Nutr. 2020, 59, 3635–3645. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Huang, X.Y.; Xiao, Y.; Jing, D.R.; Huang, Y.Z.; Chen, L.P.; Luo, D.; Chen, X.; Shen, M.X. Daily intake of soft drinks is associated with symptoms of anxiety and depression in Chinese adolescents. Public Health Nutr. 2019, 22, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Villegas, A.; Zazpe, I.; Santiago, S.; Perez-Cornago, A.; Martinez-Gonzalez, M.A.; Lahortiga-Ramos, F. Added sugars and sugar-sweetened beverage consumption, dietary carbohydrate index and depression risk in the Seguimiento Universidad de Navarra (SUN) Project. Br. J. Nutr. 2018, 119, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Wang, T.; Yu, Y.; Li, M.; Sun, X.; Song, W.; Wang, Y.; Zhang, C.; Fu, F. The etiology of poststroke-depression: A hypothesis involving HPA axis. Biomed. Pharmacother. 2022, 151, 113146. [Google Scholar] [CrossRef]
- Ren, Y.F.; Sun-Waterhouse, D.; Ouyang, F.X.; Tan, X.T.; Li, D.P.; Xu, L.H.; Li, B.; Wang, Y.L.; Li, F. Apple phenolic extracts ameliorate lead-induced cognitive impairment and depression- and anxiety-like behavior in mice by abating oxidative stress, inflammation and apoptosis via the miR-22-3p/SIRT1 axis. Food Funct. 2022, 13, 2647–2661. [Google Scholar] [CrossRef]
- Jeon, S.W.; Kim, Y.-K. Inflammation-induced depression: Its pathophysiology and therapeutic implications. J. Neuroimmunol. 2017, 313, 92–98. [Google Scholar] [CrossRef]
- Norris, G.T.; Kipnis, J. Immune cells and CNS physiology: Microglia and beyond. J. Exp. Med. 2019, 216, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Borkowski, K.; Newman, J.W.; Park, Y.S. N-3 PUFA improved post-menopausal depression induced by maternal separation and chronic mild stress through serotonergic pathway in rats-effect associated with lipid mediators. J. Nutr. Biochem. 2021, 91, 108599. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.F.; Lu, K.; Lin, P.C.; Che, H.X.; Li, H.Y.; Song, L.; Yang, X.H.; Xie, W.C. Saccharina japonica ethanol extract ameliorates depression/anxiety-like behavior by inhibiting inflammation, oxidative stress, and apoptosis in dextran sodium sulfate induced ulcerative colitis mice. Front. Nutr. 2021, 8, 784532. [Google Scholar] [CrossRef]
- Huang, F.R.; Liu, X.J.; Xu, S.; Hu, S.T.; Wang, S.S.; Shi, D.B.; Wang, K.C.; Wang, Z.X.; Lin, Q.Q.; Li, S.; et al. Prevotella histicola mitigated estrogen deficiency-induced depression via gut microbiota-dependent modulation of inflammation in ovariectomized mice. Front. Nutr. 2022, 8, 805465. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.W.; Kim, J.K.; Han, M.J.; Kim, D.H. Lacticaseibacillus paracasei NK112 mitigates Escherichia coli-induced depression and cognitive impairment in mice by regulating IL-6 expression and gut microbiota. Benef. Microbes 2021, 12, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Xu, S.; Chen, X.H.; Wang, L.; Li, J.Y.; Li, G.J.; Zhang, B.L. Antidepressant effect of EGCG through the inhibition of hippocampal neuroinflammation in chronic unpredictable mild stress-induced depression rat model. J. Funct. Foods 2020, 73, 104106. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Ou, S.S.; Fang, Y.C.; Tang, H.; Wu, T.; Chen, L.Z.; Jiang, M.; Zhou, L.Q.; Xu, J.; Guo, K.H. Lycopene protects neuroblastoma cells against oxidative damage via depression of ER stress. J. Food Sci. 2020, 85, 3552–3561. [Google Scholar] [CrossRef]
- Moosa, A.; Farzand, A.; Ahmad, T.; Khan, S.A.; Gleason, M.L.; Abbas, H.; Khan, W.A.; Jabbar, A.; Mohsan, M. First report of fusarium rot of phalsa (Grewia asiatica) caused by Fusarium solani in Pakistan. Plant Dis. 2019, 103, 2476–2477. [Google Scholar] [CrossRef]
- Imran, I.; Javaid, S.; Waheed, A.; Rasool, M.F.; Majeed, A.; Samad, N.; Saeed, H.; Alqahtani, F.; Ahmed, M.M.; Alaqil, F.A. Grewia asiatica berry juice diminishes anxiety, depression, and scopolamine-induced learning and memory impairment in behavioral experimental animal models. Front. Nutr. 2021, 7, 587367. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Sobolev, A.P.; Nabavi, S.F.; Sureda, A.; Moghaddam, A.H.; Khanjani, S.; Di Giovanni, C.; Xiao, J.B.; Shirooie, S.; Sokeng, A.J.T.; et al. Antidepressive effects of a chemically characterized maqui berry extract (Aristotelia chilensis (Molina) Stuntz) in a mouse model of post-stroke depression. Food Chem. Toxicol. 2019, 129, 434–443. [Google Scholar] [CrossRef]
- Cigliano, L.; Spagnuolo, M.S.; Boscaino, F.; Ferrandino, I.; Monaco, A.; Capriello, T.; Cocca, E.; Iannotta, L.; Treppiccione, L.; Luongo, D.; et al. Dietary supplementation with fish oil or conjugated linoleic acid relieves depression markers in mice by modulation of the nrf2 pathway. Mol. Nutr. Food Res. 2019, 63, 1900243. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.V.; D’Almeida, P.L.; Virmani, G.; Bathini, P.; Alberi, L. Effects of monoamines and antidepressants on astrocyte physiology: Implications for monoamine hypothesis of depression. J. Exp. Neurosci. 2018, 12, 1179069518789149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.M.; Ce, Q.; Jin, L.; Zheng, J.; Sun, M.; Tang, X.; Li, D.; Sun, J. Deoiled sunflower seeds ameliorate depression by promoting the production of monoamine neurotransmitters and inhibiting oxidative stress. Food Funct. 2021, 12, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Li, G.J.; Yang, J.; Wang, X.; Zhou, C.; Zheng, X.Y.; Lin, W.H. Effects of EGCG on depression-related behavior and serotonin concentration in a rat model of chronic unpredictable mild stress. Food Funct. 2020, 11, 8780–8787. [Google Scholar] [CrossRef]
- Zhang, L.L.; Yang, Z.Y.; Ren, J.N.; Fan, G.; Pan, S.Y. Dietary essential oil from navel orange alleviates depression in reserpine-treated mice by monoamine neurotransmitters. Flavour Frag. J. 2019, 34, 252–259. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, P.Y.; Pan, D.D.; Zeng, X.Q.; Guo, Y.X.; Zhao, G.S. Effect of adzuki bean sprout fermented milk enriched in gamma-aminobutyric acid on mild depression in a mouse model. J. Dairy Sci. 2021, 104, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Sahay, A.; Kale, A.; Joshi, S. Role of neurotrophins in pregnancy and offspring brain development. Neuropeptides 2020, 83, 102075. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Lu, K.H.; Lin, Y.E.; Panyod, S.; Wu, H.Y.; Chang, W.T.; Sheen, L.Y. Garlic essential oil mediates acute and chronic mild stress-induced depression in rats via modulation of monoaminergic neurotransmission and brain-derived neurotrophic factor levels. Food Funct. 2019, 10, 8094–8105. [Google Scholar] [CrossRef]
- Lim, D.W.; Han, T.; Um, M.Y.; Yoon, M.; Kim, T.E.; Kim, Y.T.; Han, D.; Lee, J.; Lee, C.H. Administration of Asian herb bennet (Geum japonicum) extract reverses depressive -like behaviors in mouse model of depression induced by corticosterone. Nutrients 2019, 11, 2841. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Jia, M.; Fu, S.; Pan, J.; Chu, C.; Liu, X.; Liu, X.; Liu, Z. (+)-Sesamin attenuates chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits via suppression of neuroinflammation. J. Nutr. Biochem. 2019, 64, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Aygun, H.; Akin, A.T.; Kizilaslan, N.; Sumbul, O.; Karabulut, D. Probiotic supplementation alleviates absence seizures and anxiety- and depression-like behavior in WAG/Rij rat by increasing neurotrophic factors and decreasing proinflammatory cytokines. Epilepsy Behav. 2022, 128, 108588. [Google Scholar] [CrossRef] [PubMed]
- Bin-Jaliah, I.; Al-Hash, F.H.; Abbas, A.M.; Nasif, K.A.; Sakr, H.F. Effect of resveratrol on neurotrophic factors and depression-like behavior in a rat model of male hypogonadism. Int. J. Pharmacol. 2021, 17, 38–47. [Google Scholar] [CrossRef]
- Bao, A.-M.; Swaab, D.F. The human hypothalamus in mood disorders: The HPA axis in the center. IBRO Rep. 2019, 6, 45–53. [Google Scholar] [CrossRef]
- Wang, C.C.; Du, L.; Shi, H.H.; Ding, L.; Yanagita, T.; Xue, C.H.; Wang, Y.M.; Zhang, T.T. Dietary EPA-enriched phospholipids alleviate chronic stress and LPS-induced depression- and anxiety-like behavior by regulating immunity and neuroinflammation. Mol. Nutr. Food Res. 2021, 65, 2100009. [Google Scholar] [CrossRef]
- Jia, D.; Dou, Y.H.; He, Y.S.; Zhou, X.X.; Gao, Y.; Ma, M.; Wu, Z.Z.; Li, W.M. Saponin extract of Baihe-Zhimu Tang ameliorates depression in chronic mild stress rats. J. Funct. Foods 2020, 68, 103905. [Google Scholar] [CrossRef]
- Choi, J.E.; Kim, E.Y.; Park, Y. N-3 PUFA improved pup separation-induced postpartum depression via serotonergic pathway regulated by miRNA. J. Nutr. Biochem. 2020, 84, 108417. [Google Scholar] [CrossRef]
- Iegaki, N.; Narita, Y.; Hattori, N.; Hirata, Y.; Ichihara, K. Royal jelly reduces depression-like behavior through possible effects on adrenal steroidogenesis in a murine model of unpredictable chronic mild stress. Biosci. Biotechnol. Biochem. 2020, 84, 606–612. [Google Scholar] [CrossRef]
- Tao, J.; Li, S.; Gan, R.-Y.; Zhao, C.-N.; Meng, X.; Li, H.-B. Targeting gut microbiota with dietary components on cancer: Effects and potential mechanisms of action. Crit. Rev. Food Sci. Nutr. 2020, 60, 1025–1037. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: A narrative review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Li, B.; Mao, Q.; Zhou, D.; Luo, M.; Gan, R.; Li, H.; Huang, S.; Saimaiti, A.; Shang, A.; Li, H. Effects of tea against alcoholic fatty liver disease by modulating gut microbiota in chronic alcohol-exposed mice. Foods 2021, 10, 1232. [Google Scholar] [CrossRef]
- Li, B.-Y.; Xu, X.-Y.; Gan, R.-Y.; Sun, Q.-C.; Meng, J.-M.; Shang, A.; Mao, Q.-Q.; Li, H.-B. Targeting gut microbiota for the prevention and management of diabetes mellitus by dietary natural products. Foods 2019, 8, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhou, D.-D.; Shang, A.; Gan, R.-Y.; Li, H.-B. Influences of food contaminants and additives on gut microbiota as well as protective effects of dietary bioactive compounds. Trends Food Sci. Technol. 2021, 113, 180–192. [Google Scholar] [CrossRef]
- Cao, S.-Y.; Zhao, C.-N.; Xu, X.-Y.; Tang, G.-Y.; Corke, H.; Gan, R.-Y.; Li, H.-B. Dietary plants, gut microbiota, and obesity: Effects and mechanisms. Trends Food Sci. Technol. 2019, 92, 194–204. [Google Scholar] [CrossRef]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Ngo, A.L.; Simopoulos, T.; Kaye, A.D.; Colontonio, M.M.; et al. Gut microbiome dysbiosis and depression: A comprehensive review. Curr. Pain Headache Rep. 2020, 24, 36. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, S.; Yang, X.; Li, W.; Si, J.; Yang, X. The antidepressant potential of Lactobacillus casei in the postpartum depression rat model mediated by the microbiota-gut-brain axis. Neurosci. Lett. 2022, 774, 136474. [Google Scholar] [CrossRef]
- Gu, F.; Wu, Y.Y.; Liu, Y.; Dou, M.; Jiang, Y.S.; Liang, H. Lactobacillus casei improves depression-like behavior in chronic unpredictable mild stress-induced rats by the BDNF-TrkB signal pathway and the intestinal microbiota. Food Funct. 2020, 11, 6148–6157. [Google Scholar] [CrossRef]
- Tian, P.J.; Wang, G.; Zhao, J.X.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef]
- Sun, Y.; Geng, W.T.; Pan, Y.J.; Wang, J.J.; Xiao, P.; Wang, Y.P. Supplementation with Lactobacillus kefiranofaciens ZW3 from Tibetan Kefir improves depression- like behavior in stressed mice by modulating the gut microbiota. Food Funct. 2019, 10, 925–937. [Google Scholar] [CrossRef]
- Yun, S.W.; Kim, J.K.; Lee, K.E.; Oh, Y.J.; Choi, H.J.; Han, M.J.; Kim, D.H. A probiotic Lactobacillus gasseri alleviates Escherichia coli-induced cognitive impairment and depression in mice by regulating IL-1 beta expression and gut microbiota. Nutrients 2020, 12, 3441. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.G.; Li, L.; Ma, S.B.; Ye, J.; Zhang, H.B.; Li, Y.T.; Sair, A.T.; Pan, J.R.; Liu, X.N.; Li, X.; et al. High-dietary fiber intake alleviates antenatal obesity-induced postpartum depression: Roles of gut microbiota and microbial metabolite short-chain fatty acid involved. J. Agric. Food Chem. 2020, 68, 13697–13710. [Google Scholar] [CrossRef]
- Song, J.; Zhou, N.A.; Ma, W.N.; Gu, X.Y.; Chen, B.Z.; Zeng, Y.; Yang, L.; Zhou, M.M. Modulation of gut microbiota by chlorogenic acid pretreatment on rats with adrenocorticotropic hormone induced depression-like behavior. Food Funct. 2019, 10, 2947–2957. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Joo, M.K.; Kim, J.K.; Jeung, W.; Kang, H.; Kim, D.H. Bifidobacteria-fermented red ginseng and its constituents ginsenoside Rd and protopanaxatriol alleviate anxiety/depression in mice by the amelioration of gut dysbiosis. Nutrients 2020, 12, 901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braidy, N.; Liu, Y. Can nicotinamide riboside protect against cognitive impairment? Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.S.; Liu, Y.; Gao, M.Q.; Xue, M.L.; Wang, Z.L.; Liang, H. Nicotinamide riboside alleviates alcohol-induced depression-like behaviours in C57BL/6J mice by altering the intestinal microbiota associated with microglial activation and BDNF expression. Food Funct. 2020, 11, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, X.J.; Ma, Y.H.; Li, X.; Zhang, J.F.; Zhao, L.C. Supplementation with soy isoflavones alleviates depression-like behaviour via reshaping the gut microbiota structure. Food Funct. 2021, 12, 4995–5006. [Google Scholar] [CrossRef]
- Hao, W.Z.; Ma, Q.Y.; Tao, G.; Huang, J.Q.; Chen, J.X. Oral coniferyl ferulate attenuated depression symptoms in mice via reshaping gut microbiota and microbial metabolism. Food Funct. 2021, 12, 12550–12564. [Google Scholar] [CrossRef]
- Chen, Y.L.; Xiao, N.; Chen, Y.X.; Chen, X.Y.; Zhong, C.F.; Cheng, Y.Y.; Du, B.; Li, P. Semen Sojae Praeparatum alters depression-like behaviors in chronic unpredictable mild stress rats via intestinal microbiota. Food Res. Int. 2021, 150, 110808. [Google Scholar] [CrossRef]
- Maehata, H.; Kobayashi, Y.; Mitsuyama, E.; Kawase, T.; Kuhara, T.; Xiao, J.Z.; Tsukahara, T.; Toyoda, A. Heat-killed Lactobacillus helveticus strain MCC1848 confers resilience to anxiety or depression-like symptoms caused by subchronic social defeat stress in mice. Biosci. Biotechnol. Biochem. 2019, 83, 1239–1247. [Google Scholar] [CrossRef]
- Chen, S.X.; Guo, W.Y.; Qi, X.X.; Zhou, J.Y.; Liu, Z.Q.; Cheng, Y.Y. Natural alkaloids from lotus plumule ameliorate lipopolysaccharide-induced depression-like behavior: Integrating network pharmacology and molecular mechanism evaluation. Food Funct. 2019, 10, 6062–6073. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.Q.; Li, Y.; Lan, T.; Wang, W.J.; Mao, X.Q.; Yu, S.Y. Prophylactic treatment of curcumin in a rat model of depression by attenuating hippocampal synaptic loss. Food Funct. 2021, 12, 11202–11213. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.-G.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health benefits and side effects of short-chain fatty acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-F.; Wang, C.-Y.; Wang, J.-H.; Wang, Q.-N.; Li, S.-J.; Wang, H.-O.; Zhou, F.; Li, J.-M. Short-chain fatty acids ameliorate depressive-like behaviors of high fructose-fed mice by rescuing hippocampal neurogenesis decline and blood-brain barrier damage. Nutrients 2022, 14, 1882. [Google Scholar] [CrossRef]
- Paderin, N.M.; Popov, S.V. The effect of dietary pectins on object recognition memory, depression-like behaviour, and IL-6 in mouse hippocampi. J. Funct. Foods 2018, 43, 131–138. [Google Scholar] [CrossRef]
- Gong, W.; Zhou, Y.; Gong, W.; Qin, X. Coniferyl ferulate exerts antidepressant effect via inhibiting the activation of NMDAR-CaMKII-MAPKs and mitochondrial apoptotic pathways. J. Ethnopharmacol. 2020, 251, 112533. [Google Scholar] [CrossRef]
- Johnston, C.S.; Jasbi, P.; Jin, Y.; Bauer, S.; Williams, S.; Fessler, S.N.; Gu, H.W. Daily vinegar ingestion improves depression scores and alters the metabolome in healthy adults: A randomized controlled trial. Nutrients 2021, 13, 4020. [Google Scholar] [CrossRef]
- Poorbaferani, F.; Rouhani, M.H.; Heidari, Z.; Poorbaferani, M.; Safavi, S.M. Flaxseed oil supplementation on severity of depression and brain-derived neurotrophic factor: A randomized, double blind placebo controlled clinical trial. Int. J. Food Prop. 2020, 23, 1518–1526. [Google Scholar] [CrossRef]
- Xue, L.L.; Zhang, J.; Shen, H.X.; Ai, L.; Wu, R.R. A randomized controlled pilot study of the effectiveness of magnolia tea on alleviating depression in postnatal women. Food Sci. Nutr. 2020, 8, 1554–1561. [Google Scholar] [CrossRef] [Green Version]
- Fisk, J.; Khalid, S.; Reynolds, S.A.; Williams, C.M. Effect of 4 weeks daily wild blueberry supplementation on symptoms of depression in adolescents. Br. J. Nutr. 2020, 124, 181–188. [Google Scholar] [CrossRef]
- Zhang, X.M.; Chen, S.B.; Zhang, M.; Ren, F.Z.; Ren, Y.M.; Li, Y.X.; Liu, N.; Zhang, Y.; Zhang, Q.; Wang, R. Effects of fermented milk containing Lacticaseibacillus paracasei strain shirota on constipation in patients with depression: A randomized, double-blind, placebo-controlled trial. Nutrients 2021, 13, 2238. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kim, S.H.; Jang, C.S.; Kim, S.I.; Kweon, C.O.; Kim, B.W.; Ryu, J.K. The combined effects of yogurt and exercise in healthy adults: Implications for biomarkers of depression and cardiovascular diseases. Food Sci. Nutr. 2018, 6, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Corbi, G.; Maes, M.; Scapagnini, G.; Davinelli, S. Exploring the impact of flavonoids on symptoms of depression: A systematic review and meta-analysis. Antioxidants 2021, 10, 1644. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Lane, M.; Rocks, T.; Ruusunen, A.; Loughman, A.; Lopresti, A.; Marshall, S.; Berk, M.; Jacka, F.; Dean, O.M. Effect of saffron supplementation on symptoms of depression and anxiety: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Koochakpoor, G.; Salari-Moghaddam, A.; Keshteli, A.H.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Dietary intake of branched-chain amino acids in relation to depression, anxiety and psychological distress. Nutr. J. 2021, 20, 11. [Google Scholar] [CrossRef]
- Kafeshani, M.; Feizi, A.; Esmaillzadeh, A.; Keshteli, A.H.; Afshar, H.; Roohafza, H.; Adibi, P. Higher vitamin B-6 intake is associated with lower depression and anxiety risk in women but not in men: A large cross-sectional study. Int. J. Vitam. Nutr. Res. 2020, 90, 484–492. [Google Scholar] [CrossRef]
- Natacci, L.; Marchioni, D.M.; Goulart, A.C.; Nunes, M.A.; Moreno, A.B.; Cardoso, L.O.; Giatti, L.; Molina, M.D.B.; Santos, I.S.; Brunoni, A.R.; et al. Omega 3 consumption and anxiety disorders: A cross-sectional analysis of the Brazilian longitudinal study of adult health (ELSA-Brasil). Nutrients 2018, 10, 663. [Google Scholar] [CrossRef] [Green Version]
- Dobersek, U.; Wy, G.; Adkins, J.; Altmeyer, S.; Krout, K.; Lavie, C.J.; Archer, E. Meat and mental health: A systematic review of meat abstention and depression, anxiety, and related phenomena. Crit. Rev. Food Sci. Nutr. 2021, 61, 622–635. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.; Liu, X.N.; Li, X.H.; Wang, Y.T.; Wang, D.N.; Kou, R.W.; Zhang, L.; Shi, R.J.; Ye, J.; Bo, X.W.; et al. Sesamol ameliorates dextran sulfate sodium-induced depression-like and anxiety-like behaviors in colitis mice: The potential involvement of the gut-brain axis. Food Funct. 2022, 13, 2865–2883. [Google Scholar] [CrossRef]
- Mudgal, J.; Mallik, S.B.; Nampoothiri, M.; Kinra, M.; Hall, S.; Grant, G.D.; Anoopkumar-Dukie, S.; Davey, A.K.; Rao, C.M.; Arora, D. Effect of coffee constituents, caffeine and caffeic acid on anxiety and lipopolysaccharide-induced sickness behavior in mice. J. Funct. Foods 2020, 64, 103638. [Google Scholar] [CrossRef]
- Cui, Y.H.; Che, Y.; Wang, H.X. Bergamot essential oil attenuate aluminum-induced anxiety-like behavior through antioxidation, anti-inflammatory and GABA regulation in rats. Food Chem. Toxicol. 2020, 145, 111766. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.; Jantsch, J.; de Oliveira, S.; Braga, M.F.; Castro, L.F.D.; Diniz, B.F.; Moreira, J.C.F.; Giovenardi, M.; Porawski, M.; Guedes, R.P. DHA/EPA supplementation decreases anxiety-like behaviour, but it does not ameliorate metabolic profile in obese male rats. Br. J. Nutr. 2022, 128, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.P.; Sun-Waterhouse, D.; Cui, C. A red pomegranate fruit extract-based formula ameliorates anxiety/depression-like behaviors via enhancing serotonin (5-HT) synthesis in C57BL/6 male mice. Food Sci. Hum. Wellness 2021, 10, 289–296. [Google Scholar] [CrossRef]
- Barbosa, M.Q.; Queiroga, R.; Bertozzo, C.; Araujo, D.F.D.; Oliveira, L.I.G.; Silva, J.Y.P.; Bomfim, M.A.D.; Guerra, G.C.B.; Costa, S.; Bessa, R.; et al. Effect of diets with goat milk fat supplemented with exercise on anxiety and oxidative stress in the brains of adult rats. Food Funct. 2018, 9, 2891–2901. [Google Scholar] [CrossRef]
- Yan, H.; Gao, H. Tangeretin improves anxiety-like behaviors in a rat model of post-traumatic stress disorder. Curr. Top. Nutraceutical Res. 2022, 20, 370–376. [Google Scholar] [CrossRef]
- Hossen, M.A.; Reza, A.; Amin, M.B.; Nasrin, M.S.; Khan, T.A.; Rajib, M.H.R.; Tareq, A.; Haque, M.A.; Rahman, M.A.; Haque, M.A. Bioactive metabolites of Blumea lacera attenuate anxiety and depression in rodents and computer-aided model. Food Sci. Nutr. 2021, 9, 3836–3851. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, M.; Cheng, S.L.; Li, F.; Zhang, B.Y.; Sun, X.M.; Hu, H.J.; Chen, L.N.; Zhao, Z.H.; Hu, H.; et al. Low-dose alcohol ameliorated high fat diet-induced anxiety-related behavior via enhancing adiponectin expression and activating the Nrf2 pathway. Food Funct. 2021, 12, 241–251. [Google Scholar] [CrossRef]
- Jabri, M.A.; Rtibi, K.; Sebai, H. Chamomile decoction mitigates high fat diet-induced anxiety-like behavior, neuroinflammation and cerebral ROS overload. Nutr. Neurosci. 2022, 25, 1350–1361. [Google Scholar] [CrossRef]
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The role of the gut microbiota in dietary interventions for depression and anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Farzi, A.; Ke, X.Q.; Yu, Y.X.; Chen, C.L.; Chen, S.; Yu, T.F.; Wang, H.F.; Li, Y.J. Oral administration of Lactococcus lactis WHH2078 alleviates depressive and anxiety symptoms in mice with induced chronic stress. Food Funct. 2022, 13, 957–969. [Google Scholar] [CrossRef]
- Jang, H.M.; Han, S.K.; Kim, J.K.; Oh, S.J.; Jang, H.B.; Kim, D.H. Lactobacillus sakei alleviates high-fat-diet-induced obesity and anxiety in mice by inducing AMPK activation and SIRT1 expression and inhibiting gut microbiota-mediated NF-kappa B activation. Mol. Nutr. Food Res. 2019, 63, 1800978. [Google Scholar] [CrossRef]
- Park, K.; Park, S.; Nagappan, A.; Ray, N.; Kim, J.; Yoon, S.; Moon, Y. Probiotic Escherichia coli ameliorates antibiotic-associated anxiety responses in mice. Nutrients 2021, 13, 811. [Google Scholar] [CrossRef]
- Tian, P.J.; Chen, Y.; Qian, X.; Zou, R.Y.; Zhu, H.Y.; Zhao, J.X.; Zhang, H.; Wang, G.; Chen, W. Pediococcus acidilactici CCFM6432 mitigates chronic stress-induced anxiety and gut microbial abnormalities. Food Funct. 2021, 12, 11241–11249. [Google Scholar] [CrossRef] [PubMed]
- Sandes, S.; Figueiredo, N.; Pedroso, S.; Sant’Anna, F.; Acurcio, L.; Abatemarco, M.; Barros, P.; Oliveira, F.; Cardoso, V.; Generoso, S.; et al. Weissella paramesenteroides WpK4 plays an immunobiotic role in gut-brain axis, reducing gut permeability, anxiety-like and depressive-like behaviors in murine models of colitis and chronic stress. Food Res. Int. 2020, 137, 109741. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Guo, W. Emotional roles of mono-aminergic neurotransmitters in major depressive disorder and anxiety disorders. Front. Psychol. 2018, 9, 2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncalves, N.G.G.; de Araujo, J.I.F.; Magalhaes, F.E.A.; Mendes, F.R.S.; Lobo, M.D.P.; Moreira, A.; Moreira, R.D. Protein fraction from Artocarpus altilis pulp exhibits antioxidant properties and reverses anxiety behavior in adult zebrafish via the serotoninergic system. J. Funct. Foods 2020, 66, 103772. [Google Scholar] [CrossRef]
- Sun, X.L.; Zhang, T.W.; Zhao, Y.; Cai, E.B.; Zhu, H.Y.; Liu, S.L. Panaxynol attenuates CUMS-induced anxiety and depressive-like behaviors via regulating neurotransmitters, synapses and the HPA axis in mice. Food Funct. 2020, 11, 1235–1244. [Google Scholar] [CrossRef]
- Roustazade, R.; Radahmadi, M.; Yazdani, Y. Therapeutic effects of saffron extract on different memory types, anxiety, and hippocampal BDNF and TNF-alpha gene expressions in sub-chronically stressed rats. Nutr. Neurosci. 2022, 25, 192–206. [Google Scholar] [CrossRef]
- Ogawa, S.; Ota, M.; Ogura, J.; Kato, K.; Kunugi, H. Effects of L-theanine on anxiety-like behavior, cerebrospinal fluid amino acid profile, and hippocampal activity in Wistar Kyoto rats. Psychopharmacology 2018, 235, 37–45. [Google Scholar] [CrossRef]
- Weiser, M.J.; Grimshaw, V.; Wynalda, K.M.; Mohajeri, M.H.; Butt, C.M. Long-term administration of queen bee acid (QBA) to rodents reduces anxiety-like behavior, promotes neuronal health and improves body composition. Nutrients 2018, 10, 13. [Google Scholar] [CrossRef]
- Nakahara, J.; Masubuchi, Y.; Takashima, K.; Takahashi, Y.; Ichikawa, R.; Nakao, T.; Koyanagi, M.; Maronpot, R.R.; Yoshida, T.; Hayashi, S.M.; et al. Continuous exposure to amorphous formula of curcumin from the developmental stage facilitates anti-anxiety-like behavior and fear-extinction learning in rats. Nutr. Res. 2021, 85, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.X.; Xiong, R.G.; Huang, S.Y.; Zhou, D.D.; Saimaiti, A.; Zhao, C.N.; Shang, A.; Zhang, Y.J.; Gan, R.Y.; Li, H.B. Effects and mechanisms of resveratrol for prevention and management of cancers: An updated review. Crit. Rev. Food Sci. Nutr. 2022, in press. [CrossRef] [PubMed]
- Xiong, R.G.; Huang, S.Y.; Wu, S.X.; Zhou, D.D.; Yang, Z.J.; Saimaiti, A.; Zhao, C.N.; Shang, A.O.; Zhang, Y.J.; Gan, R.Y.; et al. Anticancer effects and mechanisms of berberine from medicinal herbs: An update review. Molecules 2022, 27, 4523. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Huang, S.Y.; Zhou, D.D.; Xiong, R.G.; Zhao, C.N.; Fang, A.P.; Zhang, Y.J.; Li, H.B.; Zhu, H.L. Effects and mechanisms of curcumin for the prevention and management of cancers: An updated review. Antioxidants 2022, 11, 1481. [Google Scholar] [CrossRef] [PubMed]
- Namgyal, D.; Ali, S.; Hussain, M.D.; Kazi, M.; Ahmad, A.; Sarwat, M. Curcumin ameliorates the Cd-induced anxiety-like behavior in mice by regulating oxidative stress and neuro-inflammatory proteins in the prefrontal cortex region of the brain. Antioxidants 2021, 10, 1710. [Google Scholar] [CrossRef]
- Fernandez-Demeneghi, R.; Rodriguez-Landa, J.F.; Guzman-Gernimo, R.I.; Acosta-Mesa, H.G.; Meza-Alvarado, E.; Vargas-Moreno, I.; Herrera-Meza, S. Effect of blackberry juice (Rubus fruticosus L.) on anxiety-like behaviour in Wistar rats. Int. J. Food Sci. Nutr. 2019, 70, 856–867. [Google Scholar] [CrossRef]
- Eskandarzadeh, S.; Effatpanah, M.; Khosravi-Darani, K.; Askari, R.; Hosseini, A.F.; Reisian, M.; Jazayeri, S. Efficacy of a multispecies probiotic as adjunctive therapy in generalized anxiety disorder: A double blind, randomized, placebo-controlled trial. Nutr. Neurosci. 2021, 24, 102–108. [Google Scholar] [CrossRef]
- Lew, L.C.; Hor, Y.Y.; Yusoff, N.A.A.; Choi, S.B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.M.; Abdullah, M.; Zakaria, N.; et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Platero, J.L.; Cuerda-Ballester, M.; Ibanez, V.; Sancho, D.; Lopez-Rodriguez, M.M.; Drehmer, E.; Orti, J.E.D. The impact of coconut oil and epigallocatechin gallate on the levels of IL-6, anxiety and disability in multiple sclerosis patients. Nutrients 2020, 12, 305. [Google Scholar] [CrossRef] [Green Version]
- Sarris, J.; Byrne, G.J.; Cribb, L.; Oliver, G.; Murphy, J.; Macdonald, P.; Nazareth, S.; Karamacoska, D.; Galea, S.; Short, A.; et al. L-theanine in the adjunctive treatment of generalized anxiety disorder: A double-blind, randomised, placebo-controlled trial. J. Psychiatr. Res. 2019, 110, 31–37. [Google Scholar] [CrossRef]
- Young, L.M.; Pipingas, A.; White, D.J.; Gauci, S.; Scholey, A. A systematic review and meta-analysis of B vitamin supplementation on depressive symptoms, anxiety, and stress: Effects on healthy and ‘at-risk’ individuals. Nutrients 2019, 11, 2232. [Google Scholar] [CrossRef]
| Name | Study Type | Participants | Dose | Effects | Ref. |
|---|---|---|---|---|---|
| Animal Foods | |||||
| Fish | Cross-sectional study | 9183 Korean adults 19 ≤ age ≤ 64 | 4 times/week vs. <1 time/week | Associated with a lower risk of depression Total: OR, 0.52; 95% CI, 0.37–0.74 Men: OR, 0.64; 95% CI, 0.30–1.37 Women: OR, 0.44; 95% CI, 0.29–0.67; | [31] |
| Fatty fish | Cross-sectional study | 6587 participants | Second, third, fourth quintiles vs. the lowest quintiles | U-shaped relationship with depression (OR, 0.77; 95% CI, 0.63–0.94) (OR, 0.71; 95% CI, 0.58–0.87) (OR, 0.78; 95% CI, 0.64–0.96); | [32] |
| Plant foods | |||||
| Legume and nut | Cross-sectional study | 3172 participants 18 ≤ age ≤ 55 | The highest vs. the lowest quartile | No association with depression Men: OR, 0.96; 95% CI, 0.54–1.71 Women: OR, 0.98; 95% CI, 0.65–1.48; | [35] |
| Walnut | Cross-sectional study | 26,656 participants | Daily walnut consumption vs. no nut consumption | Protective effect on depression Total: OR, 0.67; 95% CI, 0.48–0.93 Men: OR, 0.72; 95% CI, 0.41–1.27 Women: OR, 0.62; 95% CI, 0.46–0.84; | [30] |
| Seaweed and mushroom fiber | Cross-sectional study | 2960 adults 19 ≤ age ≤ 64 | Seaweed fiber: ≥1.02 g/day vs. <0.31 g/day Mushroom fiber: ≥0.14 g/day vs. <0.03 g/day | Inversely associated with depressive symptoms Seaweed fiber: OR, 0.38; 95% CI, 0.20–0.72 Mushroom fiber: OR, 0.18; 95% CI, 0.01–0.37; | [29] |
| Beverages | |||||
| Green tea | Cross-sectional study | 9576 Korean adults age ≥ 19 | ≥3 cups/week vs. none or <1 cup/week | Decreased the prevalence of depression (OR, 0.79; 95% CI, 0.63–0.99); | [25] |
| Tea | Cohort study | 3177 participants age ≥ 55 | ≥3 cups/day vs. none or <1 cup/day | Associated with a lower risk of depression (OR, 0.32; 95% CI, 0.12–0.84); | [24] |
| Coffee | Cohort study | 14,413 university graduates | ≥4 cups/day vs. <1 cup/day | Associated with a lower risk of depression (HR, 0.37; 95% CI, 0.15–0.95); | [33] |
| Soft drink | Cross-sectional study | 8085 college students | >25 g sugar/day from soft drinks vs. none | Associated with a higher risk of depression (Mean difference, 0.22; 95% CI, 0.15–0.29); | [36] |
| Sugar-sweetened drink | Cohort study | 15,546 Spanish university graduates | The highest vs. the lowest quartile | No association with depression (HR, 1.12; 95% CI, 0.90–1.41); | [37] |
| Nutrients | |||||
| Carbohydrate | Two-sample Mendelian randomization analysis | 268,922 samples | NA | A causal relationship with a lower risk of depression (OR, 0.42; 95% CI, 0.28–0.62); | [34] |
| Dietary fiber | Cross-sectional study | 459 hypertensive patients | 10.5–15.4 g/day vs. ≥15.4 g/day | Associated with a higher incidence of depression (OR, 2.641; 95% CI, 1.050–6.640); | [26] |
| Selenium | Cross-sectional study | 736 Brazilian farmers 18 ≤ age ≤ 59 | ≥95.26 μg/day vs. ≤66.66 μg/day | Decreased the risk of depression (OR, 0.461; 95% CI, 0.236–0.901); | [27] |
| Name | Study Type | Participants | Dose | Effects | Ref. |
|---|---|---|---|---|---|
| Plant foods | |||||
| Legume and nut | Cross-sectional study | 3172 participants 18 ≤ age ≤ 55 | The highest vs. the lowest quartile | Protective effect on anxiety in men Men: OR, 0.34; 95% CI, 0.14–0.82 Women: OR, 1.06; 95% CI, 0.63–1.77; | [35] |
| Beverages | |||||
| Soft drinks | Cross-sectional study | 8085 college students | >25 g sugar/day from soft drinks vs. none | Associated with a higher risk of anxiety (Mean difference, 0.11; 95% CI, 0.04–0.18); | [36] |
| Nutrients | |||||
| Dietary fiber | Cross-sectional study | 459 hypertensive patients | <8.1 g/day vs. ≥15.4 g/day | Associated with a higher incidence of depression (OR, 2.757; 95% CI, 1.035–7.346); | [26] |
| n-3 fatty acids | Cross-sectional study | 12,268 adults | The highest vs. the lowest quintile | Inversely associated with anxiety EPA: OR, 0.82; 95% CI, 0.69–0.98 DHA: OR, 0.83; 95% CI, 0.69–0.98 DPA: OR, 0.82; 95% CI, 0.69–0.98; | [107] |
| Branched-chain amino acids | Cross-sectional study | 3175 Iranian adults 18 ≤ age ≤ 55 | The highest vs. the lowest tertile | Associated with a lower risk of anxiety (OR, 0.66; 95% CI, 0.47–0.91); | [105] |
| Vitamin B6 | Cross-sectional study | 3362 adults | The lowest vs. the highest tertile | Associated with a higher risk of anxiety (OR, 2.30; 95% CI, 1.19–4.46); | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-X.; Li, J.; Zhou, D.-D.; Xiong, R.-G.; Huang, S.-Y.; Saimaiti, A.; Shang, A.; Li, H.-B. Possible Effects and Mechanisms of Dietary Natural Products and Nutrients on Depression and Anxiety: A Narrative Review. Antioxidants 2022, 11, 2132. https://doi.org/10.3390/antiox11112132
Wu S-X, Li J, Zhou D-D, Xiong R-G, Huang S-Y, Saimaiti A, Shang A, Li H-B. Possible Effects and Mechanisms of Dietary Natural Products and Nutrients on Depression and Anxiety: A Narrative Review. Antioxidants. 2022; 11(11):2132. https://doi.org/10.3390/antiox11112132
Chicago/Turabian StyleWu, Si-Xia, Jiahui Li, Dan-Dan Zhou, Ruo-Gu Xiong, Si-Yu Huang, Adila Saimaiti, Ao Shang, and Hua-Bin Li. 2022. "Possible Effects and Mechanisms of Dietary Natural Products and Nutrients on Depression and Anxiety: A Narrative Review" Antioxidants 11, no. 11: 2132. https://doi.org/10.3390/antiox11112132
APA StyleWu, S.-X., Li, J., Zhou, D.-D., Xiong, R.-G., Huang, S.-Y., Saimaiti, A., Shang, A., & Li, H.-B. (2022). Possible Effects and Mechanisms of Dietary Natural Products and Nutrients on Depression and Anxiety: A Narrative Review. Antioxidants, 11(11), 2132. https://doi.org/10.3390/antiox11112132







