Modulation of NRF-2 Pathway Contributes to the Therapeutic Effects of Boswellia serrata Gum Resin Extract in a Model of Experimental Autoimmune Myocarditis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Carrageenan (CAR)-Induced Paw Edema (Preliminary Data)
2.4. Experimental Groups (Preliminary Data)
- CAR + vehicle: rats were injected with CAR and administered orally with vehicle.
- CAR + Boswellia extract (10 mg/kg): rats were injected with CAR and Boswellia extract was administered orally 30 min before CAR injection.
- CAR + Boswellia extract (50 mg/kg): rats were injected with CAR and Boswellia extract was administered orally 30 min before CAR injection.
- CAR + Boswellia extract (100 mg/kg): rats were injected with CAR and Boswellia extract was administered orally 30 min before CAR injection.
- Sham-operated groups received saline and were treated orally with vehicle or Boswellia (data not shown).
2.5. Induction of Experimental Autoimmune Myocarditis
2.6. Experimental Groups
- (1)
- Sham + vehicle: rats were injected with CFA and treated orally with vehicle every day for 21 days.
- (2)
- Sham + Boswellia gum extract: rats received CFA and were treated orally with Boswellia gum extract 100 mg/kg every day for 21 days.
- (3)
- EAM + vehicle: rats were injected with emulsified porcine myosin in CFA and treated orally with vehicle every day for 21 days.
- (4)
- EAM + Boswellia gum extract: rats were injected with emulsified porcine myosin in CFA and treated orally with Boswellia gum extract 100 mg/kg every day for 21 days. At 3 weeks post induction, (21 day), rats were killed, blood and heart tissues were collected. The route and dose administration of Boswellia gum extract were selected based on [14]. Since no significant difference was discovered between the sham + vehicle and sham + Boswellia gum extract, simply the data of sham + vehicle groups were displayed.
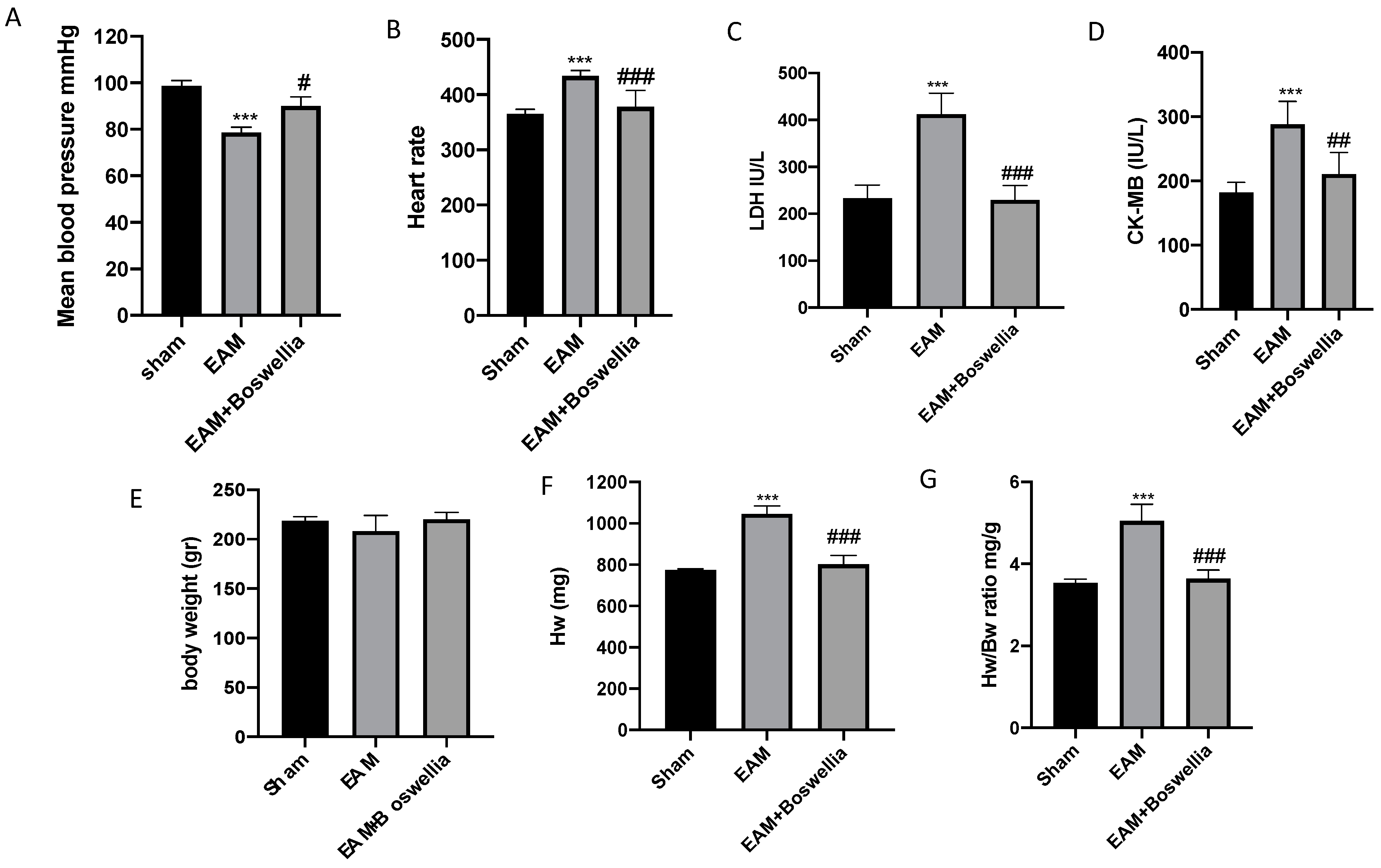
2.7. Heart Rate and Blood Pressure Measurements
2.8. Biochemical Parameters
2.9. Heart Weight, hw/Body Weight, bw (Hw/Bw)
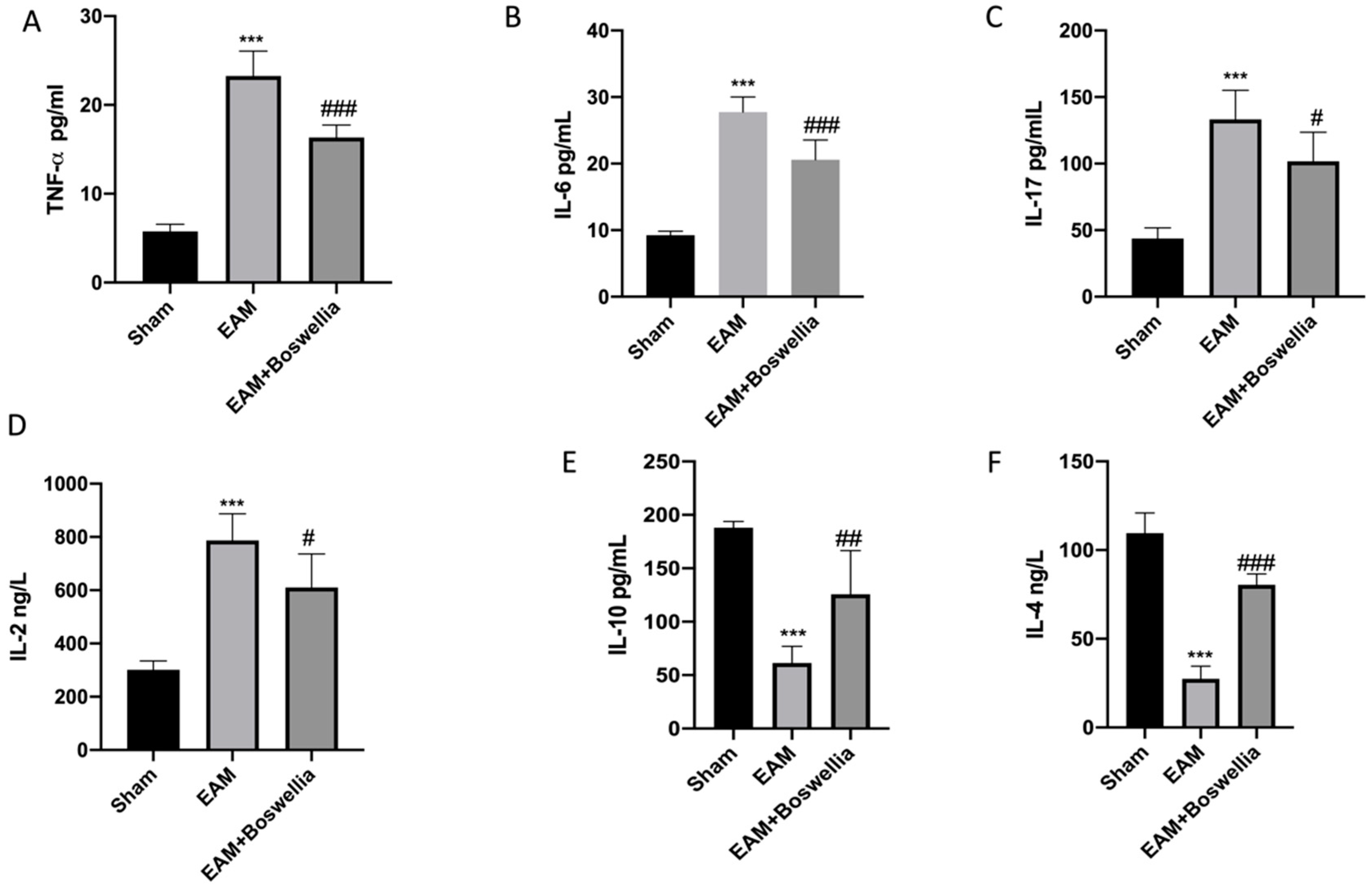
2.10. Cytokines Measurements
2.11. Macroscopic Evaluation
2.12. Histological Analysis
2.13. Immunohistochemistry for Smooth Muscle Alpha-Actin (α-Sma) and Transforming Growth Factor Beta (TGF-β) and Nuclear Factor Erythroid 2–Related Factor 2 (NRF-2)
2.14. Malondialdehyde (MDA) Levels
2.15. Determination of Nitric Oxide (NO)
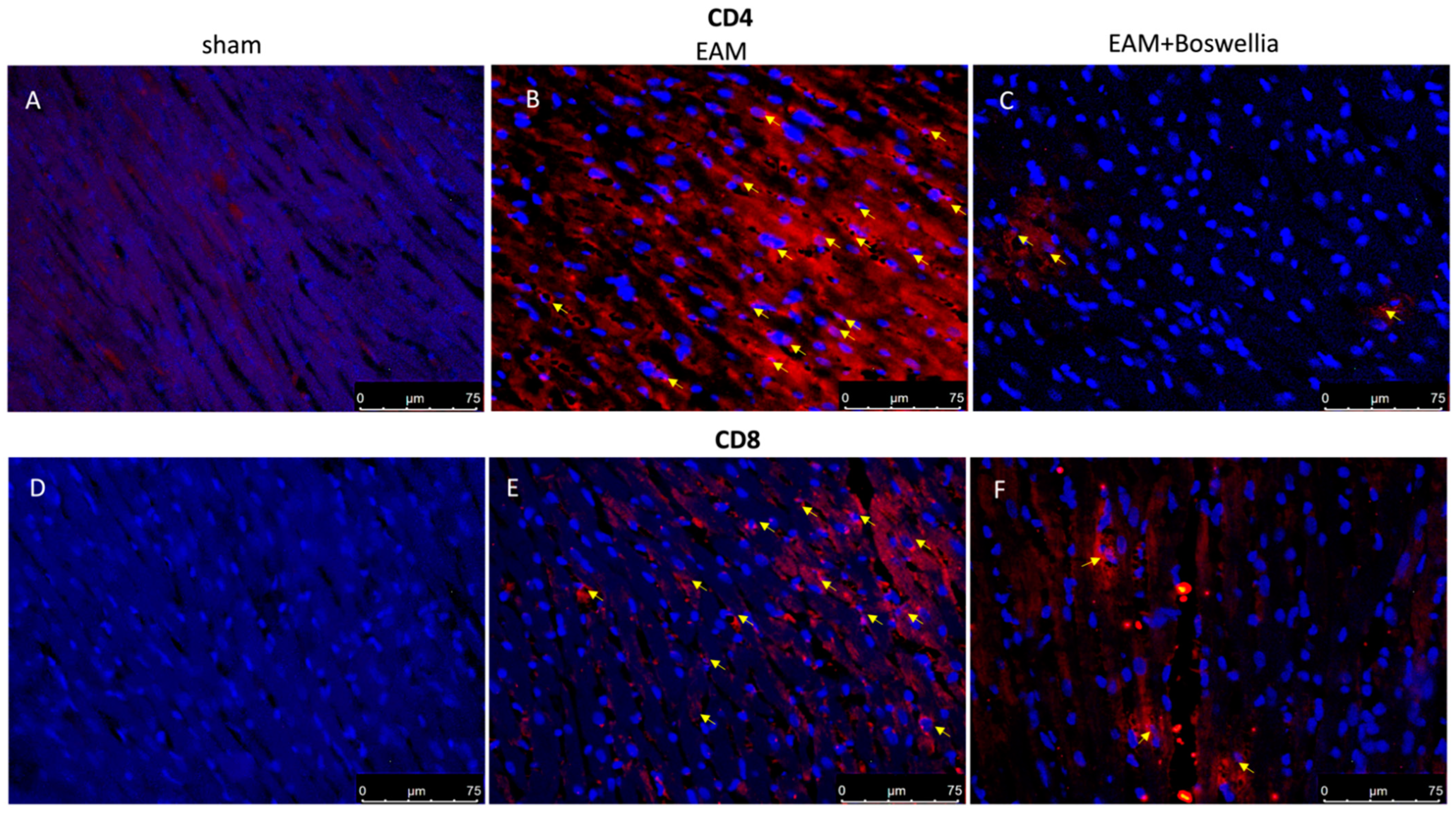
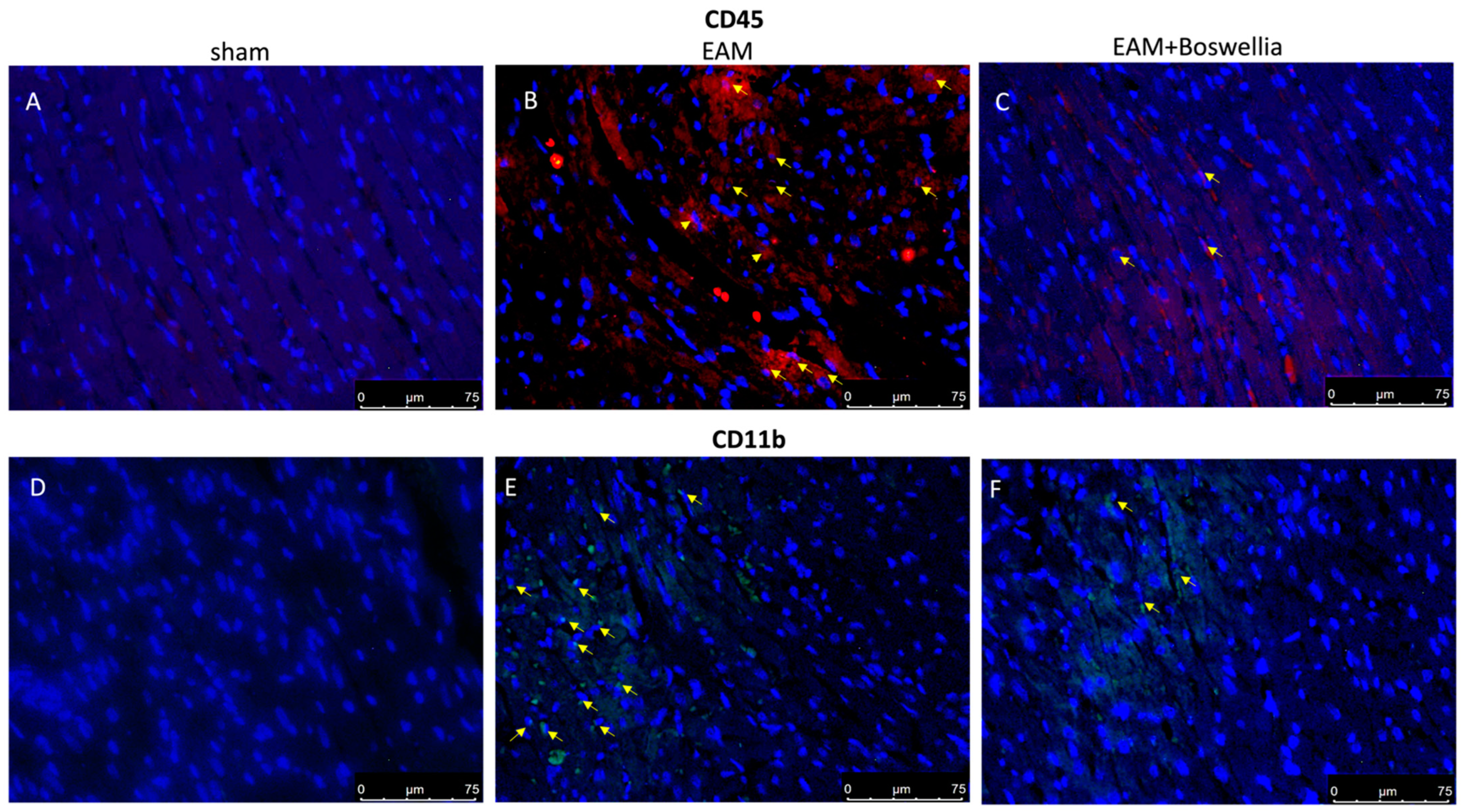
2.16. Immunofluorescence for CD4, CD8, CD45, CD11β
2.17. Western Blot for NRF-2, HO-1 and MnSOD
2.18. Statistical Evaluation
3. Results
3.1. Effects of Boswellia Gum Extract on Paw Damage: Preliminary Data
3.2. Effects of Boswellia Gum Extract on Mean Blood Pressure, Heart Rate, Biochemical Parameters, and Hw/Bw in EAM Rats
3.3. Effects of Boswellia Gum Extract on Macroscopic and Microscopic Damage and Fibrosis in EAM Rats
3.4. Effects of Boswellia Gum Extract on α-sma and TGF-β in EAM Rats
3.5. Effects of Boswellia Gum Extract on Cytokines Release in EAM Rats
3.6. Effects of Boswellia Gum Extract on CD4, CD8, CD45 and CD11β Expression in EAM Rats
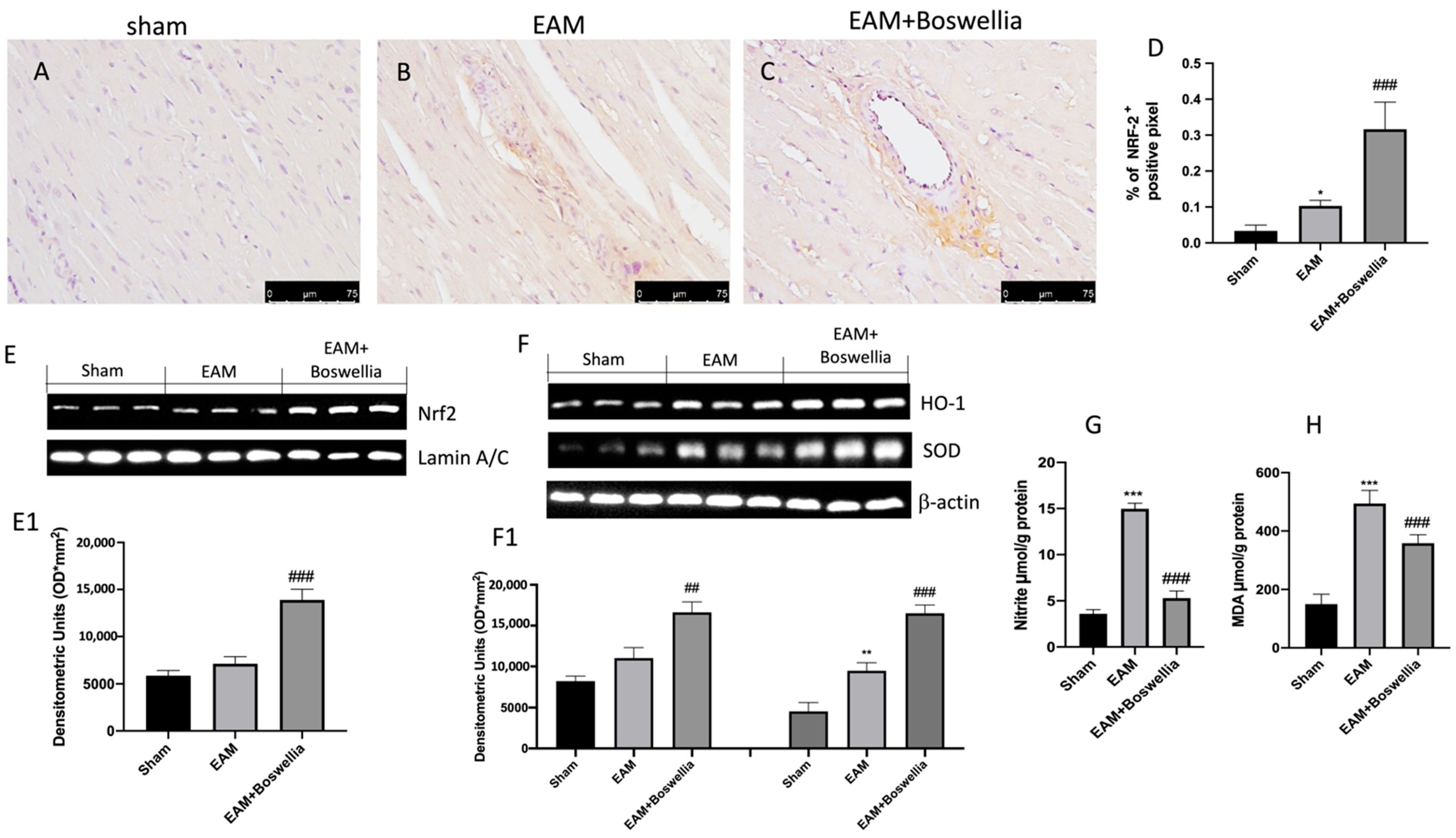
3.7. Effects of Boswellia Gum Exctract on Oxidative Stress in EAM Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, A.M.; El Fouhil, A.F.; Mohamed, R.A.; Atteya, M.; Abdel-Baky, N.A.; AlRoalle, A.H.; Aldahmash, A.M. Curcumin ameliorates experimental autoimmune acute myocarditis in rats as evidenced by decrease in thioredoxin immunoreactivity. Folia Morphol. 2015, 74, 318–324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, Z.; Kishimoto, C.; Shioji, K.; Nakamura, H.; Yodoi, J.; Sasayama, S. Temocapril treatment ameliorates autoimmune myocarditis associated with enhanced cardiomyocyte thioredoxin expression. Mol. Cell. Biochem. 2003, 248, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Wongcharoen, W.; Jai-Aue, S.; Phrommintikul, A.; Nawarawong, W.; Woragidpoonpol, S.; Tepsuwan, T.; Sukonthasarn, A.; Apaijai, N.; Chattipakorn, N. Effects of Curcuminoids on Frequency of Acute Myocardial Infarction after Coronary Artery Bypass Grafting. Am. J. Cardiol. 2012, 110, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Nakamura, H.; Shioji, K.; Tanito, M.; Oka, S.-I.; Ahsan, M.K.; Son, A.; Ishii, Y.; Kishimoto, C.; Yodoi, J. Thioredoxin-1 Ameliorates Myosin-Induced Autoimmune Myocarditis by Suppressing Chemokine Expressions and Leukocyte Chemotaxis in Mice. Circulation 2004, 110, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Draginic, N.D.; Jakovljevic, V.L.; Jeremic, J.N.; Srejovic, I.M.; Andjic, M.M.; Rankovic, M.R.; Sretenovic, J.Z.; Zivkovic, V.I.; Ljujic, B.T.; Mitrovic, S.L.; et al. Melissa officinalis L. Supplementation Provides Cardioprotection in a Rat Model of Experimental Autoimmune Myocarditis. Oxidative Med. Cell. Longev. 2022, 2022, 1344946. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R. A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J. Gastroenterol. 2010, 16, 4504–4514. [Google Scholar] [CrossRef]
- Kimmatkar, N.; Thawani, V.; Hingorani, L.; Khiyani, R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee—A randomized double blind placebo controlled trial. Phytomedicine 2003, 10, 3–7. [Google Scholar] [CrossRef]
- Hartmann, R.M.; Martins, M.I.M.; Tieppo, J.; Fillmann, H.S.; Marroni, N.P. Effect of Boswellia serrata on Antioxidant Status in an Experimental Model of Colitis Rats Induced by Acetic Acid. Am. J. Dig. Dis. 2012, 57, 2038–2044. [Google Scholar] [CrossRef]
- Krüger, P.; Daneshfar, R.; Eckert, G.P.; Klein, J.; Volmer, D.A.; Bahr, U.; Müller, W.E.; Karas, M.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Metabolism of Boswellic Acids In Vitro and In Vivo. Drug Metab. Dispos. 2008, 36, 1135–1142. [Google Scholar] [CrossRef]
- Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G.; et al. An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. Int. J. Mol. Sci. 2019, 20, 4101. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Makboul, R.M.; Al-Mokhtar, M.A.; Nicola, M.A. Polyphenol-rich Boswellia serrata gum prevents cognitive impairment and insulin resistance of diabetic rats through inhibition of GSK3β activity, oxidative stress and pro-inflammatory cytokines. Biomed. Pharmacother. 2019, 109, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Britti, D.; Crupi, R.; Impellizzeri, D.; Gugliandolo, E.; Fusco, R.; Schievano, C.; Morittu, V.M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models. BMC Vet. Res. 2017, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, L.-Q.; Li, H.-Q.; Wu, J.; Bian, N.-N.; Yan, G. Beneficial effects of andrographolide in a rat model of autoimmune myocarditis and its effects on PI3K/Akt pathway. Korean J. Physiol. Pharmacol. 2019, 23, 103–111. [Google Scholar] [CrossRef]
- Umar, S.; Umar, K.; Sarwar, A.H.; Khan, A.; Ahmad, N.; Ahmad, S.; Katiyar, C.K.; Husain, S.A.; Khan, H.A. Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomedicine 2014, 21, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, D.; Natale, S.; Gugliandolo, E.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Assessment of 2-Pentadecyl-2-oxazoline Role on Lipopolysaccharide-Induced Inflammation on Early Stage Development of Zebrafish (Danio rerio). Life 2022, 12, 128. [Google Scholar] [CrossRef]
- Lu, Y.-F.; Zhang, Q.-Y.; Wang, L.-H.; Liu, X.-Y.; Zhang, S.-X. The protective effects of taurine on experimental autoimmune myocarditis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1868–1875. [Google Scholar] [PubMed]
- Petrosino, S.; Cordaro, M.; Verde, R.; Schiano Moriello, A.; Marcolongo, G.; Schievano, C.; Siracusa, R.; Piscitelli, F.; Peritore, A.F.; Crupi, R.; et al. Oral Ultramicronized Palmitoylethanolamide: Plasma and Tissue Levels and Spinal Anti-hyperalgesic Effect. Front. Pharmacol. 2018, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Milenković, M.; Arsenović-Ranin, N.; Stojić-Vukanić, Z.; Bufan, B.; Vučićević, D.; Jančić, I. Quercetin Ameliorates Experimental Autoimmune Myocarditis in Rats. J. Pharm. Pharm. Sci. 2010, 13, 311–319. [Google Scholar] [CrossRef]
- di Paola, R.; Cordaro, M.; Crupi, R.; Siracusa, R.; Campolo, M.; Bruschetta, G.; Fusco, R.; Pugliatti, P.; Esposito, E.; Cuzzocrea, S. Protective Effects of Ultramicronized Palmitoylethanolamide (PEA-um) in Myocardial Ischaemia and Reperfusion Injury in VIVO. Shock 2016, 46, 202–213. [Google Scholar] [CrossRef]
- D’Amico, R.; Fusco, R.; Gugliandolo, E.; Cordaro, M.; Siracusa, R.; Impellizzeri, D.; Peritore, A.F.; Crupi, R.; Cuzzocrea, S.; Di Paola, R. Effects of a new compound containing Palmitoylethanolamide and Baicalein in myocardial ischaemia/reperfusion injury in vivo. Phytomedicine 2019, 54, 27–42. [Google Scholar] [CrossRef]
- Di Paola, D.; Capparucci, F.; Abbate, J.M.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Genovese, T.; Impellizzeri, D.; et al. Environmental Risk Assessment of Oxaliplatin Exposure on Early Life Stages of Zebrafish (Danio rerio). Toxics 2022, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cordaro, M.; Genovese, T.; Impellizzeri, D.; Siracusa, R.; Gugliandolo, E.; Peritore, A.; D’Amico, R.; Crupi, R.; Cuzzocrea, S.; et al. Adelmidrol: A New Promising Antioxidant and Anti-Inflammatory Therapeutic Tool in Pulmonary Fibrosis. Antioxidants 2020, 9, 601. [Google Scholar] [CrossRef]
- Hirakawa, H.; Zempo, H.; Ogawa, M.; Watanabe, R.; Suzuki, J.-I.; Akazawa, H.; Komuro, I.; Isobe, M. A DPP-4 Inhibitor Suppresses Fibrosis and Inflammation on Experimental Autoimmune Myocarditis in Mice. PLoS ONE 2015, 10, e0119360. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Impellizzeri, D.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a Palmitoylethanolamide Analogue, as a New Pharmacological Treatment for the Management of Inflammatory Bowel Disease. Mol. Pharmacol. 2016, 90, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; D’Amico, R.; Cordaro, M.; Genovese, T.; Gugliandolo, E.; Peritore, A.; Crupi, R.; Di Paola, R.; Cuzzocrea, S.; et al. Mucosa-Associated Lymphoid Tissue Lymphoma Translocation 1 Inhibitor as a Novel Therapeutic Tool for Lung Injury. Int. J. Mol. Sci. 2020, 21, 7761. [Google Scholar] [CrossRef]
- Fusco, R.; Gugliandolo, E.; Siracusa, R.; Scuto, M.; Cordaro, M.; D’Amico, R.; Evangelista, M.; Peli, A.; Peritore, A.; Impellizzeri, D.; et al. Formyl Peptide Receptor 1 Signaling in Acute Inflammation and Neural Differentiation Induced by Traumatic Brain Injury. Biology 2020, 9, 238. [Google Scholar] [CrossRef]
- Peritore, A.F.; D’Amico, R.; Siracusa, R.; Cordaro, M.; Fusco, R.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Di Paola, R.; Cuzzocrea, S.; et al. Management of Acute Lung Injury: Palmitoylethanolamide as a New Approach. Int. J. Mol. Sci. 2021, 22, 1553. [Google Scholar] [CrossRef]
- Sawant, S.; Gokulan, R.; Dongre, H.; Vaidya, M.; Chaukar, D.; Prabhash, K.; Ingle, A.; Joshi, S.; Dange, P.; Joshi, S.; et al. Prognostic role of Oct4, CD44 and c-Myc in radio–chemo-resistant oral cancer patients and their tumourigenic potential in immunodeficient mice. Clin. Oral Investig. 2016, 20, 43–56. [Google Scholar] [CrossRef]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An Open Source Plugin for the Quantitative Evaluation and Automated Scoring of Immunohistochemistry Images of Human Tissue Samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.A.; Metwally, M.E.; El-Khawanki, M.M.; Hashim, A.M. Protective effect of captopril against clozapine-induced myocarditis in rats: Role of oxidative stress, proinflammatory cytokines and DNA damage. Chem. Interact. 2014, 216, 43–52. [Google Scholar] [CrossRef]
- Di Paola, D.; Iaria, C.; Capparucci, F.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Aflatoxin B1 Toxicity in Zebrafish Larva (Danio rerio): Protective Role of Hericium erinaceus. Toxins 2021, 13, 710. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; Peritore, A.F.; D’Amico, R.; Licata, P.; Crupi, R. Evaluation of Neuroprotective Effects of Quercetin against Aflatoxin B1-Intoxicated Mice. Animals 2020, 10, 898. [Google Scholar] [CrossRef]
- Crupi, R.; Palma, E.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; Cordaro, M.; Impellizzeri, D.; De Caro, C.; Calzetta, L.; Cuzzocrea, S.; et al. Protective Effect of Hydroxytyrosol Against Oxidative Stress Induced by the Ochratoxin in Kidney Cells: In vitro and in vivo Study. Front. Veter- Sci. 2020, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Effect of PEA-OXA on neuropathic pain and functional recovery after sciatic nerve crush. J. NeuroInflamm. 2018, 15, 264. [Google Scholar] [CrossRef]
- Siracusa, R.; Paterniti, I.; Cordaro, M.; Crupi, R.; Bruschetta, G.; Campolo, M.; Cuzzocrea, S.; Esposito, E. Neuroprotective Effects of Temsirolimus in Animal Models of Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 2403–2419. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Crupi, R.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Petrosino, S.; Evangelista, M.; Di Paola, R.; et al. N-Palmitoylethanolamine-oxazoline (PEA-OXA): A new therapeutic strategy to reduce neuroinflammation, oxidative stress associated to vascular dementia in an experimental model of repeated bilateral common carotid arteries occlusion. Neurobiol. Dis. 2019, 125, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Peritore, A.F.; Crupi, R.; Scuto, M.; Gugliandolo, E.; Siracusa, R.; Impellizzeri, D.; Cordaro, M.; D’Amico, R.; Fusco, R.; Di Paola, R.; et al. The Role of Annexin A1 and Formyl Peptide Receptor 2/3 Signaling in Chronic Corticosterone-Induced Depression-Like behaviors and Impairment in Hippocampal-Dependent Memory. CNS Neurol. Disord. Drug Targets 2020, 19, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Crupi, R.; Esposito, E.; Petrosino, S.; Cuzzocrea, S. Anti-Inflammatory and Neuroprotective Effects of Co-UltraPEALut in a Mouse Model of Vascular Dementia. Front. Neurol. 2017, 8, 233. [Google Scholar] [CrossRef]
- D’Amico, R.; Cordaro, M.; Fusco, R.; Peritore, A.F.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Mandalari, G.; Caccamo, D.; Cuzzocrea, S.; et al. Consumption of Cashew (Anacardium occidentale L.) Nuts Counteracts Oxidative Stress and Tissue Inflammation in Mild Hyperhomocysteinemia in Rats. Nutrients 2022, 14, 1474. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Fusco, R.; D’Amico, R.; Peritore, A.; Gugliandolo, E.; Genovese, T.; Scuto, M.; Crupi, R.; Mandalari, G.; et al. Cashew (Anacardium occidentale L.) Nuts Counteract Oxidative Stress and Inflammation in an Acute Experimental Model of Carrageenan-Induced Paw Edema. Antioxidants 2020, 9, 660. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. Consumption of Anacardium Occidentale L. (Cashew Nuts) Inhibits Oxidative Stress through Modulation of the Nrf2/HO−1 and NF-kB Pathways. Molecules 2020, 25, 4426. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Gugliandolo, E.; Crupi, R.; Cuzzocrea, S. A new co-micronized composite containing palmitoylethanolamide and polydatin shows superior oral efficacy compared to their association in a rat paw model of carrageenan-induced inflammation. Eur. J. Pharmacol. 2016, 782, 107–118. [Google Scholar] [CrossRef]
- D’Amico, R.; Salinaro, A.T.; Fusco, R.; Cordaro, M.; Impellizzeri, D.; Scuto, M.; Ontario, M.; Dico, G.L.; Cuzzocrea, S.; Di Paola, R.; et al. Hericium erinaceus and Coriolus versicolor Modulate Molecular and Biochemical Changes after Traumatic Brain Injury. Antioxidants 2021, 10, 898. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; D’Amico, R.; Fusco, R.; Genovese, T.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Interdonato, L.; Di Paola, D.; Di Paola, R.; et al. Açai Berry Mitigates Vascular Dementia-Induced Neuropathological Alterations Modulating Nrf-2/Beclin1 Pathways. Cells 2022, 11, 2616. [Google Scholar] [CrossRef]
- Cordaro, M.; Fusco, R.; D’Amico, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Mandalari, G.; Cuzzocrea, S.; et al. Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein. Antioxidants 2020, 9, 992. [Google Scholar] [CrossRef]
- Ismail, S.; Rao, K.; Bhaskar, M. Evaluation of anti-inflammatory activity of Boswellia serrata on carrageenan induced paw edema in albino Wistar rats. Int. J. Res. Med. Sci. 2016, 4, 2980–2986. [Google Scholar] [CrossRef]
- Shimada, K.; Uzui, H.; Ueda, T.; Lee, J.-D.; Kishimoto, C. N-Acetylcysteine Ameliorates Experimental Autoimmune Myocarditis in Rats via Nitric Oxide. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 203–210. [Google Scholar] [CrossRef]
- Kim, K.-S.; Hufnagel, G.; Chapman, N.; Tracy, S. The group B coxsackieviruses and myocarditis. Rev. Med. Virol. 2001, 11, 355–368. [Google Scholar] [CrossRef]
- Leuschner, F.; Katus, H.A.; Kaya, Z. Autoimmune myocarditis: Past, present and future. J. Autoimmun. 2009, 33, 282–289. [Google Scholar] [CrossRef]
- Zaki, A.A.; Hashish, N.E.; Amer, M.A.; Lahloub, M.-F. Cardioprotective and antioxidant effects of oleogum resin “Olibanum” from Bos Boswellia carteri Birdw. (Bursearceae). Chin. J. Nat. Med. 2014, 12, 345–350. [Google Scholar] [CrossRef]
- Ammon, H.P.T. Boswellic Acids in Chronic Inflammatory Diseases. Planta Med. 2006, 72, 1100–1116. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Lou, H.-X. Bioactive Constituents of Myrrh and Frankincense, Two Simultaneously Prescribed Gum Resins in Chinese Traditional Medicine. Chem. Biodivers. 2008, 5, 540–553. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Hosseinzadeh, H.; Bideskan, A.E.; Sadeghnia, H.R. Aqueous and Ethanolic Extracts of Boswellia serrata Protect Against Focal Cerebral Ischemia and Reperfusion Injury in Rats. Phytother. Res. 2016, 30, 1954–1967. [Google Scholar] [CrossRef]
- Ranjbarnejad, T.; Saidijam, M.; Moradkhani, S.; Najafi, R. Methanolic extract of Boswellia serrata exhibits anti-cancer activities by targeting microsomal prostaglandin E synthase-1 in human colon cancer cells. Prostaglandins Other Lipid Mediat. 2017, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khajuria, A.; Taneja, S.; Khajuria, R.; Singh, J.; Johri, R.; Qazi, G. The gastric ulcer protective effect of boswellic acids, a leukotriene inhibitor from Boswellia serrata, in rats. Phytomedicine 2008, 15, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Singh, S.; Singh, G.; Khajuria, A.; Sidiq, T.; Chashoo, G.; Pagoch, S.; Kaul, A.; Saxena, A.; Johri, R.; et al. In vivo genotoxicity evaluation of a plant based antiarthritic and anticancer therapeutic agent Boswelic acids in rodents. Phytomedicine 2009, 16, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Chacko, K.M.; Aggarwal, M.L.; Bhat, B.; Khandal, R.K.; Sultana, S.; Kuruvilla, B.T. A-90 day gavage safety assessment of Boswellia serrata in rats. Toxicol. Int. 2012, 19, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.; Liu, P.P.; Cooper, L.T., Jr. Myocarditis. Lancet 2012, 379, 738–747. [Google Scholar] [CrossRef]
- Daniels, M.D.; Hyland, K.V.; Wang, K.; Engman, D.M. Recombinant cardiac myosin fragment induces experimental autoimmune myocarditis via activation of Th1 and Th17 immunity. Autoimmunity 2008, 41, 490–499. [Google Scholar] [CrossRef]
- Li, L.-Y.; Wang, X.; Zhang, T.-C.; Liu, Z.-J.; Gao, J.-Q. Cardioprotective effects of omega 3 fatty acids from fish oil and it enhances autoimmunity in porcine cardiac myosin-induced myocarditis in the rat model. Z. Nat. C 2021, 76, 407–415. [Google Scholar] [CrossRef]
- Afanasyeva, M.; Georgakopoulos, D.; Rose, N.R. Autoimmune myocarditis: Cellular mediators of cardiac dysfunction. Autoimmun. Rev. 2004, 3, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Van der Borght, K.; Scott, C.L.; Nindl, V.; Bouché, A.; Martens, L.; Sichien, D.; Van Moorleghem, J.; Vanheerswynghels, M.; De Prijck, S.; Saeys, Y.; et al. Myocardial Infarction Primes Autoreactive T Cells through Activation of Dendritic Cells. Cell Rep. 2017, 18, 3005–3017. [Google Scholar] [CrossRef] [PubMed]
- Valaperti, A.; Marty, R.R.; Kania, G.; Germano, D.; Mauermann, N.; Dirnhofer, S.; Leimenstoll, B.; Blyszczuk, P.; Dong, C.; Mueller, C.; et al. CD11b+ Monocytes Abrogate Th17 CD4+ T Cell-Mediated Experimental Autoimmune Myocarditis. J. Immunol. 2008, 180, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, S.; Saksena, A.K.; Pal, R.; Jaiswal, R.; Kumar, R. Effect of Boswellia serrata extract on acute inflammatory parameters and tumor necrosis factor-α in complete Freund’s adjuvant-induced animal model of rheumatoid arthritis. Int. J. Appl. Basic Med. Res. 2019, 9, 100–106. [Google Scholar] [CrossRef]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative Stress and Mitochondrial DNA Damage in Heart Failure. Circ. J. 2008, 72 (Suppl. A), A31–A37. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.A.; Mohamed, H.S.; Abd-Ellatief, R.B.; Gomaa, M.A. Boswellic acids/Boswellia serrata extract as a potential COVID-19 therapeutic agent in the elderly. Inflammopharmacology 2021, 29, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Barakat, B.M.; Ahmed, H.I.; Bahr, H.I.; Elbahaie, A.M. Protective Effect of Boswellic Acids against Doxorubicin-Induced Hepatotoxicity: Impact on Nrf2/HO-1 Defense Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 8296451. [Google Scholar] [CrossRef]
- Minj, E.; Upadhayay, S.; Mehan, S. Nrf2/HO-1 Signaling Activator Acetyl-11-keto-beta Boswellic Acid (AKBA)-Mediated Neuroprotection in Methyl Mercury-Induced Experimental Model of ALS. Neurochem. Res. 2021, 46, 2867–2884. [Google Scholar] [CrossRef]
- Chen, M.; Wang, M.; Yang, Q.; Wang, M.; Wang, Z.; Zhu, Y.; Zhang, Y.; Wang, C.; Jia, Y.; Li, Y.; et al. Antioxidant effects of hydroxysafflor yellow A and acetyl-11-keto-β-boswellic acid in combination on isoproterenol-induced myocardial injury in rats. Int. J. Mol. Med. 2016, 37, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, D.; Capparucci, F.; Lanteri, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Combined Toxicity of Xenobiotics Bisphenol A and Heavy Metals on Zebrafish Embryos (Danio rerio). Toxics 2021, 9, 344. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amico, R.; Fusco, R.; Cordaro, M.; Interdonato, L.; Crupi, R.; Gugliandolo, E.; Di Paola, D.; Peritore, A.F.; Siracusa, R.; Impellizzeri, D.; et al. Modulation of NRF-2 Pathway Contributes to the Therapeutic Effects of Boswellia serrata Gum Resin Extract in a Model of Experimental Autoimmune Myocarditis. Antioxidants 2022, 11, 2129. https://doi.org/10.3390/antiox11112129
D’Amico R, Fusco R, Cordaro M, Interdonato L, Crupi R, Gugliandolo E, Di Paola D, Peritore AF, Siracusa R, Impellizzeri D, et al. Modulation of NRF-2 Pathway Contributes to the Therapeutic Effects of Boswellia serrata Gum Resin Extract in a Model of Experimental Autoimmune Myocarditis. Antioxidants. 2022; 11(11):2129. https://doi.org/10.3390/antiox11112129
Chicago/Turabian StyleD’Amico, Ramona, Roberta Fusco, Marika Cordaro, Livia Interdonato, Rosalia Crupi, Enrico Gugliandolo, Davide Di Paola, Alessio Filippo Peritore, Rosalba Siracusa, Daniela Impellizzeri, and et al. 2022. "Modulation of NRF-2 Pathway Contributes to the Therapeutic Effects of Boswellia serrata Gum Resin Extract in a Model of Experimental Autoimmune Myocarditis" Antioxidants 11, no. 11: 2129. https://doi.org/10.3390/antiox11112129
APA StyleD’Amico, R., Fusco, R., Cordaro, M., Interdonato, L., Crupi, R., Gugliandolo, E., Di Paola, D., Peritore, A. F., Siracusa, R., Impellizzeri, D., Cuzzocrea, S., & Di Paola, R. (2022). Modulation of NRF-2 Pathway Contributes to the Therapeutic Effects of Boswellia serrata Gum Resin Extract in a Model of Experimental Autoimmune Myocarditis. Antioxidants, 11(11), 2129. https://doi.org/10.3390/antiox11112129











