Vitamin D Mitigates Hyperglycemia-Induced Cognition Decline in Danio rerio (Zebrafish) through the Activation of Antioxidant Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessment of Learning and Memory
2.2. Treatment with Vitamin-D and Sulforaphane
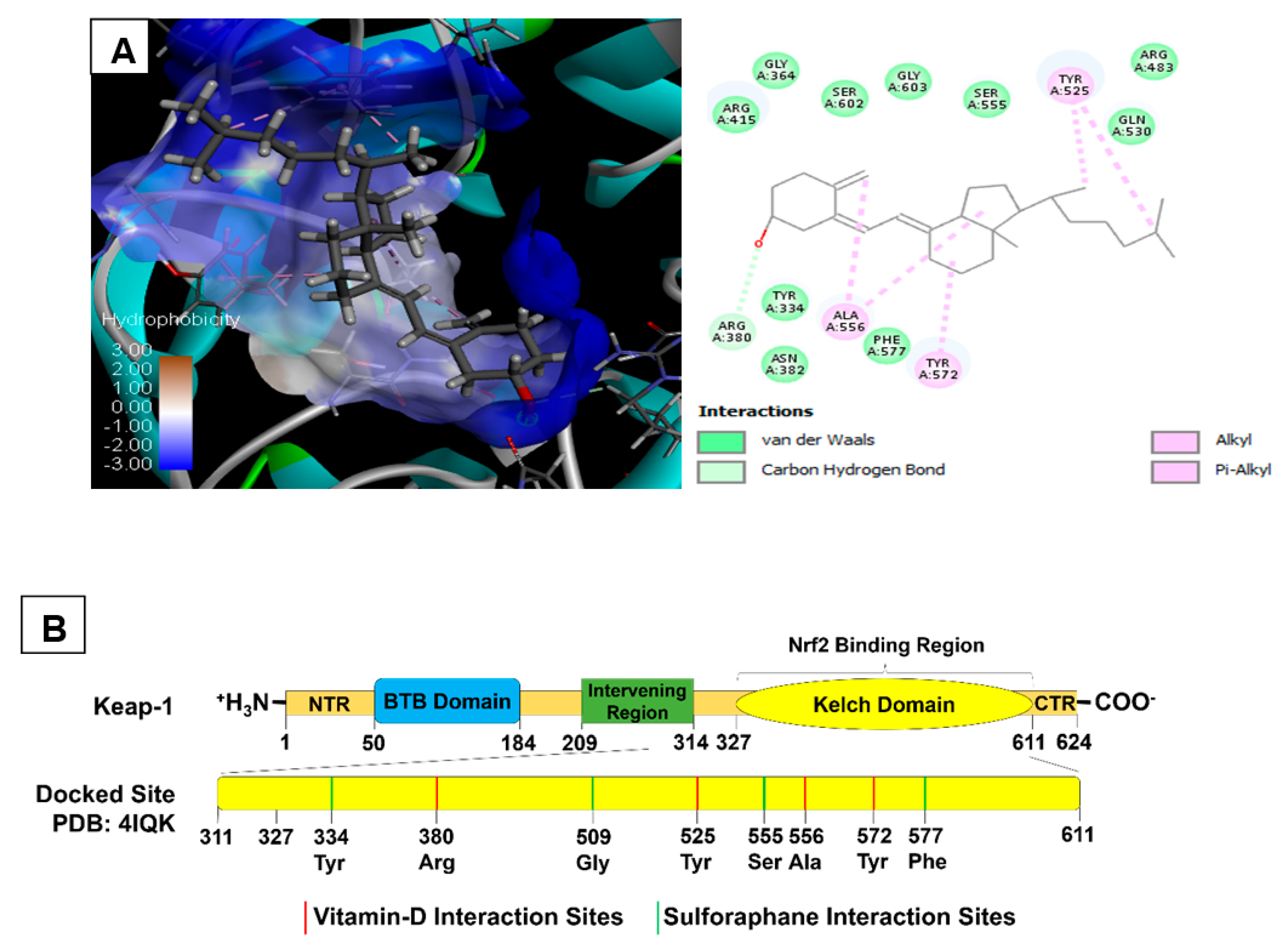
2.3. Molecular Docking of Vitamin D3 and Known Activators of Nrf2
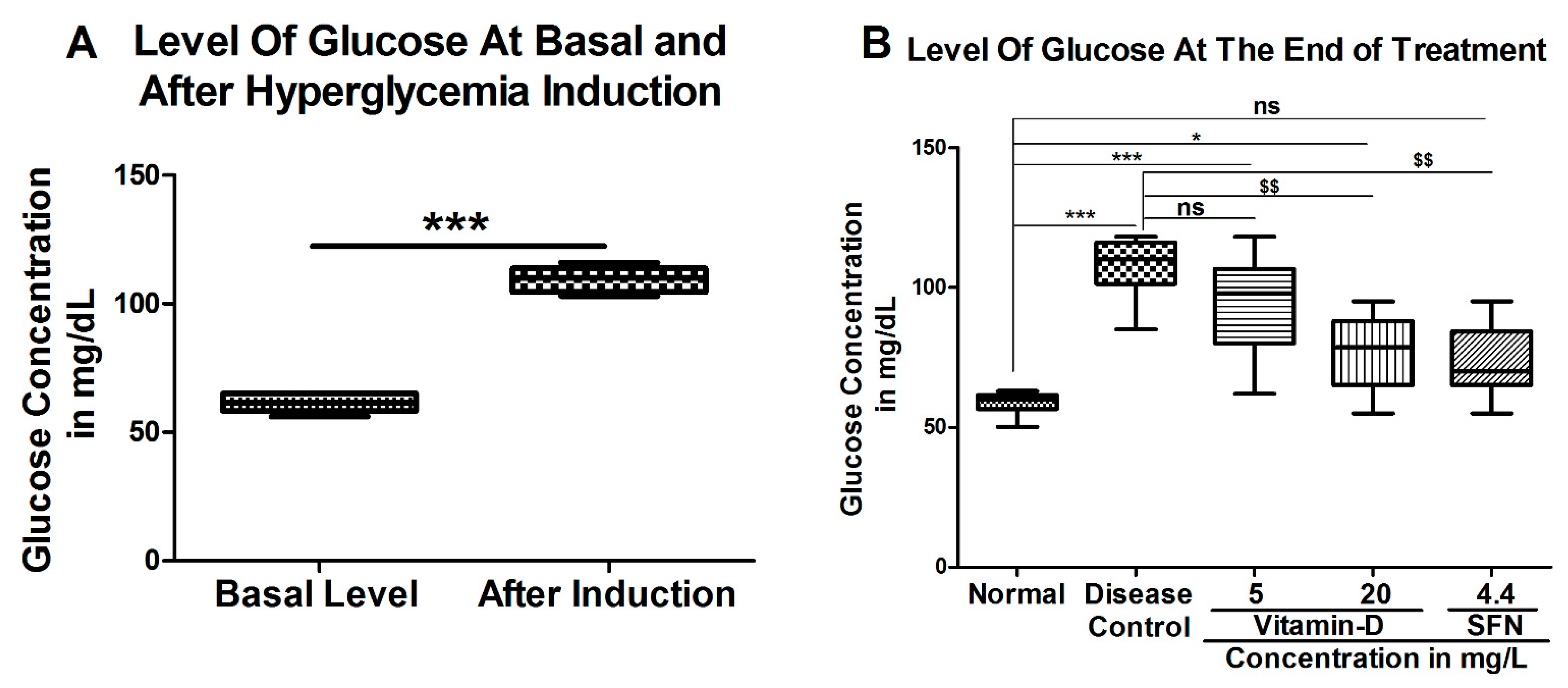
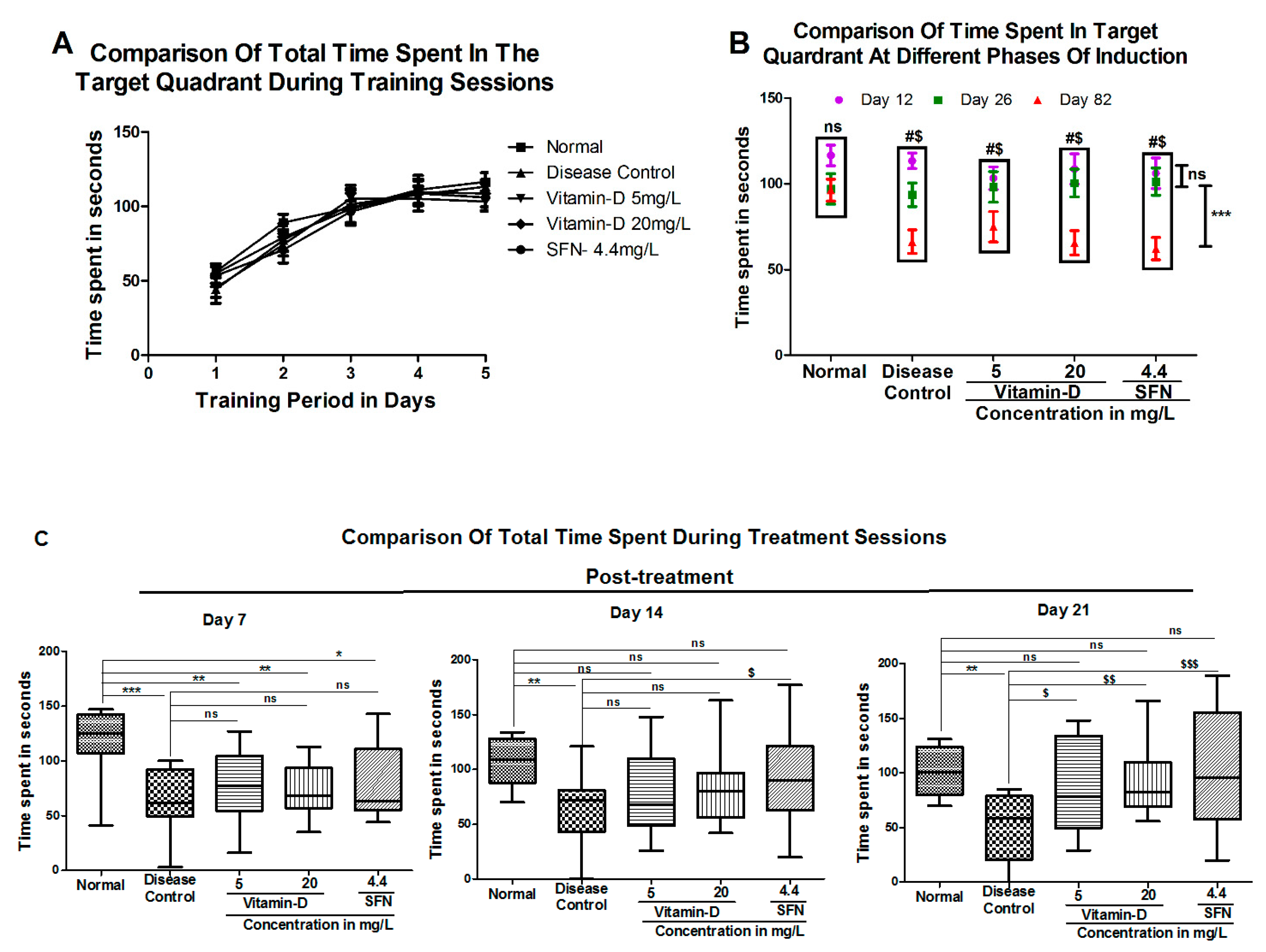
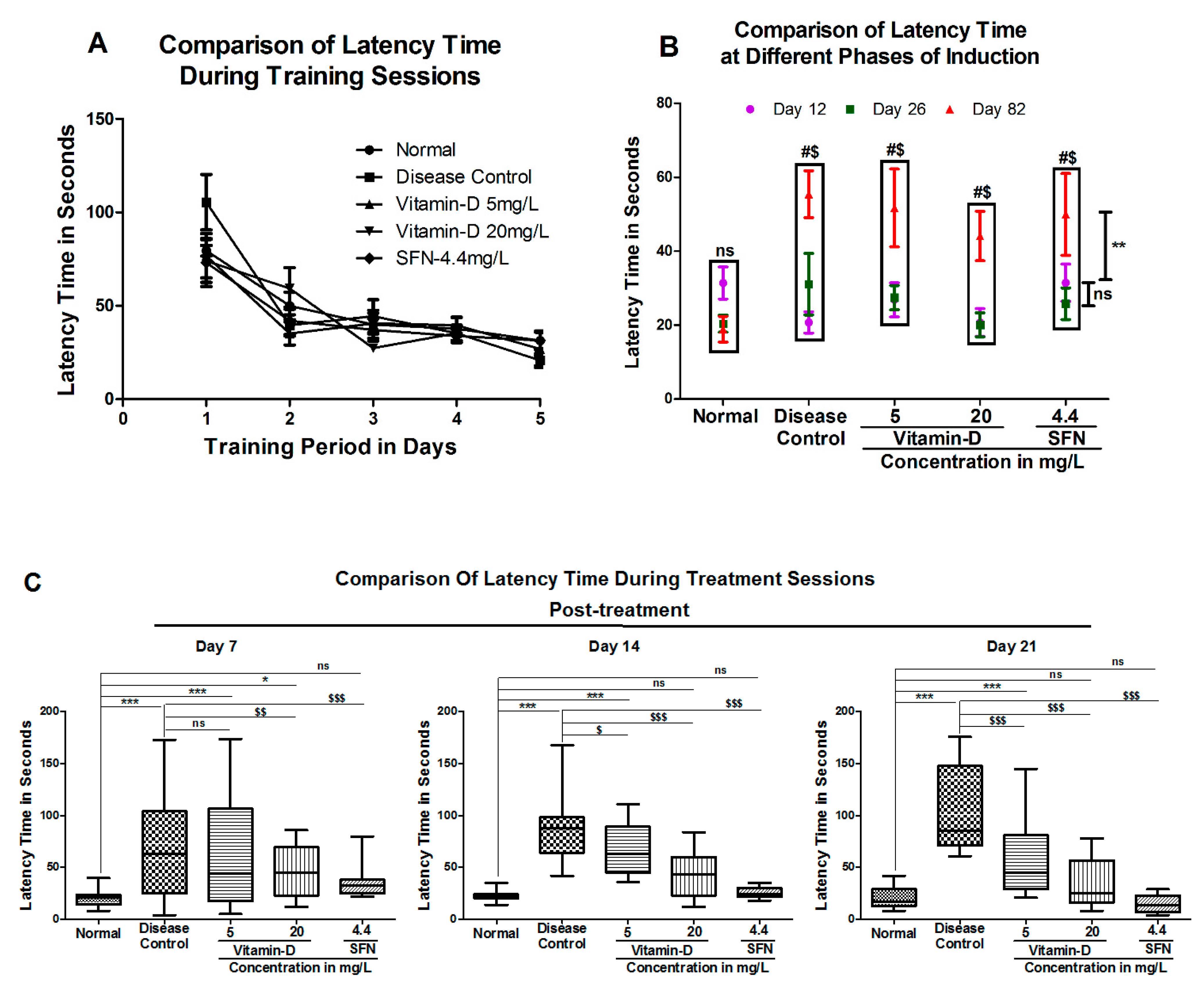
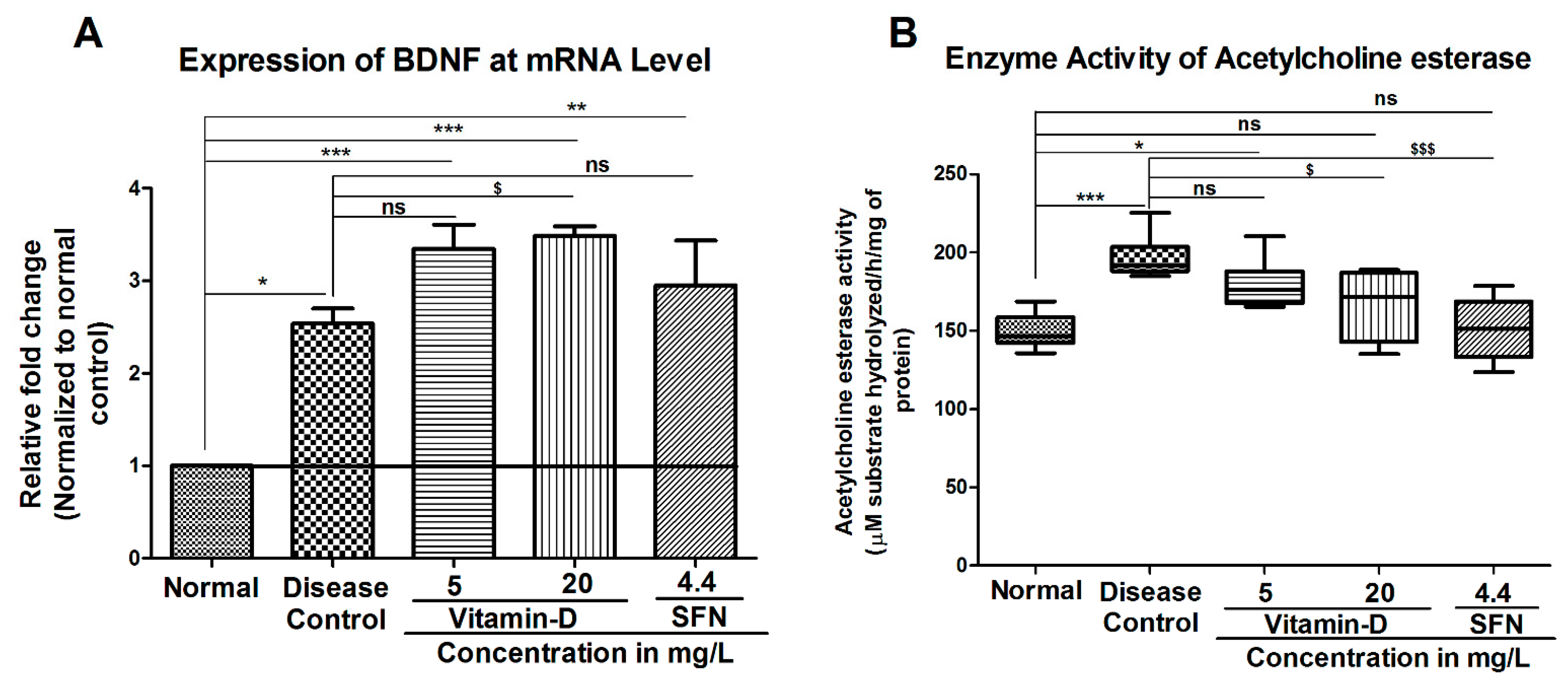
3. Results
3.1. Vitamin D and Sulforaphane Reduced the Latency Time in Hyperglycemic Zebrafish
3.2. Treatment with Vitamin-D Modulated the Expression of Brain Derived Neurotrophic Factor (BDNF) and Acetylcholine Esterase Activity in Zebrafish
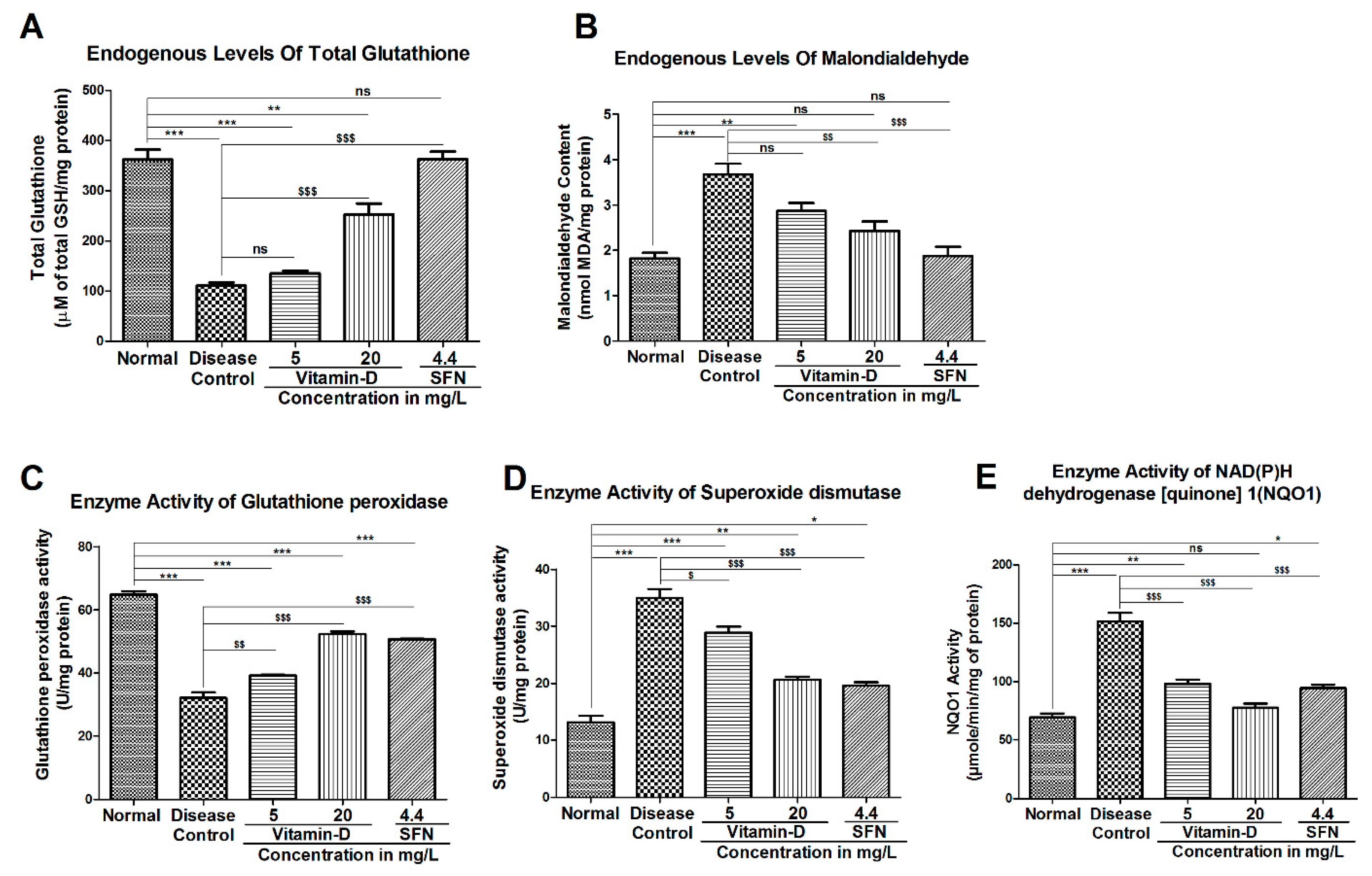
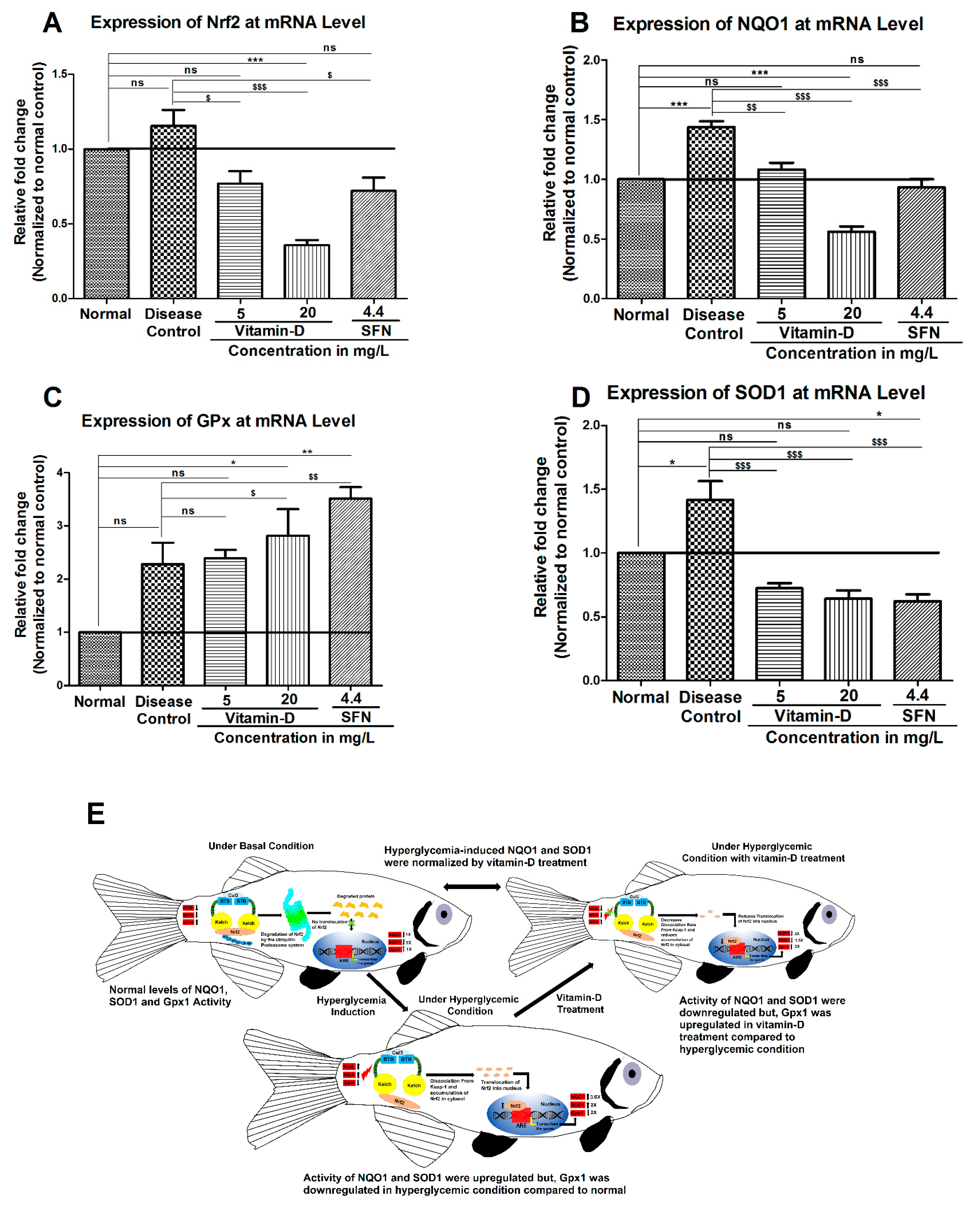
3.3. Vitamin-D and Sulforaphane Have Modulated The Hyperglycemia-Induced Oxidative Stress in Zebrafish
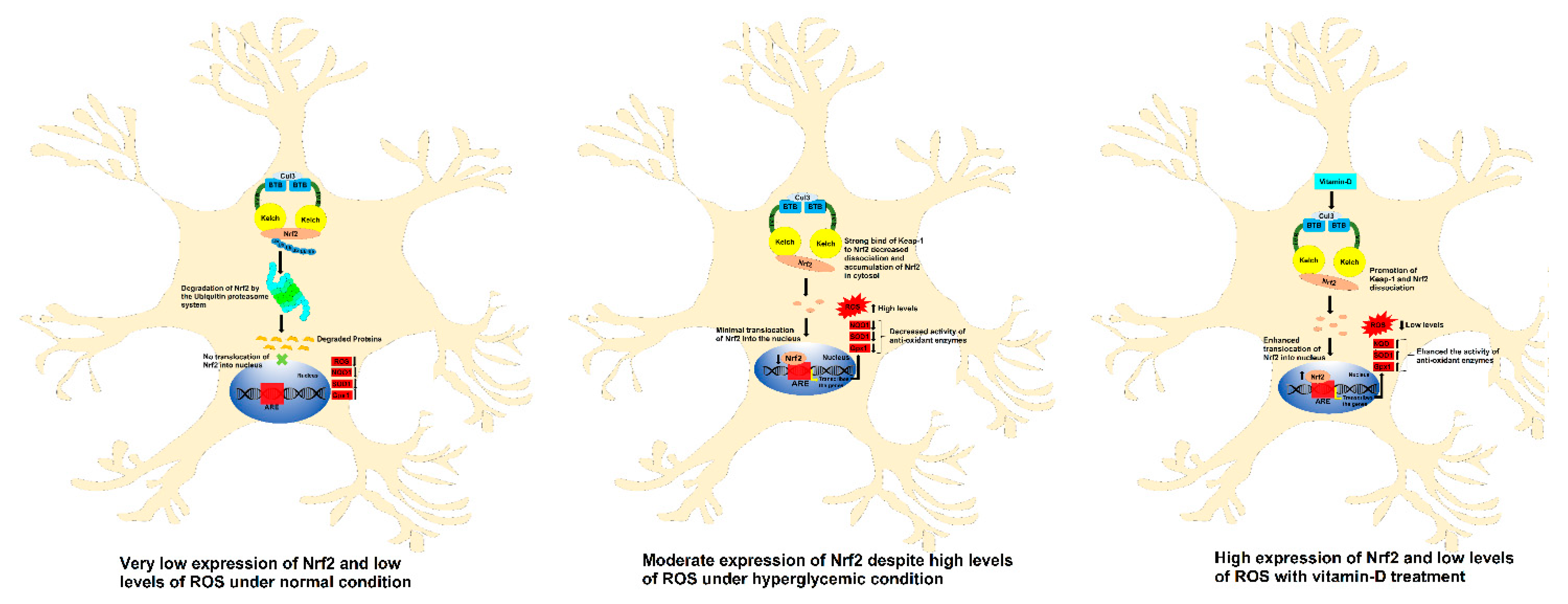
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF. IDF Diabetes Atlas Tenth Edition. Available online: www.diabetesatlas.org (accessed on 1 April 2017).
- Dyck, P.J.; Kratz, K.; Karnes, J.; Litchy, W.J.; Klein, R.; Pach, J.; Wilson, D.; O’brien, P.; Melton, L. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: The Rochester Diabetic Neuropathy Study. Neurology 1993, 43, 817. [Google Scholar] [CrossRef] [PubMed]
- Ihnat, M.; Thorpe, J.; Kamat, C.; Szabo, C.; Green, D.; Warnke, L.; Lacza, Z.; Cselenyak, A.; Ross, K.; Shakir, S. Reactive oxygen species mediate a cellular ‘memory’of high glucose stress signalling. Diabetologia 2007, 50, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Ciudin, A.; Espinosa, A.; Simo-Servat, O.; Ruiz, A.; Alegret, M.; Hernandez, C.; Boada, M.; Simo, R. Type 2 diabetes is an independent risk factor for dementia conversion in patients with mild cognitive impairment. J. Diabetes Its Complicat. 2017, 31, 1272–1274. [Google Scholar] [CrossRef]
- Güemes, A.; Georgiou, P. Review of the role of the nervous system in glucose homoeostasis and future perspectives towards the management of diabetes. Bioelectron. Med. 2018, 4, 9. [Google Scholar] [CrossRef]
- Giordani, B.; Foster, N.; Minoshima, S.; Lajiness-Oneill, R.; Koeppe, R.; Kuhl, D. Neuropsychological function and cerebral glucose utilization in isolated memory impairment and Alzheimers disease. J. Psychiatr. Res. 1999, 33, 7–16. [Google Scholar]
- Soto, M.; Cai, W.; Konishi, M.; Kahn, C.R. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proc. Natl. Acad. Sci. USA 2019, 116, 6379–6384. [Google Scholar] [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Sherin, A.; Anu, J.; Peeyush, K.; Smijin, S.; Anitha, M.; Roshni, B.; Paulose, C. Cholinergic and GABAergic receptor functional deficit in the hippocampus of insulin-induced hypoglycemic and streptozotocin-induced diabetic rats. Neuroscience 2012, 202, 69–76. [Google Scholar] [CrossRef]
- Intine, R.V.; Olsen, A.S.; Sarras, M.P., Jr. A zebrafish model of diabetes mellitus and metabolic memory. JoVE 2013, 72, e50232. [Google Scholar] [CrossRef]
- Capiotti, K.M.; Junior, R.A.; Kist, L.W.; Bogo, M.R.; Bonan, C.D.; Da Silva, R.S. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 171, 58–65. [Google Scholar] [CrossRef]
- Brown, J.; Bianco, J.I.; McGrath, J.J.; Eyles, D.W. 1, 25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci. Lett. 2003, 343, 139–143. [Google Scholar] [CrossRef]
- Moore, M.; Piazza, A.; McCartney, Y.; Lynch, M. Evidence That Vitamin D3 REVERSES Age-Related Inflammatory Changes in the Rat Hippocampus; Portland Press Ltd.: London, UK, 2005. [Google Scholar]
- Grimm, M.O.; Thiel, A.; Lauer, A.A.; Winkler, J.; Lehmann, J.; Regner, L.; Nelke, C.; Janitschke, D.; Benoist, C.; Streidenberger, O. Vitamin D and its analogues decrease amyloid-β (Aβ) formation and increase Aβ-degradation. Int. J. Mol. Sci. 2017, 18, 2764. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Gao, F.; Wu, R.; Dong, T.; Gu, C.; Lin, Q.; Zhang, Y. Vitamin D deficiency as a risk factor for dementia and Alzheimer’s disease: An updated meta-analysis. BMC Neurol. 2019, 19, 284. [Google Scholar] [CrossRef] [PubMed]
- Olsson, E.; Byberg, L.; Karlström, B.; Cederholm, T.; Melhus, H.; Sjögren, P.; Kilander, L. Vitamin D is not associated with incident dementia or cognitive impairment: An 18-y follow-up study in community-living old men. Am. J. Clin. Nutr. 2017, 105, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Fantino, B.; Gautier, J.; Beaudenon, M.; Thiery, S.; Beauchet, O. Cognitive effects of vitamin D supplementation in older outpatients visiting a memory clinic: A pre–post study. J. Am. Geriatr. Soc. 2012, 60, 793–795. [Google Scholar] [CrossRef]
- Sultan, S.; Taimuri, U.; Basnan, S.A.; Ai-Orabi, W.K.; Awadallah, A.; Almowald, F.; Hazazi, A. Low vitamin D and its association with cognitive impairment and dementia. J. Aging Res. 2020, 2020, 6097820. [Google Scholar] [CrossRef]
- Lips, P.; Eekhoff, M.; van Schoor, N.; Oosterwerff, M.; de Jongh, R.; Krul-Poel, Y.; Simsek, S. Vitamin D and type 2 diabetes. J. Steroid Biochem. Mol. Biol. 2017, 173, 280–285. [Google Scholar] [CrossRef]
- Husemoen, L.L.N.; Thuesen, B.H.; Fenger, M.; Jørgensen, T.; Glümer, C.; Svensson, J.; Ovesen, L.; Witte, D.R.; Linneberg, A. Serum 25 (OH) D and type 2 diabetes association in a general population: A prospective study. Diabetes Care 2012, 35, 1695–1700. [Google Scholar] [CrossRef]
- Knekt, P.; Laaksonen, M.; Mattila, C.; Härkänen, T.; Marniemi, J.; Heliövaara, M.; Rissanen, H.; Montonen, J.; Reunanen, A. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology 2008, 19, 666–671. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Ashraf, A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int. J. Endocrinol. 2010, 2010, 351385. [Google Scholar] [CrossRef]
- Nakai, K.; Fujii, H.; Kono, K.; Goto, S.; Kitazawa, R.; Kitazawa, S.; Hirata, M.; Shinohara, M.; Fukagawa, M.; Nishi, S. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am. J. Hypertens. 2014, 27, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Kundap, U.P.; Kumari, Y.; Othman, I.; Shaikh, M.F. Zebrafish as a model for epilepsy-induced cognitive dysfunction: A pharmacological, biochemical and behavioral approach. Front. Pharmacol. 2017, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Townsend, B.E.; Johnson, R.W. Sulforaphane induces Nrf2 target genes and attenuates inflammatory gene expression in microglia from brain of young adult and aged mice. Exp. Gerontol. 2016, 73, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Othman, I.; Shaikh, M.F. Anti-High Mobility Group Box-1 Monoclonal Antibody Attenuates Seizure-Induced Cognitive Decline by Suppressing Neuroinflammation in an Adult Zebrafish Model. Front. Pharmacol. 2021, 11, 613009. [Google Scholar] [CrossRef]
- Bishir, M.; Aslam, M.; Bhat, A.; Ray, B.; Elumalai, P.; Rashan, L.; Yang, J.; Chang, S.L.; Essa, M.M.; Sakharkar, M.K. Acetylsalicylic acid improves cognitive performance in sleep deprived adult Zebrafish (Danio rerio) model. Front. Biosci.-Landmark 2021, 26, 114–124. [Google Scholar]
- Gleeson, M.; Connaughton, V.; Arneson, L. Induction of hyperglycaemia in zebrafish (Danio rerio) leads to morphological changes in the retina. Acta Diabetol. 2007, 44, 157–163. [Google Scholar] [CrossRef]
- Connaughton, V.P.; Baker, C.; Fonde, L.; Gerardi, E.; Slack, C. Alternate immersion in an external glucose solution differentially affects blood sugar values in older versus younger zebrafish adults. Zebrafish 2016, 13, 87–94. [Google Scholar] [CrossRef]
- OECD203. 2019. Available online: https://www.oecd-ilibrary.org/environment/test-no-203-fish-acute-toxicity-test_9789264069961-en;jsessionid=U15sP-w3nD_H6GjkK1-twflC.ip-10-240-5-39 (accessed on 24 March 2020).
- Rajesh, V.; Ilanthalir, S. Cognition enhancing activity of sulforaphane against scopolamine induced cognitive impairment in zebra fish (Danio rerio). Neurochem. Res. 2016, 41, 2538–2548. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Ozdemır, G.; Ozden, M.; Maral, H.; Kuskay, S.; Cetınalp, P.; Tarkun, I. Malondialdehyde, glutathione, glutathione peroxidase and homocysteine levels in type 2 diabetic patients with and without microalbuminuria. Ann. Clin. Biochem. 2005, 42, 99–104. [Google Scholar] [CrossRef]
- Kussmaul, L.; Hirst, J. The mechanism of superoxide production by NADH: Ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl. Acad. Sci. USA 2006, 103, 7607–7612. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, H.J.; Santamaria, A.B. Direct measurement of NAD (P) H: Quinone reductase from cells cultured in microtiter wells: A screening assay for anticarcinogenic enzyme inducers. Anal. Biochem. 1988, 169, 328–336. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Odukoya, O.; Sofidiya, M.; Ilori, O.; Gbededo, M.; Ajadotuigwe, J.; Olaleye, O.; Bouskela, E.; Cyrino, F.; Marcelon, G.; Brinkhaus, B. Malondialdehyde determination as index of lipid peroxidation. Int. J. Biol. Chem 1994, 3, 281–285. [Google Scholar]
- Zilliox, L.A.; Chadrasekaran, K.; Kwan, J.Y.; Russell, J.W. Diabetes and cognitive impairment. Curr. Diabetes Rep. 2016, 16, 87. [Google Scholar] [CrossRef]
- Ngoc Hieu, B.T.; Ngoc Anh, N.T.; Audira, G.; Juniardi, S.; Liman, R.A.D.; Villaflores, O.B.; Lai, Y.-H.; Chen, J.-R.; Liang, S.-T.; Huang, J.-C. Development of a modified three-day t-maze protocol for evaluating learning and memory capacity of adult zebrafish. Int. J. Mol. Sci. 2020, 21, 1464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, L.; Yu, L.; Lin, B.; Hou, Y.; Wu, J.; Huang, Q.; Han, Y.; Guo, L.; Ouyang, Q. Acetylcholinesterase is associated with apoptosis in β cells and contributes to insulin-dependent diabetes mellitus pathogenesis. Acta Biochim. Biophys. Sin. 2012, 44, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Yoneda, M.; Nakajima, A.; Terauchi, Y. Serum butyrylcholinesterase is strongly associated with adiposity, the serum lipid profile and insulin resistance. Intern. Med. 2007, 46, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Bruce, C.R.; Carey, A.L.; Hawley, J.A.; Febbraio, M.A. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: Evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes 2003, 52, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B. Pathophysiology of oxidative stress in diabetes mellitus. J. Diabetes Its Complicat. 2001, 15, 203–210. [Google Scholar] [CrossRef]
- Savaskan, N.E.; Ufer, C.; Kühn, H.; Borchert, A. Molecular Biology of Glutathione Peroxidase 4: From Genomic Structure to Developmental Expression and Neural Function; De Gruyter Publisher: Berlin, Germany, 2007; pp. 1007–1017. [Google Scholar]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-e20. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Haffner, C.D. Linear relationships between the ligand binding energy and the activation energy of time-dependent inhibition of steroid 5α-reductase by Δ1-4-Azasteroids. J. Biol. Chem. 2001, 276, 21359–21364. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. S1), S62–S69. [Google Scholar] [CrossRef]
- Lyons, T.J.; Jenkins, A.J. Glycation, oxidation, and lipoxidation in the development of the complications of diabetes: A carbonyl stress hypothesis. Diabetes Rev. 1997, 5, 365. [Google Scholar]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Shehwana, H.; Konu, O. Comparative transcriptomics between zebrafish and mammals: A roadmap for discovery of conserved and unique signaling pathways in physiology and disease. Front. Cell Dev. Biol. 2019, 7, 5. [Google Scholar] [CrossRef]
- Ruhl, T.; Jonas, A.; Seidel, N.I.; Prinz, N.; Albayram, O.; Bilkei-Gorzo, A.; von der Emde, G. Oxidation and cognitive impairment in the aging zebrafish. Gerontology 2016, 62, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Bagle, S.; Muke, S.; Peshattiwar, V.; Kaikini, A.; Sathaye, S. Nootropic, Neuroprotective and Anti-oxidant Role of Apocynin in Scopolamine Induced Memory Deficit in a Zebrafish Model. J. Pharm. Pharm. Res. 2021, 4, 1. [Google Scholar]
- Ranjan, S.; Sharma, P.K. Study of learning and memory in type 2 diabetic model of zebrafish (Danio rerio). Endocr. Metab. Sci. 2020, 1, 100058. [Google Scholar] [CrossRef]
- Dos Santos, M.M.; de Macedo, G.T.; Prestes, A.S.; Loro, V.L.; Heidrich, G.M.; Picoloto, R.S.; Rosemberg, D.B.; Barbosa, N.V. Hyperglycemia elicits anxiety-like behaviors in zebrafish: Protective role of dietary diphenyl diselenide. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 85, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.V.; Frier, B.M.; Strachan, M.W. The relationship between type 2 diabetes and cognitive dysfunction: Longitudinal studies and their methodological limitations. Eur. J. Pharmacol. 2004, 490, 169–175. [Google Scholar] [CrossRef]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Uthaiah, C.A.; CM, R.; Madhunapantula, S.V.; Salimath, P.V.; MR, K. Comparative assessment of cognitive impairment and oxidative stress markers among vitamin D insufficient elderly patients with and without type 2 diabetes mellitus (T2DM). PLoS ONE 2022, 17, e0269394. [Google Scholar]
- Bayani, M.A.; Akbari, R.; Banasaz, B.; Saeedi, F. Status of Vitamin-D in diabetic patients. Casp. J. Intern. Med. 2014, 5, 40. [Google Scholar]
- Van der Schaft, J.; Koek, H.; Dijkstra, E.; Verhaar, H.; Van Der Schouw, Y.; Emmelot-Vonk, M. The association between vitamin D and cognition: A systematic review. Ageing Res. Rev. 2013, 12, 1013–1023. [Google Scholar] [CrossRef]
- SanMartín, C.D.; Henríquez, M.; Chacón, C.; Ponce, D.P.; Salech, F.; Rogers, N.K.; Behrens, M.I. Vitamin D increases Aβ140 plasma levels and protects lymphocytes from oxidative death in mild cognitive impairment patients. Curr. Alzheimer Res. 2018, 15, 561–569. [Google Scholar] [CrossRef]
- Mayne, P.E.; Burne, T.H. Vitamin D in synaptic plasticity, cognitive function, and neuropsychiatric illness. Trends Neurosci. 2019, 42, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Brolsma, E.M.; de Groot, L.C. Vitamin D and cognition in older adults: An update of recent findings. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.-F.; Liu, X.-Y.; Zhou, D.-H.; Du, X.; Yin, G.; Zhang, Y.; Fang, H.; Xu, G.; Soares, J.C.; Zhang, X.Y. Cognition, serum BDNF levels, and BDNF Val66Met polymorphism in type 2 diabetes patients and healthy controls. Oncotarget 2018, 9, 3653. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, L. Celecoxib alleviates memory deficits by downregulation of COX-2 expression and upregulation of the BDNF-TrkB signaling pathway in a diabetic rat model. J. Mol. Neurosci. 2017, 62, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhu, Y.; He, T.; Li, W.; Li, Q.; Miao, Y. Brain-derived neurotrophic factor inhibits hyperglycemia-induced apoptosis and downregulation of synaptic plasticity-related proteins in hippocampal neurons via the PI3K/Akt pathway. Int. J. Mol. Med. 2019, 43, 294–304. [Google Scholar] [CrossRef]
- Vincent, A.M.; Mclean, L.L.; Backus, C.; Feldman, E.L. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005, 19, 1–24. [Google Scholar] [CrossRef]
- Haider, S.; Saleem, S.; Perveen, T.; Tabassum, S.; Batool, Z.; Sadir, S.; Liaquat, L.; Madiha, S. Age-related learning and memory deficits in rats: Role of altered brain neurotransmitters, acetylcholinesterase activity and changes in antioxidant defense system. Age 2014, 36, 1291–1302. [Google Scholar] [CrossRef]
- Danışman, B.; Akçay, G.; Gökçek-Saraç, Ç.; Kantar, D.; Aslan, M.; Derin, N. The Role of Acetylcholine on the Effects of Different Doses of Sulfite in Learning and Memory. Neurochem. Res. 2022, 47, 3331–3343. [Google Scholar] [CrossRef]
- Balint, T.; Szegletes, T.; Szegletes, Z.; Halasy, K.; Nemcsók, J. Biochemical and subcellular changes in carp exposed to the organophosphorus methidathion and the pyrethroid deltamethrin. Aquat. Toxicol. 1995, 33, 279–295. [Google Scholar] [CrossRef]
- Lima, L.A.; Lopes, M.J.P.; Costa, R.O.; Lima, F.A.V.; Neves, K.R.T.; Calou, I.B.; Andrade, G.M.; Viana, G.S. Vitamin D protects dopaminergic neurons against neuroinflammation and oxidative stress in hemiparkinsonian rats. J. Neuroinflamm. 2018, 15, 249. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Mesgari-Abbasi, M.; Nameni, G.; Hajiluian, G.; Shahabi, P. The effects of vitamin D administration on brain inflammatory markers in high fat diet induced obese rats. BMC Neurosci. 2017, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Noberasco, G.; Odetti, P.; Boeri, D.; Maiello, M.; Adezati, L. Malondialdehyde (MDA) level in diabetic subjects. Relationship with blood glucose and glycosylated hemoglobin. Biomed. Pharmacother. 1991, 45, 193–196. [Google Scholar] [CrossRef]
- Nellore, J.; Pauline, P.; Mohanan, S.P.; Ravikumar, R.; Pillai, R.C. Vinca rosea normalizes oxidative stress and inhibits hyperglycemia induced increase in VEGF in zebrafish retina. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 927–936. [Google Scholar]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Koh, Y.H.; Park, Y.S.; Fujiwara, N.; Sakiyama, H.; Misonou, Y.; Ookawara, T.; Suzuki, K.; Honke, K.; Taniguchi, N. Oxidative Stress Caused by Inactivation of Glutathione Peroxidase and Adaptive Responses; De Gruyter: Berlin, Germany, 2003. [Google Scholar]
- Kakkar, R.; Kalra, J.; Mantha, S.V.; Prasad, K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol. Cell. Biochem. 1995, 151, 113–119. [Google Scholar] [CrossRef]
- Patel, U.N.; Patel, U.D.; Khadayata, A.V.; Vaja, R.K.; Patel, H.B.; Modi, C.M. Assessment of neurotoxicity following single and co-exposure of cadmium and mercury in adult zebrafish: Behavior alterations, oxidative stress, gene expression, and histological impairment in brain. Water Air Soil Pollut. 2021, 232, 340. [Google Scholar] [CrossRef]

| Gene | Forward Sequence (5′ → 3′) | Reverse Sequence (5′ → 3′) | Product Length (bp) | Annealing Temperature (°C) | Accession Number |
|---|---|---|---|---|---|
| NRF2 | GAGCGGGAGAAATCACACAGAATG | CAGGAGCTGCATGCACTCATCG | 82 | 65 | AF057040 |
| GPX1 | CAGATGAACGAGCTCCACAG | CCATTCACTTCCAGCTTCTCC | 185 | 58.5 | NM_001007281 |
| NQO1 | CGAGATGTTGCAGTTCAGGC | ATCGACCCTCTTTCCATGCA | 175 | 53 | NM_205542 |
| SOD1 | CGCACTTCAACCCTCATGAC | TGAATCACCATGGTCCTCCC | 137 | 50 | NM_131294 |
| BDNF | CGCTCACCATGTCATCCAAC | TCTGCGATATTCGTCCGCTC | 173 | 58.5 | NM_131595.2 |
| β-ACTIN | CGAGCAGGAGATGGGAACC | CAACGGAAACGCTCATTGC | 102 | 60 | AF057040 |
| Selected Biomolecules | Protein & PDB ID | Docking Score | Nature of Interaction(s) | Active Site Amino Acids |
|---|---|---|---|---|
| Cholecalciferol | Keap1 (4IQK) | −56.45 | Carbon Hydrogen Bond | Arg380 |
| Alkyl | Ala556, Tyr525, Tyr572 | |||
| Sulforaphane | −24.82 | Carbon Hydrogen Bond | Ser555, Gly509 | |
| Pi-sulfur | Phe577, Tyr334 | |||
| Pterostilbene | −37.79 | Carbon Hydrogen Bond | Arg530, Gln530 | |
| Pi-Alkyl | Tyr572, Tyr525 | |||
| Conventional Hydrogen bond | Ser336, Ser508 | |||
| Bardoxolone | −16.79 | Conventional Hydrogen bond | Tyr572, Asn382, Arg380, Arg415 | |
| Pi-Alkyl | Tyr334, Arg415 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uthaiah, C.A.; Devaru, N.C.; Shivakumar, N.H.; R, R.; Madhunapantula, S.V. Vitamin D Mitigates Hyperglycemia-Induced Cognition Decline in Danio rerio (Zebrafish) through the Activation of Antioxidant Mechanisms. Antioxidants 2022, 11, 2114. https://doi.org/10.3390/antiox11112114
Uthaiah CA, Devaru NC, Shivakumar NH, R R, Madhunapantula SV. Vitamin D Mitigates Hyperglycemia-Induced Cognition Decline in Danio rerio (Zebrafish) through the Activation of Antioxidant Mechanisms. Antioxidants. 2022; 11(11):2114. https://doi.org/10.3390/antiox11112114
Chicago/Turabian StyleUthaiah, Chinnappa A., Nandini C. Devaru, Nandini H. Shivakumar, Rajalakshmi R, and SubbaRao V. Madhunapantula. 2022. "Vitamin D Mitigates Hyperglycemia-Induced Cognition Decline in Danio rerio (Zebrafish) through the Activation of Antioxidant Mechanisms" Antioxidants 11, no. 11: 2114. https://doi.org/10.3390/antiox11112114
APA StyleUthaiah, C. A., Devaru, N. C., Shivakumar, N. H., R, R., & Madhunapantula, S. V. (2022). Vitamin D Mitigates Hyperglycemia-Induced Cognition Decline in Danio rerio (Zebrafish) through the Activation of Antioxidant Mechanisms. Antioxidants, 11(11), 2114. https://doi.org/10.3390/antiox11112114






