1. Introduction
The consumption of chicken has recently increased worldwide due to its relatively lower fat content, high nutritional value and distinct flavor. However, broilers are exposed to frequent challenges such as pathogenic infection, drug abuse and other external elements in modern intensive production, which could induce oxidative damage and immunological stress [
1,
2]. Under a stress response, birds are prone to infecting intestinal diseases, which impairs the body’s health and production performance and further leads to a decline in the efficiency of poultry production [
3,
4]. Lipopolysaccharide (LPS) is one of the components that make up the outer membrane of gram-negative bacteria that could lead to acute systemic inflammation and trigger an imbalance between oxidation and antioxidant defense systems in broilers [
2,
5]. The injection of LPS in broilers is considered to be a suitable experimental model for investigating systemic inflammation response and oxidative damage in poultry science [
5,
6]. LPS could cause damage to normal nutrient absorption and mucosal integrity in the small intestine and interfere with the structure and metabolism of cecal microflora in broilers, which could lead to compromised growth performance [
7,
8]. However, in the European Union and some other countries including China, the use of antibiotic growth promoters has been forbidden in livestock feed. LPS-induced excessive immune response and oxidative damage of broilers could be alleviated by feed additives including vitamins and plant extracts, etc. [
9,
10]. Obviously, it is important to develop a novel and effective feed additive for regulating the immune status and antioxidant capacity of broilers under stress responses.
In recent years, 25-hydroxycholecalciferol (25OHD
3) has been becoming more popular based on its commercial production and higher bioavailability. In comparison with vitamin D
3, adding 25OHD
3 to diets could avoid a 25-hydroxylation reaction due to the presence of a hydroxyl group, which indicates that exogenous supplementation of 25OHD
3 is directly used by animals in a ready-to-use active form [
11]. Except for the traditional role of maintaining optimal calcium and phosphorus homeostasis, vitamin D has beneficial effects on modulating the body’s immunity, antioxidant status and gut health [
12,
13,
14]. Moreover, 25OHD
3 could regulate the microbial community in the hindgut of monogastric animals [
15]. However, whether 25OHD
3 exerts beneficial effects on blood antioxidant capacity, immune status, gut barrier function and the microbiota of broilers under the LPS challenge is not fully understood.
It is important to highlight that LPS was used as a model of health challenges and does not necessarily represent a potential problem for broiler chick production like bacterial, fungal, or viral infections released in inflammatory diseases. The principal hypothesis of the study is to show that 25OHD3 has antioxidant, immunological and gut health properties when broilers are challenged with LPS. Therefore, this study was conducted to evaluate the effects of 25OHD3 on the antioxidant potential, immune function, intestinal barrier and microbiota of broilers under the LPS challenge.
4. Discussion
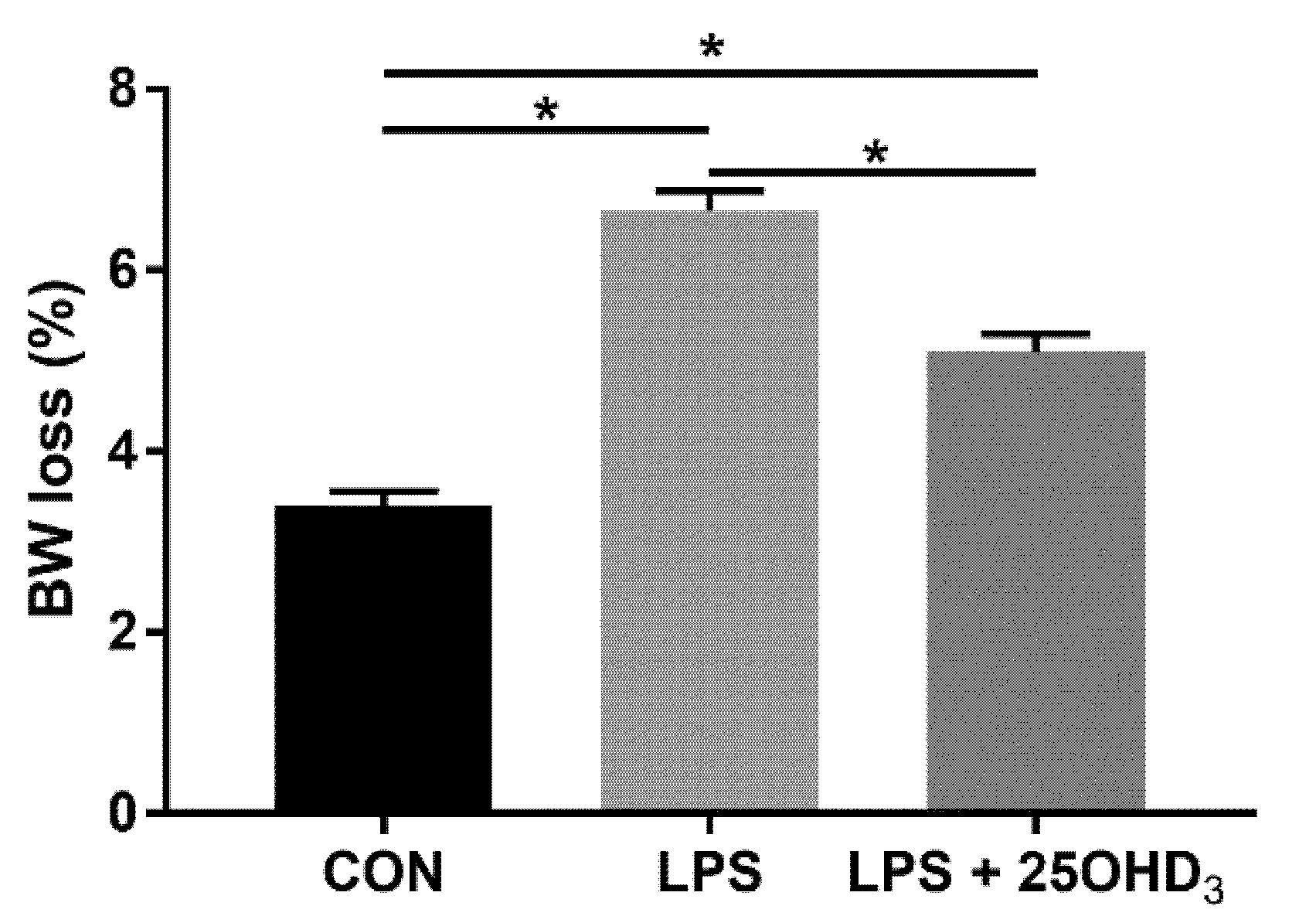
Live BW loss due to enhanced catabolism and decreased anabolism under stress response is of economic importance in poultry production and has been considered a predictable indicator of stress in broilers [
21,
22]. Recent studies have demonstrated that 25OHD
3 could improve the performance of broilers [
16,
23]. Our study showed that 25OHD
3 supplementation decreased the BW loss of broilers compared with the LPS group, indicating that 25OHD
3 had a stronger positive effect on the improvement of performance in broilers under LPS stimulation. The findings of this present study substantiate the results that 25OHD
3 supplementation alleviated the compromised performance of broilers during an experimental LPS injection [
9]. The changes in BW loss among the three treatments may be related to the changes in antioxidant status, immunity, intestinal barrier function and the microbial composition of broilers. Therefore, we further determined the effects of adding 25OHD
3 to diets on antioxidant potential, immune status and intestinal function of broilers under the LPS challenge to explore the underlying mechanisms.
In the poultry industry, broilers inevitably face the oxidative stress associated with bacteria or their products such as LPS, which could impair health status [
2]. Oxidative stress refers to the unbalanced status between the generation rate of reactive oxygen species (ROS) and their clearing by the body’s antioxidant system [
24]. Serum antioxidant status could indicate the host’s resistibility to oxidative damage, and a higher degree of antioxidant potential contributes to relieving oxidative stress [
25]. Wu et al. [
26] reported that LPS disturbed the balance status between oxidant and antioxidant systems, which led to oxidative damage to broilers. The previous study showed that 25OHD
3 could maintain the delicate balance between the oxidant and antioxidant systems of broilers by modulating the nonenzymatic antioxidant defense system reflected by T-AOC and serum activities of antioxidant enzymes including CAT and GSH-Px [
16]. This study showed that the LPS challenge significantly led to oxidative stress in broilers due to a decrease in serum activities of T-AOC, CAT and SOD, whereas, dietary 25OHD
3 supplementation significantly alleviated the LPS-induced decrease in the serum activity of SOD. Based on this result, we concluded that 25OHD
3 alleviates LPS-induced oxidative stress of broilers by stimulating the non-enzymatic and enzymatic antioxidant defensive systems.
The morphological structure is a major indicator to assess the absorption ability and mucosal integrity of the small intestine [
18]. Morphological changes in the small intestine, such as villus atrophy and crypt hyperplasia, indicate nutrient malabsorption and growth retardation in animals [
27]. Microbial challenges and their products such as LPS could induce marked changes in the morphological structure including shorter villus height and a lower ratio of villus height to crypt depth in the small intestine [
2,
28], and these changes, in turn, lead to growth retardation in broilers. The present study showed that 25OHD
3 supplementation significantly alleviated the LPS-induced decrease in villus height of the jejunum. Higher villus height caused by 25OHD
3 supplementation reflected the greater ability of nutrient digestion and absorption, thereby increasing performance in broilers. It has been acknowledged that putrescine has a certain modulatory effect on the potential mode of vitamin D regulating intestinal morphology and function [
29]. 1,25-dihydroxycholecalciferol [1,25(OH)
2D
3] is a pleiotropic steroid hormone that could improve the activities of spermidine N-acetyltransferase and ornithine decarboxylase used to catalyze the production of putrescine [
30,
31]. One possibility is that an increased 25OHD
3 level in serum may result in a higher 1,25(OH)
2D
3 level, and the other possible mode is that 25OHD
3 may act directly on vitamin D receptor (VDR) in a VDR independent action of 1,25(OH)
2D
3 [
32], although its affinity for VDR is less than 1,25(OH)
2D
3. However, the exact mechanism behind increased intestinal morphology caused by 25OHD
3 supplementation needs further research.
The intestinal epithelial barrier consists of epithelial cells and intercellular multiprotein complexes including ZO-1, occludin and claudin, which could prevent pathogens, antigens and toxins from passing through the intestinal lumen into the circulating system [
33]. Tight junction proteins are critical for regulating intestinal permeability and maintaining the integrity of the intestinal tissue. Moreover, DAO, D-lactate and endotoxin are considered as important indicators related to intestinal permeability, and their serum concentrations could indicate the intestinal barrier integrity [
34]. Chen et al. [
28] reported that the intraperitoneal LPS challenge impaired the intestinal barrier of broilers based on increased serum D-lactate levels and lower mRNA abundances of intestinal tight junction proteins. Our study showed that 25OHD
3 had the potential to alleviate the LPS-induced increase in serum D-lactate level, resulting in lower intestinal permeability and improved intestinal barrier in broilers. The present results also showed that the inclusion of 25OHD
3 markedly promoted Occludin mRNA expression in the mucosa of the jejunum and ileum compared with LPS group, which, once again, indicated an improved intestinal barrier. Overall, adding 25OHD
3 to diets could relieve intestinal barrier injury induced by the LPS challenge. Compared with vitamin D
3, 25OHD
3 could enhance 1α-hydroxylase activity in broilers. 1,25(OH)
2D
3 has been shown to protect the intestinal barrier from damage caused by the LPS challenge [
35]. The protective effects of 1,25(OH)
2D
3 on the intestinal barrier are mediated by VDR, and the receptor might play a vital role in the main pathway which modulates all of the factors including inflammation and microbiota composition [
35,
36].
The inflammatory cytokines have important effects on regulating intestinal immune response and are taken as important mediators for preventing or being susceptible to infection and certain intestinal disorders. The present study showed that the LPS challenge enhanced serum contents of TNF-α and IL-1β in comparison with the CON group. Our results from intestinal mucosa also showed that broilers in the LPS group had higher concentrations of TNF-α and IFN-γ. Our findings were similar to the previous results of Chen et al. [
28] that LPS stimulation enhanced the concentrations of pro-inflammatory cytokines in serum and the intestinal mucosa of broilers. The pro-inflammatory cytokines have been shown to impair the intestinal tight junction and disrupt the intestinal barrier [
37,
38]. Therefore, lower contents of pro-inflammatory cytokines in the LPS + 25-D group may be part of the reason for improving the intestinal barrier of broilers. In addition, our findings suggested that 25OHD
3 markedly enhanced the serum IgG level in broilers compared to the LPS group. Serum immunoglobulin levels reflect the immune function of animals to a certain extent, which could help relieve immunological stress, promote health status and improve growth performance [
39,
40]. In animals, 1α-hydroxylase has been identified in T-cells, B-cells, macrophages and the small intestine [
41,
42,
43,
44]. It was reported that immune cells and the small intestine may show a greater response to this secondary metabolite of vitamin D, 25OHD
3 [
45], which partly explained the improved humoral immunity of broilers. Overall, these results suggested that 25OHD
3 could promote the immunity of broilers after LPS stimulation by changing the production of inflammatory cytokines and immunoglobulins.
The cecal microbiota regulates nutritional metabolism and immune function, such as nitrogen cycling, providing their host with vitamins, amino acids and SCFA, and competitively preventing pathogenic infections [
46]. The microbial community in the cecum of broilers could be affected by several factors, such as diet compositions, feed additives, raising conditions and pathogenic microorganisms [
47]. In particular, the LPS challenge could result in significant changes in the cecal microbiota of broilers [
4,
7]. An adequate vitamin D level is considered as an essential factor for improving microbial composition in the intestine [
48]. The α-diversity is taken as the diversity of the intestinal microbiota within one sample, including species diversity (Shannon and Simpson) and species richness (Chao and Ace) [
15], and high α-diversity helps maintain stable gut ecosystems that inhibit pathogen proliferation [
49]. Our study showed that 25OHD
3 alleviated the LPS-induced decrease in the Shannon index of cecal digesta, revealing that 25OHD
3 could improve intestinal immunity and barrier function in broilers under the LPS challenge. The β-diversity shown by the PCA and UPGMA tree also suggested that cecal bacteria responded differently to different experimental groups. At the phylum level, the main bacteria in the cecal digesta were Firmicutes and Proteobacteria, which were consistent with the previous results described by Lucke et al. [
7]. Firmicutes are closely related to energy metabolism and anti-inflammatory response, and a lower abundance of Firmicutes has a relationship with the occurrence of intestinal inflammation [
50,
51]. Many pathogens, including
Salmonella,
Escherichia,
Helicobacter and
Pseudomonas, belong to the Proteobacteria phylum [
52]. Our findings demonstrated that the LPS challenge tended to decrease Firmicutes abundance and increase Proteobacteria abundance in the cecal digesta, indicating that the LPS challenge disrupted the intestinal microbial community and impaired the intestinal homeostasis of broilers. Down to the genus level, 25OHD
3 supplementation enhanced
Lactobacillus abundance and decreased
Lachnoclostridium abundance in the cecal digesta compared with the LPS group.
Lactobacillus is regarded as probiotic bacteria, which plays important role in inhibiting inflammatory response by mediating the production of cytokines, improving intestinal barrier function by stimulating the tight junction integrity and combating infection by pathogens such as
Salmonella [
53,
54]. In this study, the correlation analysis showed that BW loss and serum contents of D-lactate, IL-1β and TNF-α were negatively correlated with cecal
Lactobacillus abundance, confirming the beneficial effects of
Lactobacillus on gut barrier function and performance. Wu et al. [
55] demonstrated that the
Lactobacillus species, including
L. rhamnosus and
L. plantarum, increased the expression and activity of VDR in the intestinal epithelial cells, and
Lactobacillus was depleted in the feces of VDR
–/– mice. Therefore, higher
Lactobacillus abundance may be caused by the vitamin D/VDR signaling pathway, but the underlying mechanism needs further research.
Lachnoclostridium has been reported to act as a pathogenic source of inflammatory disease [
56], which could disrupt intestinal health and impair performance in animals. The correlation analysis also showed that BW loss and serum contents of TNF-α, IL-1β and D-lactate were positively correlated with cecal
Lachnoclostridium abundance, highlighting the negative effects of
Lachnoclostridium on gut barrier function and performance. Tangestani et al. [
48] suggested that vitamin D may connect to some genera of the Lachnospiaceae family such as
Blautia. The beneficial genus
Blautia has been shown to produce SCFA, and it is positively correlated with metabolic homeostasis [
57]. Our findings suggested that adding 25OHD
3 to diets markedly promoted the relative abundance of
Blautia compared with the CON and LPS groups, and no clear difference was discovered between the CON and LPS groups. These results indicated that the changes in
Blautia abundance were not related to the LPS challenge and were only associated with 25OHD
3 supplementation. SCFA, including acetate, propionate and butyrate, have beneficial effects on alleviating intestinal inflammation and preventing the imbalance of the intestinal flora. SCFA, especially butyrate, could stimulate the growth of beneficial bacteria including Lactobacillus in poultry, which in turn promotes an increase in SCFA, improves the intestinal barrier function and inhibits the proliferation of pathogenic bacteria including
Salmonella and
E. coli [
58,
59]. The previous study also suggested that propionate and butyrate suppressed the LPS-induced release of TNF-α and inhibited the downstream pathway of NF-κB signaling [
60]. The present study showed that 25OHD
3 had the potential to prevent the LPS-induced decrease in propionate, isobutyrate, butyrate and total SCFA, which may be partly responsible for the improved immune responses and enhanced barrier function in broilers under stress response. Overall, 25OHD
3 could alleviate LPS-induced intestinal injury via modulating the intestinal bacterial compositions and metabolites of broilers.