Effect of Tomato Pomace Addition on Chemical, Technological, Nutritional, and Sensorial Properties of Cream Crackers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cream Crackers Preparation
2.2.2. Pasting Properties
2.2.3. Physical Characteristics
2.2.4. Chemical Characteristics
2.2.5. Tocols and Carotenoids
2.2.6. Free and Bound Phenolic Compounds
2.2.7. Antioxidant Capacity
2.2.8. Sensory Analysis
2.2.9. Statistical Data Analysis
3. Results and Discussion
3.1. Rheological Properties
3.2. Physical Characteristics
3.3. Chemical Characteristics
3.4. Carotenoids and Tocols
3.5. Phenolic Compounds
3.6. Antioxidant Capacity
3.7. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqbal, A.; Schulz, P.; Rizvi, S.S.H. Valorization of bioactive compounds in fruit pomace from agro-fruit industries: Present insights and future challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- European Commission. Communication form the Commission to the European Parlament, the Council, the European Economic and Social Committee and the Committee of the Regions—An EU Action Plan for the Circular Economy; European Commission: Brussels, Belgium, 2015.
- Campos, D.A.; Ricardo, G.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of fruit industrial by-products—A case study on circular economy approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data (accessed on 22 February 2022).
- Zuorro, A.; Fidaleo, M.; Lavecchia, R. Enzyme-assisted extraction of lycopene from tomato processing waste. Enzym. Microb. Technol. 2011, 49, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Jaiswal, A.K. Exploitation of food industry waste for high-value products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, A.; Di Prima, R.; Fongaro, L.; Cappa, C.; Lucisano, M. Tocols, carotenoids, heat damage and technological quality of diced tomatoes processed in different industrial lines. LWT Food Sci. Technol. 2017, 83, 254–261. [Google Scholar] [CrossRef]
- Dorais, M.; Ehret, D.L.; Papadopoulos, A.P. Tomato (Solanum lycopersicum) health components: From the seed to the consumer. Phytochem. Rev. 2008, 7, 231–250. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Mehta, D.; Prasad, P.; Sangwan, R.S.; Yadav, S.K. Tomato processing byproduct valorization in bread and muffin: Improvement in physicochemical properties and shelf life stability. J. Food Sci. Technol. 2018, 55, 2560–2568. [Google Scholar] [CrossRef]
- Özbek, Z.A.; Çelik, K.; Ergönül, P.G.; Hepçimen, A.Z. A promising food waste for food fortification: Characterization of dried tomato pomace and its cold pressed oil. J. Food Chem. Nanotechnol. 2020, 6, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Capanoglu, E.; Beekwilder, J.; Boyacioglu, D.; Hall, R.; De Vos, R. Changes in antioxidant and metabolite profiles during production of tomato paste. J. Agric. Food Chem. 2008, 56, 964–973. [Google Scholar] [CrossRef]
- Lazos, E.S.; Tsaknis, J.; Lalas, S. Characteristics and composition of tomato seed oil. Grasas Aceites 1998, 49, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Isik, F.; Topkaya, C. Effects of tomato pomace supplementation on chemical and nutritional properties of crackers. Ital. J. Food Sci. 2016, 28, 525–535. [Google Scholar] [CrossRef]
- Altan, A.; McCarthy, K.L.; Maskan, M. Evaluation of snack foods from barley-tomato pomace blends by extrusion processing. J. Food Eng. 2008, 84, 231–242. [Google Scholar] [CrossRef]
- Salem, R.B.; El-Sahy, K.M.; Sulieman, A.; Gouda, M.R. Use of tomato pomace, mango seeds kernel and pomegranate peels powders for the production of functional biscuits. Zagazig J. Agric. Res. 2020, 47, 1011–1023. [Google Scholar] [CrossRef]
- Alazb, R.B.; El-Sahy, M.K.; Sulieman, M.A.E.-R.; Youssif, M.R. Physicochemical and organoleptic characteristics of cakes supplemented with tomato pomace, mango seeds kernel and pomegranate peels powders. Plant Arch. 2021, 21, 432–439. [Google Scholar] [CrossRef]
- Nakov, G.; Ivanova, N. The effect of different methods for production of crackers on their physical and sensory characteristics. Technol. Acta 2020, 13, 41–45. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO 665:2020); International Standard—Oilseeds—Determination of Moisture and Volatile Matter Content. International Organization for Standardization: Vernier, Switzerland, 2020.
- International Organization for Standardization (ISO 5984:2022); International Standard—Animal Feeding Stuffs—Determination of Crude Ash. International Organization for Standardization: Vernier, Switzerland, 2022.
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.J. Protein determination—Method matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization (ISO 6492:1999); International Standard—Animal Feeding Stuffs—Determination of Fat Content. International Organization for Standardization: Vernier, Switzerland, 1999.
- AACC Approved Methods of Analysis, 11th Ed. Method 32-07.01. Determination of Soluble, Insoluble, and Total Dietary Fiber in Foods and Food Products. Approved 16 October 1991. Cereals & Grains Association: St. Paul, MN, USA, 1999. Available online: https://www.cerealsgrains.org/resources/Methods/Pages/32Fiber.aspx (accessed on 1 October 2022).
- Panfili, G.; Fratianni, A.; Irano, M. Normal-phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J. Agric. Food Chem. 2003, 51, 3940–3944. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A. Tocols stability during bread, water biscuit and pasta processing from wheat flours. J. Cereal Sci. 2010, 52, 254–259. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A.; Pompei, C. Carotenoids evolution during pasta, bread and water biscuit preparation from wheat flours. Food Chem. 2010, 121, 746–751. [Google Scholar] [CrossRef]
- Hidalgo, A.; Ferraretto, A.; De Noni, I.; Bottani, M.; Cattaneo, S.; Galli, S.; Brandolini, A. Bioactive compounds and antioxidant properties of pseudocereals-enriched water biscuits and their in vitro digestates. Food Chem. 2018, 240, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 2015, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandolini, A.; Glorio-Paulet, P.; Estivi, L.; Locatelli, N.; Córdova-Ramos, J.S.; Hidalgo, A. Tocopherols, carotenoids and phenolics changes during Andean lupin (Lupinus mutabilis Sweet) seeds processing. J. Food Compos. Anal. 2022, 106, 104335. [Google Scholar] [CrossRef]
- Dueñas, M.; Hernández, T.; Estrella, I.; Fernández, D. Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chem. 2009, 117, 599–607. [Google Scholar] [CrossRef]
- Zalewski, K.; Nitkiewicz, B.; Stolarski, M.; Amarowicz, R.; Okorskid, A. The influence of exogenous methyl jasmonate on the germination and, content and composition of flavonoids in extracts from seedlings of yellow and narrow-leafed lupine. J. Food Compos. Anal. 2020, 87, 103398. [Google Scholar] [CrossRef]
- Estivi, L.; Grassi, S.; Briceño-Berrú, L.; Glorio-Paulet, P.; Camarena, F.; Hidalgo, A.; Brandolini, A. Free phenolic compounds, antioxidant capacity and FT-NIR survey of debittered Lupinus mutabilis seeds. Processes 2022, 10, 1637. [Google Scholar] [CrossRef]
- Condori, M.V.; Chagman, G.P.; Barriga Sanchez, M.E.; Vilchez, L.F.V.; Ursetta, S.; Guevara, A.; Hidalgo, A. Effect of tomato (Solanum lycopersicum L.) lycopene-rich extract on the kinetics of rancidity and shelf-life of linseed (Linum usitatissimum L.) oil. Food Chem. 2020, 302, 125327. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Siebenhandl, S.; Grausgruber, H.; Pellegrini, N.; Del Rio, D.; Fogliano, V.; Pernice, R.; Berghofer, E. Phytochemical profile of main antioxidants in different fractions of purple and blue wheat, and black barley. J. Agric. Food Chem. 2007, 55, 8541–8547. [Google Scholar] [CrossRef]
- Alfonsi, A.; Coles, D.; Hasle, C.; Koppel, J.; Ladikas, M.; Schmucker von Koch, J.; Schroeder, D.; Sprumont, D.; Verbeke, W.; Zaruk, D. Guidance Note: Ethics and Food-Related Research; European Commission Ethics Review Sector: Brussels, Belgium, 2012. [Google Scholar]
- Majzoobi, M.; Ghavi, F.S.; Farahnaky, A.; Jamalian, J.; Mesbahi, G. Effect of tomato pomace powder on the physicochemical properties of flat bread (Barbari bread). J. Food Process. Preserv. 2011, 35, 247–256. [Google Scholar] [CrossRef]
- Ray, S.; Saha, R.; Raychaudhuri, U.; Chakraborty, R. Different quality characteristics of tomato (Solanum lycopersicum) as a fortifying ingredient in food products: A review. Tech. Sci. 2016, 19, 199–213. [Google Scholar]
- Nakov, G.; Brandolini, A.; Hidalgo, A.; Ivanova, N.; Stamatovska, V.; Dimov, I. Effect of grape pomace powder addition on chemical, nutritional and technological properties of cakes. LWT Food Sci. Technol. 2020, 134, 109950. [Google Scholar] [CrossRef]
- Mironeasa, S.; Gabriela, G. Dough rheological behavior and microstructure characterization of composite dough with wheat and tomato seed flours. Foods 2019, 8, 626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sogi, D.S.; Sidhu, J.S.; Arora, M.S.; Garg, S.K.; Bawa, A.S. Effect of tomato seed meal supplementation on the dough and bread characteristics of wheat (PBW 343) flour. Int. J. Food Prop. 2002, 5, 563–571. [Google Scholar] [CrossRef]
- Bhat, A.M.; Ahsan, H. Physico-Chemical Characteristics of Cookies Prepared with Tomato Pomace Powder. J. Food Process. Technol. 2015, 7, 1–4. [Google Scholar] [CrossRef]
- Chung, H.J. Quality attributes of cookies prepared with tomato powder. J. Food Sci. Nutr. 2007, 12, 229–233. [Google Scholar] [CrossRef]
- Bhat, A.M.; Ahsan, H.; Masoodi, L.; bin Hameed, O.; Saleem, R. Tomato pomace as a functional ingredient in cookie making. Food Sci. Res. J. 2017, 8, 254–259. [Google Scholar] [CrossRef]
- Nour, V.; Ionica, M.E.; Trandafir, I. Bread enriched in lycopene and other bioactive compounds by addition of dry tomato waste. J. Food Sci. Technol. 2015, 52, 8260–8267. [Google Scholar] [CrossRef] [Green Version]
- Jukić, M.; Lukinac, J.; Čuljak, J.; Pavlović, M.; Šubarić, D.; Koceva Komlenić, D. Quality evaluation of biscuits produced from composite blends of pumpkin seed oil press cake and wheat flour. Int. J. Food Sci. Technol. 2019, 54, 602–609. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Sambanthamurthi, R.; Tan, Y.; Sundram, K.; Abeywardena, M.; Sambandan, T.G.; Rha, C.; Sinskey, A.J.; Subramaniam, K.; Leow, S.S.; Hayes, K.C.; et al. Oil palm vegetation liquor: A new source of phenolic bioactives. Br. J. Nutr. 2011, 106, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Betoret, E.; Rosell, C.M. Enrichment of bread with fruits and vegetables: Trends and strategies to increase functionality. Cereal Chem. 2020, 97, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Ripari, V.; Bai, Y.; Gänzle, M.G. Metabolism of phenolic acids in whole wheat and rye malt sourdoughs. Food Microbiol. 2019, 77, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Peng, H. Bioaccessibility and bioavailability of phenolic compounds. J. Food Bioact. 2018, 4, 11–68. [Google Scholar] [CrossRef] [Green Version]
- Yagci, S.; Calıskan, R.; Gunes, Z.S.; Capanoglu, E.; Tomas, M. Impact of tomato pomace powder added to extruded snacks on the in vitro gastrointestinal behaviour and stability of bioactive compounds. Food Chem. 2022, 368, 130847. [Google Scholar] [CrossRef] [PubMed]




| 0% TP | 4% TP | 6% TP | 8% TP | 10% TP | |
|---|---|---|---|---|---|
| Peak viscosity (BU) | 895.0 ± 12.7 d | 898.5 ± 2.1 d | 946.0 ± 5.7 c | 971.0 ± 5.7 b | 1024.0 ± 5.7 a |
| Breakdown (BU) | 297.0 ± 4.2 d | 308.0 ± 2.8 d | 345.0 ± 2.8 c | 390.0 ± 14.1 b | 422.5 ± 10.6 a |
| Final viscosity (BU) | 1042.5 ± 14.9 a | 1047.5 ± 14.9 bc | 1058.0 ± 4.2 bc | 1068.0 ± 2.8 b | 1147.0 ± 10.0 a |
| Pasting temperature (°C) | 60.7 ± 0.6 b | 61.3 ± 0.3 ab | 61.3 ± 0.1 ab | 61.5 ± 0.5 ab | 61.8 ± 0.2 a |
| 0% TP | 4% TP | 6% TP | 8% TP | 10% TP | |
|---|---|---|---|---|---|
| Colour | |||||
| Dough | |||||
| L* | 68.5 ± 2.5 a | 68.1 ± 2.5 a | 65.6 ± 2.2 b | 62.8 ± 2.8 ab | 58.1 ± 1.3 c |
| a* | −0.4 ± 0.04 e | 5.4 ± 0.9 d | 8.1 ± 0.3 c | 9.7 ± 0.8 b | 10.9 ± 0.9 a |
| b* | 19.7 ± 0.3 d | 25.7 ± 0.4 c | 27.8 ± 0.4 b | 28.3 ± 0.1 ab | 28.4 ± 0.5 a |
| Crackers | |||||
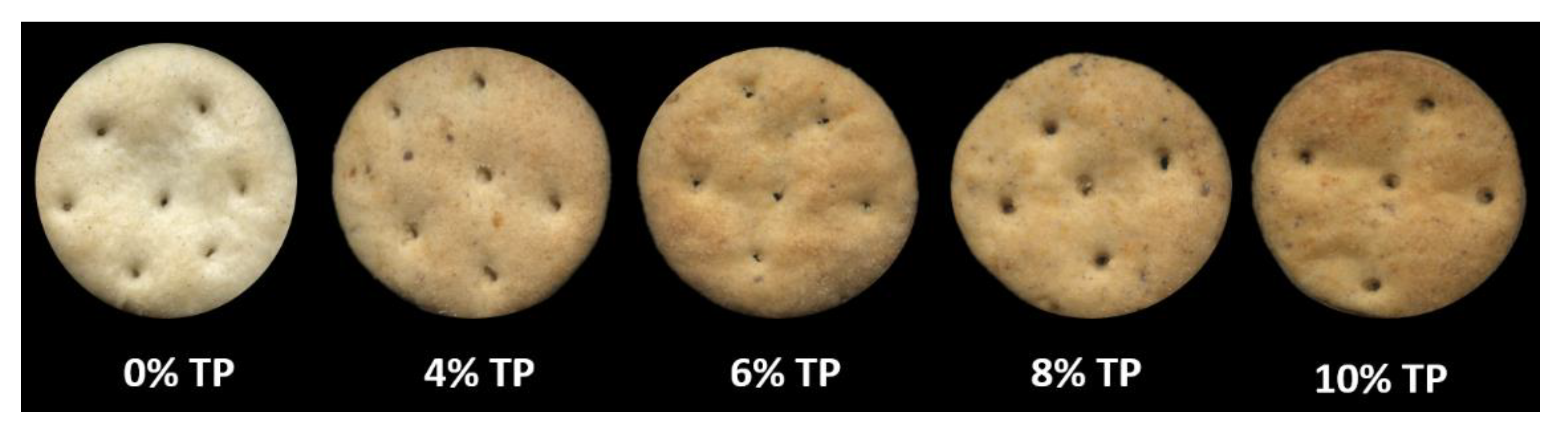
| L* | 69.7 ± 0.8 a | 66.1 ± 1.2 b | 61.9 ± 0.7 c | 61.0 ± 1.6 c | 58.4 ± 2.3 d |
| a* | −0.7 ± 0.03 a | 3.8 ± 0.7 a | 6.8 ± 1.2 a | 7.3 ± 0.3 b | 7.6 ± 1.6 c |
| b* | 16.9 ± 0.2 a | 23.9 ± 0.6 ab | 25.3 ± 2.3 bc | 26.9 ± 1.1 c | 27.5 ± 1.2 d |
| Physical and textural characteristics | |||||
| Width (mm) | 35.8 ± 0.0 a | 35.1 ± 3.0 a | 34.9 ± 2.0 a | 34.3 ± 0.7 a | 34.5 ± 1.8 a |
| Thickness (mm) | 8.4 ± 0.9 a | 7.8 ± 0.0 ab | 7.6 ± 0.4 ab | 6.7 ± 0.2 bc | 6.1 ± 0.1 c |
| Spread factor | 4.3 ± 0.5 b | 4.5 ± 0.4 b | 4.6 ± 0.5 b | 5.2 ± 0.3 ab | 5.7 ± 0.2 a |
| Volume (cm3) | 7.1 ± 0.1 a | 4.9 ± 0.1 b | 4.6 ± 0.3 b | 3.7 ± 0.1 c | 3.5 ± 0.0 c |
| Specific volume (cm3/g) | 1.8 ± 0.1 a | 1.7 ± 0.0 ab | 1.5 ± 0.01 bc | 1.5 ± 0.1 c | 1.4 ± 0.1 c |
| Hardness (N) | 65.4 ± 9.5 a | 42.7 ± 5.0 b | 36.4 ± 3.5 bc | 30.0 ± 2.7 cd | 26.3 ± 4.4 d |
| Snapping force (N) | 4.8 ± 2.6 d | 8.0 ± 1.9 c | 10.4 ± 1.7 bc | 12.0 ± 1.9 ab | 13.8 ± 3.0 a |
| 0% TP | 4% TP | 6% TP | 8% TP | 10% TP | |
|---|---|---|---|---|---|
| Palmitic | 20.28 ± 0.01 d | 20.54 ± 0.01 c | 20.58 ± 0.07 c | 20.88 ± 0.01 b | 21.35 ± 0.01 a |
| Stearic | 4.16 ± 0.00 e | 4.18 ± 0.00 d | 4.54 ± 0.01 c | 4.77 ± 0.00 b | 4.92 ± 0.02 a |
| Oleic | 31.09 ± 0.01 d | 32.04 ± 0.01 c | 32.06 ± 0.11 c | 32.19 ± 0.01 b | 33.10 ± 0.01 a |
| Linoleic | 38.60 ± 0.14 d | 39.24 ± 0.02 c | 39.31 ± 0.01 c | 40.66 ± 0.01 b | 41.74 ± 0.01 a |
| MUFA | 33.52 ± 0.01 e | 33.99 ± 0.01 d | 34.28 ± 0.02 c | 33.92 ± 0.11 b | 33.75 ± 0.01 a |
| PUFA | 40.64 ± 0.16 d | 40.41 ± 0.01 c | 40.21 ± 0.01 c | 40.78 ± 0.02 b | 40.94 ± 0.02 a |
| SFA | 25.83 ± 0.01 d | 25.62 ± 0.01 c | 25.53 ± 0.01 b | 25.52 ± 0.09 a | 25.35 ± 0.01 a |
| WF | TP | 0% | 4% | 6% | 8% | 10% | |
|---|---|---|---|---|---|---|---|
| Carotenoids | |||||||
| Lutein | 1.26 ± 0.06 | 4.14 ± 0.24 | 0.53 ± 0.03 | 0.64 ± 0.07 | 0.59 ± 0.05 | 0.57 ± 0.02 | 0.59 ± 0.01 |
| Zeaxanthin | 0.06 ± 0.00 | 0.36 ± 0.03 | 0.02 c ± 0.00 | 0.04 b ± 0.01 | 0.04 b ± 0.01 | 0.04 ab ± 0.00 | 0.05 a ± 0.00 |
| 15-cis-neurosporene | nd | 20.59 ± 1.43 | nd d | 2.24 c ± 0.22 | 2.46 bc ± 0.19 | 2.77 b ± 0.11 | 3.47 a ± 0.19 |
| δ-carotene-isomer | nd | 10.00 ± 1.04 | nd | 1.56 ± 0.03 | 1.61 ± 0.02 | 1.63 ± 0.07 | 1.69 ± 0.02 |
| trans-lycopene | nd | 11.89 ± 0.75 | nd c | 2.35 b ± 0.09 | 2.49 b ± 0.04 | 2.53 b ± 0.09 | 2.76 a ± 0.00 |
| Tocols | |||||||
| α-tocopherol | nd | 55.30 ± 5.19 | 18.52 c ± 0.45 | 21.40 b ± 1.27 | 23.65 a ± 0.92 | 24.10 a ± 0.28 | 25.46 a ± 0.17 |
| α-tocotrienol | nd | nd | 7.76 ± 0.62 | 9.10 ± 0.71 | 8.83 ± 0.91 | 8.64 ± 0.91 | 8.81 ± 0.04 |
| β-tocopherol | nd | 3.24 ± 0.38 | 2.53 ± 0.05 | 2.05 ± 0.34 | 3.03 ± 0.90 | 2.25 ± 0.81 | 2.24 ± 0.18 |
| β-tocotrienol | 13.00 ± 0.85 | nd | 13.21 ± 0.29 | 14.51 ± 1.24 | 14.15 ± 1.43 | 14.47 ± 0.92 | 14.36 ± 0.11 |
| γ-tocopherol | nd | 116.65 ± 4.03 | nd d | 2.17 c ± 0.37 | 2.80 b ± 0.25 | 3.94 a ± 0.03 | 4.55 a ± 0.01 |
| δ-tocopherol | nd | nd | 15.57 ± 0.24 | 16.09 ± 0.41 | 15.43 ± 1.30 | 15.20 ± 1.18 | 15.02 ± 0.14 |
| δ-tocotrienol | nd | 3.47 ± 0.14 | nd | 0.90 ± 0.09 | 1.55 ± 0.32 | 1.60 ± 0.10 | 1.19 ± 0.30 |
| WF | TP | 0% | 4% | 6% | 8% | 10% | |
|---|---|---|---|---|---|---|---|
| Soluble free | |||||||
| Phenolic acids | |||||||
| 4OH-benzoic acid der | 87.60 ± 4.23 | 94.78 ± 8.48 | 92.90 ± 9.21 | 91.06 ± 3.40 | 92.21 ± 8.44 | ||
| Coumaric acid | 6.84 ± 0.10 | 0.40 d ± 0.02 | 0.57 c ± 0.01 | 0.74 b ± 0.02 | 0.92 a ± 0.01 | ||
| Coumaric acid der | 49.97 ± 2.15 | 3.09 d ± 0.11 | 4.27 c ± 0.08 | 5.52 b ± 0.10 | 6.35 a ± 0.01 | ||
| Ferulic acid | 28.22 ± 1.13 | 2.55 c ± 0.03 | 4.30 b ± 0.04 | 4.28 b ± 0.00 | 4.44 b ± 0.12 | 5.13 a ± 0.08 | |
| Gallic acid | 81.98 ± 2.98 | 9.93 d ± 0.17 | 70.99 c ± 2.80 | 124.81 b ± 7.23 | 160.65 a ± 4.49 | 173.12 a ± 10.93 | |
| Syringic acid | 42.53 ± 0.80 | ||||||
| Flavonoids | |||||||
| Naringenin | 39.02 ± 0.14 | 2.96 c ± 0.24 | 5.20 b ± 0.10 | 6.98 a ± 0.31 | 7.69 a ± 0.35 | ||
| Naringenin der | 33.78 ± 0.27 | 0.73 d ± 0.01 | 1.28 c ± 0.04 | 1.84 b ± 0.13 | 2.39 a ± 0.01 | ||
| Quercitin der | 56.74 ± 0.24 | 1.86 d ± 0.02 | 3.23 c ± 0.11 | 4.15 b ± 0.04 | 5.00 a ± 0.20 | ||
| Rutin | 489.26 ± 6.50 | 9.81 d ± 0.03 | 17.26 c ± 0.18 | 23.76 b ± 0.55 | 30.20 a ± 1.17 | ||
| Insoluble bound | |||||||
| Phenolic acids | |||||||
| Caffeic acid | 29.75 ± 0.18 | ||||||
| Coumaric acid | 0.16 ± 0.01 | 5.54 ± 0.65 | 0.16 c ± 0.01 | 0.36 b ± 0.03 | 0.44 b ± 0.04 | 0.53 a ± 0.03 | 0.58 a ± 0.05 |
| Coumaric acid der | 0.26 ± 0.01 | ||||||
| Ferulic acid | 67.28 ± 4.21 | 11.47 ± 0.92 | 67.35 ± 0.44 | 63.93 ± 4.79 | 67.65 ± 3.09 | 65.33 ± 2.86 | 64.25 ± 0.51 |
| Ferulic acid der | 5.79 ± 0.38 | 6.16 ± 0.02 | 5.88 ± 0.22 | 6.09 ± 0.03 | 5.91 ± 0.03 | 5.83 ± 0.41 | |
| Flavonoids | |||||||
| Naringenin | 172.46 ± 11.81 | 3.92 c ± 0.31 | 6.05 b ± 0.50 | 6.86 ab ± 0.67 | 8.10 a ± 0.41 | ||
| Naringenin der | 65.47 ± 2.29 | 4.23 b ± 0.07 | 5.50 a ± 0.53 | 5.47 a ± 0.06 | 6.37 a ± 0.40 | ||
| Quercitin der | 98.14 ± 6.23 | 5.77 c ± 0.57 | 7.46 c ± 0.59 | 10.28 b ± 1.07 | 13.24 a ± 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakov, G.; Brandolini, A.; Estivi, L.; Bertuglia, K.; Ivanova, N.; Jukić, M.; Komlenić, D.K.; Lukinac, J.; Hidalgo, A. Effect of Tomato Pomace Addition on Chemical, Technological, Nutritional, and Sensorial Properties of Cream Crackers. Antioxidants 2022, 11, 2087. https://doi.org/10.3390/antiox11112087
Nakov G, Brandolini A, Estivi L, Bertuglia K, Ivanova N, Jukić M, Komlenić DK, Lukinac J, Hidalgo A. Effect of Tomato Pomace Addition on Chemical, Technological, Nutritional, and Sensorial Properties of Cream Crackers. Antioxidants. 2022; 11(11):2087. https://doi.org/10.3390/antiox11112087
Chicago/Turabian StyleNakov, Gjore, Andrea Brandolini, Lorenzo Estivi, Katia Bertuglia, Nastia Ivanova, Marko Jukić, Daliborka Koceva Komlenić, Jasmina Lukinac, and Alyssa Hidalgo. 2022. "Effect of Tomato Pomace Addition on Chemical, Technological, Nutritional, and Sensorial Properties of Cream Crackers" Antioxidants 11, no. 11: 2087. https://doi.org/10.3390/antiox11112087
APA StyleNakov, G., Brandolini, A., Estivi, L., Bertuglia, K., Ivanova, N., Jukić, M., Komlenić, D. K., Lukinac, J., & Hidalgo, A. (2022). Effect of Tomato Pomace Addition on Chemical, Technological, Nutritional, and Sensorial Properties of Cream Crackers. Antioxidants, 11(11), 2087. https://doi.org/10.3390/antiox11112087











