Antiglycation and Antioxidant Effect of Nitroxyl towards Hemoglobin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
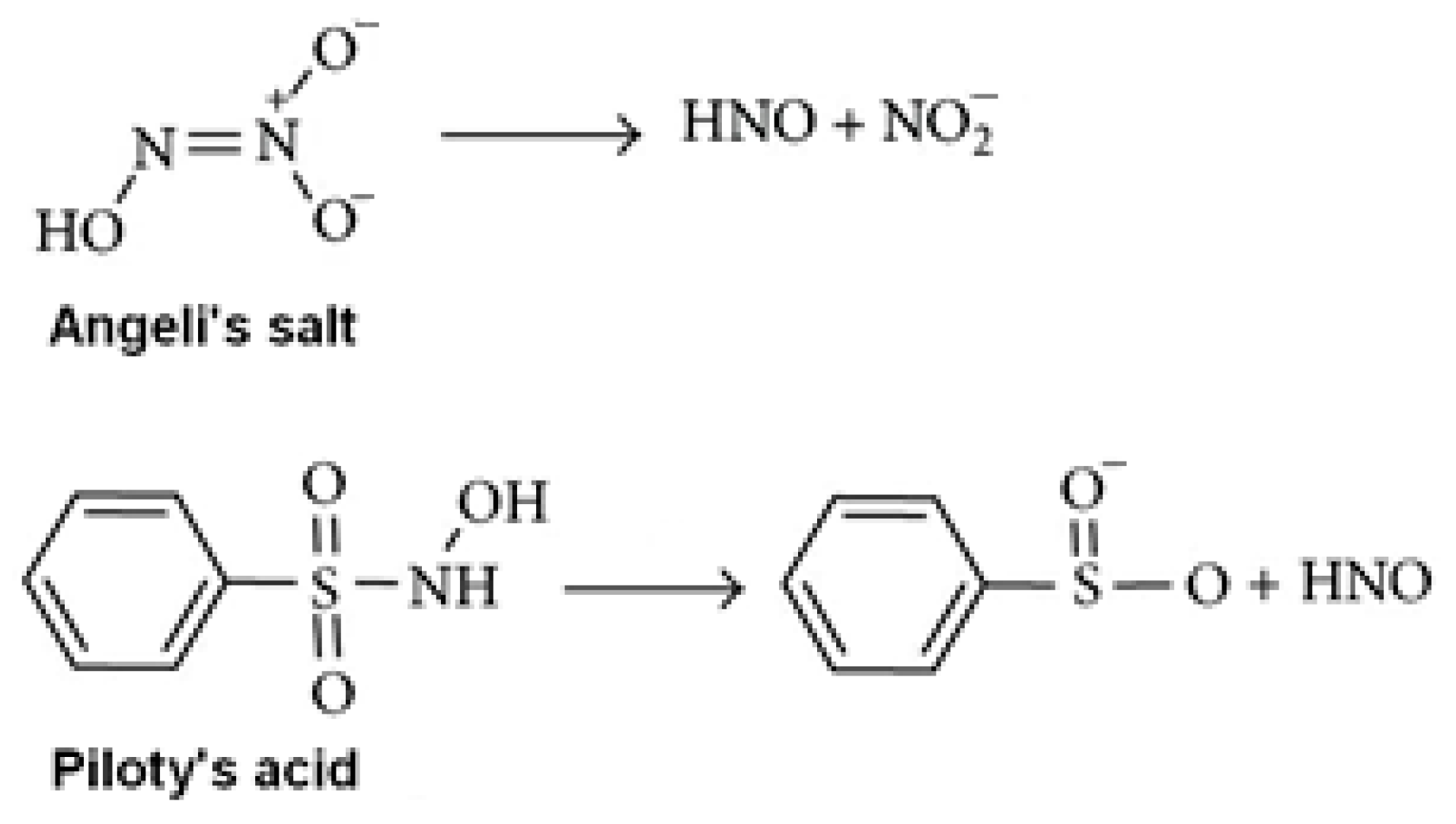
2.2. Hb Modification with MG. Studying the Effect of Angeli’s Salt and Aminoguanidine
2.3. Measurements of Hb-Bound AGEs
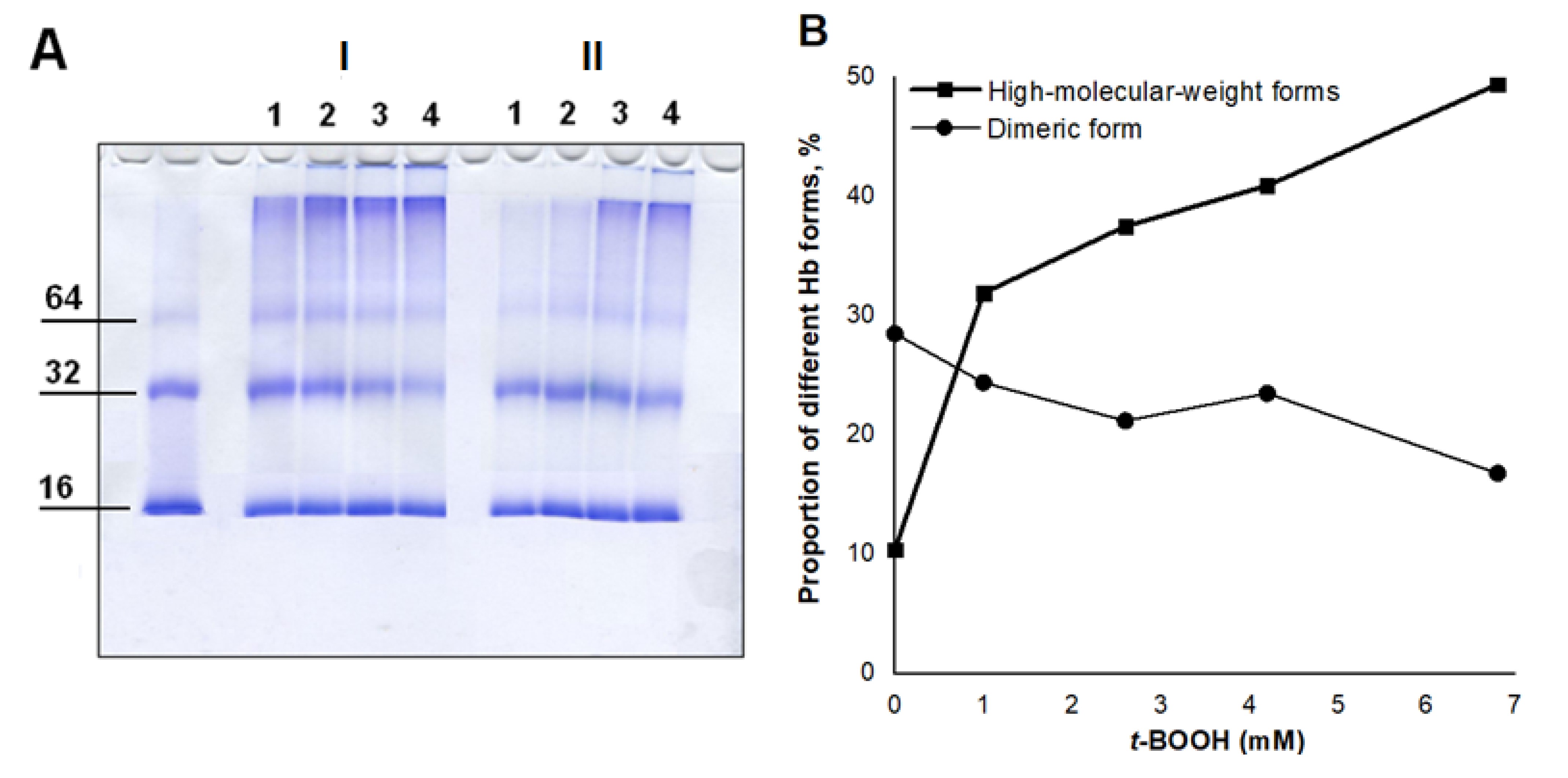
2.4. SDS-Electrophoresis
2.5. Gel Chromatography
2.6. Oxidative Modification of Hemoglobin by tert-Butyl Hydroperoxide
2.7. Measurements of Protein Carbonyls
2.8. Measurements of the Heme Group
2.9. Evaluation of Antioxidant/Antiradical Properties of Nitroxyl
2.9.1. Luminol-Dependent Chemiluminescence
2.9.2. Obtaining and Registration of Oxoferrylhemoglobin
2.9.3. TMPD Oxidation
2.9.4. Detecting ROS with a Fluorescent Probe
2.9.5. UV-Visible Spectrophotometry
2.10. Statistical Analysis
3. Results
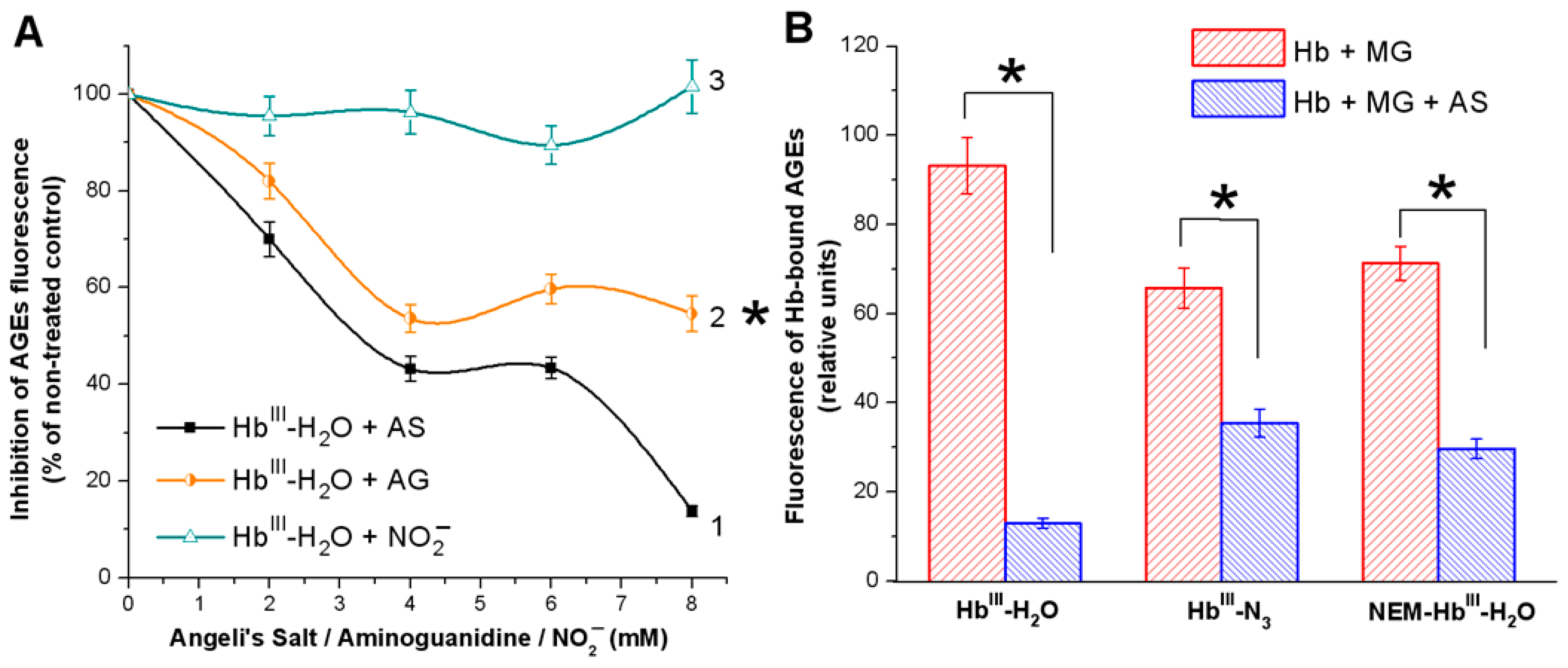
3.1. Effect of Nitroxyl on Non-Enzymatic Hb Glycation
3.1.1. Spectrofluorescence Analysis of AGE Formation
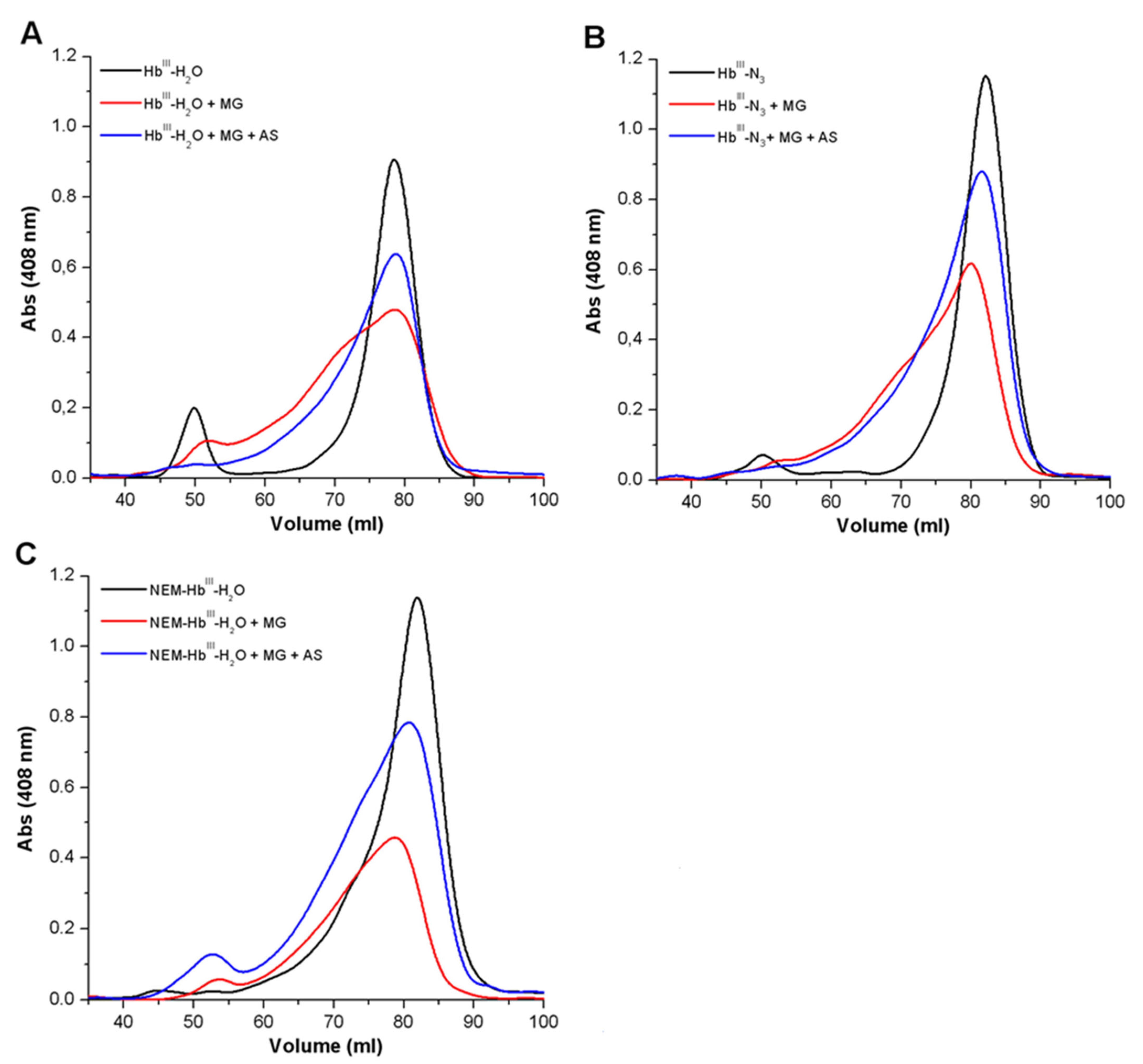
3.1.2. Analysis of the Formation of Cross-Linked Hb
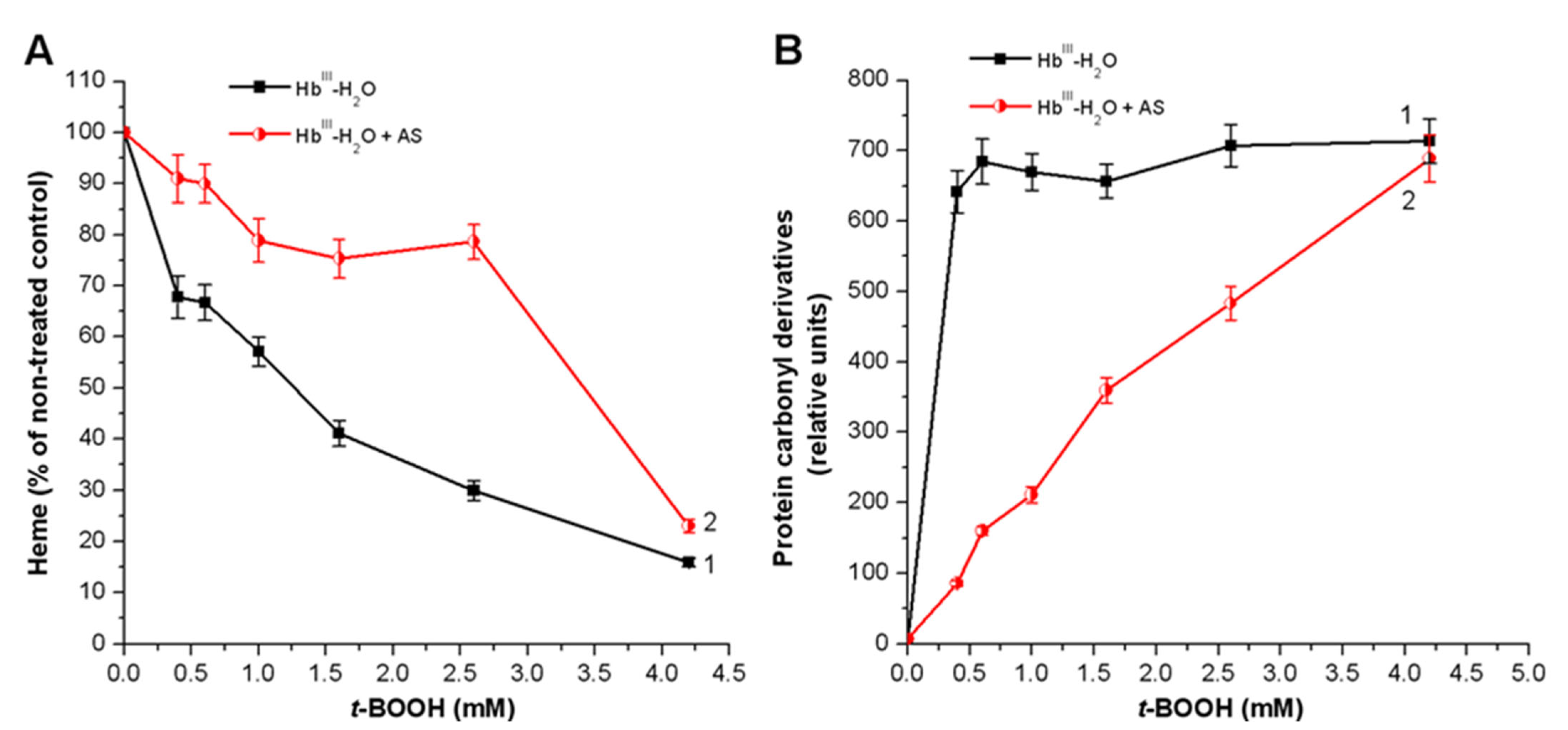
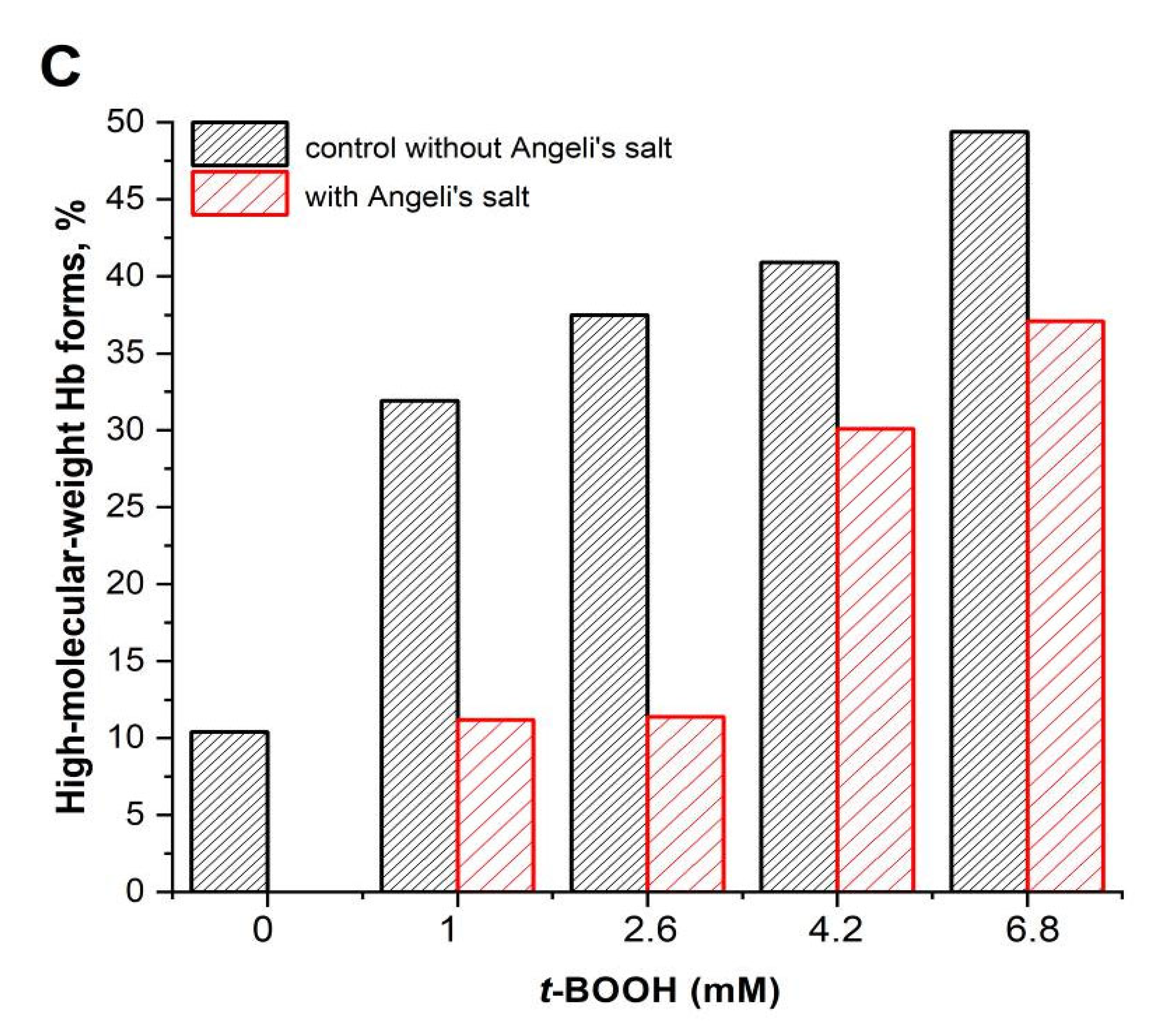
3.2. Effect of Nitroxyl on Hb Oxidative Modification in Reaction with t-BOOH
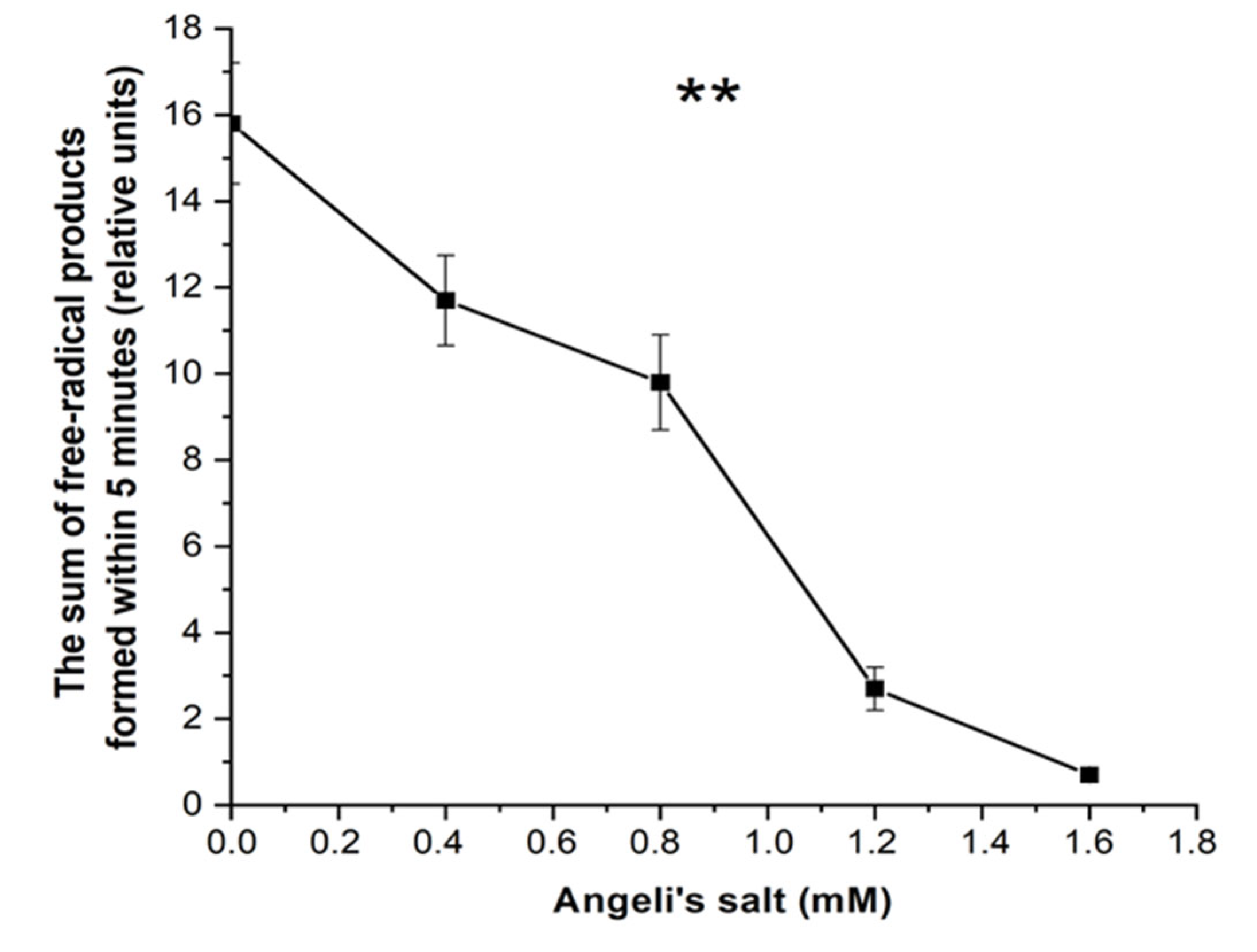
3.3. Effect of Nitroxyl on Free Radical Products Formation in Hb Reactions with MG and t-BOOH
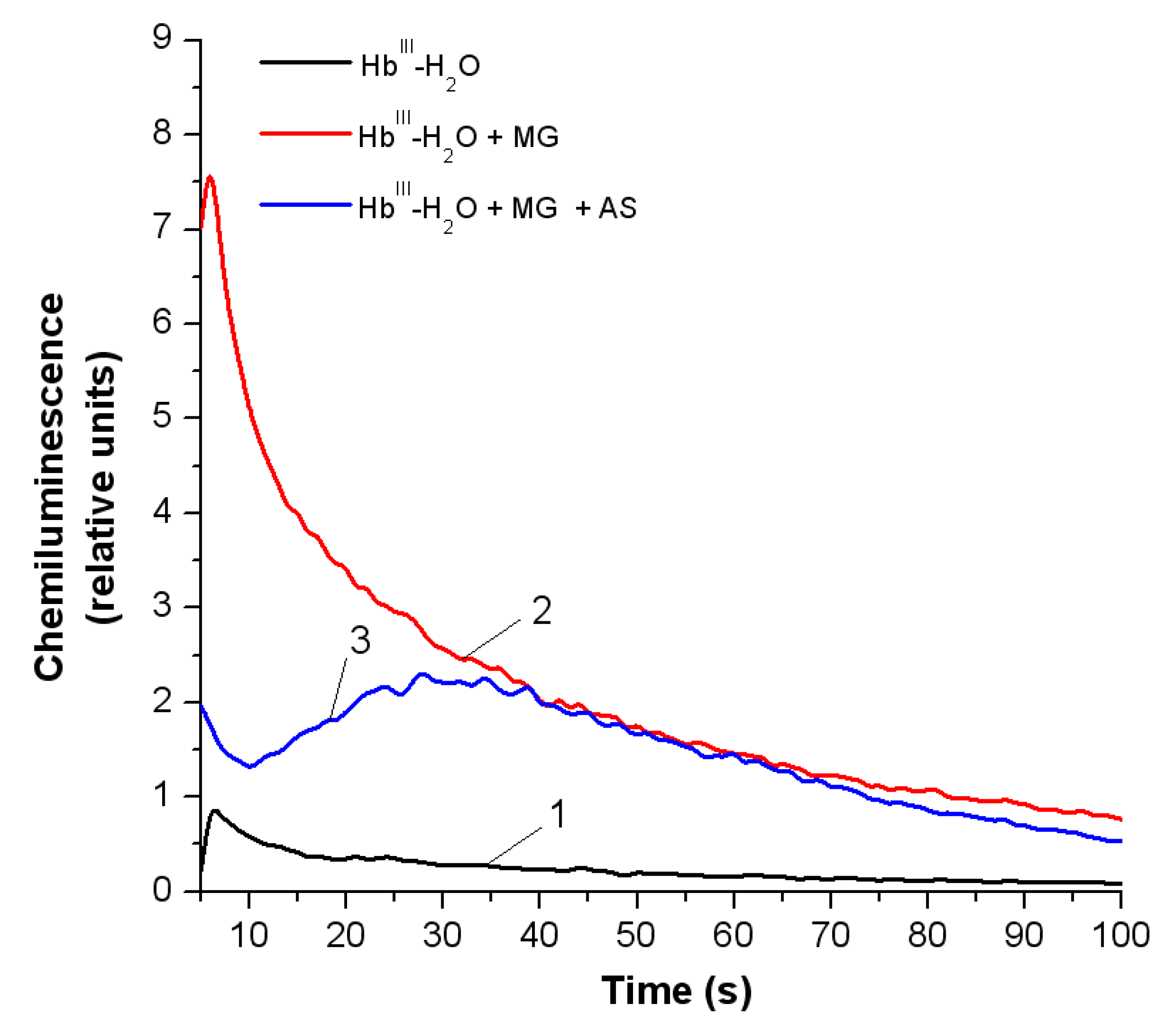
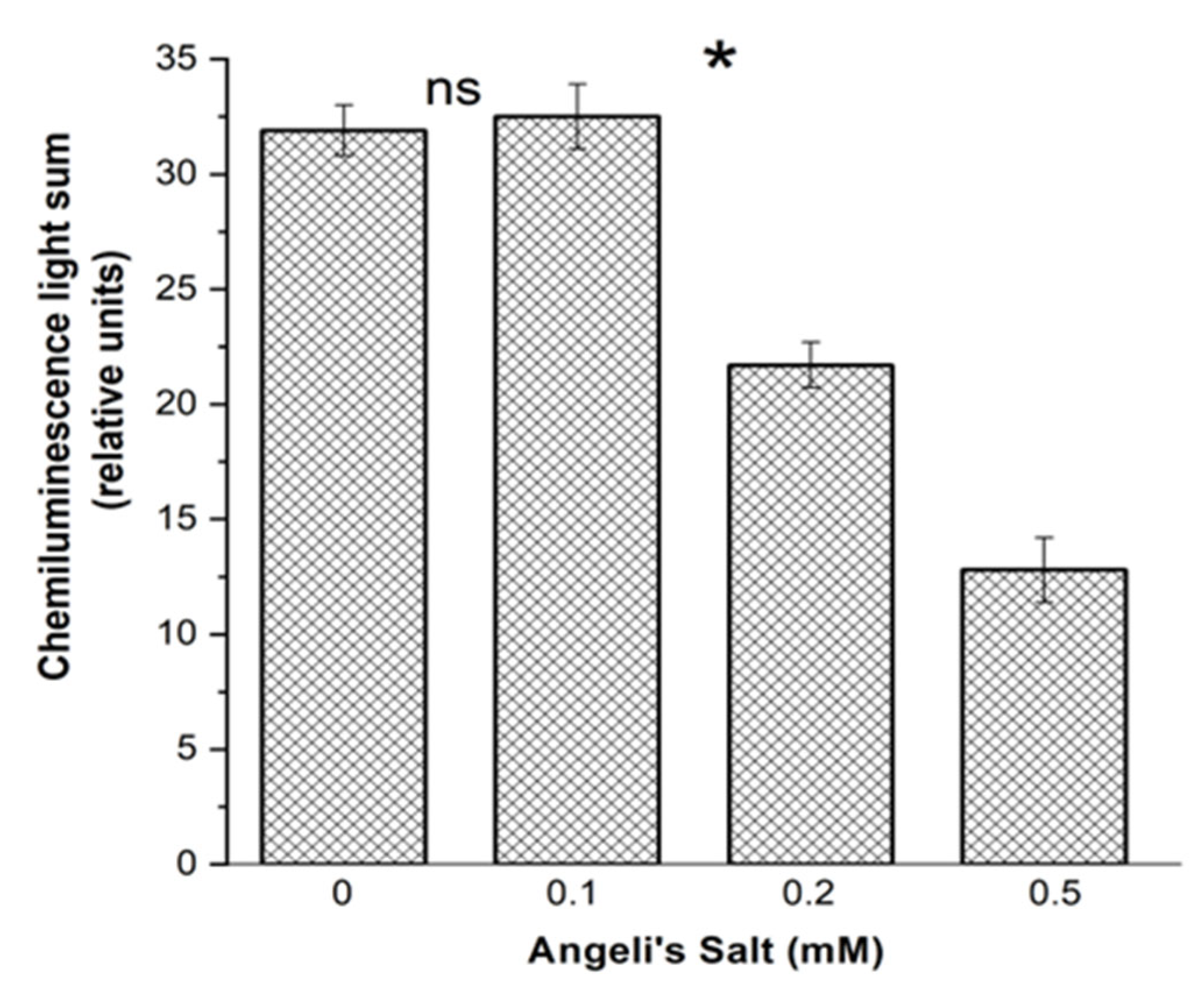
3.3.1. Luminol-Dependent Chemiluminescence
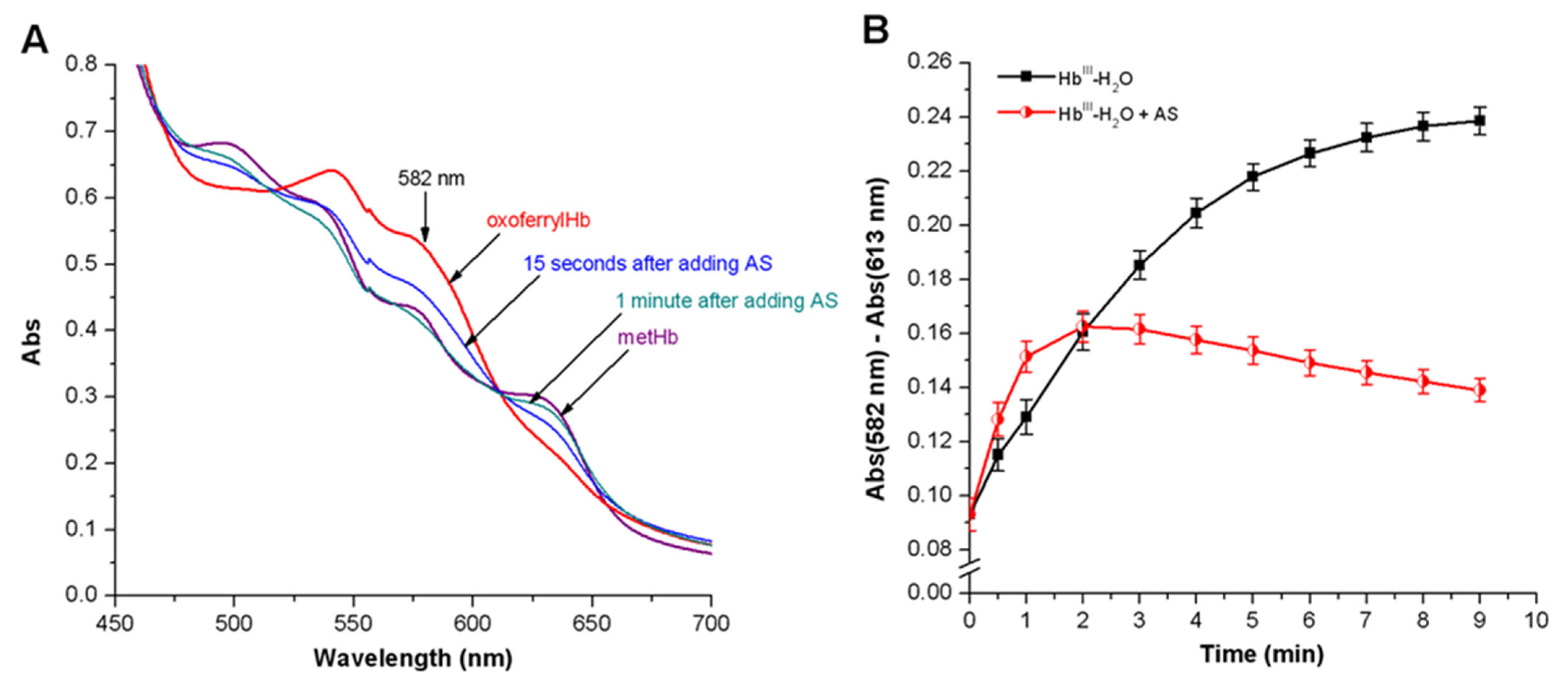
3.3.2. Peroxyl Radicals’ Formation and Reduction of OxoferrylHb
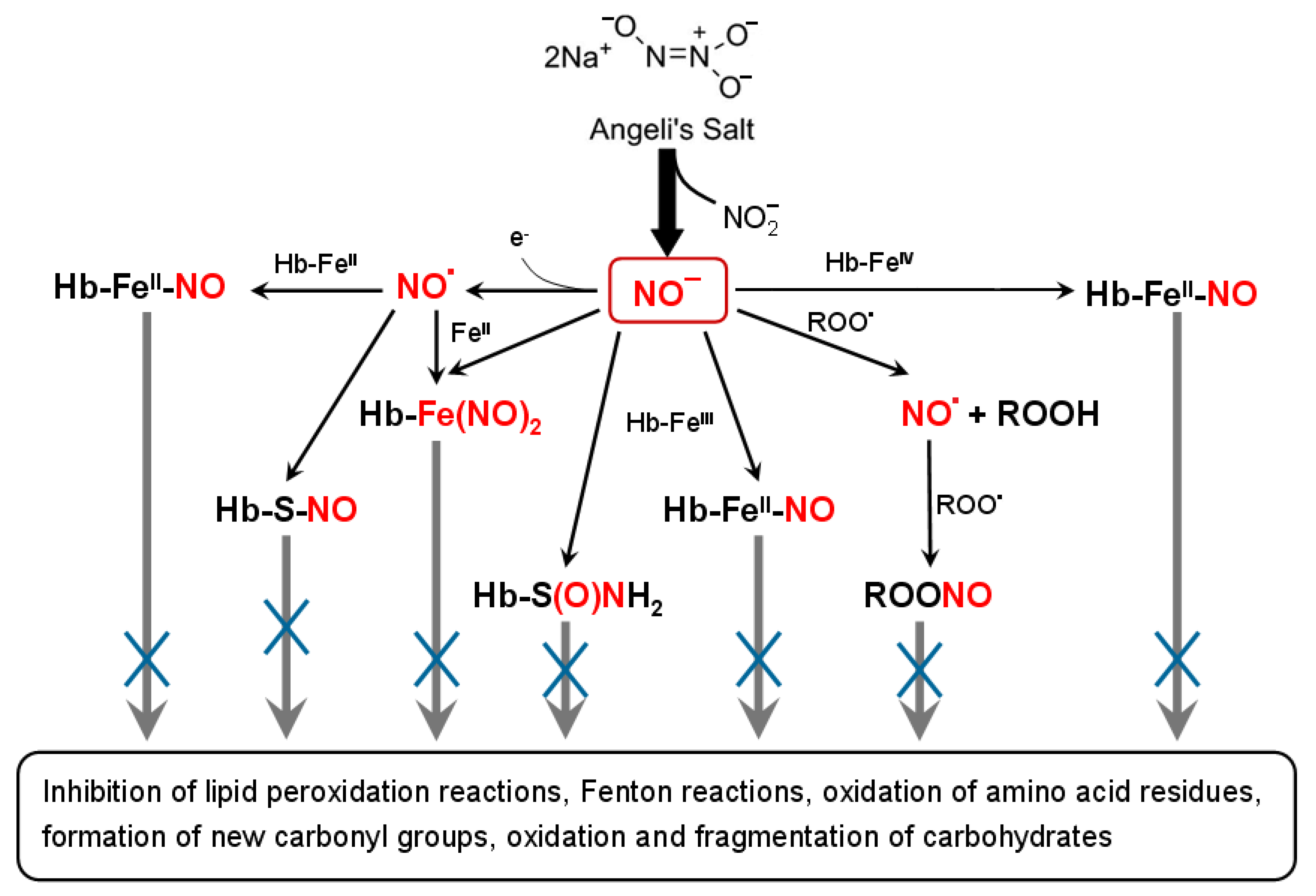
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabbani, N.; Thornalley, P.J. The dicarbonyl proteome: Proteins susceptible to dicarbonyl glycation at functional sites in health, aging, and disease. Ann. N. Y. Acad. Sci. 2008, 1126, 124–127. [Google Scholar] [CrossRef]
- Iram, A.; Alam, T.; Khan, J.M.; Khan, T.A.; Khan, R.H.; Naeem, A. Molten globule of hemoglobin proceeds into aggregates and advanced glycated end products. PLoS ONE 2013, 8, e72075. [Google Scholar] [CrossRef]
- Banerjee, S.; Banerjee, S. Glyoxal modification mediates conformational alterations in silk fibroin: Induction of fibrillation with amyloidal features. J. Biosci. 2020, 45, e142. [Google Scholar] [CrossRef]
- Farhan, S.S.F.; Hussain, S.A. Advanced glycation end products (AGEs) and their soluble receptors (sRAGE) as early predictors of reno-vascular complications in patients with uncontrolled type 2 diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2457–2461. [Google Scholar] [CrossRef]
- Egaña-Gorroño, L.; López-Díez, R.; Yepuri, G.; Ramirez, L.S.; Reverdatto, S.; Gugger, P.F.; Shekhtman, A.; Ramasamy, R.; Schmidt, A.M. Receptor for advanced glycation end products (RAGE) and mechanisms and therapeutic opportunities in diabetes and cardiovascular disease: Insights from human subjects and animal models. Front. Cardiovasc. Med. 2020, 7, e37. [Google Scholar] [CrossRef]
- Fournet, M.; Bonté, F.; Desmoulière, A. Glycation damage: A possible hub for major pathophysiological disorders and aging. aging and disease. Aging Dis. 2018, 9, 880–900. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef]
- Yim, H.S.; Kang, S.O.; Hah, Y.C.; Chock, P.B.; Yim, M.B. Free radicals generated during the glycation reaction of amino acids by methylglyoxal. A model study of protein-cross-linked free radicals. J. Biol. Chem. 1995, 270, 28228–28233. [Google Scholar] [CrossRef] [PubMed]
- Kalapos, M.P. The tandem of free radicals and methylglyoxal. Chem. Biol. Interact. 2008, 171, 251–271. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.; Moosavi-Movahedi, A.A.; Habibi-Rezaei, M.; Shourian, M.; Ghourchian, H.; Ahmad, F.; Farhadi, M.; Saboury, A.A.; Sheibani, N. Hemoglobin fructation promotes heme degradation through the generation of endogenous reactive oxygen species. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Kosmachevskaya, O.V.; Shumaev, K.B.; Nasybullina, E.I.; Topunov, A.F. Formation of nitri-and nitrosylhemoglobin in systems modeling the Maillard reaction. Clin. Chem. Lab. Med. 2014, 52, 161–168. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2021, 5, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.M. The chemistry of nitroxyl (HNO) and implications in biology. Coord. Chem. Rev. 2005, 249, 433–455. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Bartberger, M.D.; Dutton, A.S.; Paolocci, N.; Wink, D.A.; Houk, K.N. The physiological chemistry and biological activity of nitroxyl (HNO): The neglected, misunderstood and enigmatic nitrogen oxide. Chem. Res. Toxicol. 2005, 18, 790–801. [Google Scholar] [CrossRef]
- Shafirovich, V.; Lymar, S.V. Nitroxyl and its anion in aqueous solutions: Spin states, protic equilibria, and reactivities toward oxygen and nitric oxide. Proc. Natl. Acad. Sci. USA 2002, 99, 7340–7345. [Google Scholar] [CrossRef] [PubMed]
- Fukuto, J.M.; Bianco, C.L.; Chavez, T.A. Nitroxyl (HNO) signaling. Free Radic. Biol. Med. 2009, 47, 1318–1324. [Google Scholar] [CrossRef]
- Suarez, S.A.; Neuman, N.I.; Munoz, M.; Alvarez, L.; Bikiel, D.E.; Brondino, C.D.; Ivanovic-Burmazovic, I.; Miljkovic, J.; Filipovic, M.R.; Marti, M.A.; et al. Nitric oxide is reduced to HNO by proton-coupled nucleophilic attack by ascorbate, tyrosine, and other alcohols. A new route to HNO in biological media? J. Am. Chem. Soc. 2015, 137, 4720–4727. [Google Scholar] [CrossRef] [PubMed]
- Smulik-Izydorczyk, R.; Dębowska, K.; Rostkowski, M.; Adamus, J.; Michalski, R.; Sikora, A. Kinetics of azanone (HNO) reactions with thiols: Effect of pH. Cell. Biochem. Biophys. 2021, 79, 845–856. [Google Scholar] [CrossRef]
- Lopez, B.E.; Shinyashiki, M.; Han, T.H.; Fukuto, J.M. Antioxidant actions of nitroxyl (HNO). Free Radic. Biol. Med. 2007, 42, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Shumaev, K.B.; Gorudko, I.V.; Kosmachevskaya, O.V.; Grigoryeva, D.V.; Panasenko, O.M.; Vanin, A.F.; Topunov, A.F.; Terekhova, M.S.; Sokolov, A.V.; Cherenkevich, S.N.; et al. Protective effect of dinitrosyl iron complexes with glutathione in red blood cell lysis induced by hypochlorous acid. Oxid. Med. Cell. Longev. 2019, 2019, e2798154. [Google Scholar] [CrossRef] [PubMed]
- Shumaev, K.B.; Dudylina, A.L.; Ivanova, M.V.; Pugachenko, I.S.; Ruuge, E.K. Dinitrosyl iron complexes: Formation and antiradical action in heart mitochondria. BioFactors 2018, 44, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kosmachevskaya, O.V.; Nasybullina, E.I.; Shumaev, K.B.; Novikova, N.N.; Topunov, A.F. Protective effect of dinitrosyl iron complexes bound with hemoglobin on oxidative modification by peroxynitrite. Int. J. Mol. Sci. 2021, 22, 13649. [Google Scholar] [CrossRef] [PubMed]
- DuMond, J.F.; King, S.B. The chemistry of nitroxyl-releasing compounds. Antioxid. Redox Signal. 2011, 14, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Fukuto, J.M. A recent history of nitroxyl chemistry, pharmacology and therapeutic potential. Br. J. Pharmacol. 2019, 176, 135–146. [Google Scholar] [CrossRef]
- Asahi, K.; Ichimori, K.; Nakazawa, H.; Izuhara, Y.; Inagi, R.; Watanabe, T.; Miyata, T.; Kurokawa, K. Nitric oxide inhibits the formation of advanced glycation end products. Kidney Int. 2000, 58, 1780–1787. [Google Scholar] [CrossRef]
- Nasybullina, E.I.; Pugachenko, I.S.; Kosmachevskaya, O.V.; Topunov, A.F. The influence of nitroxyl on Escherichia coli cells grown under carbonyl stress conditions. Appl. Biochem. Microbiol. 2022, 58, 575–581. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Novikova, N.N.; Topunov, A.F. Carbonyl stress in red blood cells and hemoglobin. Antioxidants 2021, 10, 253. [Google Scholar] [CrossRef]
- Switzer, C.H.; Flores-Santana, W.; Mancardi, D.; Basudhar, D.; Ridnour, L.A.; Miranda, K.M.; Fukuto, J.M.; Paolocci, N.; Wink, D.A. The emergence of nitroxyl (HNO) as a pharmacological agent. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 835–840. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef]
- Feng, L.; Zhou, S.; Gu, L.; Gell, D.A.; Mackay, J.P.; Weiss, M.J.; Gow, A.J.; Shi, Y. Structure of oxidized α-haemoglobin bound to AHSP reveals a protective mechanism for haem. Nature 2005, 435, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Nagaraj, R.H.; Grandhee, S.K.; Odetti, P.; Lapolla, A.; Fogart, J.; Monnier, V.M. Pentosidine: A molecular marker for the cumulative damage to proteins in diabetes, aging, and uremia. Diabetes Metab. Rev. 1991, 7, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Liu, J.; Dong, L.; Wang, X.; Zhang, X. Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed. Pharmacother. 2021, 140, e111750. [Google Scholar] [CrossRef] [PubMed]
- Mukunda, D.C.; Joshi, V.K.; Chandra, S.; Siddaramaiah, M.; Rodrigues, J.; Gadag, S.; Nayak, U.Y.; Mazumder, N.; Satyamoorthy, K.; Mahato, K.K. Probing nonenzymatic glycation of proteins by deep ultraviolet light emitting diode induced autofluorescence. Int. J. Biol. Macromol. 2022, 213, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.; Parravano, M.; Micheli, A.; Gaddini, L.; Matteucci, A.; Mallozzi, C.; Facchiano, F.; Malchiodi-Albedi, F.; Pricci, F. A quick, simple method for detecting circulating fluorescent advanced glycation end-products: Correlation with in vitro and in vivo non-enzymatic glycation. Metabolism 2017, 71, 64–69. [Google Scholar] [CrossRef]
- Kong, X.; Ma, M.-Z.; Huang, K.; Qin, L.; Zhang, H.-M.; Yang, Z.; Li, X.-Y.; Su, Q. Increased plasma levels of the methylglyoxal in patients with newly diagnosed type 2 diabetes. J. Diabetes 2014, 6, 535–540. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Yurek-George, A.; Argirov, O.K. Kinetics and mechanism of the reaction of aminoguanidine with the alpha-oxoaldehydes glyoxal, rnethylglyoxal and 3-deoxyglucosone under physiological conditions. Biochem. Pharmacol. 2000, 60, 55–65. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, M.S.; Alouffi, S.; Khan, S.; Khan, M.; Akashah, R.; Faisal, M.; Shahab, U. Gold nanoparticle-bioconjugated aminoguanidine inhibits glycation reaction: An in vivo study in a diabetic. Animal model. Biomed Res. Int. 2021, 2021, e5591851. [Google Scholar] [CrossRef]
- Shoman, M.E.; Aly, O.M. Nitroxyl (HNO): A reduced form of nitric oxide with distinct chemical, pharmacological, and therapeutic properties. Oxid. Med. Cell. Longev. 2016, 2016, e4867124. [Google Scholar] [CrossRef]
- Pasten, C.; Lozano, M.; Rocco, J.; Carrión, F.; Alvarado, C.; Liberona, J.; Michea, L.; Irarrázabal, C.E. Aminoguanidine prevents the oxidative stress, inhibiting elements of inflammation, endothelial activation, mesenchymal markers, and confers a renoprotective effect in renal ischemia and reperfusion injury. Antioxidants 2021, 10, 1724. [Google Scholar] [CrossRef]
- Velagic, A.; Qin, C.; Woodman, O.L.; Horowitz, J.D.; Ritchie, R.H.; Kemp-Harper, B.K. Nitroxyl: A novel strategy to circumvent diabetes associated impairments in nitric oxide signaling. Front. Pharmacol. 2020, 11, e727. [Google Scholar] [CrossRef] [PubMed]
- Shumaev, K.B.; Gubkina, S.A.; Kumskova, E.M.; Shepel’kova, G.S.; Ruuge, E.K.; Lankin, V.Z. Superoxide formation as a result of interaction of L-lysine with dicarbonyl compounds and its possible mechanism. Biochemistry 2009, 74, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yim, M.B.; Chock, P.B.; Yim, H.S.; Kang, S.O. Oxidation-reduction properties of methylglyoxal-modified protein in relation to free radical generation. J. Biol. Chem. 1998, 273, 25272–25278. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Rifkind, J.M. Heme degradation by reactive oxygen species. Antioxid. Redox Signal. 2004, 6, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Svistunenko, D.A. Reaction of haem containing proteins and enzymes with hydroperoxides: The radical view. Biochim. Biophys. Acta 2005, 1707, 127–155. [Google Scholar] [CrossRef] [PubMed]
- Reeder, B.J. Redox and peroxidase activities of the hemoglobin superfamily: Relevance to health and disease. Antioxid. Redox Signal. 2017, 26, 763–776. [Google Scholar] [CrossRef]
- Svistunenko, D.A.; Dunne, J.; Fryer, M.; Nicholls, P.; Reeder, B.J.; Wilson, M.T.; Bigotti, M.G.; Cutruzzolà, F.; Cooper, C.E. Comparative study of tyrosine radicals in hemoglobin and myoglobins treated with hydrogen peroxide. Biophys. J. 2002, 83, 2845–2855. [Google Scholar] [CrossRef]
- Reeder, B.J.; Svistunenko, D.A.; Cooper, C.E.; Wilson, M.T. The radical and redox chemistry of myoglobin and hemoglobin: From in vitro studies to human pathology. Antioxid. Redox Signal. 2004, 6, 954–966. [Google Scholar] [CrossRef]
- Vlasova, I.I. Peroxidase activity of human hemoproteins: Keeping the fire under control. Molecules 2018, 23, 2561. [Google Scholar] [CrossRef]
- Wilson, M.T.; Reeder, B.J. The peroxidatic activities of Myoglobin and Hemoglobin, their pathological consequences and possible medical interventions. Mol. Asp. Med. 2022, 84, e101045. [Google Scholar] [CrossRef] [PubMed]
- Karoui, H.; Chalier, F.; Finet, J.-P.; Tordo, P. DEPMPO: An efficient tool for the coupled ESR-spin trapping of alkylperoxyl radicals in water. Org. Biomol. Chem. 2011, 9, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Hogg, N.; Kalyanaraman, B. Nitric oxide and lipid peroxidation. Biochim. Biophys. Acta Bioenerg. 1999, 1411, 378–384. [Google Scholar] [CrossRef]
- Rubbo, H.; Radi, R.; Anselmi, D.; Kirk, M.; Barnes, S.; Butler, J.; Eiserich, J.P.; Freeman, B.A. Nitric oxide reaction with lipid peroxyl radicals spares α-tocopherol during lipid peroxidation. J. Biol. Chem. 2000, 275, 10812–10818. [Google Scholar] [CrossRef] [PubMed]
- Homma, T.; Kobayashi, S.; Conrad, M.; Konno, H.; Yokoyama, C.; Fujii, J. Nitric oxide protects against ferroptosis by aborting the lipid peroxidation chain reaction. Nitric. Oxide 2021, 115, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Faingold, I.I.; Poletaeva, D.A.; Soldatova, Y.V.; Smolina, A.V.; Pokidova, O.V.; Kulikov, A.V.; Sanina, N.A.; Kotelnikova, R.A. Effects of albumin-bound nitrosyl iron complex with thiosulfate ligands on lipid peroxidation and activities of mitochondrial enzymes in vitro. Nitric Oxide 2021, 117, 46–52. [Google Scholar] [CrossRef]
- Yeh, W.; Hsia, S.; Lee, W.; Wu, C. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Mridula, S.; Masroor, W.S.; Xavier, M.; Hui, T.W.; Chan, H.K.; Chirara, K.; Nwabueze, O.P. Antioxidant and anti-advanced glycation end products formation properties of palmatine. J. Pharm. Pharmacogn. Res. 2021, 9, 366–378. [Google Scholar] [CrossRef]
- Reddy, V.P.; Garrett, M.R.; Ferry, G.; Smith, M.A. Carnosine: A versatile antioxidant and antiglycating agent. Sci. Aging Knowl. Environ. 2005, 2005, pe12. [Google Scholar] [CrossRef] [PubMed]
- Menini, S.; Iacobini, C.; Fantauzzi, C.B.; Pugliese, G. L-carnosine and its derivatives as new therapeutic agents for the prevention and treatment of vascular complications of diabetes. Curr. Med. Chem. 2020, 27, 1744–1763. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Guo, R.; Wang, Q.; Zhang, D. Antioxidants inhibit advanced glycosylation end-product-induced apoptosis by downregulation of miR-223 in human adipose tissue-derived stem cells. Sci. Rep. 2016, 6, 23021. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Hanbaue, I.; Krishna, M.C.; DeGraff, W.; Gamson, J.; Mitchell, J.B. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc. Natl. Acad. Sci. USA 1993, 90, 9813–9817. [Google Scholar] [CrossRef] [PubMed]
- Hummel, S.G.; Fischer, A.J.; Martin, S.M.; Schafer, F.Q.; Buettner, G.R. Nitric oxide as a cellular antioxidant: A little goes a long way. Free Radic. Biol. Med. 2006, 40, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.Q.; Irvine, J.C.; Cao, A.H.; Alexander, A.E.; Love, J.E.; Patel, R.; McMullen, J.R.; Kaye, D.M.; Kemp-Harper, B.K.; Ritchie, R.H. Nitroxyl (HNO) Stimulates Soluble guanylyl cyclase to suppress cardiomyocyte hypertrophy and superoxide generation. PLoS ONE 2012, 7, e34892. [Google Scholar] [CrossRef]
- Shiva, S.; Crawford, J.H.; Ramachandran, A.; Ceaser, E.K.; Hillson, T.; Brookes, P.S.; Patel, R.P.; Darley-Usmar, V.M. Mechanisms of the interaction of nitroxyl with mitochondria. Biochem. J. 2004, 379 Pt 2, 359–366. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Carrington, S.J. HNO signaling mechanisms. Antioxid. Redox Signal. 2011, 14, 1649–1657. [Google Scholar] [CrossRef]
- Donzelli, S.; Espey, M.G.; Thomas, D.; Mancardi, D. Discriminating formation of HNO from other reactive nitrogen oxide species. Free Radic. Biol. Med. 2006, 40, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Sauerland, M.; Mertes, R.; Morozzi, C.; Eggler, A.L.; Gamon, L.F.; Davies, M.J. Kinetic assessment of Michael addition reactions of alpha, beta-unsaturated carbonyl compounds to amino acid and protein thiols. Free Radic. Biol. Med. 2021, 169, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bari, S.E.; Martí, M.A.; Amorebieta, V.T.; Estrin, D.A.; Doctorovich, F. Fast nitroxyl trapping by ferric porphyrins. J. Am. Chem. Soc. 2003, 125, 15272–15273. [Google Scholar] [CrossRef]
- Grachev, D.I.; Shumaev, K.B.; Kosmachevskaya, O.V.; Topunov, A.F.; Ruuge, E.K. Nitrosyl comlexes of hemoglobin in various model systems. Biophysics 2021, 66, 897–904. [Google Scholar] [CrossRef]
- Gorbunov, N.V.; Osipov, A.N.; Day, B.W.; Zayas-Rivera, B.; Kagan, V.E.; Elsayed, N.M. Reduction of ferrylmyoglobin and ferrylhemoglobin by nitric oxide: A protective mechanism against ferrylhemoprotein-induced oxidations. Biochemistry 1995, 34, 6689–6699. [Google Scholar] [CrossRef] [PubMed]
- Baron, C.P.; Mǿller, J.S.; Skibsted, L.; Andersen, H.J. Nitrosylmyoglobin as antioxidant—Kinetics and proposed mechanism for reduction of hydroperoxides. Free Radic. Res. 2007, 41, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.M.; Nagasawa, H.T.; Toscano, J.P. Donors of HNO. Curr. Top. Med. Chem. 2005, 5, 649–664. [Google Scholar] [CrossRef]
- Flores-Santana, W.; Switzer, C.; Ridnour, L.A.; Basudhar, D.; Mancardi, D.; Donzelli, S.; Thomas, D.D.; Miranda, K.M.; Fukuto, J.M.; Wink, D. A Comparing the chemical biology of NO and HNO. Arch. Pharm. Res. 2009, 32, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Farmer, P.J.; Sulc, F. Coordination chemistry of the HNO ligand with hemes and synthetic coordination complexes. J. Inorg. Biochem. 2005, 99, 166–184. [Google Scholar] [CrossRef]
- Espey, M.G.; Miranda, K.M.; Thomas, D.D.; Wink, D.A. Ingress and reactive chemistry of nitroxyl-derived species within human cells. Free Radic. Biol. Med. 2002, 33, 827–834. [Google Scholar] [CrossRef]
- Flores-Santana, W.; Salmon, D.J.; Donzelli, S.; Switzer, C.H.; Basudhar, D.; Ridnour, L.; Cheng, R.; Glynn, S.A.; Paolocci, N.; Fukuto, J.M.; et al. The specificity of nitroxyl chemistry is unique among nitrogen oxides in biological systems. Antioxid. Redox Signal. 2011, 14, 1659–1674. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.L.; Lumsden, N.G.; Farry, J.; Jefferis, A.-M.; Kemp-Harper, B.K.; Chin-Dusting, J.P.F. Nitroxyl: A vasodilator of human vessels that is not susceptible to tolerance. Clin. Sci. 2015, 129, 179–187. [Google Scholar] [CrossRef]
- Keceli, G.; Majumdar, A.; Thorpe, C.N.; Jun, S.; Tocchetti, C.G.; Lee, D.I.; Mahaney, J.E.; Paolocci, N.; Toscano, J.P. Nitroxyl (HNO) targets phospholamban cysteines 41 and 46 to enhance cardiac function. J. Gen. Physiol. 2019, 151, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.L.; Irvine, J.C.; Tare, M.; Apostolopoulos, J.; Favaloro, J.L.; Triggle, C.R.; Kemp-Harper, B.K. A role for nitroxyl (HNO) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br. J. Pharmacol. 2009, 157, 540–550. [Google Scholar] [CrossRef]
- Muronetz, V.I.; Melnikova, A.K.; Barinova, K.V.; Schmalhausen, E.V. Inhibitors of glyceraldehyde 3-phosphate dehydrogenase and unexpected effects of its reduced activity. Biochemistry 2019, 84, 1268–1279. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmachevskaya, O.V.; Nasybullina, E.I.; Pugachenko, I.S.; Novikova, N.N.; Topunov, A.F. Antiglycation and Antioxidant Effect of Nitroxyl towards Hemoglobin. Antioxidants 2022, 11, 2007. https://doi.org/10.3390/antiox11102007
Kosmachevskaya OV, Nasybullina EI, Pugachenko IS, Novikova NN, Topunov AF. Antiglycation and Antioxidant Effect of Nitroxyl towards Hemoglobin. Antioxidants. 2022; 11(10):2007. https://doi.org/10.3390/antiox11102007
Chicago/Turabian StyleKosmachevskaya, Olga V., Elvira I. Nasybullina, Igor S. Pugachenko, Natalia N. Novikova, and Alexey F. Topunov. 2022. "Antiglycation and Antioxidant Effect of Nitroxyl towards Hemoglobin" Antioxidants 11, no. 10: 2007. https://doi.org/10.3390/antiox11102007
APA StyleKosmachevskaya, O. V., Nasybullina, E. I., Pugachenko, I. S., Novikova, N. N., & Topunov, A. F. (2022). Antiglycation and Antioxidant Effect of Nitroxyl towards Hemoglobin. Antioxidants, 11(10), 2007. https://doi.org/10.3390/antiox11102007





