Carbon Black Functionalized with Naturally Occurring Compounds in Water Phase for Electrochemical Sensing of Antioxidant Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Chemicals, and Samples
2.2. Apparatus
2.3. CB Water-Phase Nanodispersion/Functionalization and CB-AuNP Self-Assembling
2.4. Physicochemical Characterization
2.5. Electrochemical Characterization and Measurements
3. Results and Discussion
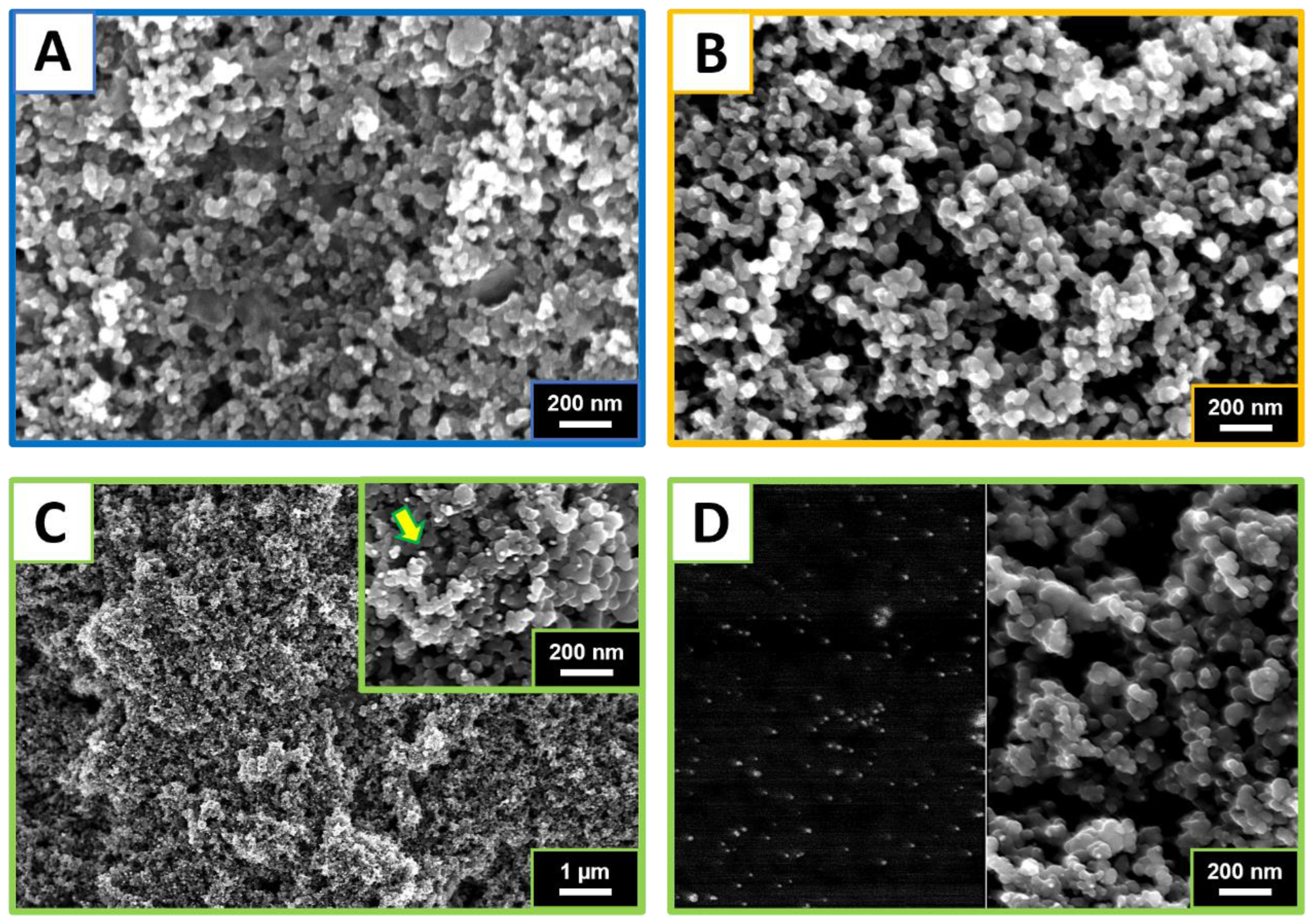
3.1. Water-Phase Carbon Black Production and AuNP Self-Assembling
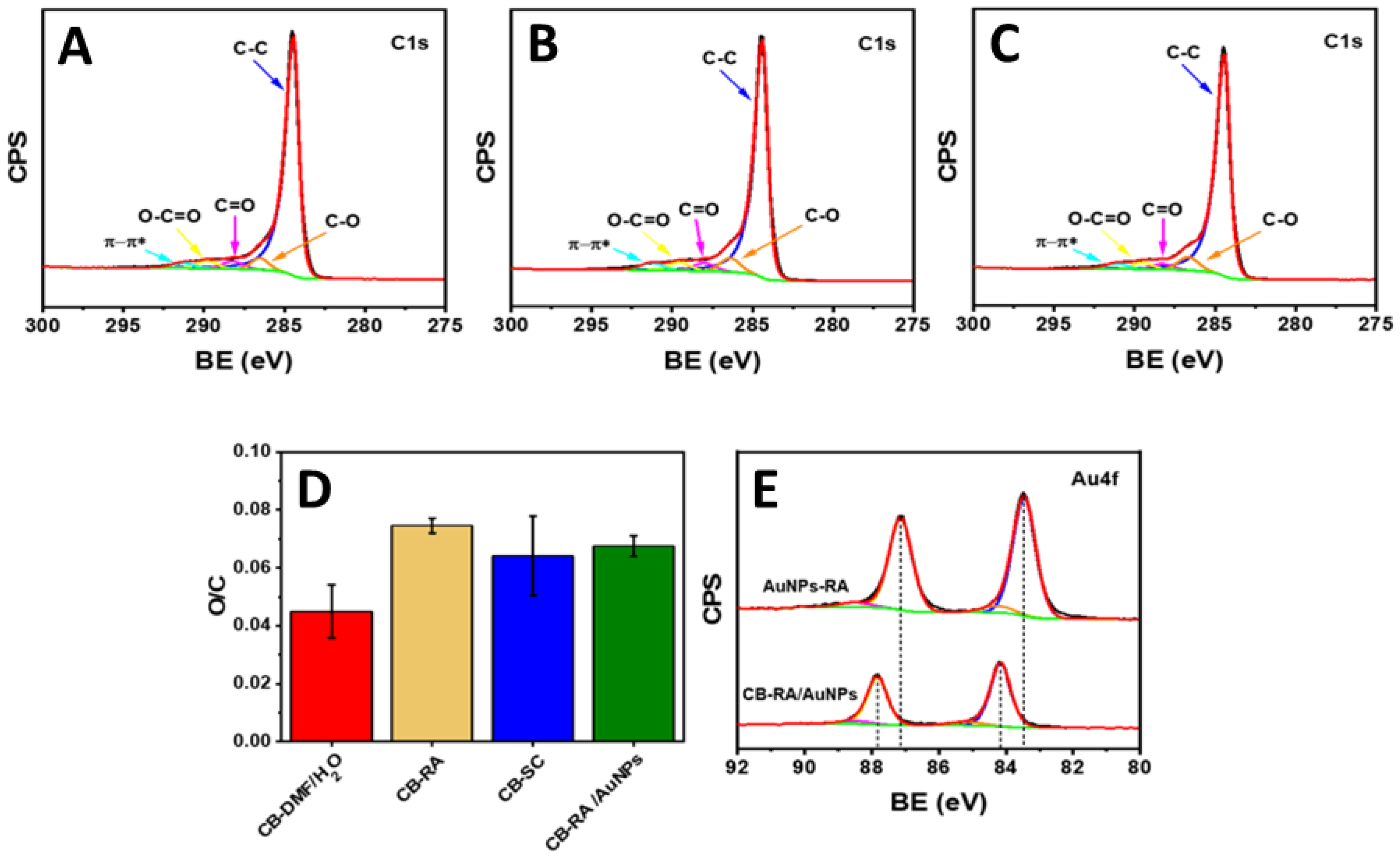
3.2. Morphological and Chemical Characterization
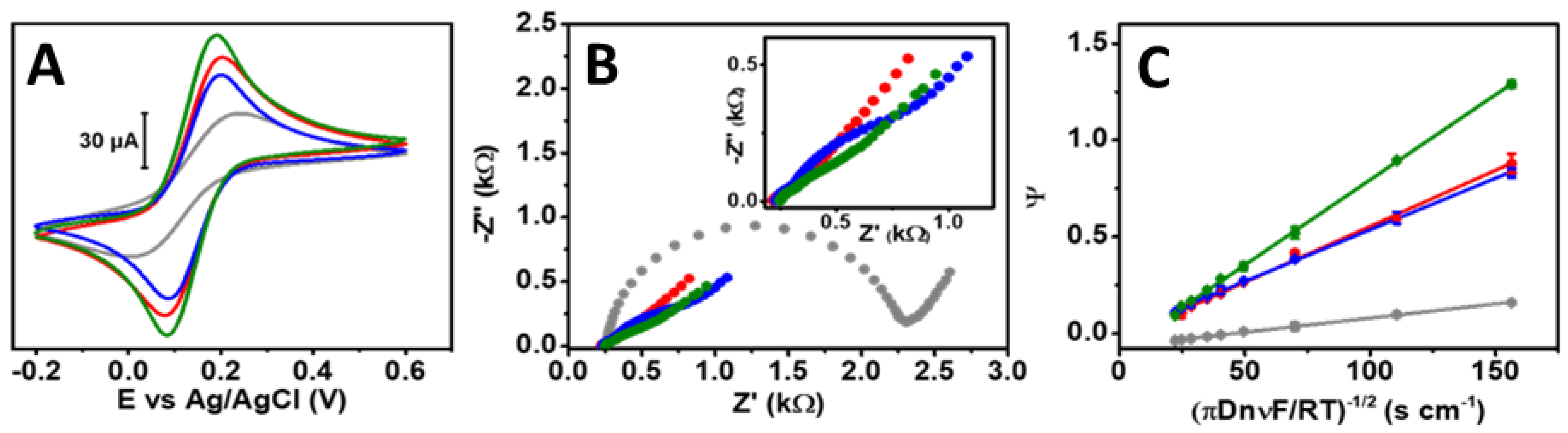
3.3. Electrochemical Characterization
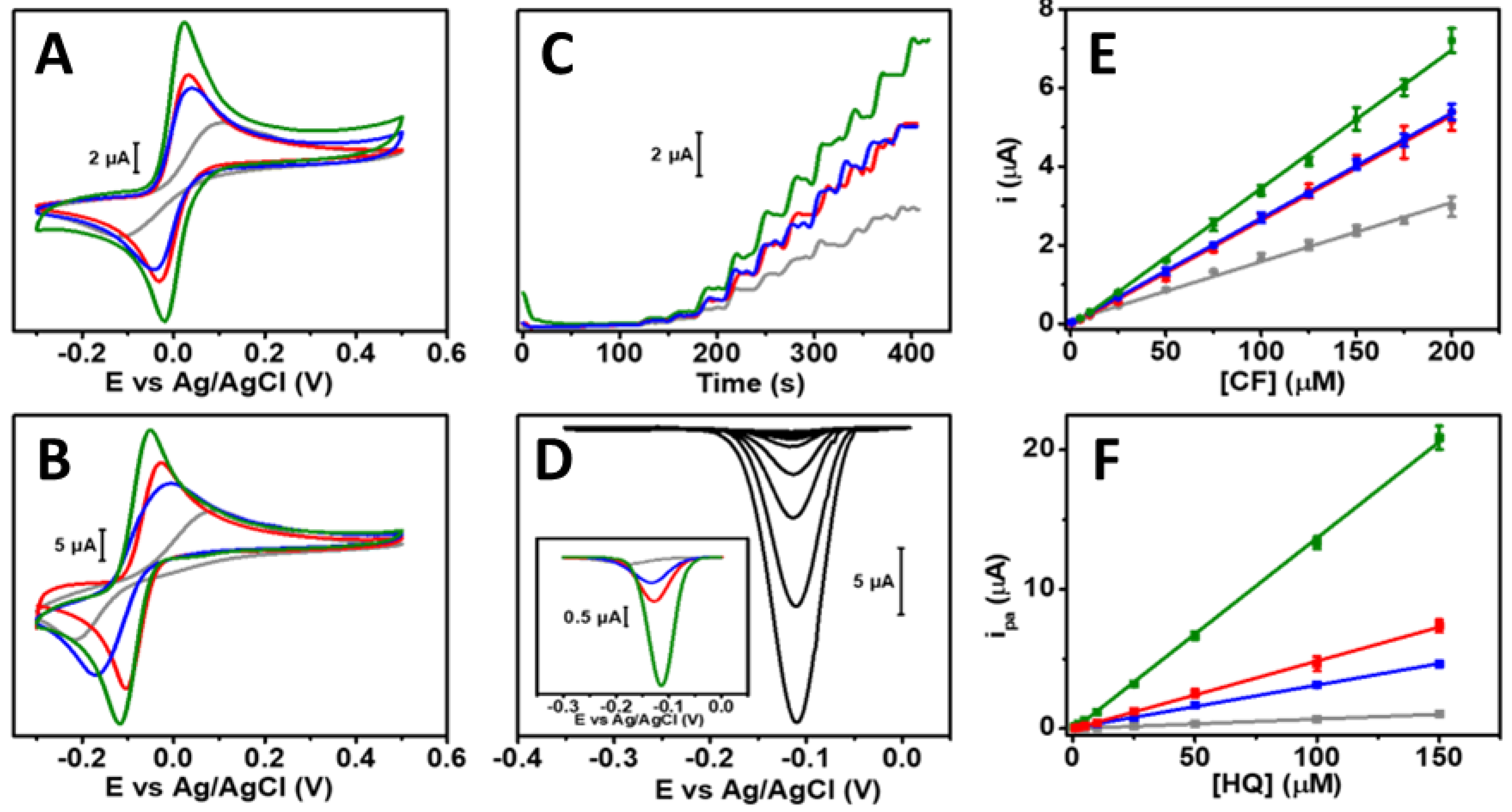
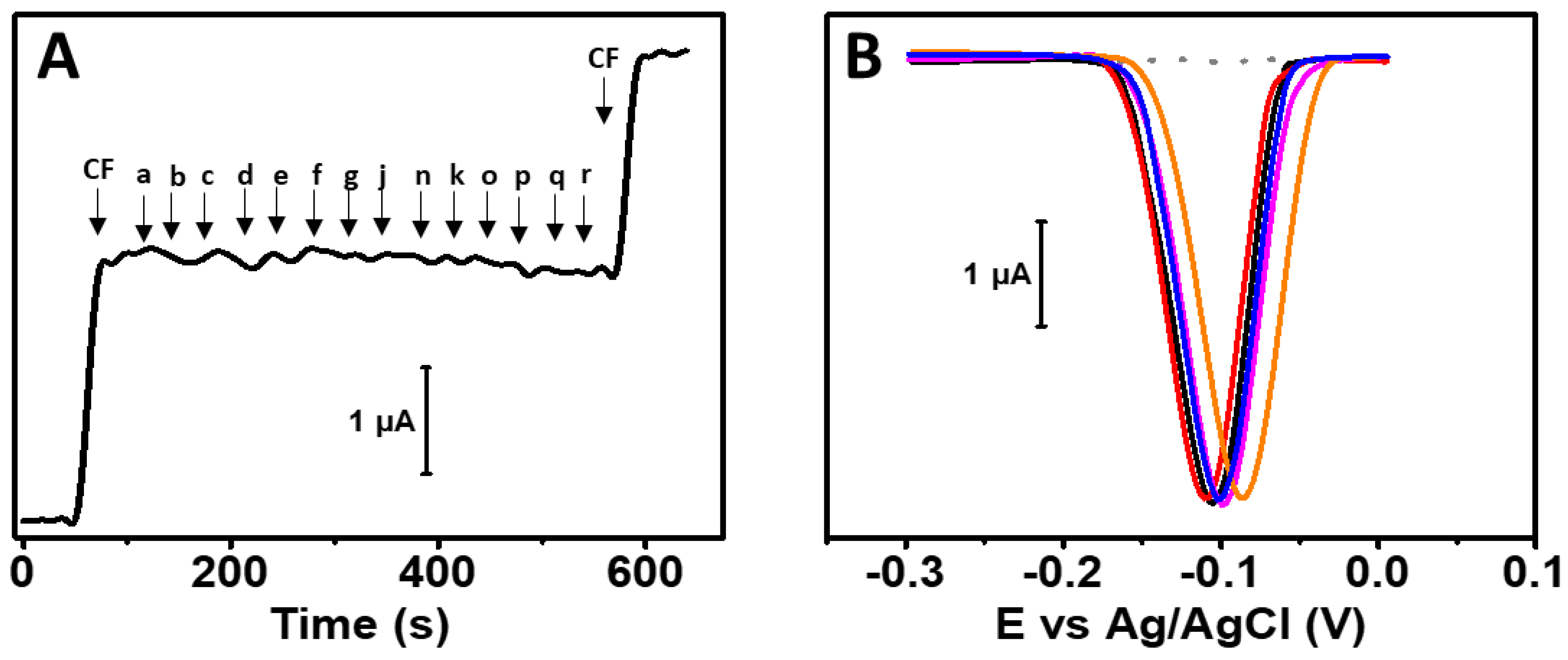
3.4. Electroanalytical Applications
3.5. Sample Analysis and Selectivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J. Fullerenes in Electrochemical Catalytic and Affinity Biosensing: A Review. J. Carbon Res. 2017, 3, 21. [Google Scholar] [CrossRef]
- Coroş, M.; Pruneanu, S.; Stefan-van Staden, R.-I. Review—Recent Progress in the Graphene-Based Electrochemical Sensors and Biosensors. J. Electrochem. Soc. 2020, 167, 037528. [Google Scholar] [CrossRef]
- Rivas, G.A.; Rodríguez, M.C.; Rubianes, M.D.; Gutierrez, F.A.; Eguílaz, M.; Dalmasso, P.R.; Primo, E.N.; Tettamanti, C.; Ramírez, M.L.; Montemerlo, A.; et al. Carbon Nanotubes-Based Electrochemical (Bio)Sensors for Biomarkers. Appl. Mater. Today 2017, 9, 566–588. [Google Scholar] [CrossRef]
- Khodabakhshi, S.; Fulvio, P.F.; Andreoli, E. Carbon Black Reborn: Structure and Chemistry for Renewable Energy Harnessing. Carbon 2020, 162, 604–649. [Google Scholar] [CrossRef]
- Mazzaracchio, V.; Tomei, M.R.; Cacciotti, I.; Chiodoni, A.; Novara, C.; Castellino, M.; Scordo, G.; Amine, A.; Moscone, D.; Arduini, F. Inside the Different Types of Carbon Black as Nanomodifiers for Screen-Printed Electrodes. Electrochim. Acta 2019, 317, 673–683. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Mazzaracchio, V.; Scognamiglio, V.; Amine, A.; Moscone, D. Carbon Black as an Outstanding and Affordable Nanomaterial for Electrochemical (Bio)Sensor Design. Biosens. Bioelectron. 2020, 156, 112033. [Google Scholar] [CrossRef]
- Talarico, D.; Arduini, F.; Constantino, A.; Del Carlo, M.; Compagnone, D.; Moscone, D.; Palleschi, G. Carbon Black as Successful Screen-Printed Electrode Modifier for Phenolic Compound Detection. Electrochem. Commun. 2015, 60, 78–82. [Google Scholar] [CrossRef]
- Arduini, F.; di Nardo, F.; Amine, A.; Micheli, L.; Palleschi, G.; Moscone, D. Carbon Black-Modified Screen-Printed Electrodes as Electroanalytical Tools. Electroanalysis 2012, 24, 743–751. [Google Scholar] [CrossRef]
- Arduini, F.; Majorani, C.; Amine, A.; Moscone, D.; Palleschi, G. Hg2+ Detection by Measuring Thiol Groups with a Highly Sensitive Screen-Printed Electrode Modified with a Nanostructured Carbon Black Film. Electrochim. Acta 2011, 56, 4209–4215. [Google Scholar] [CrossRef]
- Della Pelle, F.; Angelini, C.; Sergi, M.; del Carlo, M.; Pepe, A.; Compagnone, D. Nano Carbon Black-Based Screen Printed Sensor for Carbofuran, Isoprocarb, Carbaryl and Fenobucarb Detection: Application to Grain Samples. Talanta 2018, 186, 389–396. [Google Scholar] [CrossRef]
- Della Pelle, F.; Blandón-Naranjo, L.; Alzate, M.; del Carlo, M.; Compagnone, D. Cocoa Powder and Catechins as Natural Mediators to Modify Carbon-Black Based Screen-Printed Electrodes. Application to Free and Total Glutathione Detection in Blood. Talanta 2020, 207, 120349. [Google Scholar] [CrossRef] [PubMed]
- Della Pelle, F.; Del Carlo, M.; Sergi, M.; Compagnone, D.; Escarpa, A. Press-Transferred Carbon Black Nanoparticles on Board of Microfluidic Chips for Rapid and Sensitive Amperometric Determination of Phenyl Carbamate Pesticides in Environmental Samples. Microchim. Acta 2016, 183, 3143–3149. [Google Scholar] [CrossRef]
- Della Pelle, F.; Di Battista, R.; Vázquez, L.; Palomares, F.J.; Del Carlo, M.; Sergi, M.; Compagnone, D.; Escarpa, A. Press-Transferred Carbon Black Nanoparticles for Class-Selective Antioxidant Electrochemical Detection. Appl. Mater. Today 2017, 9, 29–36. [Google Scholar] [CrossRef]
- Della Pelle, F.; Vázquez, L.; Del Carlo, M.; Sergi, M.; Compagnone, D.; Escarpa, A. Press-Printed Conductive Carbon Black Nanoparticle Films for Molecular Detection at the Microscale. Chem. A Eur. J. 2016, 22, 12761–12766. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, J.F.; Della Pelle, F.; Rojas, D.; Compagnone, D.; Escarpa, A. Xurography-Enabled Thermally Transferred Carbon Nanomaterial-Based Electrochemical Sensors on Polyethylene Terephthalate-Ethylene Vinyl Acetate Films. Anal. Chem. 2020, 92, 13565–13572. [Google Scholar] [CrossRef]
- Cinti, S.; Minotti, C.; Moscone, D.; Palleschi, G.; Arduini, F. Fully Integrated Ready-to-Use Paper-Based Electrochemical Biosensor to Detect Nerve Agents. Biosens. Bioelectron. 2017, 93, 46–51. [Google Scholar] [CrossRef]
- Deroco, P.B.; Fatibello-Filho, O.; Arduini, F.; Moscone, D. Electrochemical Determination of Capsaicin in Pepper Samples Using Sustainable Paper-Based Screen-Printed Bulk Modified with Carbon Black. Electrochim. Acta 2020, 354, 136628. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Silva, T.A.; Vicentini, F.C.; Fatibello-Filho, O. Effect of Carbon Black Functionalization on the Analytical Performance of a Tyrosinase Biosensor Based on Glassy Carbon Electrode Modified with Dihexadecylphosphate Film. Enzym. Microb. Technol. 2018, 116, 41–47. [Google Scholar] [CrossRef]
- Sakthivel, R.; Annalakshmi, M.; Chen, S.-M.; Kubendhiran, S. Efficient Electrochemical Detection of Lethal Environmental Pollutant Hydroquinone Based on Functionalized Carbon Black/Polytyramine/Gold Nanoparticles Nanocomposite. J. Electrochem. Soc. 2019, 166, B680–B689. [Google Scholar] [CrossRef]
- Sakthivel, R.; Kubendhiran, S.; Chen, S.M.; Ranganathan, P.; Rwei, S.P. Functionalized Carbon Black Nanospheres Hybrid with MoS2 Nanoclusters for the Effective Electrocatalytic Reduction of Chloramphenicol. Electroanalysis 2018, 30, 1820–1828. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Cheng, W.T. Fabrication and Stabilization of Oxidized Carbon Black Nanoparticle Dispersion in Aqueous Solution for Photothermal Conversion Enhancement. ACS Omega 2021, 6, 3693–3700. [Google Scholar] [CrossRef]
- Lin, S.; Blankschtein, D. Role of the Bile Salt Surfactant Sodium Cholate in Enhancing the Aqueous Dispersion Stability of Single-Walled Carbon Nanotubes: A Molecular Dynamics Simulation Study. J. Phys. Chem. B 2010, 114, 15616–15625. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.J.; Lin, S.; Strano, M.S.; Blankschtein, D. Understanding the Stabilization of Single-Walled Carbon Nanotubes and Graphene in Ionic Surfactant Aqueous Solutions: Large-Scale Coarse-Grained Molecular Dynamics Simulation-Assisted DLVO Theory. J. Phys. Chem. C 2015, 119, 1047–1060. [Google Scholar] [CrossRef]
- Qurat Ul Ain, B.; Silveri, F.; Della Pelle, F.; Scroccarello, A.; Zappi, D.; Cozzoni, E.; Compagnone, D. Water-Phase Exfoliated Biochar Nano Fi Bers from Eucalyptus Scraps for Electrode Modi Fi Cation and Conductive Film Fabrication. ACS Sustain. Chem. Eng. 2021, 9, 13988–13998. [Google Scholar] [CrossRef]
- Lotya, M.; King, P.J.; Khan, U.; De, S.; Coleman, J.N. High-Concentration, Surfactant-Stabilized Graphene Dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Navik, R.; Gai, Y.; Wang, W.; Zhao, Y. Curcumin-Assisted Ultrasound Exfoliation of Graphite to Graphene in Ethanol. Ultrason. Sonochem. 2018, 48, 96–102. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, L.; Shao, X.; Liu, X.; Zhao, S.; Xie, S.; Xin, Z. Tannic Acid-Assisted Green Fabrication of Functionalized Graphene towards Its Enhanced Compatibility in NR Nanocomposite. Polym. Test. 2018, 70, 396–402. [Google Scholar] [CrossRef]
- Zhao, S.; Xie, S.; Zhao, Z.; Zhang, J.; Li, L.; Xin, Z. Green and High-Efficiency Production of Graphene by Tannic Acid-Assisted Exfoliation of Graphite in Water. ACS Sustain. Chem. Eng. 2018, 6, 7652–7661. [Google Scholar] [CrossRef]
- Yu, Z.; Shi, Z.; Xu, H.; Ma, X.; Tian, M.; Yin, J. Green Chemistry: Co-Assembly of Tannin-Assisted Exfoliated Low-Defect Graphene and Epoxy Natural Rubber Latex to Form Soft and Elastic Nacre-like Film with Good Electrical Conductivity. Carbon 2017, 114, 649–660. [Google Scholar] [CrossRef]
- Rojas, D.; Della Pelle, F.; Silveri, F.; Ferraro, G.; Fratini, E.; Compagnone, D. Phenolic Compounds as Redox-Active Exfoliation Agents for Group VI Transition Metal Dichalcogenides. Mater. Today Chem. 2022, 26, 101122. [Google Scholar] [CrossRef]
- Silveri, F.; Della Pelle, F.; Rojas, D.; Ferraro, G.; Bukhari, Q.U.A.; Fratini, E.; Compagnone, D. (+)-Catechin-Assisted Graphene Production by Sonochemical Exfoliation in Water. A New Redox-Active Nanomaterial for Electromediated Sensing. Microchim. Acta 2021, 188, 369. [Google Scholar] [CrossRef] [PubMed]
- Kubendhiran, S.; Sakthivel, R.; Chen, M.; Bhuvanenthiran, M.; Chen, T.-W. Innovative Strategy Based on a Novel Carbon-Black−β-Cyclodextrin Nanocomposite for the Simultaneous. Anal. Chem. 2018, 90, 6283–6291. [Google Scholar] [CrossRef] [PubMed]
- Vieira Jodar, L.; Orzari, L.O.; Storti Ortolani, T.; Assumpção, M.H.M.T.; Vicentini, F.C.; Janegitz, B.C. Electrochemical Sensor Based on Casein and Carbon Black for Bisphenol A Detection. Electroanalysis 2019, 31, 2162–2170. [Google Scholar] [CrossRef]
- Fu, S.; Ma, X.; Wang, S.; Zha, Q.; Wen, W.; Hu, B. Surfactant-Assisted Carbon Black for the Electrochemical Detection of Endocrine Disruptors. Surf. Interfaces 2021, 24, 101128. [Google Scholar] [CrossRef]
- Arduini, F.; Zanardi, C.; Cinti, S.; Terzi, F.; Moscone, D.; Palleschi, G.; Seeber, R. Effective Electrochemical Sensor Based on Screen-Printed Electrodes Modified with a Carbon Black-Au Nanoparticles Composite. Sens. Actuators B Chem. 2015, 212, 536–543. [Google Scholar] [CrossRef]
- Ben Messaoud, N.; Ait Lahcen, A.; Dridi, C.; Amine, A. Ultrasound Assisted Magnetic Imprinted Polymer Combined Sensor Based on Carbon Black and Gold Nanoparticles for Selective and Sensitive Electrochemical Detection of Bisphenol A. Sens. Actuators B Chem. 2018, 276, 304–312. [Google Scholar] [CrossRef]
- Jebril, S.; Cubillana-Aguilera, L.; Palacios-Santander, J.M.; Dridi, C. A Novel Electrochemical Sensor Modified with Green Gold Sononanoparticles and Carbon Black Nanocomposite for Bisphenol A Detection. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2021, 264, 114951. [Google Scholar] [CrossRef]
- Mattos, G.J.; Moraes, J.T.; Barbosa, E.C.M.; Camargo, P.H.C.; Dekker, R.F.H.; Barbosa-Dekker, A.M.; Sartori, E.R. Laccase Stabilized on β-D-Glucan Films on the Surface of Carbon Blackgold Nanoparticles A New Platform for Electrochemical Biosensing. Bioelectrochemistry 2019, 129, 116–123. [Google Scholar] [CrossRef]
- Cinti, S.; Santella, F.; Moscone, D.; Arduini, F. Hg2+ Detection Using a Disposable and Miniaturized Screen-Printed Electrode Modified with Nanocomposite Carbon Black and Gold Nanoparticles. Environ. Sci. Pollut. Res. 2016, 23, 8192–8199. [Google Scholar] [CrossRef]
- Cinti, S.; Politi, S.; Moscone, D.; Palleschi, G.; Arduini, F. Stripping Analysis of As(III) by Means of Screen-Printed Electrodes Modified with Gold Nanoparticles and Carbon Black Nanocomposite. Electroanalysis 2014, 26, 931–939. [Google Scholar] [CrossRef]
- Fakude, C.T.; Arotiba, O.A.; Mabuba, N. Electrochemical Aptasensing of Cadmium (II) on a Carbon Black-Gold Nano-Platform. J. Electroanal. Chem. 2020, 858, 113796. [Google Scholar] [CrossRef]
- Yammouri, G.; Mohammadi, H.; Amine, A. A Highly Sensitive Electrochemical Biosensor Based on Carbon Black and Gold Nanoparticles Modified Pencil Graphite Electrode for MicroRNA-21 Detection. Chem. Afr. 2019, 2, 291–300. [Google Scholar] [CrossRef]
- Silva, L.P.; Silva, T.A.; Moraes, F.C.; Fatibello-Filho, O. A Voltammetric Sensor Based on a Carbon Black and Chitosan-Stabilized Gold Nanoparticle Nanocomposite for Ketoconazole Determination. Anal. Methods 2021, 13, 4495–4502. [Google Scholar] [CrossRef]
- Kim, Y.; Jo, A.; Ha, Y.; Lee, Y.; Lee, D.; Lee, Y.; Lee, C. Highly Dispersive Gold Nanoparticles on Carbon Black for Oxygen and Carbon Dioxide Reduction. Electroanalysis 2018, 30, 2861–2868. [Google Scholar] [CrossRef]
- Scalera, F.; Monteduro, A.G.; Maruccio, G.; Blasi, L.; Gervaso, F.; Mazzotta, E.; Malitesta, C.; Piccirillo, C. Sustainable Chitosan-Based Electrical Responsive Scaffolds for Tissue Engineering Applications. Sustain. Mater. Technol. 2021, 28, e00260. [Google Scholar] [CrossRef]
- Bilgi, M.; Sahin, E.M.; Ayranci, E. Sensor and Biosensor Application of a New Redox Mediator: Rosmarinic Acid Modified Screen-Printed Carbon Electrode for Electrochemical Determination of NADH and Ethanol. J. Electroanal. Chem. 2018, 813, 67–74. [Google Scholar] [CrossRef]
- David, I.G.; Popa, D.E.; Buleandra, M.; Cheregi, M.C. Electrochemical Methods and (Bio) Sensors for Rosmarinic Acid Investigation. Chemosensors 2020, 8, 74. [Google Scholar] [CrossRef]
- Gil, E.; Enache, T.; Oliveira-Brett, A. Redox Behaviour of Verbascoside and Rosmarinic Acid. Comb. Chem. High Throughput Screen. 2013, 16, 92–97. [Google Scholar] [CrossRef]
- Sharp, M.; Petersson, M.; Edstrom, K. Preliminary Determinations of Electron Transfer Kinetics Involving Ferrocene Covalently Attached to a Platinum Surface. J. Electroanal. Chem. Interfacial Electrochem. 1979, 95, 123–130. [Google Scholar] [CrossRef]
- Wang, Y.; Laborda, E.; Ward, K.R.; Tschulik, K.; Compton, R.G. A Kinetic Study of Oxygen Reduction Reaction and Characterization on Electrodeposited Gold Nanoparticles of Diameter between 17 Nm and 40 Nm in 0.5 M Sulfuric Acid. Nanoscale 2013, 5, 9699–9708. [Google Scholar] [CrossRef]
- Palanisamy, S.; Thirumalraj, B.; Chen, S.M. Electrochemical Fabrication of Gold Nanoparticles Decorated on Activated Fullerene C60: An Enhanced Sensing Platform for Trace Level Detection of Toxic Hydrazine in Water Samples. RSC Adv. 2015, 5, 94591–94598. [Google Scholar] [CrossRef]
- Baghayeri, M.; Ansari, R.; Nodehi, M.; Veisi, H. Designing and Fabrication of a Novel Gold Nanocomposite Structure: Application in Electrochemical Sensing of Bisphenol A. Int. J. Environ. Anal. Chem. 2018, 98, 874–888. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, S.; Tang, J.; Zhang, Y.; Cui, Y.; Su, C.; Qu, Y.; Wei, L.; Cao, H.; Quan, J. Adsorptive Performance of Coal Based Magnetic Activated Carbon for Perfluorinated Compounds from Treated Landfill Leachate Effluents. Process Saf. Environ. Prot. 2018, 117, 383–389. [Google Scholar] [CrossRef]
- Scroccarello, A.; Molina-Hernández, B.; Della Pelle, F.; Ciancetta, J.; Ferraro, G.; Fratini, E.; Valbonetti, L.; Chaves Copez, C.; Compagnone, D. Effect of Phenolic Compounds-Capped AgNPs on Growth Inhibition of Aspergillus niger. Colloids Surf. B Biointerfaces 2021, 199, 111533. [Google Scholar] [CrossRef]
- Sainato, M.; Strambini, L.M.; Rella, S.; Mazzotta, E.; Barillaro, G.; Marsilio Strambini, L.; Barillaro, G. Sub-Parts Per Million NO2 Chemi-Transistor Sensors Based on Composite Porous Silicon/Gold Nanostructures Prepared by Metal-Assisted Etching. ACS Appl. Mater. Interfaces 2015, 7, 7136–7145. [Google Scholar] [CrossRef]
- Naskar, B.; Mondal, S.; Moulik, S.P. Amphiphilic Activities of Anionic Sodium Cholate (NaC), Zwitterionic 3-[(3-Cholamidopropyl)Dimethylammonio]-1-Propanesulfonate (CHAPS) and Their Mixtures: A Comparative Study. Colloids Surf. B Biointerfaces 2013, 112, 155–164. [Google Scholar] [CrossRef]
- Samyn, L.M.; Suresh Babu, R.; Devendiran, M.; de Barros, A.L.F. One-Step Electropolymerization of Methylene Blue Films on Highly Flexible Carbon Fiber Electrode as Supercapacitors. Micro Nano Syst. Lett. 2021, 9, 3. [Google Scholar] [CrossRef]
- Velusamy, V.; Arshak, K.; Yang, C.F.; Yu, L.; Korostynska, O.; Adley, C. Comparison between DNA Immobilization Techniques on a Redox Polymer Matrix. Am. J. Anal. Chem. 2011, 2, 392–400. [Google Scholar] [CrossRef]
- Blandón-Naranjo, L.; Della Pelle, F.; Vázquez, M.V.; Gallego, J.; Santamaría, A.; Alzate-Tobón, M.; Compagnone, D. Electrochemical Behaviour of Microwave-Assisted Oxidized MWCNTs Based Disposable Electrodes: Proposal of a NADH Electrochemical Sensor. Electroanalysis 2018, 30, 509–516. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications. Russ. J. Electrochem. 2002, 38, 1364–1365. [Google Scholar]
- Nicholson, R.S. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Della Pelle, F.; Rojas, D.; Silveri, F.; Ferraro, G.; Fratini, E.; Scroccarello, A.; Escarpa, A.; Compagnone, D. Class-Selective Voltammetric Determination of Hydroxycinnamic Acids Structural Analogs Using a WS2/Catechin-Capped AuNPs/Carbon Black–Based Nanocomposite Sensor. Microchim. Acta 2020, 187, 296. [Google Scholar] [CrossRef]
- Hyde, M.E.; Davies, T.J.; Compton, R.G. Fabrication of Random Assemblies of Metal Nanobands: A General Method. Angew. Chem. Int. Ed. 2005, 44, 6491–6496. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic Acid Derivatives, Analogs and Applications: A Patent Review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Enguita, F.J.; Leitão, A.L. Hydroquinone: Environmental Pollution, Toxicity, and Microbial Answers. Biomed. Res. Int. 2013, 2013, 542168. [Google Scholar] [CrossRef]
- Li, S.J.; Xing, Y.; Wang, G.F. A Graphene-Based Electrochemical Sensor for Sensitive and Selective Determination of Hydroquinone. Microchim. Acta 2012, 176, 163–168. [Google Scholar] [CrossRef]
- Pogacean, F.; Coroş, M.; Magerusan, L.; Rosu, M.-C.; Socaci, C.; Gergely, S.; Stefan-van Staden, R.-I.; Moldovan, M.; Sarosi, C.; Pruneanu, S. Sensitive Detection of Hydroquinone Using Exfoliated Graphene-Au/Glassy Carbon Modified Electrode. Nanotechnology 2017, 29, 095501. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, H.; Yang, J.; Shi, Z.; Tan, Y.; Jin, J.; Wang, R.; Zhang, S.; Wang, J. Biochar Decorated with Gold Nanoparticles for Electrochemical Sensing Application. Electrochim. Acta 2018, 261, 464–473. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Voltamperometric Sensors and Biosensors Based on Carbon Nanomaterials Used for Detecting Caffeic Acid—A Review. Int. J. Mol. Sci. 2020, 21, 9275. [Google Scholar] [CrossRef]

| Caffeic Acid Equivalents | Hydroquinone | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | Added | Found | Recovery | RSD | Samples | Added | Found | Recovery | RSD |
| (µM) | (µM) | (%) | (%) | (µM) | (µM) | (%) | (%) | ||
| Thyme | - | 41.2 | - | 5.1 | Irrigation | - | - | - | - |
| 10 | 51.6 | 104.1 | 4.0 | 0.5 | 0.5 | 101.6 | 5.6 | ||
| 20 | 61.4 | 101.3 | 5.7 | 5 | 5.1 | 101.4 | 4.2 | ||
| 30 | 72.1 | 102.9 | 2.3 | 10 | 10.4 | 103.6 | 3.5 | ||
| Sage | - | 96.0 | - | 2.9 | River mouth | - | - | - | - |
| 10 | 107.0 | 110.2 | 3.3 | 0.5 | 05 | 97.6 | 4.8 | ||
| 20 | 115.5 | 97.5 | 3.2 | 5 | 5.1 | 102.9 | 2.9 | ||
| 30 | 125.7 | 99.0 | 4.4 | 10 | 10.2 | 101.9 | 3.7 | ||
| Mint | - | 105.5 | - | 4.1 | Well | - | - | - | - |
| 10 | 116.8 | 112.8 | 5.4 | 0.5 | 0.5 | 97.1 | 6.0 | ||
| 20 | 127.3 | 108.9 | 4.3 | 5 | 4.8 | 97.5 | 3.1 | ||
| 30 | 135.4 | 99.7 | 3.4 | 10 | 9.7 | 97.0 | 5.9 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveri, F.; Della Pelle, F.; Scroccarello, A.; Mazzotta, E.; Di Giulio, T.; Malitesta, C.; Compagnone, D. Carbon Black Functionalized with Naturally Occurring Compounds in Water Phase for Electrochemical Sensing of Antioxidant Compounds. Antioxidants 2022, 11, 2008. https://doi.org/10.3390/antiox11102008
Silveri F, Della Pelle F, Scroccarello A, Mazzotta E, Di Giulio T, Malitesta C, Compagnone D. Carbon Black Functionalized with Naturally Occurring Compounds in Water Phase for Electrochemical Sensing of Antioxidant Compounds. Antioxidants. 2022; 11(10):2008. https://doi.org/10.3390/antiox11102008
Chicago/Turabian StyleSilveri, Filippo, Flavio Della Pelle, Annalisa Scroccarello, Elisabetta Mazzotta, Tiziano Di Giulio, Cosimino Malitesta, and Dario Compagnone. 2022. "Carbon Black Functionalized with Naturally Occurring Compounds in Water Phase for Electrochemical Sensing of Antioxidant Compounds" Antioxidants 11, no. 10: 2008. https://doi.org/10.3390/antiox11102008
APA StyleSilveri, F., Della Pelle, F., Scroccarello, A., Mazzotta, E., Di Giulio, T., Malitesta, C., & Compagnone, D. (2022). Carbon Black Functionalized with Naturally Occurring Compounds in Water Phase for Electrochemical Sensing of Antioxidant Compounds. Antioxidants, 11(10), 2008. https://doi.org/10.3390/antiox11102008










