Abstract
The aim of this study was to produce a fresh ovine pressed cheese within Pecorino “Primosale” typology with the addition of citrus essential oils (EOs). For this purpose, ewe’s pasteurized milk was added with EOs from the peel of lemons, oranges and tangerines. Seven cheese productions were performed at the pilot plant scale level, including one control production (CP) without the addition of EOs and six experimental productions obtained by the addition of two EO concentrations (100 and 200 µL/L) to milk. The acidification process was obtained by means of the starter cultures Lactococcus lactis CAG4 and PON36. All cheeses showed levels of lactic acid bacteria (LAB) around 109 CFU/g, indicating that citrus EOs did not negatively influence the starter evolution. The addition of citrus EOs did not determine significant variations for dry matter, fat and protein percentages but increased the antioxidant capacity of all the experimental cheeses of about 50% in comparison to the control trial. The citrus EOs impacted cheese VOCs, especially for terpene class (limonene, β-pinene, myrcene, carene, linalool and α-terpineol). The sensory evaluation showed that cheeses enriched with 100 µL/L of citrus EOs were mostly appreciated by the panelists.
1. Introduction
In the past few years, the negative consumers’ acceptance for synthetic chemical preservatives determined an increasing interest in natural alternative preservatives to produce food with long shelf-lives, high nutritional values and sensory characteristics [1]. Therefore, food industries and academic researchers focused their attention on the use of active compounds derived from plant and fruit byproducts [2]. This strategy fits well within the European Green Deal aimed at highlighting environmental sustainability through the reuse of food wastes of processing industries for a clean and circular economy [3]. In this context, particular attention has been paid to plant essential oils (EOs) obtained by steam distillation; dry distillation or a suitable mechanical process of different parts of the plants, including the leaves, flowers, peels, barks, seeds and roots [4].
EOs are a mixture of secondary bioactive metabolites, such as phenylpropenes, terpenes and other volatile constituents [5]. These substances are natural aromatic and volatile liquids known since ancient times for their flavoring properties [6] and antimicrobial properties [7]. Nowadays, the positive effects of EOs to exert antioxidant, anti-inflammatory and antispasmodic actions are well-known [8]. Although some essential oils may cause risk to human health [9], most of them are harmlessness and classified as Generally Recognized As Safe (GRAS) by the U.S. Food and Drug Administration, and their use is allowed in all foods [10]. Among the raw plant materials used for the extraction of EOs, the byproducts of the citrus industry have been extensively considered [11].
The region of Sicily is a leader in the cultivation of several citrus genotypes (pummelo, grapefruit, orange, kumquat, mandarin and lemon) [12], and their fruits are consumed fresh or processed into juices and candied items [13]. The main waste generated during the processing of fruits is represented by epicarp or flavedo [14], unexploited byproducts in juice production [15]. However, this byproduct contains numerous oil glands filled with EOs [16], which have a wide spectrum of applications, including pharmaceutical, cosmetic, agricultural and food formulations [17].
The use of citrus EOs in dairy production is not new; so far, this application has only been performed for bovine milk-derived cheeses in order to improve their microbiological stability [18,19]. To our knowledge, no studies regarding the addition of citrus EOs to ewes’ milk cheeses are present in the literature. Generally, Sicilian fresh-pressed cheese made from ewes’ milk are referred to as Pecorino “Primosale” cheeses [20], which are commonly consumed after a very short ripening time [21].
The purpose of the present research was to produce, for the first time, a new typology of Pecorino “Primosale” cheese with the addition of three industrial citrus EOs extracted from oranges, lemons and tangerines and Lactococcus lactis starter cultures. The specific objectives of the present study were to: (i) evaluate the in vitro inhibitory activity of citrus EOs against starter cultures; (ii) characterize the citrus EOs for their chemical compositions; (iii) monitor the ability of starter cultures to drive the fermentation process in the presence of citrus Eos and (iv) characterize the final cheeses in terms of their physicochemical traits, antioxidant capacity, volatile organic compounds and sensory characteristics.
2. Materials and Methods
2.1. Raw Materials and Starter Cultures
Three industrial citrus EOs extracted by cold pressing from the peels of oranges (Citrus sinensis (L.) Osbeck), lemons (Citrus limon (L.) Osbeck) and tangerines (Citrus reticulata Blanco) were provided by the “EuroFood S.r.l.” facility located in Capo d’Orlando (Messina, Italy). Whole ewes’ milk used for cheese production was obtained exclusively from Valle del Belìce breed sheep. Two strains of Lactococcus lactis subsp. lactis (CAG4 and PON36) belonging to the culture collection of the Department of Agricultural, Food and Forest Sciences (University of Palermo, Italy), previously isolated from raw ewes’ cheese productions and evaluated for their dairy traits [22,23], were used as the fermenting agents.
2.2. Inhibitory Assay
In order to evaluate the suitability of citrus EOs to produce Primosale cheese, their inhibitory activity was tested against the two Lc. lactis starter strains. Following the methodology described by Gaglio et al. [24], citrus EOs were tested against a cell density of approximately 107 colony-forming units (CFU)/mL of each Lc. lactis strain in Media 17 (M17) soft agar (0.7% w/v) (Biotec, Grosseto, Italy) applying the paper disc diffusion method. Sterile water was used as the negative control, while streptomycin (10% w/v) as the positive control [25]. The inhibitory activity was evaluated after incubation at 30 °C for 24 h and was scored positive only in the case of a definite clear area surrounding the paper discs. This test was carried out in duplicate.
2.3. Development of Natural Milk Starter Culture
The Natural Milk Starter Culture (NMSC) was prepared according to Gaglio et al. [26]. Briefly, the strains Lc. lactis CAG4 and PON36 were reactivated in M17 broth (Biotec) incubated at 30 °C for 24 h. After growth, the cells were centrifuged at 10,000× g for 5 min, washed and resuspended in Ringer’s solution (Sigma-Aldrich, Milan, Italy). Washed cells were then inoculated into ovine whole-fat UHT milk (Leeb Vital, Wartberg an der Krems, Austria) at a final concentration of about 106 CFU/mL. After incubation at 30 °C for 24 h, the NMSC containing the multi-strain culture was used for cheese making.
2.4. Cheese Productions and Sample Collection
The experimental cheese productions were performed under controlled conditions at a dairy pilot plant of the dairy factory “Biopek”, located in Gibellina (Trapani, Italy). The experimental plan included a control production (CP) and two experimental productions (EP), one for each concentration of citrus EOs (100 and 200 µL/L) added to ewes’ milk; the EO concentrations were chosen based on the previous study of Marcial et al. [27]. A total of seven productions were planned (Table 1).

Table 1.
Cheese productions.
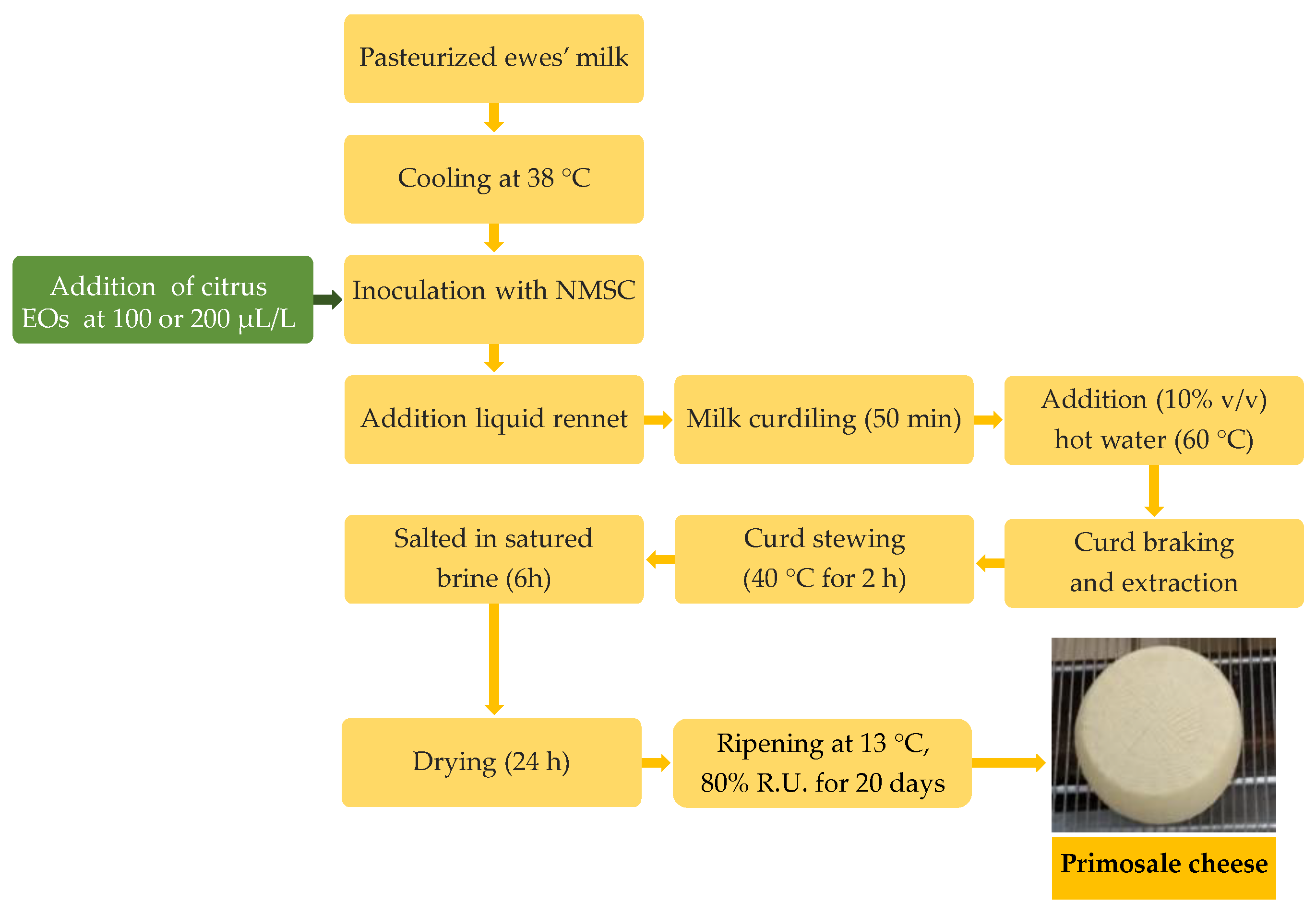
Cheese production was performed by applying the technology for the “Primosale” pressed cheese type (Figure 1).
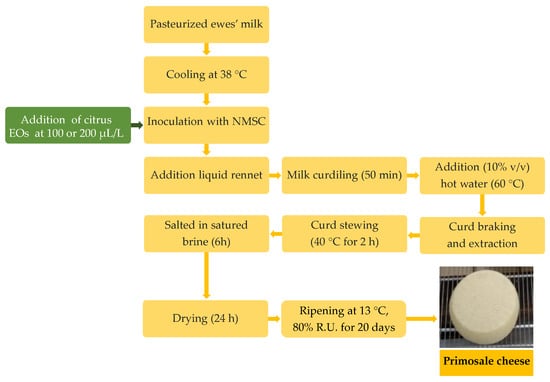
Figure 1.
Flow diagrams of Primosale cheese production enriched with citrus essential oils. Abbreviations: EOs, essential oils; NMSC, natural milk starter cultures.
Briefly, each trial was performed in plastic vats with 25 L pasteurized (72 °C for 15 s) whole ewe’s milk. After cooling at 38 °C, the bulk milk was inoculated with the NMSC (250 mL) and added to 100 or 200 µL/L of each citrus EOs prior the liquid rennet (Clerici Sacco International, Cadorago, Italy) addition (3 mL). After coagulation, the curd was cut to the dimension of rice grains, hand-pressed into cylindrical, perforated plastic molds and subjected to stewing at 40 °C for 2 h. The cheeses were salted in saturated brine for 6 h and ripened for 20 d in a storage chamber at 13 °C and 80% relative humidity (RH). Cheese productions were carried out in duplicate in two consecutive weeks. Samples of raw milk, pasteurized milk, inoculated milk, curds and the final cheeses were collected for analysis.
2.5. Microbiological Analyses
Milk samples were directly serially diluted (1:10) in Ringer’s solution (Sigma-Aldrich, Milan, Italy) into 10-mL/volume test tubes, while the curd and cheese samples (10 g) were first, homogenized in sodium citrate (2% w/v) solution by means of BagMixer® 400 (Interscience, Saint Nom, France) at the highest speed for 2 min and then serially diluted in Ringer’s solution. The dilutions of raw milk and pasteurized milk were plated on agar media to analyze the total mesophilic microorganisms (TMM), and the main pro-technological (lactic acid bacteria (LAB), including enterococci); spoilage (Pseudomonas spp.) and pathogenic (members of the Enterobacteriaceae family, coagulase-positive staphylococci (CPS), Listeria monocytogenes, Escherichia coli and Salmonella spp.) bacterial groups following the approach of Gaglio et al. [28].
2.6. Isolation, Typing and Identification of LAB Resistant to the Pasteurization Process
After growth, all colonies of presumptive LAB sharing different morphologies in terms of the shape, size, color, edge, characteristics of the surface and elevation were isolated from the highest cell densities of pasteurized bulk milk. All isolates were preliminary characterized by Gram determination applying the method described by Gregersen [29] and catalase test, determined as reported by Koneman et al. [30]. Only Gram-positive cultures negative for catalase expression were purified by consecutive subculturing and differentiated by randomly amplified polymorphic DNA (RAPD)-PCR analysis applying the protocol described by Gaglio et al. [31]. Gelcompare II software version 6.5 (Applied-Maths, Sint-Martens-Latem, Belgium) was used to analyze the resulting RAPD profiles. All different strains were identified at the species level by 16S rRNA gene sequencing following the approach applied by Gaglio et al. [32]. The sequences were compared with those available in the GenBank/EMBL/DDBJ (http://www.ncbi.nlm.nih.gov, accessed on 14 September 2022) [33] and EzTaxon-e (http://eztaxon-e.ezbiocloud.net/, accessed on 14 September 2022) databases [34].
2.7. Starter Cultures Recognition
All presumptive LAB collected during cheese making from inoculated milk with NMSC until the final cheeses were analyzed at the strain level by means of RAPD-PCR in order to monitor the dominance of lactococci inoculated as starter cultures (Lc. lactis CAG4 and PON36) over LAB resistant to pasteurization.
2.8. Physicochemical Analysis of Cheeses
Cheeses were sampled and evaluated for their physicochemical traits. Cheeses were assessed for external and internal colors, measured in duplicate by a Minolta Chroma Meter CR-300 (Minolta, Osaka, Japan) using the illuminant C. The results are expressed as lightness (L*, from 0 = black to 100 = white), redness (a*, from green = −a to red = +a) and yellowness (b*, from blue = −b to yellow = +b), according to the CIE L*a*b* system [35].
Cheese hardness was evaluated with an Instron 5564 tester (Instron, Trezzano sul Naviglio, Milan, Italy) measuring the maximum resistance to compression (compressive stress, N/mm2) of samples (2 cm × 2 cm × 2 cm) kept at room temperature (22 °C).
Water activity (aw) was determined by a HygroPalm water activity indicator (Rotronic, Bassersdrof, Germany), according to ISO 21807 [36].
The cheese samples were freeze-dried according to the method reported by Rashidinejad et al. [37] and analyzed for the contents of dry matter (DM) [38], fat [39] and ash [40], whereas protein (% DM) was calculated by difference (100—fat % DM—ash % DM).
2.9. Antioxidant Capacity of Cheeses
Cheese samples were analyzed in duplicate for their antioxidant properties, determining the total polyphenols and the Trolox equivalent antioxidant capacity (TEAC).
Total polyphenols were determined by the Folin–Ciocalteau colorimetric method [41], with gallic acid as the standard. Briefly, 100 µL of extracted sample were mixed with 900 µL of distilled water and 500 µL of Folin–Ciocalteau reagent diluted in distilled water to a concentration of 1 N. Then, 2.5 mL of a 20% (w/v) sodium carbonate aqueous solution were added, and the mixture was vortexed for 30 s and incubated for 40 min in the dark at room temperature. The absorbance of the samples was read at 725 nm with the HUCH DR3900 spectrophotometer. Aqueous solutions of gallic acid with different concentrations (0–1 mg/mL) were used for the calibration curve (R2 = 0.99). The results were expressed as gallic acid equivalents (g GAE/kg DM).
The TEAC, a discoloration test measuring the radical scavenging ability of samples using ABTS radical cation (ABTS • +) and Trolox as the standard [42], was performed to evaluate the antioxidant activity of cheese extracts as described by Bonanno et al. [43], with some modifications. Briefly, the radical cation ABTS was obtained by reacting a 14 mM ABTS aqueous solution with an equal volume of 4.9 mM potassium persulfate and incubating the mixture for 16 h in the dark at room temperature before use. For the assay, the ABTS solution was diluted with 5 mM phosphate-buffered saline (PBS) (pH 7.4) to an absorbance of 0.795 (±0.02) at 734 nm. The absorbance of a mixture of 10.5 µL of PBS with 1.5 mL of a diluted ABTS solution was read at 734 nm after incubation for 6 min at 30 °C. In the same way, 10.5 µL of each extracted sample were mixed with 1.5 mL diluted ABTS radical cation solution, and the absorbance read at 734 nm after incubation at 30 °C for 6 min was used to calculate the percentage decrease of the absorbance due to decolorization in comparison with the absorbance read with PBS. A Trolox solution in PBS, between 0 and 2.5 mM, was used to develop a calibration curve (R2 = 0.99), and the results were expressed as mmol Trolox/kg DM.
The oxidative stability of cheese fat was evaluated by determining in duplicate the peroxide value (POV, mEq O2/kg fat) as the index of primary lipid oxidation [44], and the thiobarbituric acid-reactive substances (TBARs) as products of secondary lipid oxidation expressed as μg of malonylaldehyde (MDA)/kg DM, according to the methods proposed by Tarladgis et al. [45] and modified by Mele et al. [46]. Briefly, 2 g of freeze-dried cheese were mixed with 8 mL of phosphate buffer aqueous solution (pH 7) and homogenized by an Art-Miccra D-8 high-speed homogenizer (Art Labortechnik, Heitersheim, Germany). After the addition of 30% (v/v) trichloroacetic acid aqueous solution (2 mL), the sample was vortexed for a few seconds and filtered with Whatman No. 1 filter paper. An aqueous solution of 0.02 M thiobarbituric acid (5 mL) was added to 5 mL of filtrate, placed in a hot water bath at 90 °C for 20 min and then refrigerated. After centrifugation at 4500 rpm for 5 min, the absorbance of the supernatant was read at 530 nm using a Hach DR/4000 U spectrophotometer. To quantify TBARs, 1,1,3,3-tetramethoxypropane solutions at concentrations between 0.016 and 0.165 µg/mL were used for the calibration curve (R2 = 0.99).
2.10. Volatile Organic Compounds
Volatile organic compositions of the citrus EOs and cheese samples (CP and EP) were performed by GC/MS analysis. The citrus essential oils were directly diluted with hexane (1:100 ratio), while the cheese samples (2 g) were finely chopped. All samples were placed in a glass vial for the headspace solid-phase microextraction (SPME). SPME (DVB/CAR/PDMS, 50 mm, Supelco, Bellefonte, PA, USA) fiber was exposed to the samples under continuous stirring at 60 °C for 15 min. After sampling, the volatile components were desorbed for 1 min at 250 °C through a GC split-less injector with a SPME inlet liner. GC analyses were performed on an Agilent 6890 gas chromatograph coupled with a mass selective detector (Agilent 5975 c) using a DB-624 capillary column (Agilent Technologies, Santa Clara, CA, USA, 60 m, 0.25 mm, 1.40 µm). Helium was used as the carrier gas with an ionization voltage of 70 eV at a flow rate of 1 mL/min. The oven temperature program was 5 min isotherm at 40 °C, followed by a linear temperature increase of 5 °C min up to 200 °C, where it was held for 2 min. The transfer line temperature was 230 °C, and the scanning range of the GC/MS was 40–400 amu. The volatile components of the samples were identified by comparing their mass spectra to the NIST Library and confirmed by comparison with the Kovat’s Index.
2.11. Sensory Evaluation
Sensory evaluation of CP and EP cheeses was performed by a descriptive panel of 15 assessors (eight women and seven men aged between 20 and 62 years old). The judges were trained specifically following the ISO 8589 [47] indications and were asked to score 16 descriptors, including color, structure uniformity, intensity of odor, unpleasant odor, intensity of aroma, salty, sweet, acid, bitter, spicy, unpleasant aroma, adhesiveness, hardness, humidity, taste persistency and overall acceptability. The assessors scored the level of each attribute with a mark on a 7-point hedonic scale (0 = extremely low; 7 = extremely high).
2.12. Statistical Analyses
One-Way Analysis of Variance (ANOVA) was applied to identify the differences among the microbiological and physicochemical data. Tukey’s test was applied for pairwise and multiple mean comparisons (statistical significance p < 0.05). The values of VOCs emitted from citrus EOs were graphically represent as a heat map generated using ascendant hierarchical clustering. The VOC concentrations were graphically represented by a color change from yellow (lowest concentration) to red (highest concentration). Two-factor analysis of variance (ANOVA), with judges (i = 1, ..., 15) and cheeses (j = 1, ..., 7) as the fixed factors, was applied to evaluate the discrimination efficiency of the sensory attributes for each panelist. t-test with significance levels of p < 0.05 was used to compare the least square means (LSM). Statistical processing of microbiological and sensory data, as well as the graphic constructions of VOCs emitted from citrus EOs was performed with XLStat software version 2020.3.1 for Excel (Addinsoft, New York, NY, USA), while the physicochemical data were analyzed using the generalized linear model (GLM) procedure in SAS 9.2 software (SAS Institute Inc., Campus Drive Cary, NC, USA).
3. Results and Discussion
3.1. Suitability of Citrus EOs for Cheese Production
The antimicrobial activity of EOs obtained from the citrus genotypes (oranges, lemons and tangerines) cultivated in Sicily against the main human pathogens, including E. coli, L. monocytogenes, Salmonella spp. and Staphylococcus aureus bacteria, is well-known [48,49]. In our work, we tested the three industrial citrus EOs extracted from oranges, lemons and tangerines against LAB, which are necessary to drive the fermentation process and to transform milk into cheese [50]. The citrus EOs tested did not inhibit the growth of the two strains of Lc. lactis (CAG4 and PON36) selected as starter cultures (results not shown). Thus, these results confirmed the suitability of citrus EOs for Primosale cheese production, because their presence does not interfere with Lactococcus development.
3.2. Evolution of Microbial Population during Cheese Making
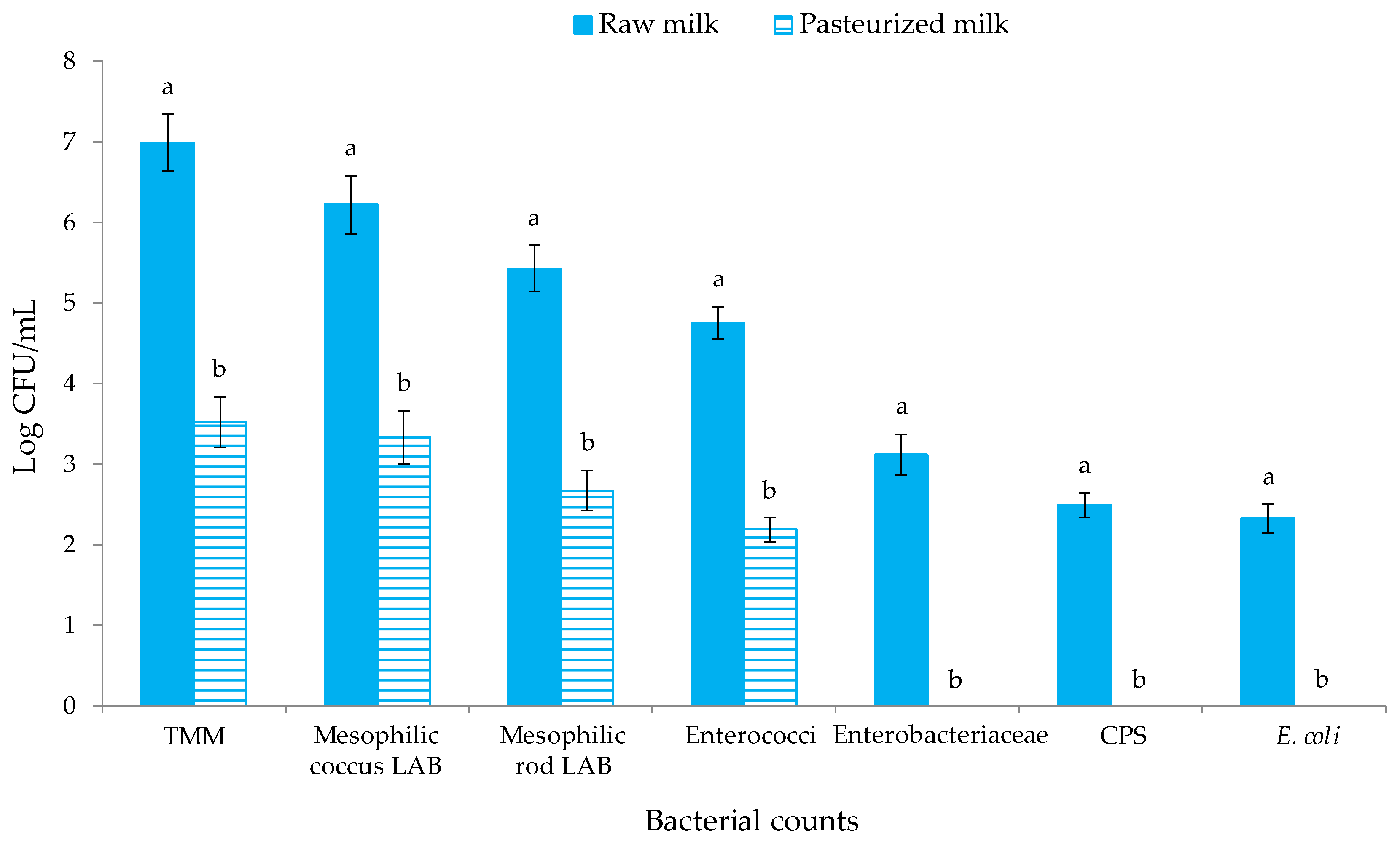
The levels of the different microbial groups investigated in ewes’ milk before and after pasteurization are reported in Figure 2. The presence of Salmonella spp. and L. monocytogenes was never found (for this reason, these results are not reported in Figure 2).
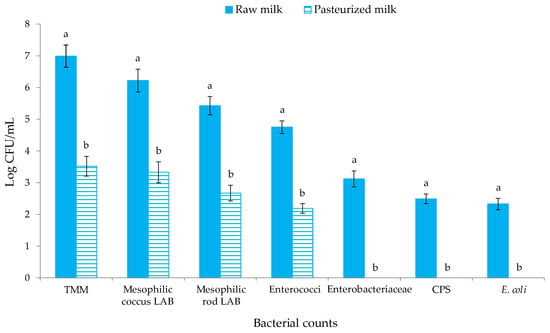
Figure 2.
Microbial counts (Log CFU/mL) of raw and pasteurized milk samples. Abbreviations: TMM, total mesophilic microorganisms; CPS, coagulase-positive staphylococci; E., Escherichia. The results indicate the mean values and standard deviation of four plate counts (carried out in duplicate for two independent productions). Different superscript letters indicate significant differences in the microbial concentrations according to Tukey’s test between the raw and pasteurized milk samples for p < 0.05.
Statistically significant differences (p < 0.05) were observed among the raw and pasteurized milk for the levels of all the microbial group objects of investigation. Ewes’ milk before pasteurization hosted levels of TMM of 6.99 CFU/mL, which is higher than the European limit of <500.000 CFU/mL for raw ewes’ milk [51]. High levels of TMM are often detected in raw ewe’s milk from the Valle del Belìce breed [52,53], and this is imputable to the microbial contamination of the udder surface occurring during the milking procedures or during transport or to the growth of indigenous milk microorganisms during storage [54]. Mesophilic coccus LAB were found at 106 CFU/mL, while mesophilic rod LAB were one Log cycle lower. After pasteurization, the levels of TMM, mesophilic coccus, rod LAB and enterococci, decreased by about three Log cycles, showing the ability of some indigenous milk bacteria to survive until the pasteurization process [55,56]. The levels of undesired microbial groups, such as members of the Enterobacteriaceae family, CPS and E. coli in raw milk, were in the range 102–103 CFU/mL and decreased below the detection limit in pasteurized milk. A similar trend was previously observed for ewes’ milk by Barbaccia et al. [57,58]. The analysis of milk after each citrus EO and NMSC addition showed levels of mesophilic coccus LAB above 7 Log CFU/mL, confirming that Lc. lactis inoculums occurred at 107 CFU/mL.
Table 2 reports the levels of TMM and Lc. lactis, added as a starter culture, on curds and cheeses samples.

Table 2.
Growth of starter LAB during cheese production.
After curdling, TMM and Lc. lactis were counted at 108 CFU/g, while, in the final control and experimental cheeses, their levels were above 9.0 Log CFU/g. A similar trend was previously reported by Marcial et al. [27] for bovine pressed cheeses produced with an oregano EO addition and using selected LAB strains as fermenting agents. These results highlighted that the addition of citrus EOs did not alter the microbiological parameters of the final cheeses.
3.3. Composition of Thermoduric LAB Populations
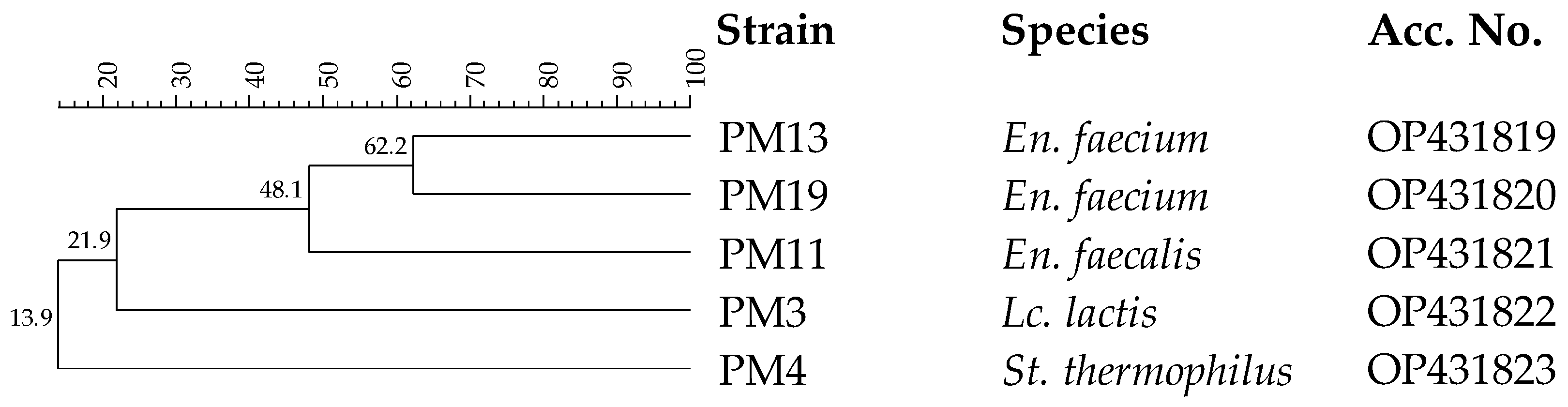
Thirty-three isolates from pasteurized milk were considered presumptive LAB, being Gram-positive and catalase-negative. After purification, all isolates were analyzed by RAPD-PCR in order to distinguish the different strains. The dendrogram reported in Figure 3 shows five different strains.
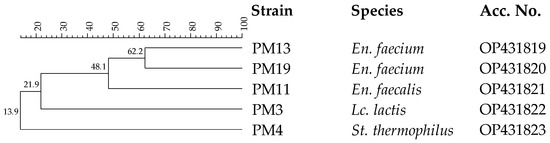
Figure 3.
Dendrogram obtained with combined RAPD-PCR patterns generated with three primers for thermoduric indigenous milk LAB strains isolated from pasteurized milk. Abbreviations: En., Enterococcus; Lc., Lactococcus; St., Streptococcus.
These strains resistant to the pasteurization process were analyzed by 16S rRNA gene sequencing. This analysis confirmed their inclusion in the LAB group, since they were identified as Enterococcus faecalis, Enterococcus faecium, Lc. lactis and Streptococcus thermophilus. These species are typical of dairy environments [48]. In particular, Lc. lactis and St. thermophilus are components of the starter LAB (SLAB) responsible for lactic fermentation, while En. faecalis and En. faecium to the non-starter LAB (NSLAB) community associated with the development of typical sensory traits [59]. The ability of En. faecalis, En. faecium and St. thermophilus to survive the pasteurization process is well-known [60,61], while Lc. lactis is a species sensitive to heat treatments [62], and for this reason, its presence in pasteurized ewes’ milk could be imputable to post-pasteurization contamination [63].
3.4. Persistence of the Added Starter LAB Strains
The dominance of Lc. lactis CAG4 and PON36 during cheese making was evaluated by means of RAPD profile comparisons. This technique, commonly used to monitor the LAB starter cultures in dairy production [64,65], showed the dominance of the added strains over those that survived the thermal treatment both in the control and experimental productions, excluding any negative influence of EOs.
3.5. Physicochemical Characterisation of Cheeses
The physical properties and the chemical compositions of the cheeses are reported in Table 3.

Table 3.
Physicochemical traits of Primosale cheeses.
The effect of production was statistically significant for all cheese color indexes. Indeed, the external and internal surfaces of cheeses performed with lemon and tangerine EOs at both levels showed lower values of lightness (L*) and redness (a*) and a corresponding higher yellow index (b*) in comparison with the other cheeses. This effect is presumably related to the transfer of a larger amount of carotenoid pigments that, among those isolable from the respective EOs [66], were able to induce a higher yellow color. Overall, the indexes of internal colors recorded in CP cheeses were within the ranges observed for Primosale cheeses [26,43].
The hardness of the pastes was comparable among the cheeses from different productions, apart from the highest consistency recorded for the cheeses of EPO100 production, which differed significantly from the EPT100 cheeses. No explanation based on variations in chemical components, as increasing the humidity or fat reduction, was found to justify this increase; however, the major consistency of the EPO100 cheeses corresponded to their tendency towards a higher adhesiveness perceived at the sensory level.
The chemical composition of the cheeses in terms of the DM, fat and protein, as well as aw, were not affected by the addition of citrus EOs. Indeed, a slightly higher ash content emerged in EPO200 cheeses, with a significant difference in EPT200 cheeses, which can be attributed to the major ash content of orange EOs or to a greater amount of salt absorbed by cheeses. Additionally, the chemical components of the Primosale cheeses ranged into the levels found by other investigations [26,67,68].
3.6. Antioxidant Capacity of Cheeses
The results of the antioxidant capacity of the cheese analyses are reported in Table 4.

Table 4.
Antioxidant capacity of the Primosale cheeses.
As expected, the cheeses from all the experimental productions displayed a higher antioxidant capacity, expressed as TEAC, than the control cheeses, without differences between the Eos and their addition levels. This result confirmed the well-known antioxidant properties of citrus EOs [69], mainly due to their components such as terpenes and phenolic compounds. For this reason, citrus EOs are commonly incorporated into foods to prevent oxidation, to increase the shelf life and to provide health benefits [70]. However, the total polyphenols content did not differ significantly among the experimental and control productions. Thus, the improvement of the antioxidant activity of cheeses fortified with citrus EOs is mainly attributable to other antioxidant molecules, especially terpenes. Furthermore, the antioxidant activity of Eos also derives from the synergic actions of various molecules [71]. Although limonene was the major terpene in all the experimental cheeses, its antioxidant contribution is reported to be limited [72].
Although the inclusion of Eos was able to improve the antioxidant capacity of the experimental cheeses, it was not effective in preserving the cheese fat from oxidation. Indeed, the primary lipid oxidation, expressed as the peroxide value, was lower only in the cheeses fortified with lemon EOs, whereas TBARS, indicating the secondary lipid oxidation, was higher in the experimental cheeses than in the control cheese, although the orange and tangerine EOs induced a better protection from oxidation when used at the highest level. Based on these results, the potential of citrus EOs as sources of antioxidant compounds to preserve cheese fat from oxidation needs to be further investigated, while also considering the maintenance of oxidative stability during the storage time.
3.7. Volatile Organic Compounds Emitted from Citrus EOs and Cheeses
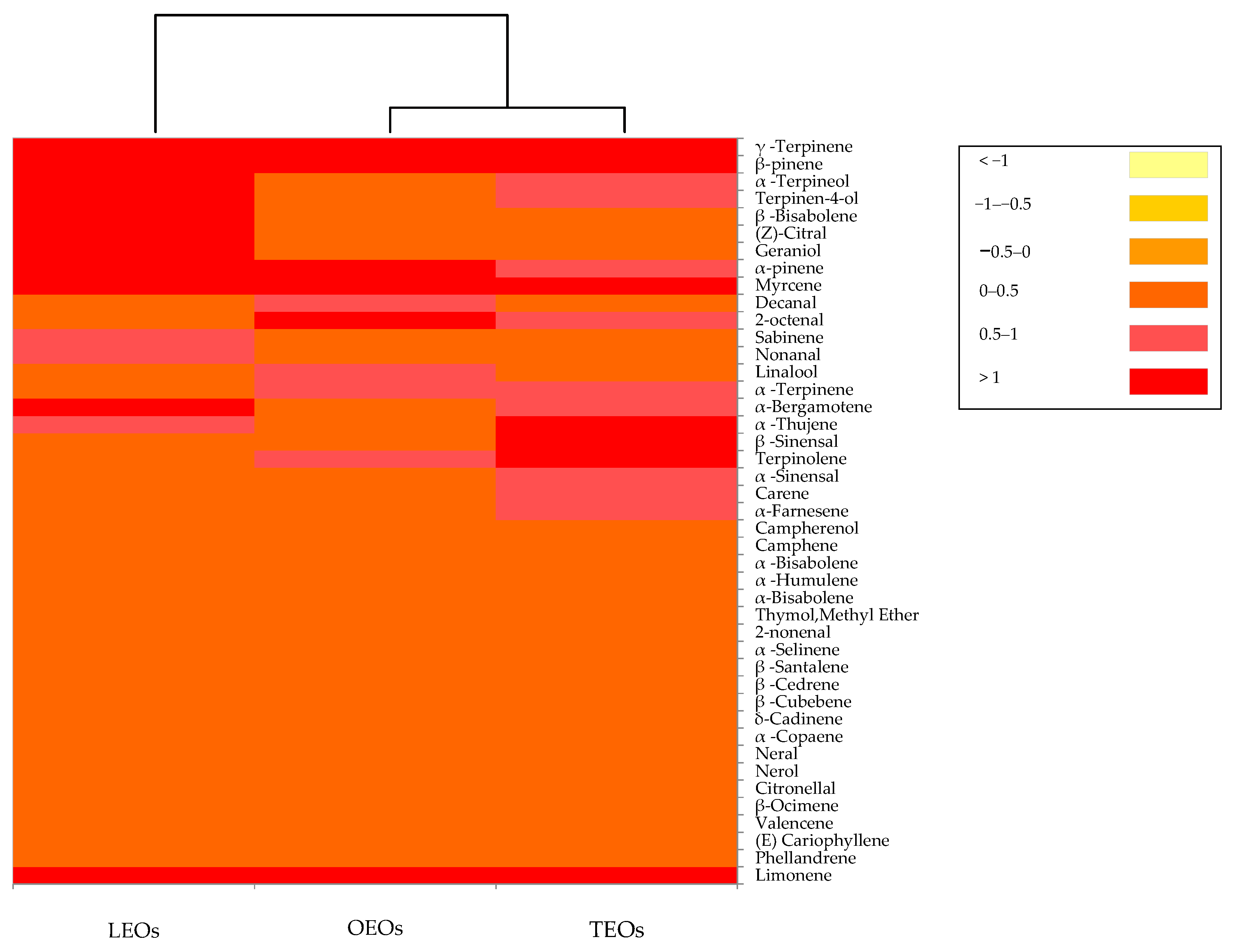
The volatile profiles generated by the citrus EOs are reported in Figure 4.
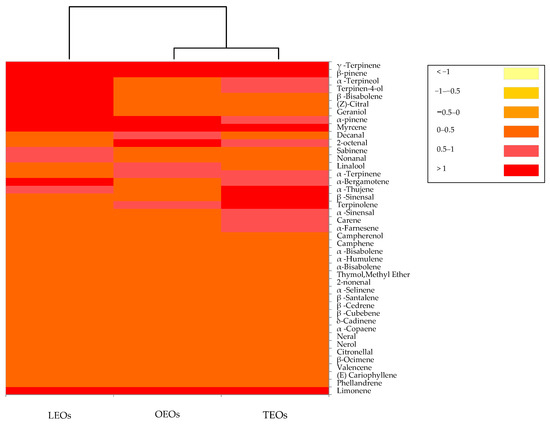
Figure 4.
Distribution of the volatile organic compounds among the citrus EOs. The heat map plot depicts the relative concentration of each VOC. Abbreviations: LEOs, lemon essential oils; OEOs, orange essential oils; TEOs, tangerine essential oils.
The dendrogram resulting from the cluster analysis and the heat map showed the formation of two clusters. The orange and tangerine EOs clustered together into one main group, while the lemon EOs were in a separate single cluster. In particular, in lemon, orange and tangerine Eos, a total of 32, 31 and 26 volatile compounds were identified, respectively. Three phytochemical groups, including monoterpene hydrocarbons, oxygenated monoterpenes and sesquiterpene, were recognized in all three citrus EOs. The monoterpene hydrocarbons constitute the most representative group of volatile compounds, with 94% in the orange EOs, 92% in the tangerine Eos and 87% in the lemon EOs. In all three EOs, the most quantitatively relevant compounds were limonene, β-pinene and γ-terpinene. Limonene, the major compound of the present study, is a cyclic monoterpene with a lemon-like odor and is considered to be the major chemical constituents in various EOs of citrus species [73,74]. In addition to limonene, all three citrus EOs were characterized by the presence of linalool and citral. These components are reported to exert an antimicrobial activity towards bacteria and fungi [75,76]. Basically, the volatile compounds identified in citrus EOs analyzed in this study were similar to those found by other authors [74,77].
Table 5 reports the VOCs emitted from cheeses sample with and without EO additions. The control cheese profile was characterized by 20 compounds belonging to the classes of acids, ketones, aldehydes, alcohols and monoterpene. The main acids identified were hexanoic, butyric and 2-hydroxy4-methyl-pentanoic acids. These compounds contribute to the formation of cheese flavor both directly and indirectly as precursors of odor-active compounds such as ketones and aldehydes [78,79]. Hexenal and heptanal were the main aldehydes detected in the control cheese, and the main alcohol was 1-butanol-3-methyl. Similar volatile compound profiles were also observed in other cheeses produced from sheep’s milk [26,53,80]. The cheeses processed with citrus EO additions emitted 24 VOCs in the presence of lemon EOs and 23 in the presence of orange, as well as tangerine, EOs. Cheeses produced with citrus EOs differed from the control cheeses, especially for the limonene, β-pinene, myrcene, carene, linalool and α-terpineol concentrations. No statistically significant differences were found among the experimental cheeses produced with the addition of 100 and 200 µL/L of citrus EOs. The most abundant VOC found in all the enriched cheeses with citrus EOs was limonene, which showed a slight increase with the increasing percentage of citrus EOs added to milk. The only terpene found both in the control and experimental cheese is α-pinene, commonly present in cheese as a result of pasture feeding [81,82]. The monoterpene hydrocarbons constitute the most representative group of VOCs in experimental cheeses. In particular, in cheeses enriched with 100 and 200 µL/L of lemon, orange and tangerine Eos, monoterpene hydrocarbons accounted for 82%, 83% and 85%, respectively. This class of compounds is commonly found in cheeses enriched with different types of essential oils [83,84]. The higher carryovers observed for monoterpene hydrocarbons with respect to the oxygenated compounds may be explained by possible interactions with fat, carbohydrate and protein matrices in cheese [85,86,87]. Specifically, hydroxyl groups can interact with the receptor groups of proteins, such as NH and CO, determining the higher retention rate for oxygenated compounds in the food matrix, restricting their ability to be transferred [88].

Table 5.
Volatile organic compounds emitted Primosale cheeses.
3.8. Sensory Aspects of Cheeses
The results of the sensory evaluation are reported in Table 6. This evaluation is commonly used to determine the tasters’ acceptance of a new food product before marketing [89].

Table 6.
Evaluation of the sensory attributes of Primosale cheeses.
In this study, this analysis showed that the addition of citrus EOs led to the production of Primosale cheeses characterized by a sensory profile not particularly different from that generally recognized for traditional Primosale cheeses. In particular, except for the intensity of odor, intensity of aroma and taste persistency that were scored different for the cheeses, all other sensory traits evaluated were not influenced by the addition of citrus EOs. In detail, the intensity of odor and of aroma and taste persistency increased with the concentration of citrus EOs added, confirming the previous findings of Marcial et al. [27], who tested different concentrations of oregano EO to fortify a traditional Argentinean fresh bovine cheese. Although it is well-known the EOs have a strong flavor that can alter the sensory acceptability of dairy products [90], our results highlighted that the cheeses produced with 100 µL/L of citrus EOs were the most appreciated ones by the panelists.
4. Conclusions
This study provided an analysis of the microbiological, physicochemical and sensory characteristics of a Primosale cheese processed with citrus EOs extracted from peels of lemons, oranges and tangerines. The enrichment of milk at 100 and 200 µL/L did not negatively influence the fermentation activity of the two Lc. lactis (CAG4 and PON36) used as the starter cultures. The addition of citrus EOs to milk did not affect the chemical compositions of the Primosale cheeses while determining a relevant increase of the antioxidant capacity. The volatile profiles of the cheeses enriched with citrus EOs were impacted with monoterpene hydrocarbons and oxygenated monoterpenes compounds. The sensory evaluation indicated that Primosale cheeses enriched with 100 µL/L of lemon, orange and tangerine EOs were characterized by the highest overall acceptability. These results clearly highlighted that the addition of citrus EOs to milk determined the production of novel fresh cheeses able to enlarge the Sicilian ewes’ milk dairy products portfolio.
Author Contributions
Conceptualization, L.S. and R.G. (Raimondo Gaglio); methodology, E.P., A.B., G.M. (Giancarlo Moschetti) and R.G. (Raimondo Gaglio); software, A.A., A.B. and R.G. (Raimondo Gaglio); validation, A.B., L.S. and R.G. (Raimondo Gaglio); formal analysis, G.B., M.P., M.B., A.I., G.M. (Giuseppe Maniaci) and R.G. (Rosa Guarcello); investigation, G.B., M.P., M.B., A.I., G.M. (Giuseppe Maniaci) and R.G. (Rosa Guarcello); resources, N.F.; data curation, M.B., A.A., A.B. and R.G. (Raimondo Gaglio); writing—original draft preparation, M.B., A.B. and R.G. (Raimondo Gaglio); writing—review and editing, L.S. and R.G. (Raimondo Gaglio); project administration, N.F. and funding acquisition, N.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the project for industrial research “Integrated approach to product development innovations in the leading sectors of the Sicilian agri-food sector” Prog. F/050267/03/X32-COR 109494-CUP: B78I17000260008 of the Ministry of the Economic Development, General Management for Business Incentives.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data included in this study are available upon request by contacting the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guzik, P.; Szymkowiak, A.; Kulawik, P.; Zając, M. Consumer attitudes towards food preservation methods. Foods 2022, 11, 1349. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Tóth, G. Circular economy and its comparison with 14 other business sustainability movements. Resources 2019, 8, 159. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, 1231–1249. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Guenther, E.; Althausen, D. The Essential Oils, 3rd ed.; Van Nostrand Company: New York, NY, USA, 1948. [Google Scholar]
- Boyle, W. Spices and essential oils as preservatives. Amer. Perfumer Essential Oil Rev. 1955, 66, 25–28. [Google Scholar]
- Worwood, V.A. The Complete Book of Essential Oils and Aromatherapy, Revised and Expanded: Over 800 Natural, Nontoxic, and Fragrant Recipes to Create Health, Beauty, and Safe Home and Work Environments; New World Library: Novato, CA, USA, 2016. [Google Scholar]
- Dreger, M.; Wielgus, K. Application of essential oils as natural cosmetic preservatives. Herba Pol. 2013, 59, 142–156. [Google Scholar] [CrossRef]
- FDA (Food and Drug Administration) USA. Substances Generally Recognized as Safe. Available online: https://www.federalregister.gov/d/2016-19164/p-1 (accessed on 1 August 2022).
- Blanco Tirado, C.; Stashenko, E.E.; Combariza, M.Y.; Martinez, J.R. Comparative study of Colombian citrus oils by high-resolution gas chromatography and gas chromatography–mass spectrometry. J. Chromatogr. A 1995, 697, 501–513. [Google Scholar] [CrossRef]
- Raimondo, M.; Caracciolo, F.; Cembalo, L.; Chinnici, G.; Pecorino, B.; D’Amico, M. Making virtue out of necessity: Managing the citrus waste supply chain for bioeconomy applications. Sustainability 2018, 10, 4821. [Google Scholar] [CrossRef]
- Del Bravo, F.; Finizia, A.; Moriello, M.S.L.; Ronga, M. Report Ministero delle Politiche Agricole Alimentari e Forestali. La Competitività Della Filiera Agrumicola in Italia Aprile 2020. Available online: https://www.ismea.it/istituto-di-servizi-per-il-mercato-agricolo-alimentare (accessed on 1 August 2022).
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Gervasi, L.; Tundis, R.; Loizzo, M.R. Chemical compositions and antioxidant activities of essential oils, and their combinations, obtained from flavedo by-product of seven cultivars of Sicilian Citrus aurantium L. Molecules 2022, 27, 1580. [Google Scholar] [CrossRef]
- Moshonas, M.G.; Shaw, P.E. Composition of essence oil from overripe oranges. J. Agric. Food Chem. 1979, 27, 1337–1339. [Google Scholar] [CrossRef]
- Chanthaphon, S.; Chanthachum, S.; Hongpattarakere, T. Antimicrobial activities of essential oils and crude extracts from tropical Citrus spp. against food-related microorganisms. Songklanakarin J. Sci. Technol. 2008, 30, 125–131. [Google Scholar]
- Palazzolo, E.; Laudicina, V.A.; Germanà, M.A. Current and potential use of citrus essential oils. Curr. Org. Chem. 2013, 17, 3042–3049. [Google Scholar] [CrossRef]
- Gammariello, D.; Di Giulio, S.; Conte, A.; Del Nobile, M.A. Effects of natural compounds on microbial safety and sensory quality of Fior di Latte cheese, a typical Italian cheese. J. Dairy Sci. 2008, 91, 4138–4146. [Google Scholar] [CrossRef]
- Gammariello, D.; Attanasio, M.; Del Nobile, M.A.; Conte, A. Chitosan and lemon extract applied during Giuncata cheese production to improve the microbiological stability. Appl. Sci. 2021, 11, 7446. [Google Scholar] [CrossRef]
- Giammanco, G.M.; Pepe, A.; Aleo, A.; D’Agostino, V.; Milone, S.; Mammina, C. Microbiological quality of Pecorino Siciliano “Primosale” cheese on retail sale in the street markets of Palermo, Italy. New Microbiol. 2011, 34, 179–185. [Google Scholar]
- Fusaro, I.; Giammarco, M.; Vaintrub, M.O.; Chincarini, M.; Manetta, A.C.; Mammi, L.E.M.; Polmonari, A.; Formigoni, A.; Vignola, G. Effects of three different diets on the fatty acid profile and sensory properties of fresh Pecorino cheese “Primo Sale”. Asian-Australas J. Anim. Sci. 2020, 33, 1991–1998. [Google Scholar] [CrossRef]
- Settanni, L.; Gaglio, R.; Guarcello, R.; Francesca, N.; Carpino, S.; Sannino, C.; Todaro, M. Selected lactic acid bacteria as a hurdle to the microbial spoilage of cheese: Application on a traditional raw ewes’ milk cheese. Int. Dairy J. 2013, 32, 126–132. [Google Scholar] [CrossRef]
- Gaglio, R.; Francesca, N.; Di Gerlando, R.; Cruciata, M.; Guarcello, R.; Portolano, B.; Moschetti, G.; Settanni, L. Identification, typing and investigation of the dairy characteristics of lactic acid bacteria isolated from “Vastedda della valle del Belìce” cheeses. Dairy Sci. Technol. 2014, 94, 157–180. [Google Scholar] [CrossRef]
- Gaglio, R.; Barbera, M.; Aleo, A.; Lommatzsch, I.; La Mantia, T.; Settanni, L. Inhibitory activity and chemical characterization of Daucus carota subsp. maximus essential oils. Chem. Biod. 2017, 14, e1600477. [Google Scholar]
- Miceli, A.; Aleo, A.; Corona, O.; Sardina, M.T.; Mammina, C.; Settanni, L. Antibacterial activity of Borago officinalis and Brassica juncea aqueous extracts evaluated in vitro and in situ using different food model systems. Food Control 2014, 40, 157–164. [Google Scholar] [CrossRef]
- Gaglio, R.; Barbaccia, P.; Barbera, M.; Restivo, I.; Attanzio, A.; Maniaci, G.; Di Grigoli, A.; Francesca, N.; Tesoriere, L.; Bonanno, A.; et al. The use of winery by-products to enhance the functional aspects of the fresh ovine “Primosale” cheese. Foods 2021, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Marcial, G.E.; Gerez, C.L.; De Kairuz, M.N.; Araoz, V.C.; Schuff, C.; De Valdez, G.F. Influence of oregano essential oil on traditional Argentinean cheese elaboration: Effect on lactic starter cultures. Rev. Argent. Microbiol. 2016, 48, 229–235. [Google Scholar] [CrossRef]
- Gaglio, R.; Restivo, I.; Barbera, M.; Barbaccia, P.; Ponte, M.; Tesoriere, L.; Bonanno, A.; Attanzio, A.; Di Grigoli, A.; Francesca, N.; et al. Effect on the antioxidant, lipoperoxyl radical scavenger capacity, nutritional, sensory and microbiological traits of an ovine stretched cheese produced with grape pomace powder addition. Antioxidants 2021, 10, 306. [Google Scholar] [CrossRef]
- Gregersen, T. Rapid method for distinction of Gram-negative from Gram-positive bacteria. Eur. J. Appl. Microbiol. Biotechnol. 1978, 5, 123–127. [Google Scholar] [CrossRef]
- Koneman, E.W.; Allen, S.D.; Janda, W.M.; Schreckenberger, P.C.; Winn, W.C. The nonfermentative gram-negative bacilli. In Color Atlas and Textbook of Diagnostic Microbiology, 7th ed.; Koneman, E.W., Allen, S.D., Janda, W.M., Schreckkenberger, P.C., Winn, W.C., Eds.; Lippincott: Philadelphia, PA, USA, 1997; pp. 253–320. [Google Scholar]
- Gaglio, R.; Francesca, N.; Di Gerlando, R.; Mahony, J.; De Martino, S.; Stucchi, C.; Moschetti, G.; Settanni, L. Enteric bacteria of food ice and their survival in alcoholic beverages and soft drinks. Food Microbiol. 2017, 67, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, R.; Cruciata, M.; Di Gerlando, R.; Scatassa, M.L.; Mancuso, I.; Sardina, M.T.; Moschetti, G.; Portolano, B.; Settanni, L. Microbial activation of wooden vats used for traditional cheese production and evolution of the neo-formed biofilms. Appl. Environ. Microbiol. 2016, 82, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W. Grapped BLAST and PSI BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Lee, J.H.; Jung, Y.; Kim, M.; Kim, S.; Kim, B.K.; Lim, Y.W. EzTaxon: A web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int. J. Syst. Evol. Microbiol. 2007, 57, 2259–2261. [Google Scholar] [CrossRef] [PubMed]
- CIE (Commission Internationale de l’Eclairage). Colorimetry; Commission Internationale de l’Eclairage: Vienna, Austria, 1986; Volume CIE 15.2. [Google Scholar]
- ISO (International Organization for Standardization). Microbiology of Food and Animal Feeding Stuffs. Determination of Water Activity; International Standardization Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Rashidinejad, A.E.; Birch, J.; Sun-Waterhouse, D.; Everett, D.W. Effects of catechin on the phenolic content and antioxidant properties of low-fat cheese. Int. J. Food Sci. Technol. 2013, 48, 2448–2455. [Google Scholar] [CrossRef]
- Standard FIL-IDF 4A; Cheese and Processed Cheese Product. Determination of the Total Solids Content. International Dairy Federation: Brussels, Belgium, 1982.
- Standard FIL-IDF 5B; Cheese and Processed Cheese Product. Determination of Fat Content-Gravimetric Method. International Dairy Federation: Brussels, Belgium, 1986.
- Standard FIL-IDF 27; Determination of the Ash Content of Processed Cheese Products. International Dairy Federation: Brussels, Belgium, 1964.
- ISO (International Organization for Standardization). Determination of Substances Characteristic of Green and Black Tea. Part 1: Content of Total Polyphenols in Tea. Colorimetric Method Using Folin-Ciocalteu Reagent; International Standardization Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bonanno, A.; Di Grigoli, A.; Vitale, F.; Di Miceli, G.; Todaro, M.; Alabiso, M.; Gargano, M.L.; Venturella, G.; Anike, F.N.; Isikhuemhen, O.S. Effects of feeding diets supplemented with medicinal mushrooms myceliated grains on some production, health and oxidation traits of dairy ewes. Int. J. Med. Mushrooms 2019, 21, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Standard FIL-IDF 74A; Determination of the Peroxide Value. International Dairy Federation: Brussels, Belgium, 1991.
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L., Jr. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 1960, 37, 44–48. [Google Scholar] [CrossRef]
- Mele, M.; Contarini, G.; Cercaci, L.; Serra, A.; Buccioni, A.; Povolo, M.; Conte, G.; Funaro, A.; Banni, S.; Lercker, G.; et al. Enrichment of pecorino cheese with conjugated linoleic acid by feeding dairy ewes with extruded linseed: Effect on fatty acid and triglycerides composition and on oxidative stability. Int. Dairy J. 2011, 21, 365–372. [Google Scholar] [CrossRef]
- ISO (International Organization for Standardization). Sensory Analysis e General Guidance for the Design of Test Rooms; International Standardization Organization: Geneva, Switzerland, 2007; Volume ISO 8589. [Google Scholar]
- Settanni, L.; Randazzo, W.; Palazzolo, E.; Moschetti, M.; Aleo, A.; Guarrasi, V.; Mammina, C.; San Biagio, P.L.; Marra, F.P.; Moschetti, G.; et al. Seasonal variations of antimicrobial activity and chemical composition of essential oils extracted from three Citrus limon L. Burm. cultivars. Nat. Prod. Res. 2014, 28, 383–391. [Google Scholar] [CrossRef]
- Randazzo, W.; Jiménez-Belenguer, A.; Settanni, L.; Perdones, A.; Moschetti, M.; Palazzolo, E.; Guarrasi, V.; Vargas, M.; Germanà, M.A.; Moschetti, G. Antilisterial effect of citrus essential oils and their performance in edible film formulations. Food Control 2016, 59, 750–758. [Google Scholar] [CrossRef]
- Settanni, L.; Moschetti, G. Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol. 2010, 27, 691–697. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004, laying down specific hygiene rules for food of animal origin. Off. J. Eur. Union 2005, 139, 55–206. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R0853&qid=1454951893891&from=EN (accessed on 22 August 2022).
- Gaglio, R.; Todaro, M.; Scatassa, M.L.; Franciosi, E.; Corona, O.; Mancuso, I.; Di Gerlando, R.; Cardamone, C.; Settanni, L. Transformation of raw ewes’ milk applying “Grana” type pressed cheese technology: Development of extra-hard “Gran Ovino” cheese. Int. J. Food Microbiol. 2019, 307, 108277. [Google Scholar] [CrossRef]
- Gaglio, R.; Cruciata, M.; Scatassa, M.L.; Tolone, M.; Mancuso, I.; Cardamone, C.; Corona, O.; Todaro, M.; Settanni, L. Influence of the early bacterial biofilms developed on vats made with seven wood types on PDO Vastedda della valle del Belìce cheese characteristics. Int. J. Food Microbiol. 2019, 291, 91–103. [Google Scholar] [CrossRef]
- Franciosi, E.; Settanni, L.; Cologna, N.; Cavazza, A.; Poznanski, E. Microbial analysis of raw cows’ milk used for cheese-making: Influence of storage treatments on microbial composition and other technological traits. World J. Microbiol. Biotechnol. 2011, 2, 171–180. [Google Scholar] [CrossRef]
- Rynne, N.M.; Beresford, T.P.; Kelly, A.L.; Guinee, T.P. Effect of milk pasteurisation temperature on age-related changes in lactose metabolism, pH and the growth of non-starter lactic acid bacteria in half-fat Cheddar cheese. Food Chem. 2007, 100, 375–382. [Google Scholar] [CrossRef]
- Gaglio, R.; Gentile, C.; Bonanno, A.; Vintaloro, L.; Perrone, A.; Mazza, F.; Barbaccia, P.; Settanni, L.; Di Grigoli, A. Effect of saffron addition on the microbiological, physicochemical, antioxidant and sensory characteristics of yoghurt. Int. J. Dairy Technol. 2019, 72, 208–217. [Google Scholar] [CrossRef]
- Barbaccia, P.; Busetta, G.; Matraxia, M.; Sutera, A.M.; Craparo, V.; Moschetti, G.; Francesca, N.; Settanni, L.; Gaglio, R. Monitoring commercial starter culture development in presence of red grape pomace powder to produce polyphenol-enriched fresh ovine cheeses at industrial scale level. Fermentation 2021, 7, 35. [Google Scholar] [CrossRef]
- Barbaccia, P.; Busetta, G.; Barbera, M.; Alfonzo, A.; Garofalo, G.; Francesca, N.; Moscarelli, A.; Moschetti, G.; Settanni, L.; Gaglio, R. Effect of grape pomace from red cultivar ’Nero d’Avola’ on the microbiological, physicochemical, phenolic profile and sensory aspects of ovine Vastedda-like stretched cheese. J. Appl. Microbiol. 2021, 133, 130–144. [Google Scholar] [CrossRef]
- Grujović, M.Ž.; Mladenović, K.G.; Semedo-Lemsaddek, T.; Laranjo, M.; Stefanović, O.D.; Kocić-Tanackov, S.D. Advantages and disadvantages of non starter lactic acid bacteria from traditional fermented foods: Potential use as starters or probiotics. Compr. Rev. Food Sci. 2022, 21, 1537–1567. [Google Scholar] [CrossRef]
- McAuley, C.M.; Gobius, K.S.; Britz, M.L.; Craven, H.M. Heat resistance of thermoduric enterococci isolated from milk. Int. J. Food Microbiol. 2012, 154, 162–168. [Google Scholar] [CrossRef]
- Delgado, S.; Rachid, C.T.; Fernández, E.; Rychlik, T.; Alegría, Á.; Peixoto, R.S.; Mayo, B. Diversity of thermophilic bacteria in raw, pasteurized and selectively-cultured milk, as assessed by culturing, PCR-DGGE and pyrosequencing. Food Microbiol. 2013, 36, 103–111. [Google Scholar] [CrossRef]
- Sorhaug, T.; Stepaniak, L. Psychrotrophs and their enzymes in milk and dairy products: Quality aspects. Trends Food Sci. Technol. 1997, 8, 35–40. [Google Scholar] [CrossRef]
- Dumalisile, P.; Witthuhn, R.C.; Britz, T.J. Impact of different pasteurization temperatures on the survival of microbial contaminants isolated from pasteurized milk. Int. J. Dairy Technol. 2005, 58, 74–82. [Google Scholar] [CrossRef]
- Gaglio, R.; Scatassa, M.L.; Cruciata, M.; Miraglia, V.; Corona, O.; Di Gerlando, R.; Portolano, B.; Moschetti, G.; Settanni, L. In vivo application and dynamics of lactic acid bacteria for the four-season production of Vastedda-like cheese. Int. J. Food Microbiol. 2014, 177, 37–48. [Google Scholar] [CrossRef]
- Fusco, V.; Quero, G.M.; Poltronieri, P.; Morea, M.; Baruzzi, F. Autochthonous and probiotic lactic acid bacteria employed for production of “advanced traditional cheeses”. Foods 2019, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Myrtsi, E.D.; Koulocheri, S.D.; Evergetis, E.; Haroutounian, S.A. Agro-industrial co-products upcycling: Recovery of carotenoids and fine chemicals from Citrus sp. juice industry co-products. Ind. Crops Prod. 2022, 186, 115190. [Google Scholar] [CrossRef]
- Bonanno, A.; Di Grigoli, A.; Mazza, F.; De Pasquale, C.; Giosuè, C.; Vitale, F.; Alabiso, M. Effects of ewes grazing sulla or ryegrass pasture for different daily durations on forage intake, milk production and fatty acid composition of cheese. Animal 2016, 10, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Alabiso, M.; Scatassa, M.L.; Di Grigoli, A.; Mazza, F.; Maniaci, G.; Bonanno, A. Effect of the inclusion of fresh lemon pulp in the diet of lactating ewes on the properties of milk and cheese. Anim. Feed Sci. Technol. 2017, 225, 213–223. [Google Scholar] [CrossRef]
- Lin, X.; Cao, S.; Sun, J.; Lu, D.; Zhong, B.; Chun, J. The chemical compositions, and antibacterial and antioxidant activities of four types of citrus essential oils. Molecules 2021, 26, 3412. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Soto, E.; Cenobio-Galindo, A.D.J.; Espino-Manzano, S.O.; Franco-Fernández, M.J.; Ludeña-Urquizo, F.E.; Jiménez-Alvarado, R.; Zepeda-Velázquez, A.P.; Campos-Montiel, R.G. The Addition of microencapsulated or nanoemulsified bioactive compounds influences the antioxidant and antimicrobial activities of a fresh cheese. Molecules 2021, 26, 2170. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Małgorzata, O. Does antioxidant properties of the main component of essential oil reflect its antioxidant properties? The comparison of antioxidant properties of essential oils and their main components. Nat. Prod. Res. 2014, 28, 1952–1963. [Google Scholar] [CrossRef]
- Yi, F.P.; Jin, R.Y.; Sun, J.; Ma, B.D.; Bao, X.L. Evaluation of mechanical-pressed essential oil from Nanfeng mandarin (Citrus reticulata Blanco cv. Kinokuni) as a food preservative based on antimicrobial and antioxidant activities. LWT-Food Sci. Technol. 2018, 95, 346–353. [Google Scholar] [CrossRef]
- Paw, M.; Begum, T.; Gogoi, R.; Pandey, S.K.; Lal, M. Chemical composition of Citrus limon L. Burmf peel essential oil from North East India. J. Essent. Oil Bear. Plants 2020, 23, 337–344. [Google Scholar] [CrossRef]
- Denkova-Kostova, R.; Teneva, D.; Tomova, T.; Goranov, B.; Denkova, Z.; Shopska, V.; Slavchev, A.; Hristova-Ivanova, Y. Chemical composition, antioxidant and antimicrobial activity of essential oils from tangerine (Citrus reticulata L.), grapefruit (Citrus paradisi L.), lemon (Citrus lemon L.) and cinnamon (Cinnamomum zeylanicum Blume). Z. Naturforsch. C. J. Biosci. 2021, 76, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Alma, M.H.; Nitz, S.; Kollmannsberger, H.; Digrak, M.; Efe, F.T.; Yilmaz, N. Chemical composition and antimicrobial activity of the essential oils from the gum of Turkish Pistachio (Pistacia vera L.). J. Agric. Food Chem. 2004, 52, 3911–3914. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B.; Akpulat, H.A.; Sokmen, M.; Daferera, D.; Yumrutas, O.; Aydin, E.; Polissiou, M.; Sokem, A. Screening of the antioxidative and antimicrobial properties of the essential oils of Pimpinella anisetum and Pimpinella flabellifolia from Turkey. Food Chem. 2006, 97, 719–724. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Smit, G.; Smit, B.A.; Engels, W.J. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef]
- Thierry, A.; Collins, Y.F.; Mukdsi, M.C.A.; McSweeney, P.L.H.; Wilkinson, M.G.; Spinnler, H.E. Lipolysis and metabolism of fatty acids in cheese. In Cheese: Chemistry, Physics and Microbiology; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 423–444. [Google Scholar]
- Kirmaci, H.A.; Hayaloğlu, A.A.; Özer, H.B.; Atasoy, A.F.; Levent, O. Effects of wild-type starter culture (artisanal strains) on volatile profile of Urfa cheese made from ewe milk. Int. J. Food Prop. 2015, 18, 1915–1929. [Google Scholar] [CrossRef]
- Gil, K.A.; Jerković, I.; Marijanović, Z.; Manca, M.L.; Caddeo, C.; Tuberoso, C.I.G. Evaluation of an innovative sheep cheese with antioxidant activity enriched with different thyme essential oil lecithin liposomes. LWT-Food Sci. Technol. 2022, 154, 112808. [Google Scholar] [CrossRef]
- Coda, R.; Brechany, E.; De Angelis, M.; De Candia, S.; Di Cagno, R.; Gobbetti, M. Comparison of the compositional, microbiological, biochemical, and volatile profile characteristics of nine Italian ewes’ milk cheeses. J. Dairy Sci. 2006, 89, 4126–4143. [Google Scholar] [CrossRef]
- Licon, C.C.; Moro, A.; Librán, C.M.; Molina, A.M.; Zalacain, A.; Berruga, M.I.; Carmona, M. Volatile transference and antimicrobial activity of cheeses made with ewes’ milk fortified with essential oils. Foods 2020, 9, 35. [Google Scholar] [CrossRef]
- Moro, A.; Librán, C.M.; Berruga, M.I.; Carmona, M.; Zalacain, A. Dairy matrix effect on the transference of rosemary (Rosmarinus officinalis) essential oil compounds during cheese making. J. Sci Food Agric. 2015, 95, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, B.; Estrella, I.; Hernandez, M.T. Interaction of low molecular weight phenolics with proteins (BSA). J. Food Sci. 2000, 65, 617–621. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods–a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef]
- Khorshidian, N.; Yousefi, M.; Khanniri, E.; Mortazavian, A.M. Potential application of essential oils as antimicrobial preservatives in cheese. Innov. Food Sci. Emerg. Technol. 2018, 45, 62–72. [Google Scholar] [CrossRef]
- Vivek, K.; Subbarao, K.V.; Routray, W.; Kamini, N.R.; Dash, K.K. Application of fuzzy logic in sensory evaluation of food products: A comprehensive study. Food Bioproc. Technol. 2020, 13, 1–29. [Google Scholar] [CrossRef]
- Ahmed, L.I.; Ibrahim, N.; Abdel-Salam, A.B.; Fahim, K.M. Potential Application of ginger, clove and thyme essential oils to improve soft cheese microbial safety and sensory characteristics. Food Biosci. 2021, 42, 101–177. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).