Salt Stress Induces Changes in Physiological Characteristics, Bioactive Constituents, and Antioxidants in Kenaf (Hibiscus cannabinus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Salt Treatments
2.2. Chemicals and Reagents
2.3. Parameters for Plant Growth
2.4. Determination of Mineral Contents
2.5. Quantification of Proline, Total Soluble Sugar, and Protein Contents
2.6. ROS Determination—Hydrogen Peroxide (H2O2) and Superoxide Anion (O2•−)
2.7. Measurement of the Lipid Peroxidation–MDA Content
2.8. Preparation of Ethanol Extracts
2.9. Determination of Total Phenolic and Flavonoid Contents
2.10. Determination of Total Saponin Content
2.11. Gas Chromatography–Mass Spectrometry (GC–MS) Identification of Volatile Compounds
2.12. Detection of Antioxidant Activity
2.13. Statistical Analysis
3. Results
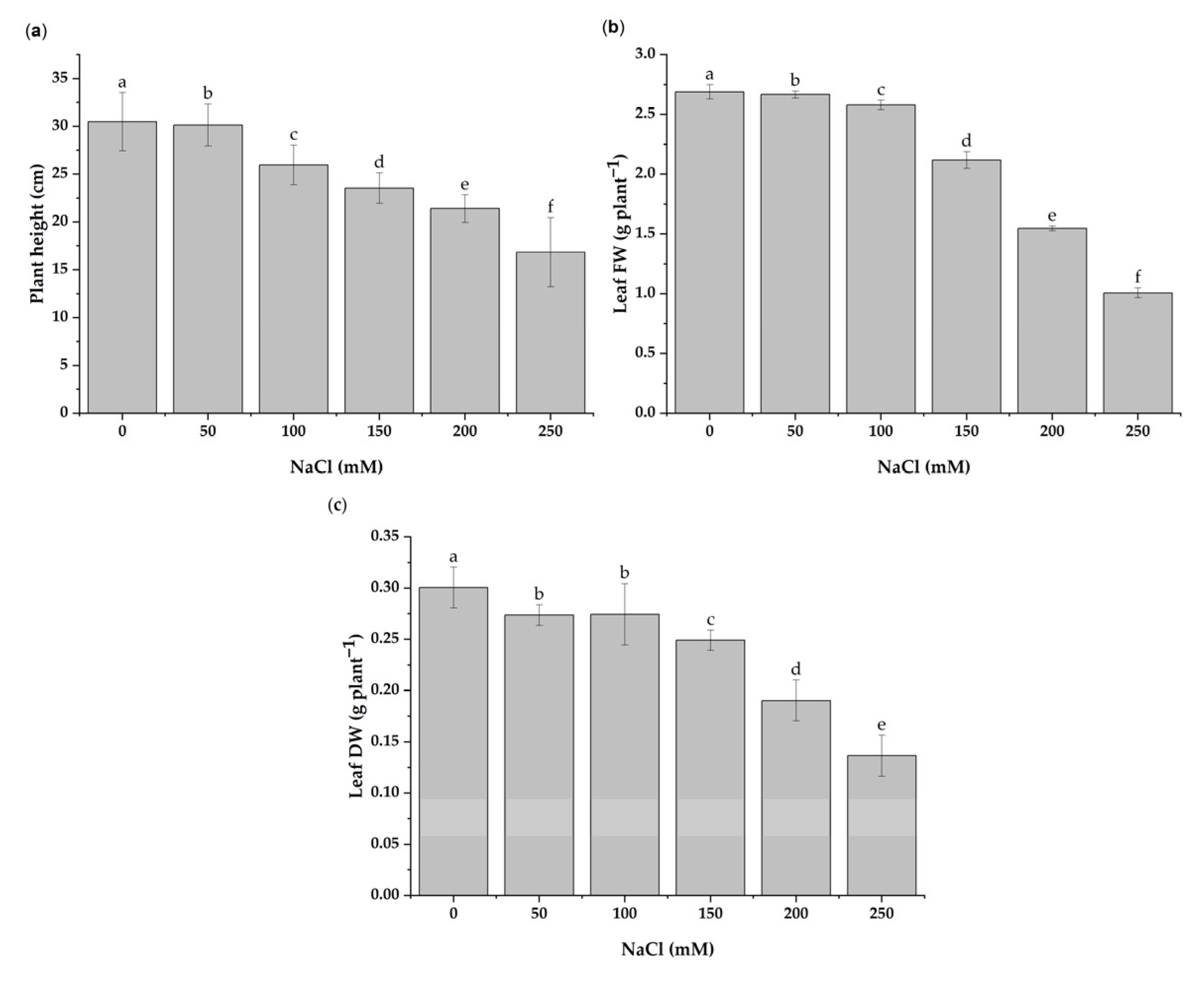
3.1. Effect of Salt Stress on Growth Parameters
3.2. Impact of Salt Stress on Minerals in Leaves
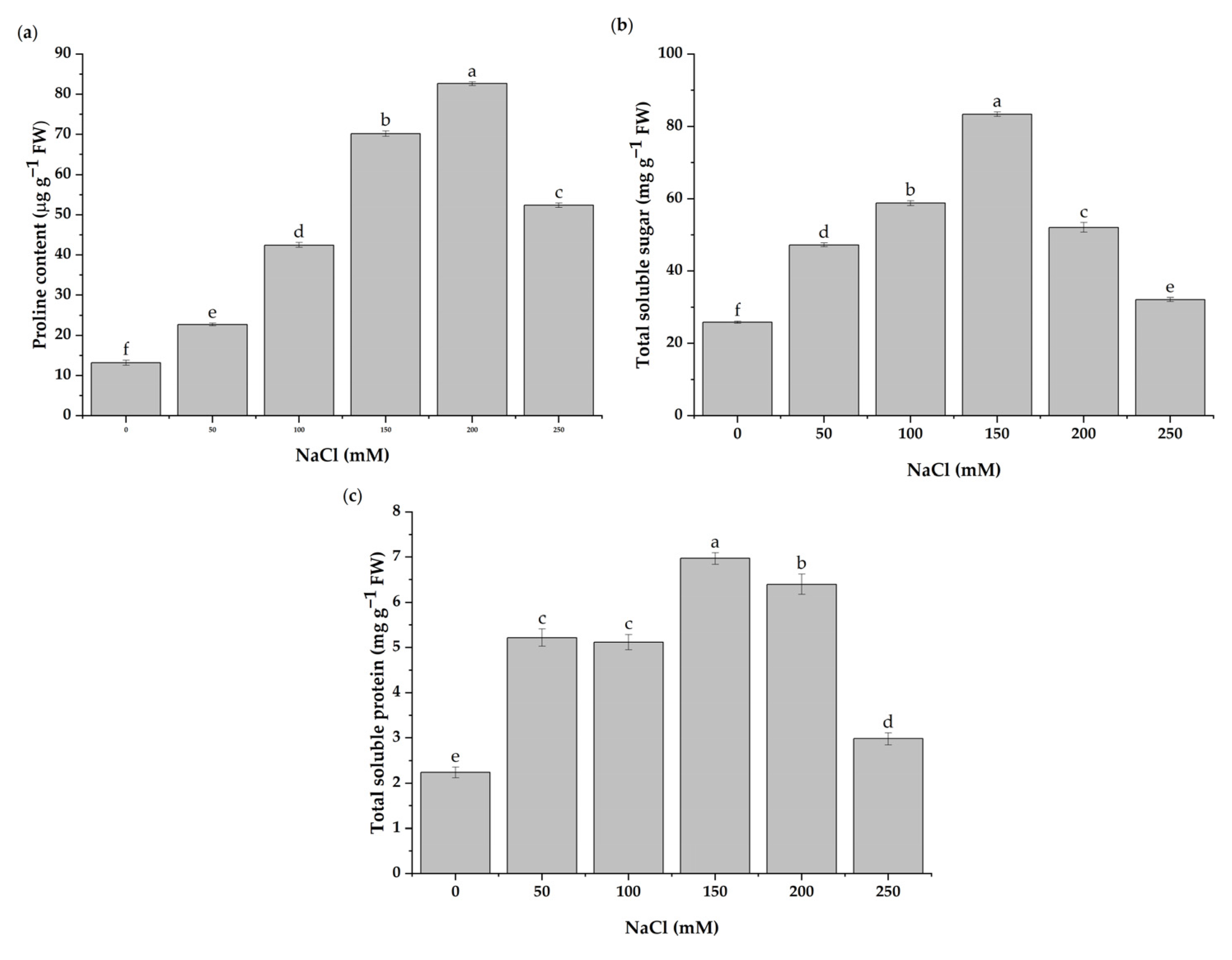
3.3. Alterations of Proline, Total Soluble Sugar, and Soluble Protein Contents under Salt Stress
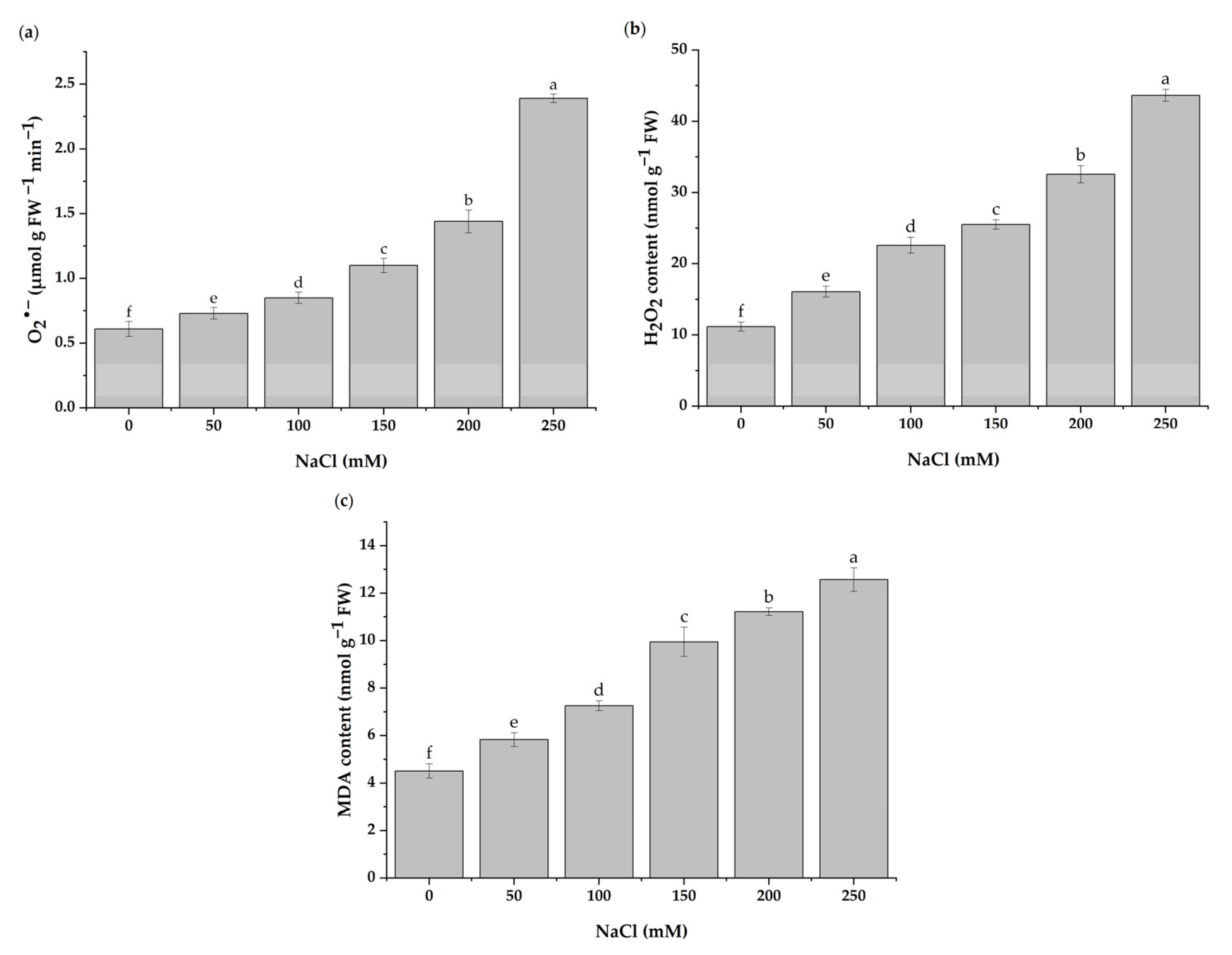
3.4. ROS Detection and Lipid Peroxidation
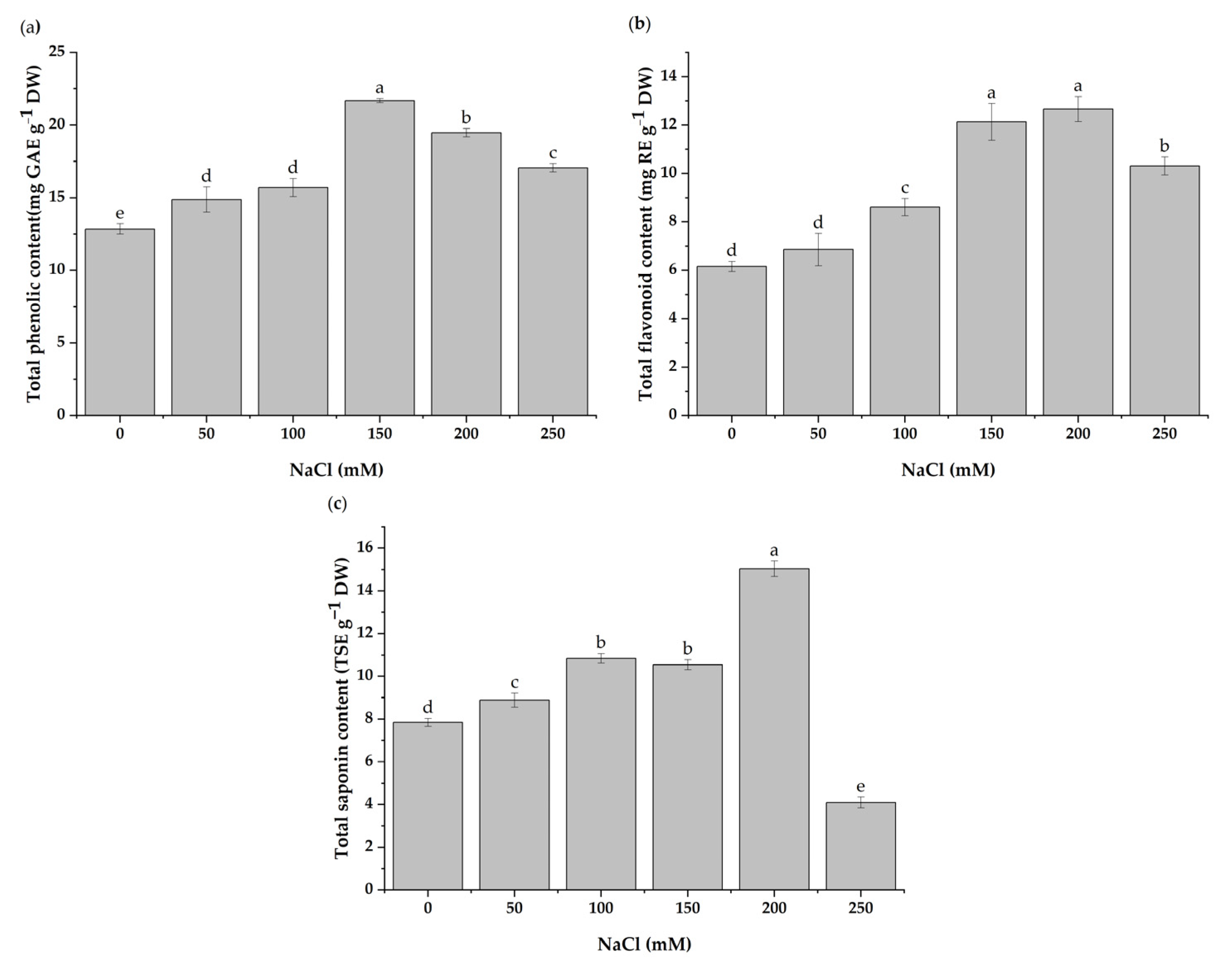
3.5. Total Contents of Phenolics, Flavonoids, and Saponins
3.6. Alterations in the Compositions of Volatile Compounds under Salt Stress
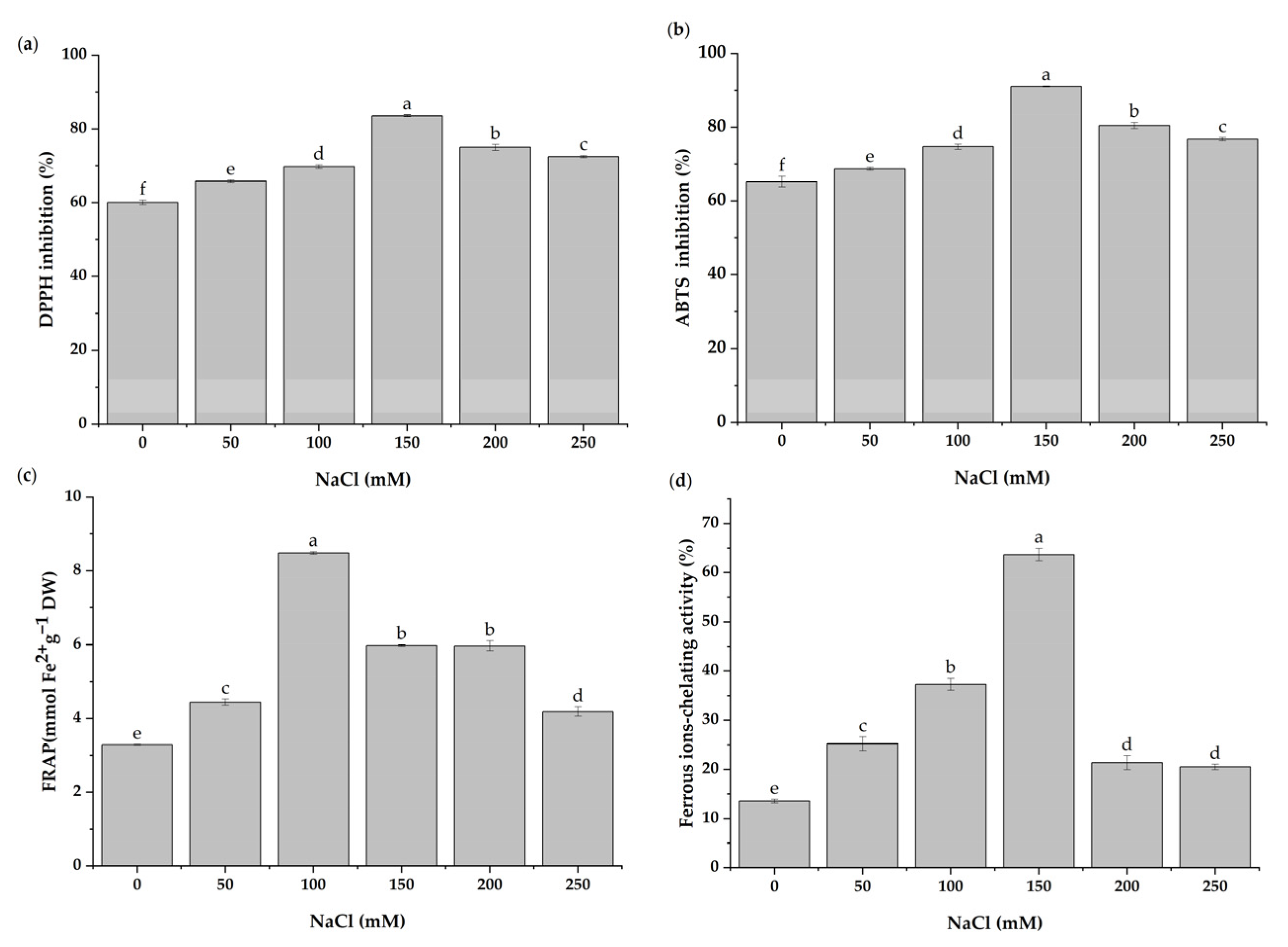
3.7. In Vitro Antioxidant Activities under Salt Stress
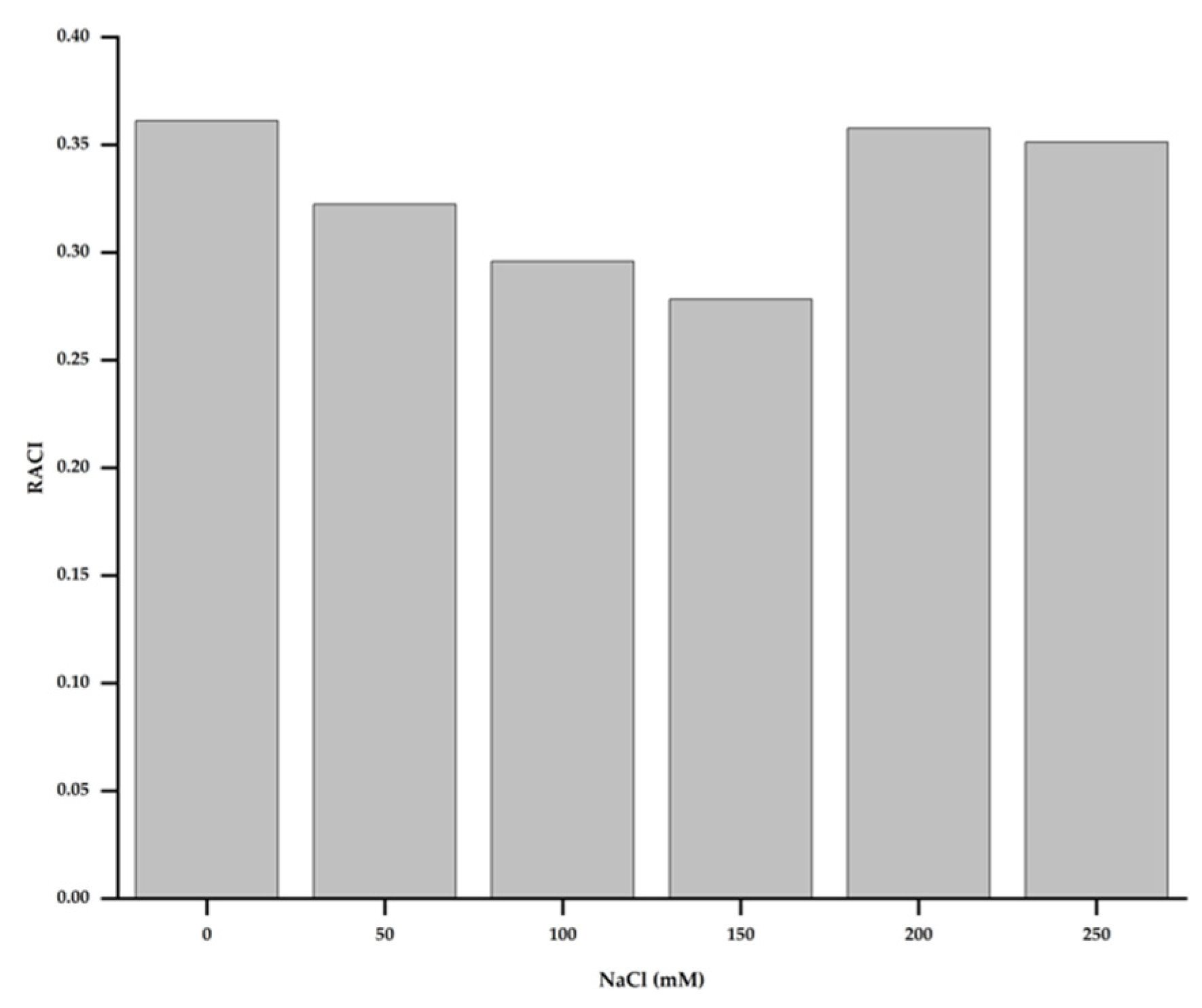
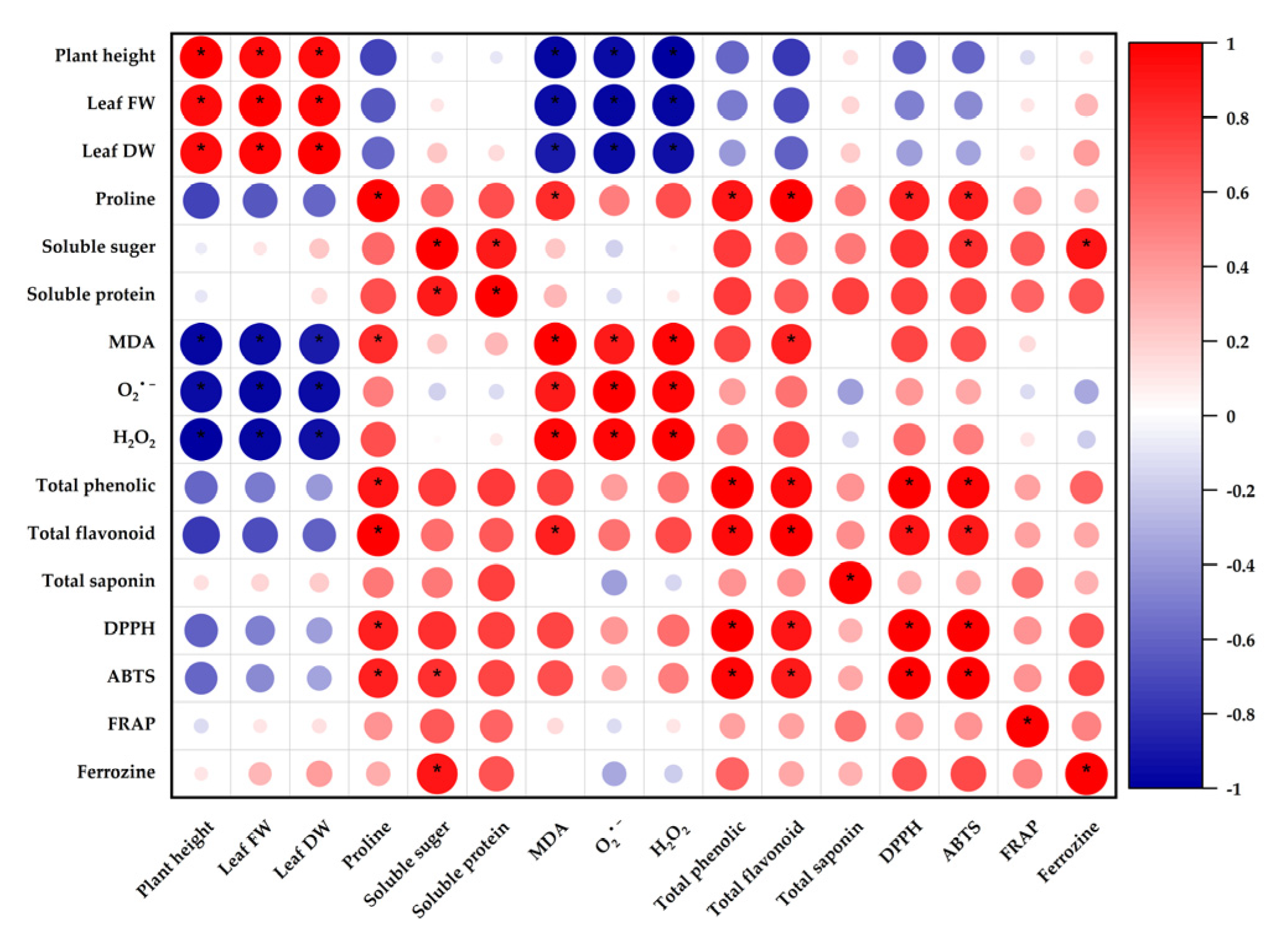
3.8. Correlation Analysis of Physiological and Biochemical Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kashif, M.H.; Tang, D.; Li, Z.; Wei, F.; Liang, Z.; Chen, P. Comparative Cytological and Gene Expression Analysis Reveals Potential Metabolic Pathways and Target Genes Responsive to Salt Stress in Kenaf (Hibiscus cannabinus L.). J. Plant Growth Regul. 2020, 39, 1245–1260. [Google Scholar] [CrossRef]
- Munns, R.; Day, D.A.; Fricke, W.; Watt, M.; Arsova, B.; Barkla, B.J.; Bose, J.; Byrt, C.S.; Chen, Z.; Foster, K.J.; et al. Energy Costs of Salt Tolerance in Crop Plants. New Phytol. 2020, 225, 1072–1090. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pan, T.; Allakhverdiev, S.I.; Yu, M.; Shabala, S. Crop Halophytism: An Environmentally Sustainable Solution for Global Food Security. Trends Plant Sci. 2020, 25, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Li-ping, L.; Xiao-hua, L.; Hong-bo, S.; Zhao-Pu, L.; Ya, T.; Quan-suo, Z.; Jun-qin, Z. Ameliorants Improve Saline–Alkaline Soils on a Large Scale in Northern Jiangsu Province, China. Ecol. Eng. 2015, 81, 328–334. [Google Scholar] [CrossRef]
- Rahneshan, Z.; Nasibi, F.; Moghadam, A.A. Effects of Salinity Stress on Some Growth, Physiological, Biochemical Parameters and Nutrients in Two Pistachio (Pistacia vera L.) Rootstocks. J. Plant Interact. 2018, 13, 73–82. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, Y.; Ji, Y.; Jiang, Z.; Wang, L. Effects of Salt Stress on Ion Content, Antioxidant Enzymes and Protein Profile in Different Tissues of Broussonetia papyrifera. S. Afr. J. Bot. 2013, 85, 1–9. [Google Scholar] [CrossRef]
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Liu, Z.; Ke, W.; Hou, H. Effect of Salt Stress on Growth, Physiological Parameters, and Ionic Concentration of Water Dropwort (Oenanthe javanica) Cultivars. Front. Plant Sci. 2021, 12, 15. [Google Scholar] [CrossRef]
- Singh, M. Plant Tolerance Mechanism Against Salt Stress: The Nutrient Management Approach. Biochem. Pharm. 2014, 3, e165. [Google Scholar] [CrossRef]
- Rahman, A.; Hossain, M.S.; Mahmud, J.-A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Manganese-Induced Salt Stress Tolerance in Rice Seedlings: Regulation of Ion Homeostasis, Antioxidant Defense and Glyoxalase Systems. Physiol. Mol. Biol. Plants 2016, 22, 291–306. [Google Scholar] [CrossRef]
- Ali, Q.; Daud, M.K.; Haider, M.Z.; Ali, S.; Rizwan, M.; Aslam, N.; Noman, A.; Iqbal, N.; Shahzad, F.; Deeba, F.; et al. Seed Priming by Sodium Nitroprusside Improves Salt Tolerance in Wheat (Triticum aestivum L.) by Enhancing Physiological and Biochemical Parameters. Plant Physiol. Biochem. 2017, 119, 50–58. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of Individual and Combined Effects of Salinity and Drought on Physiological, Nutritional and Biochemical Properties of Cabbage (Brassica oleracea Var Capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and Salinity Stress Alters ROS Accumulation, Water Retention, and Osmolyte Content in Sorghum Plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mostofa, M.G.; Das, A.K.; Anik, T.R.; Keya, S.S.; Ahsan, S.M.; Khan, M.A.R.; Ahmed, M.; Rahman, M.A.; Hossain, M.M.; et al. Ethanol Positively Modulates Photosynthetic Traits, Antioxidant Defense and Osmoprotectant Levels to Enhance Drought Acclimatization in Soybean. Antioxidants 2022, 11, 516. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, S.; Di Marco, G.; Bruno, L.; Gismondi, A.; Canini, A. Investigating the Drought and Salinity Effect on the Redox Components of Sulla coronaria (L.) Medik. Antioxidants 2021, 10, 1048. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the Physiological and Biochemical Impacts of Salt Stress on Plant Growth and Development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Chen, C.; Wang, C.; Liu, Z.; Liu, X.; Zou, L.; Shi, J.; Chen, S.; Chen, J.; Tan, M. Variations in Physiology and Multiple Bioactive Constituents under Salt Stress Provide Insight into the Quality Evaluation of Apocyni Veneti Folium. Int. J. Mol. Sci. 2018, 19, 3042. [Google Scholar] [CrossRef]
- Pang, J.; Cuin, T.; Shabala, L.; Zhou, M.; Mendham, N.; Shabala, S. Effect of Secondary Metabolites Associated with Anaerobic Soil Conditions on Ion Fluxes and Electrophysiology in Barley Roots. Plant Physiol. 2007, 145, 266–276. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Chen, W.; Feng, C.; Guo, W.; Shi, D.; Yang, C. Comparative Effects of Osmotic-, Salt- and Alkali Stress on Growth, Photosynthesis, and Osmotic Adjustment of Cotton Plants. Photosynthetica 2011, 49, 417–425. [Google Scholar] [CrossRef]
- Kobaisy, M.; Tellez, M.R.; Webber, C.L.; Dayan, F.E.; Schrader, K.K.; Wedge, D.E. Phytotoxic and Fungitoxic Activities of the Essential Oil of Kenaf (Hibiscus cannabinus L.) Leaves and Its Composition. J. Agric. Food Chem. 2001, 49, 3768–3771. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of Salt Stress on Plant Growth, Antioxidant Capacity, Glandular Trichome Density, and Volatile Exudates of Schizonepeta Tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.C.; Bustos-Sanmamed, P.; Clemente, M.R.; Becana, M. Effects of Salt Stress on the Expression of Antioxidant Genes and Proteins in the Model Legume Lotus Japonicus. New Phytol. 2009, 181, 851–859. [Google Scholar] [CrossRef]
- Li, H. RNA-Seq for Comparative Transcript Profiling of Kenaf under Salinity Stress. J. Plant Res. 2017, 8, 365–372. [Google Scholar] [CrossRef]
- Kim, D.-G.; Ryu, J.; Lee, M.-K.; Kim, J.M.; Ahn, J.-W.; Kim, J.-B.; Kang, S.-Y.; Bae, C.-H.; Kwon, S.-J. Nutritional Properties of Various Tissues from New Kenaf Cultivars. J. Crop Sci. Biotechnol. 2018, 21, 229–239. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Maganha, E.G.; da Costa Halmenschlager, R.; Rosa, R.M.; Henriques, J.A.; de Paula Ramos, A.L.; Saffi, J. Pharmacological Evidences for the Extracts and Secondary Metabolites from Plants of the Genus Hibiscus. Food Chem. 2010, 118, 1–10. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Ding, S.; Deng, Y.; Wang, X. Skin-Care Effects of Dandelion Leaf Extract and Stem Extract: Antioxidant Properties, Tyrosinase Inhibitory and Molecular Docking Simulations. Ind. Crops Prod. 2018, 111, 238–246. [Google Scholar] [CrossRef]
- Sim, Y.Y.; Jess Ong, W.T.; Nyam, K.L. Effect of Various Solvents on the Pulsed Ultrasonic Assisted Extraction of Phenolic Compounds from Hibiscus cannabinus L. Leaves. Ind. Crops Prod. 2019, 140, 111708. [Google Scholar] [CrossRef]
- Zhao, S.; Li, X.; Cho, D.; Arasu, M.; Al-Dhabi, N.; Park, S. Accumulation of Kaempferitrin and Expression of Phenyl-Propanoid Biosynthetic Genes in Kenaf (Hibiscus cannabinus). Molecules 2014, 19, 16987–16997. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kwon, S.-J.; Ahn, J.-W.; Jo, Y.D.; Kim, S.H.; Jeong, S.W.; Lee, M.K.; Kim, J.-B.; Kang, S.-Y. Phytochemicals and Antioxidant Activity in the Kenaf Plant (Hibiscus cannabinus L.). J. Plant Biotechnol. 2017, 44, 191–202. [Google Scholar] [CrossRef]
- Survay, N. New Genera of Flavonols and Flavonol Derivatives As Therapeutic Molecules. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 1–18. [Google Scholar] [CrossRef]
- Byju, K.; Vasundhara, G.; Anuradha, V.; Nair, S.M.; Kumar, N.C. Presence of Phytol, a Precursor of Vitamin E in Chaetomorpha Antinnina. Mapana J. Sci. 2013, 12, 57–65. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A Phytochemical and Pharmacological Review. Food Chem. 2014, 165, 424–443. [Google Scholar]
- Rajaram, S. Health Benefits of Plant-Derived α-Linolenic Acid. Am. J. Clin. Nutr. 2014, 100, 443S–448S. [Google Scholar] [CrossRef]
- Agbor, G.; Oben, J.; Blaise, N.; Takala, J.; Jeanne, N. Hepatoprotective Activity of Hibiscus cannabinus (Linn.) Against Carbon Tetrachloride and Paracetamol Induced Liver Damage in Rats. Pak. J. Biol. Sci. 2005, 8, 1397–1401. [Google Scholar] [CrossRef]
- Pradeep, K.; Gagandeep, K.; Nanjaian, M. Antihyperlipidemic Effect of Hydroalcoholic Extract of Kenaf (Hibiscus cannabinus L.) Leaves in High Fat Diet Fed Rats. Ann. Biol. Res. 2010, 1, 174–181. [Google Scholar]
- Lee, Y.G.; Byeon, S.E.; Kim, J.Y.; Lee, J.Y.; Rhee, M.H.; Hong, S.; Wu, J.C.; Lee, H.S.; Kim, M.J.; Cho, D.H.; et al. Immunomodulatory Effect of Hibiscus Cannabinus Extract on Macrophage Functions. J. Ethnopharmacol. 2007, 113, 62–71. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants and Developmental Regulators: Relative Significance in Plants and Humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef]
- Jin, C.H.; Ghimeray, A.; Wang, L.; Xu, M.L.; Piao, J.P.; Cho, D.H. Far Infrared Assisted Kenaf Leaf Tea Preparation and Its Effect on Phenolic Compounds, Antioxidant and ACE Inhibitory Activity. J. Med. Plant Res. 2013, 7, 1121–1128. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of Salinity Stress on the Physiological Characteristics, Phenolic Compounds and Antioxidant Activity of Thymus vulgaris L. and Thymus Daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; A. A. Mohamed, I.; Wang, Z.; Khatab, A.; Sherif, A.; Ahmad, H.; Khan, M.N.; Hassan, H.M.; Elrewainy, I.M.; et al. Antioxidative and Metabolic Contribution to Salinity Stress Responses in Two Rapeseed Cultivars during the Early Seedling Stage. Antioxidants 2021, 10, 1227. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, L.; Cai, Z.; Chen, C.; Liu, Z.; Liu, X.; Zou, L.; Chen, J.; Tan, M.; Wei, L.; et al. Dynamic Variations in Multiple Bioactive Constituents under Salt Stress Provide Insight into Quality Formation of Licorice. Molecules 2019, 24, 3670. [Google Scholar] [CrossRef]
- Deng, Y.; Li, D.; Huang, Y.; Huang, S. Physiological Response to Cadmium Stress in Kenaf (Hibiscus cannabinus L.) Seedlings. Ind. Crops Prod. 2017, 107, 453–457. [Google Scholar] [CrossRef]
- Wei, F.; Tang, D.; Li, Z.; Kashif, M.H.; Khan, A.; Lu, H.; Jia, R.; Chen, P. Molecular Cloning and Subcellular Localization of Six HDACs and Their Roles in Response to Salt and Drought Stress in Kenaf (Hibiscus cannabinus L.). Biol Res. 2019, 52, 20. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants without Soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar]
- Hseu, Z.-Y. Evaluating Heavy Metal Contents in Nine Composts Using Four Digestion Methods. Bioresour. Technol. 2004, 95, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Du Laing, G.; Tack, F.M.G.; Verloo, M.G. Performance of Selected Destruction Methods for the Determination of Heavy Metals in Reed Plants (Phragmites australis). Anal. Chim. Acta 2003, 497, 191–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Michel, D.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Guy, C.; Haskell, D.; Neven, L.; Klein, P.; Smelser, C. Hydration-State-Responsive Protein Link Cold and Drought Stress in Spinach. Planta 1992, 188, 265–270. [Google Scholar] [CrossRef]
- Okuda, T.; Matsuda, Y.; Yamanaka, A.; Sagisaka, S. Abrupt Increase in the Level of Hydrogen Peroxide in Leaves of Winter Wheat Is Caused by Cold Treatment. Plant Physiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef]
- Bu, R.; Xie, J.; Yu, J.; Liao, W.; Xiao, X.; Lv, J.; Wang, C.; Ye, J.; Calderón-Urrea, A. Autotoxicity in Cucumber (Cucumis sativus L.) Seedlings Is Alleviated by Silicon through an Increase in the Activity of Antioxidant Enzymes and by Mitigating Lipid Peroxidation. J. Plant Biol. 2016, 59, 247–259. [Google Scholar] [CrossRef]
- Lang, D.; Yu, X.; Jia, X.; Li, Z.; Zhang, X. Methyl Jasmonate Improves Metabolism and Growth of NaCl-Stressed Glycyrrhiza Uralensis Seedlings. Sci. Hortic. 2020, 266, 109287. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplast I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Panayiotou, C.; Tzortzakis, N. Nitrogen and Phosphorus Levels Affected Plant Growth, Essential Oil Composition and Antioxidant Status of Lavender Plant (Lavandula angustifolia Mill.). Ind. Crops Prod. 2016, 83, 577–586. [Google Scholar] [CrossRef]
- He, J.; Wu, Z.; Zhang, S.; Zhou, Y.; Zhao, F.; Peng, Z.; Hu, Z. Optimization of Microwave-Assisted Extraction of Tea Saponin and Its Application on Cleaning of Historic Silks. J. Surfact Deterg. 2014, 17, 919–928. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Comparative Study Regarding the Chemical Composition and Biological Activity of Pine (Pinus nigra and P. sylvestris) Bark Extracts. Antioxidants 2021, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Muscolo, A.; Calderaro, A.; Papalia, T.; Settineri, G.; Mallamaci, C.; Panuccio, M.R. Soil Salinity Improves Nutritional and Health Promoting Compounds in Three Varieties of Lentil (Lens Culinaris Med.). Food Biosci. 2020, 35, 100571. [Google Scholar] [CrossRef]
- James, R.A.; Blake, C.; Byrt, C.S.; Munns, R. Major Genes for Na+ Exclusion, Nax1 and Nax2 (Wheat HKT1;4 and HKT1;5), Decrease Na+ Accumulation in Bread Wheat Leaves under Saline and Waterlogged Conditions. J. Exp. Bot. 2011, 62, 2939–2947. [Google Scholar] [CrossRef]
- Zong, J.-W.; Zhang, Z.-L.; Huang, P.-L.; Chen, N.-Y.; Xue, K.-X.; Tian, Z.-Y.; Yang, Y.-H. Growth, Physiological, and Photosynthetic Responses of Xanthoceras Sorbifolium Bunge Seedlings Under Various Degrees of Salinity. Front. Plant Sci. 2021, 12, 730737. [Google Scholar] [CrossRef]
- Oueslati, S.; Karray-Bouraoui, N.; Attia, H.; Rabhi, M.; Ksouri, R.; Lachaal, M. Physiological and Antioxidant Responses of Mentha Pulegium (Pennyroyal) to Salt Stress. Acta Physiol Plant 2010, 32, 289–296. [Google Scholar] [CrossRef]
- Bettaieb Rebey, I.; Bourgou, S.; Rahali, F.Z.; Msaada, K.; Ksouri, R.; Marzouk, B. Relation between Salt Tolerance and Biochemical Changes in Cumin (Cuminum cyminum L.) Seeds. J. Food Drug Anal. 2017, 25, 391–402. [Google Scholar] [CrossRef]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.; Dreyer, I. The Twins K+ and Na+ in Plants? J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I. Regulation of Potassium Transport in Plants under Hostile Conditions: Implications for Abiotic and Biotic Stress Tolerance. Physiol Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef]
- Mahouachi, J. Long-Term Salt Stress Influence on Vegetative Growth and Foliar Nutrient Changes in Mango (Mangifera indica L.) Seedlings. Sci. Hortic. 2018, 234, 95–100. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The Response of Salinity Stress-Induced A. Tricolor to Growth, Anatomy, Physiology, Non-Enzymatic and Enzymatic Antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in Plants under Abiotic Stresses: New Insights into a Classical Phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Al Hassan, M.; Morosan, M.; López-Gresa, M.; Prohens, J.; Vicente, O.; Boscaiu, M. Salinity-Induced Variation in Biochemical Markers Provides Insight into the Mechanisms of Salt Tolerance in Common (Phaseolus vulgaris) and Runner (P. coccineus) Beans. Int. J. Mol. Sci. 2016, 17, 1582. [Google Scholar] [CrossRef]
- Sharif, P.; Seyedsalehi, M.; Paladino, O.; Van Damme, P.; Sillanpää, M.; Sharifi, A.A. Effect of Drought and Salinity Stresses on Morphological and Physiological Characteristics of Canola. Int. J. Environ. Sci. Technol. 2018, 15, 1859–1866. [Google Scholar] [CrossRef]
- Ibrahimova, U.F. The Effect of Nacl on Some Physiological and Biochemical Parameters in Triticum aestivum L. Genotypes. Plant Physiol. 2019, 24, 370–375. [Google Scholar] [CrossRef]
- El-Badri, A.M.A. Modulation of Salinity Impact on Early Seedling Stage via Nano-Priming Application of Zinc Oxide on Rapeseed (Brassica napus L.). Plant Physiol. Biochem. 2021, 17, 370–375. [Google Scholar] [CrossRef]
- Alam, P.; Albalawi, T.H.; Altalayan, F.H.; Bakht, A.; Ahanger, M.A.; Raja, V.; Ashraf, M.; Ahmad, P. 24-Epibrassinolide (EBR) Confers Tolerance against NaCl Stress in Soybean Plants by Up-Regulating Antioxidant System, Ascorbate-Glutathione Cycle, and Glyoxalase System. Biomolecules 2019, 21, 640. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Li, Z.; Hu, Y.; Chang, M.; Kashif, M.H.; Tang, M.; Luo, D.; Cao, S.; Lu, H.; Zhang, W.; Huang, Z.; et al. 5-Azacytidine Pre-Treatment Alters DNA Methylation Levels and Induces Genes Responsive to Salt Stress in Kenaf (Hibiscus cannabinus L.). Chemosphere 2021, 271, 129562. [Google Scholar] [CrossRef] [PubMed]
- Petridis, A.; Therios, I.; Samouris, G.; Tananaki, C. Salinity-Induced Changes in Phenolic Compounds in Leaves and Roots of Four Olive Cultivars (Olea europaea L.) and Their Relationship to Antioxidant Activity. Environ. Exp. Bot. 2012, 79, 37–43. [Google Scholar] [CrossRef]
- Navarro, J.; Flores, P.; Garrido, C.; Martínez, V. Changes in the Contents of Antioxidant Compounds in Pepper Fruits at Different Ripening Stages, as Affected by Salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Salem, N.; Msaada, K.; Dhifi, W.; Limam, F.; Marzouk, B. Effect of Salinity on Plant Growth and Biological Activities of Carthamus tinctorius L. Extracts at Two Flowering Stages. Acta Physiol. Plant 2014, 36, 433–445. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Chaabani, S.; Roula, R.; Muscolo, A. Bio-Priming Mitigates Detrimental Effects of Salinity on Maize Improving Antioxidant Defense and Preserving Photosynthetic Efficiency. Plant Physiol. Biochem. 2018, 132, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of Salinity Stress on Growth and Metabolomic Profiling of Cucumis sativus and Solanum lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef]
- Caravaca, A.M.G.; Iafelice, G.; Lavini, A.; Pulvento, C.; Caboni, M.; Marconi, E. Phenolic Compounds and Saponins in Quinoa Samples (Chenopodium Quinoa Willd.) Grown under Different Saline and Nonsaline Irrigation Regimens. J. Agric. Food Chem. 2012, 60, 4620–4627. [Google Scholar] [CrossRef]
- Rajesh, K.D.; Vasantha, S.; Panneerselvam, A.; Valsala Rajesh, N.; Jeyathilakan, N. Phytochemical analysis, in vitro antioxidant potential and gas chromatography-mass spectrometry studies of Dicranopteris linearis. Asian J. Pharm. Clin. Res. 2016, 220, 220–225. [Google Scholar] [CrossRef]
- Faridha Begum, I.; Mohankumar, R.; Jeevan, M.; Ramani, K. GC–MS Analysis of Bio-Active Molecules Derived from Paracoccus Pantotrophus FMR19 and the Antimicrobial Activity Against Bacterial Pathogens and MDROs. Indian J. Microbiol. 2016, 56, 426–432. [Google Scholar] [CrossRef]
- Al-Abd, N.M.; Mohamed Nor, Z.; Mansor, M.; Azhar, F.; Hasan, M.S.; Kassim, M. Antioxidant, Antibacterial Activity, and Phytochemical Characterization of Melaleuca Cajuputi Extract. BMC Complement. Altern. Med. 2015, 15, 385. [Google Scholar] [CrossRef]
- Abubacker, M.N.; Kamala Devi, P. In Vitro Antifungal Potentials of Bioactive Compound Oleic Acid, 3-(Octadecyloxy) Propyl Ester Isolated from Lepidagathis Cristata Willd. (Acanthaceae) Inflorescence. Asian Pac. J. Trop. Biomed. 2014, 4, S661–S664. [Google Scholar] [CrossRef]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.; Karaman, R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.J.; Soszynski, A.; Martins, A.; Rauter, A.; Neng, N.; Nogueira, J.; Varela, J.; Barreira, L.; Custódio, L. Unravelling the Antioxidant Potential and the Phenolic Composition of Different Anatomical Organs of the Marine Halophyte Limonium Algarvense. Ind. Crops Prod. 2015, 77, 315–322. [Google Scholar] [CrossRef]

| Treatments (mM) | N (mg/g DW) | K (mg/g DW) | Ca (mg/g DW) | Mg (mg/g DW) | p (mg/g DW) | Fe (mg/g DW) |
|---|---|---|---|---|---|---|
| 0 | 63.07 ± 0.04 a | 29.65 ± 0.22 a | 9.59 ± 0.32 a | 1.14 ± 0.01 a | 3.59 ± 0.05 a | 0.13 ± 0.00 b |
| 50 | 62.13 ± 0.04 a | 27.72 ± 0.02 d | 7.38 ± 0.14 b | 1.08 ± 0.01 a, b | 3.31 ± 0.03 b | 0.17 ± 0.00 a |
| 100 | 57.43 ± 0.45 b | 29.00 ± 0.19 a, b | 5.26 ± 0.06 c | 1.01 ± 0.03 b | 2.80 ± 0.09 c | 0.10 ± 0.00 c |
| 150 | 53.40 ± 1.33 c | 28.87 ± 0.90 a–c | 4.15 ± 0.13 d | 0.90 ± 0.07 c | 2.10 ± 0.05 d | 0.09 ± 0.00 c |
| 200 | 48.80 ± 0.36 d | 27.95 ± 0.10 c, d | 2.78 ± 0.04 e | 0.86 ± 0.00 c | 2.05 ± 0.05 d | 0.08 ± 0.00 c |
| 250 | 47.40 ± 0.46 e | 28.50 ± 0.09 b–d | 5.10 ± 0.06 c | 0.88 ± 0.02 c | 2.16 ± 0.07 d | 0.09 ± 0.00 c |
| No. | Name of the Compound | Content (%) | |||||
|---|---|---|---|---|---|---|---|
| NaCl Concentration (mM) | |||||||
| 0 | 50 | 100 | 150 | 200 | 250 | ||
| 1 | Methyl linolelaidate | 4.36 ± 0.49 b | 3.43 ± 0.30 c | 2.92 ± 0.36 d | 9.15 ± 0.68 a | 1.87 ± 0.19 e | nd |
| 2 | Phytol | 18.64 ± 1.60 a | 2.32 ± 0.45 c | 2.09 ± 0.15 d | 5.73 ± 0.10 b | nd | nd |
| 3 | Oleamide | 9.36 ± 0.64 c | 10.86 ± 0.10 b | 5.02 ± 0.07 f | 16.32 ± 1.18 a | 7.92 ± 0.1 d | 6.12 ± 0.05 e |
| 4 | Alterungsschutzmittel BKF | 7.30 ± 0.74 b | 6.25 ± 0.15 c | 2.40 ± 0.17 e | 10.30 ± 0.15 a | 5.01 ± 0.20 d | 2.06 ± 0.03 d |
| 5 | Diisooctyl phthalate | 2.52 ± 0.70 c | 5.25 ± 0.73 a | 0.96 ± 0.14 d | Nd | 4.10 ± 0.89 b | 3.08 ± 0.23 c |
| 6 | Phthalic acid, heptyl undecyl ester | 6.70 ± 1.38 d | 6.55 ± 0.12 e | 68.50 ± 2.50 a | 11.43 ± 0.59 b | 9.96 ± 1.54 c | 4.76 ± 0.44 f |
| 7 | Heptacosane | nd | nd | nd | Nd | 1.55 ± 0.91 b | 2.78 ± 0.66 a |
| 8 | 1-Octadecanesulphonyl chloride | nd | nd | nd | Nd | 0.34 ± 0.14 | nd |
| 9 | α-Glyceryl linolenate | 2.72 ± 0.10 c | 23.36 ± 0.94 b | 0.68 ± 0.04 d | 0.29 ± 0.06 e | nd | 42.85 ± 1.10 a |
| 10 | β-Monoolein | 2.06 ± 0.20 c | 4.60 ± 0.14 b | 0.57 ± 0.10 d | 13.96 ±1.50 a | nd | 0.55 ± 0.12 e |
| 11 | 1-Heptacosanol | 18.23 ±1.92 b | 3.08 ± 0.32 e | 4.61 ± 0.34 d | Nd | 19.47 ± 1.44 a | 13.97 ± 0.95 c |
| 12 | 12-Methyl-E,E-2,13-octadecadien-1-ol | 8.27 ± 0.15 b | 20.73 ± 2.00 a | 2.90 ± 0.52 d | Nd | 4.35 ± 0.16 c | 1.84 ± 0.15 e |
| 13 | Oleic acid, 3-(octadecyloxy)propyl ester | 3.57 ± 0.03 e | 6.16 ± 0.86 c | 5.98 ± 0.12 d | 31.67 ± 1.56 b | 40.02 ± 0.10 a | 2.87 ± 0.56 f |
| 14 | cis-Vaccenic acid | 6.80 ± 0.25 a | 1.64 ± 0.14 b | 0.57 ± 0.08 d | 1.16 ± 0.04 c | nd | nd |
| 15 | Tetratetracontane | nd | nd | nd | nd | 0.71 ± 0.14 b | 0.89 ± 0.59 a |
| 16 | Ethyl iso-allocholate | 1.81 ± 0.03 a | 0.50 ± 0.01 b | 0.23 ± 0.09 c | nd | nd | 0.15 ± 0.04 d |
| 17 | β-Sitosterol | 0.92 ± 0.04 c | 2.13 ± 0.13 b | 0.22 ± 0.01 e | nd | 0.34 ± 0.05 d | 9.48 ± 0.50 a |
| 18 | β-Viscol | 1.45 ± 0.05 a | 1.12 ± 0.15 b | 0.28 ± 0.02 c | nd | 0.15 ± 0.02 e | 0.16 ± 0.02 d |
| 19 | Unknown | 3.90 ± 0.15 b | 0.43 ± 0.04 e | 1.93 ± 0.15 d | nd | 3.32 ± 0.18 c | 5.07 ± 0.25 a |
| 20 | Unknown | 1.39 ± 0.14 c | 1.60 ± 0.24 b | 0.15 ± 0.05 e | nd | 0.89 ± 0.14 d | 3.62 ± 0.19 a |
| Total | 100 | 100 | 100 | 100 | 99.90 | 100 | |
| Total identified classes | |||||||
| Alkanes | 0.00 c | 0.00 c | 0.00 c | 0.00 c | 2.60 ± 0.21 b | 3.67 ± 3.25 a | |
| Esters | 19.41 ± 1.78 f | 44.10 ± 2.62 e | 78.65 ± 0.48 a | 66.50 ± 2.24 b | 51.85 ± 0.99 c | 51.03 ± 0.79 d | |
| Phenols | 7.30 ± 0.74 b | 6.25 ± 0.15 c | 2.40 ± 0.17 e | 10.30 ± 0.15 a | 5.01 ± 0.61 d | 1.91 ± 0.03 f | |
| Amide | 9.36 ± 0.64 c | 10.86 ± 0.10 b | 5.02 ± 0.07 f | 16.32 ± 1.18 a | 7.92 ± 0.10 d | 6.12 ± 0.01 e | |
| Alcohols | 27.42 ± 1.06 a | 25.94 ± 1.13 b | 7.73 ± 0.12 e | 0.00 f | 24.16 ± 1.61 d | 25.29 ± 0.21 c | |
| Terpenes | 20.09 ± 1.60 a | 3.44 ± 0.45 c | 2.37 ± 0.15 d | 5.73 ± 0.42 b | 0.15 ± 0.001 e | 0.16 ± 0.002 f | |
| Steroid derivative | 1.81 ± 0.03 a | 0.50 ± 0.01 b | 0.23 ± 0.09 c | 0.00 e | 0.00 e | 0.15 ± 0.003 d | |
| Others | 14.61 ± 0.20 a | 8.92 ± 0.14 c | 3.61 ± 0.04 e | 1.16 ± 0.06 f | 8.31 ± 0.02 d | 11.77 ± 0.14 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birhanie, Z.M.; Yang, D.; Luan, M.; Xiao, A.; Liu, L.; Zhang, C.; Biswas, A.; Dey, S.; Deng, Y.; Li, D. Salt Stress Induces Changes in Physiological Characteristics, Bioactive Constituents, and Antioxidants in Kenaf (Hibiscus cannabinus L.). Antioxidants 2022, 11, 2005. https://doi.org/10.3390/antiox11102005
Birhanie ZM, Yang D, Luan M, Xiao A, Liu L, Zhang C, Biswas A, Dey S, Deng Y, Li D. Salt Stress Induces Changes in Physiological Characteristics, Bioactive Constituents, and Antioxidants in Kenaf (Hibiscus cannabinus L.). Antioxidants. 2022; 11(10):2005. https://doi.org/10.3390/antiox11102005
Chicago/Turabian StyleBirhanie, Ziggiju Mesenbet, Dawei Yang, Mingbao Luan, Aiping Xiao, Liangliang Liu, Chao Zhang, Ashok Biswas, Susmita Dey, Yong Deng, and Defang Li. 2022. "Salt Stress Induces Changes in Physiological Characteristics, Bioactive Constituents, and Antioxidants in Kenaf (Hibiscus cannabinus L.)" Antioxidants 11, no. 10: 2005. https://doi.org/10.3390/antiox11102005
APA StyleBirhanie, Z. M., Yang, D., Luan, M., Xiao, A., Liu, L., Zhang, C., Biswas, A., Dey, S., Deng, Y., & Li, D. (2022). Salt Stress Induces Changes in Physiological Characteristics, Bioactive Constituents, and Antioxidants in Kenaf (Hibiscus cannabinus L.). Antioxidants, 11(10), 2005. https://doi.org/10.3390/antiox11102005










