The Prevention of Ischemia-Reperfusion Injury in Elderly Rats after Lower Limb Tourniquet Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Ischemia Induction and Maintenance
2.2. Biochemical Analyses
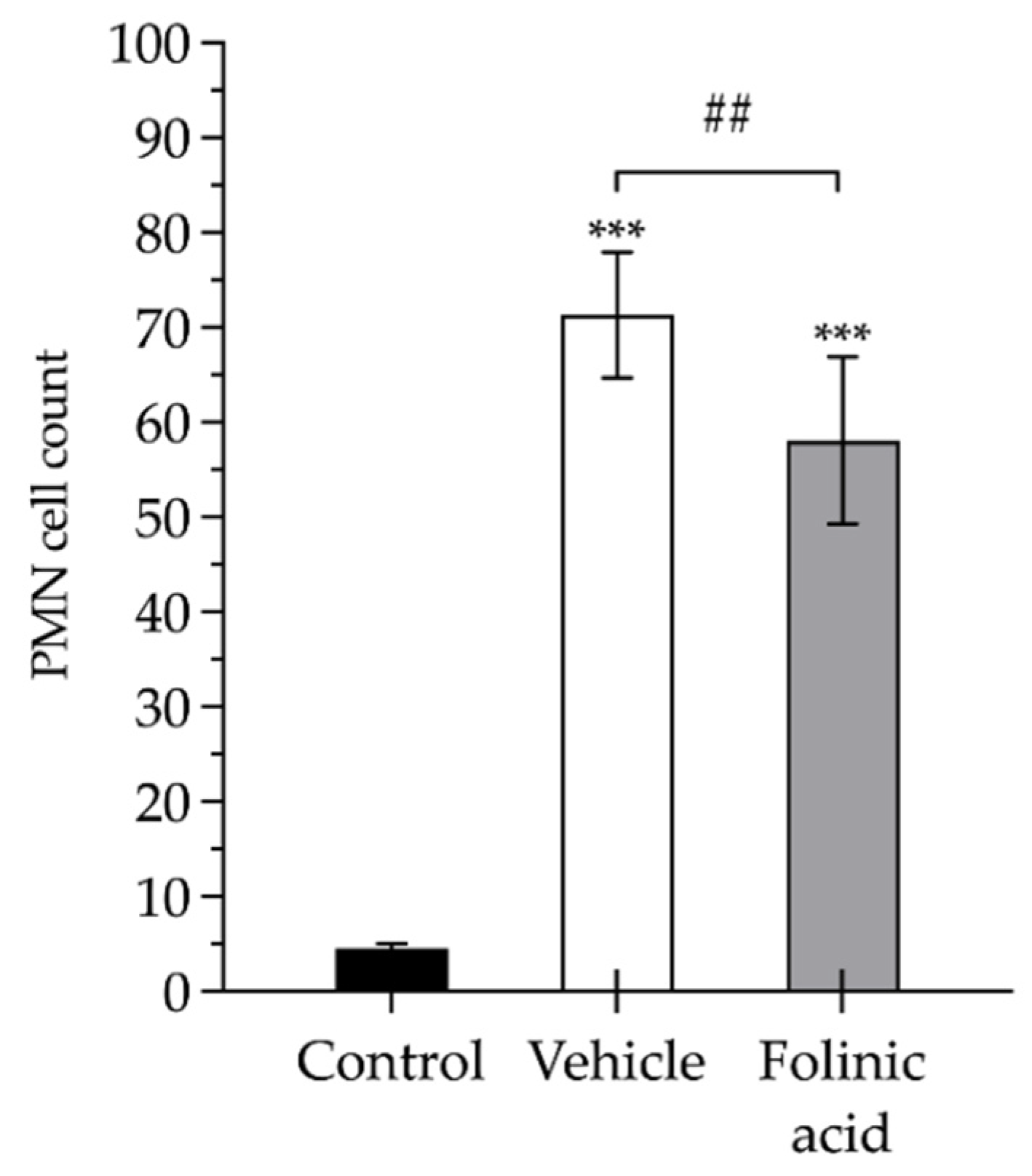
2.3. Pathological Studies
2.4. Functional Study: Rotarod Test
2.5. Euthanasia
2.6. Statistical Studies
3. Results
3.1. Biochemical Analyses
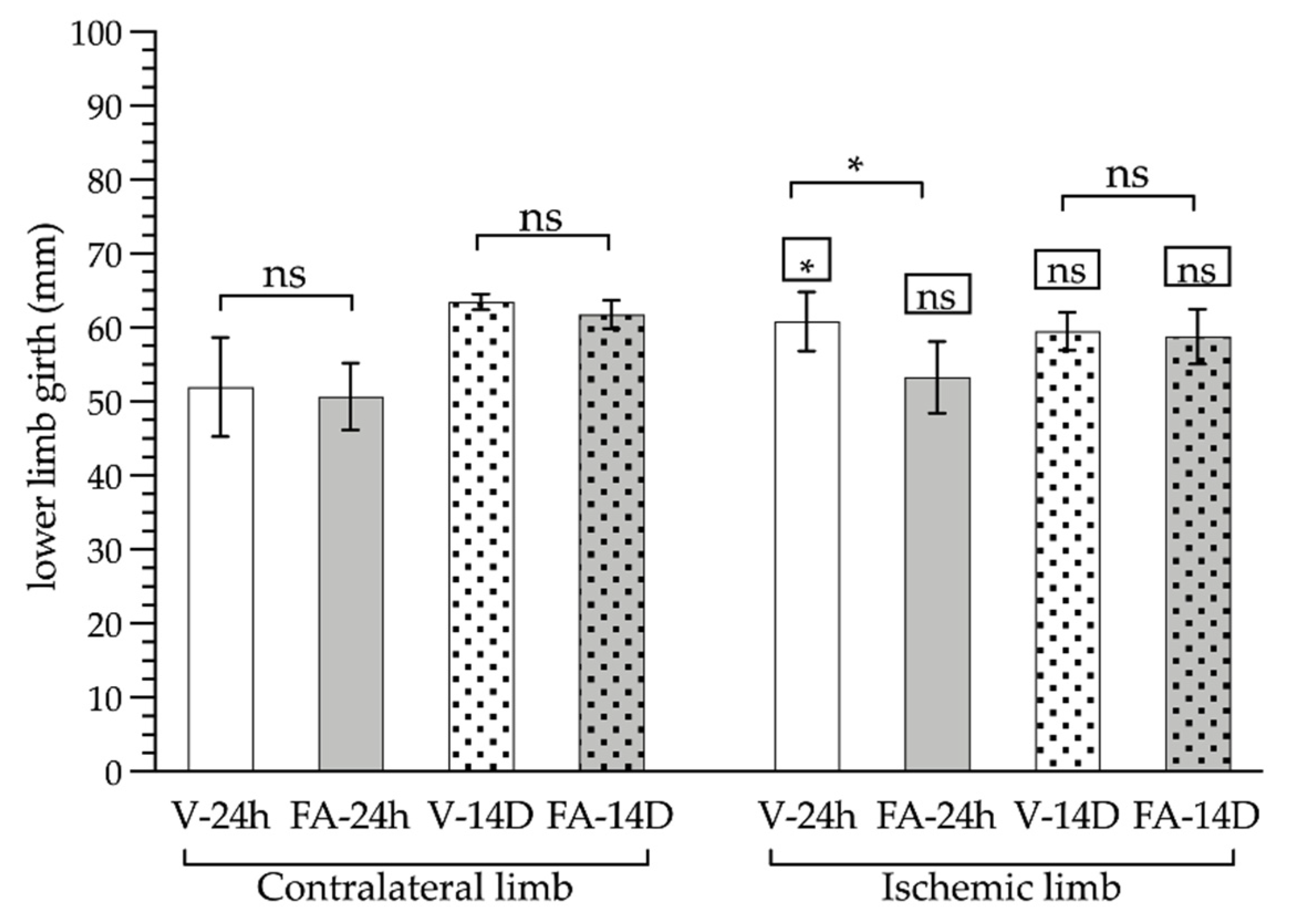
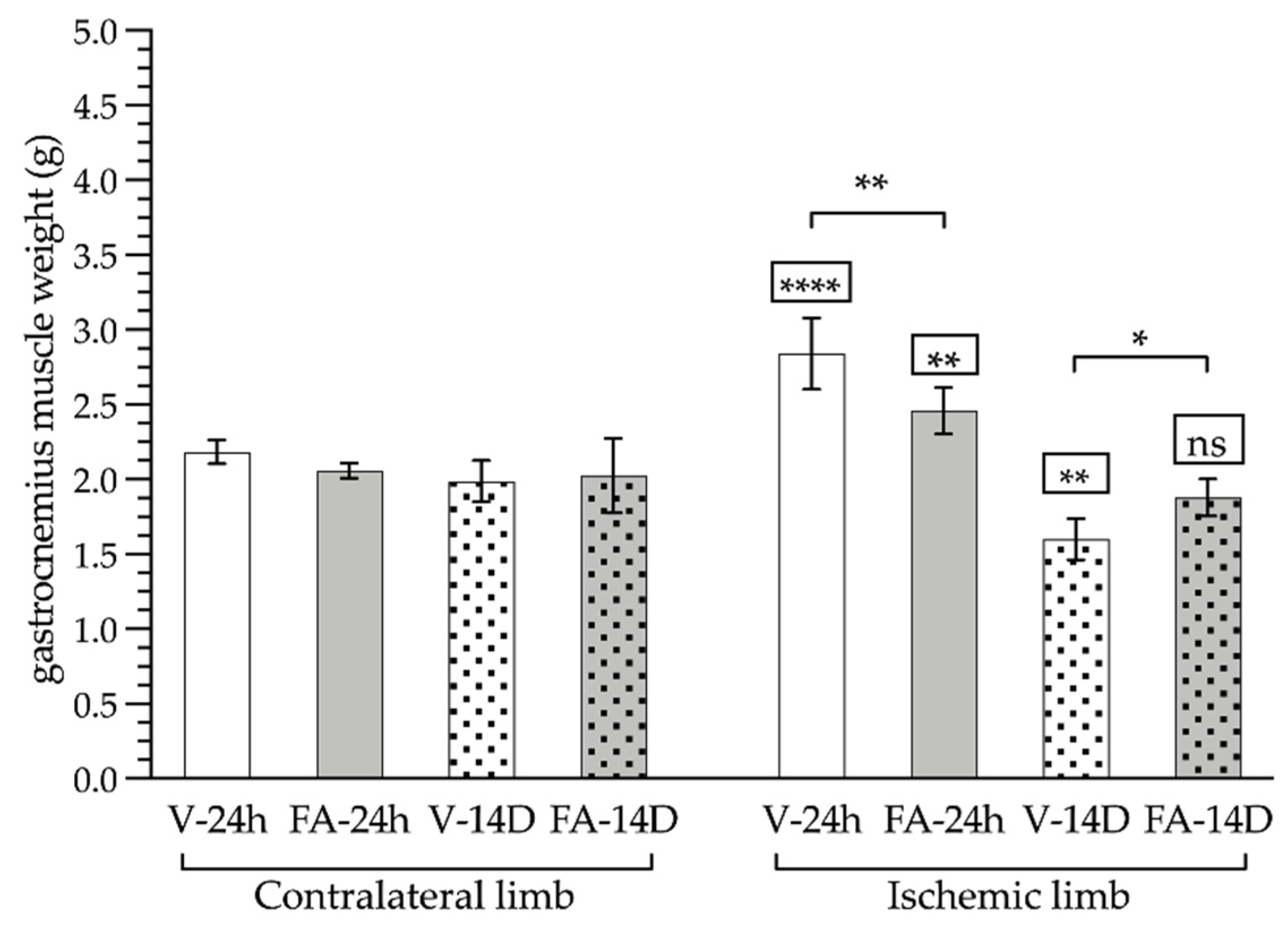
3.2. Gastrocnemius Muscle Analyses: Macroscopic and Microscopic
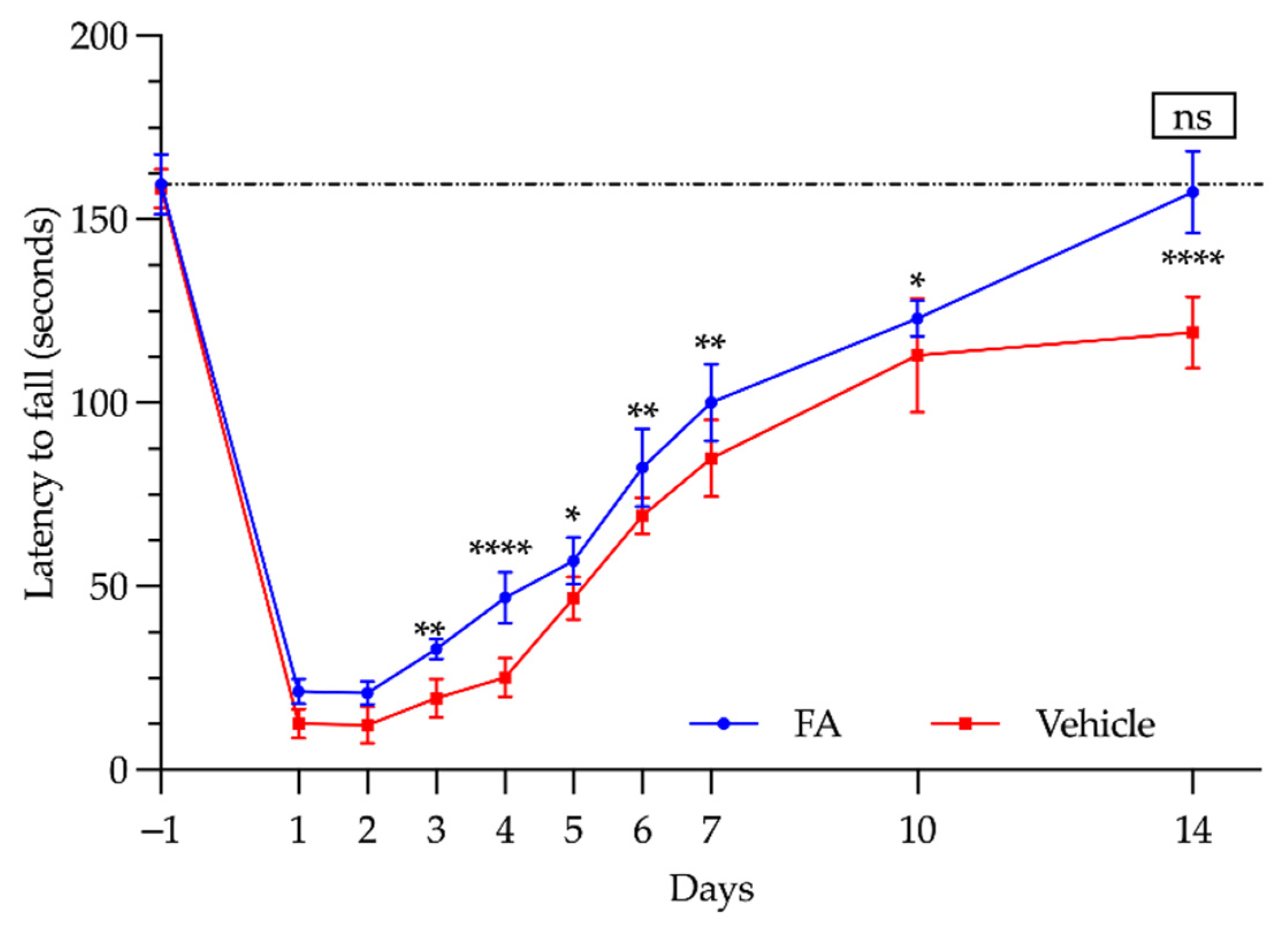
3.3. Functional Study: Rotarod Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Kumar, K.; Railton, C.; Tawfic, Q. Tourniquet Application during Anesthesia: “What We Need to Know?”. J. Anaesthesiol. Clin. Pharmacol. 2016, 32, 424–430. [Google Scholar] [CrossRef] [PubMed]
- McEwen, J.A.; Auchinleck, G.F. Advances in Surgical Tourniquets. AORN J. 1982, 36, 889–896. [Google Scholar] [CrossRef]
- McEwen, J.A. Introduction to the Best Tourniquets|Tourniquets.Org. Available online: https://tourniquets.org/introduction-to-the-best-tourniquets/ (accessed on 23 May 2022).
- Estebe, J.P.; Davies, J.M.; Richebe, P. The Pneumatic Tourniquet: Mechanical, Ischaemia-Reperfusion and Systemic Effects. Eur. J. Anaesthesiol. 2011, 28, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Pedowitz, R.A.; Gershuni, D.H.; Schmidt, A.H.; Fridén, J.; Rydevik, B.L.; Hargens, A.R. Muscle Injury Induced beneath and Distal to a Pneumatic Tourniquet: A Quantitative Animal Study of Effects of Tourniquet Pressure and Duration. J. Hand Surg. 1991, 16, 610–621. [Google Scholar] [CrossRef]
- Mingo-Robinet, J.; Castañeda-Cabrero, C.; Alvarez, V.; León Alonso-Cortés, J.M.; Monge-Casares, E. Tourniquet-Related Iatrogenic Femoral Nerve Palsy after Knee Surgery: Case Report and Review of the Literature. Case Rep. Orthop. 2013, 2013, 368290. [Google Scholar] [CrossRef]
- Anzell, A.R.; Maizy, R.; Przyklenk, K.; Sanderson, T.H. Mitochondrial Quality Control and Disease: Insights into Ischemia-Reperfusion Injury. Mol. Neurobiol. 2018, 55, 2547–2564. [Google Scholar] [CrossRef]
- Toyokuni, S. Reactive Oxygen Species-Induced Molecular Damage and Its Application in Pathology. Pathol. Int. 1999, 49, 91–102. [Google Scholar] [CrossRef]
- Carden, D.L.; Granger, D.N. Pathophysiology of Ischaemia-Reperfusion Injury. J. Pathol. 2000, 190, 255–266. [Google Scholar] [CrossRef]
- Collard, C.D.; Lekowski, R.; Jordan, J.E.; Agah, A.; Stahl, G.L. Complement Activation Following Oxidative Stress. Mol. Immunol. 1999, 36, 941–948. [Google Scholar] [CrossRef]
- Panés, J.; Perry, M.; Granger, D.N. Leukocyte-Endothelial Cell Adhesion: Avenues for Therapeutic Intervention. Br. J. Pharmacol. 1999, 126, 537–550. [Google Scholar] [CrossRef]
- Konrad, G.; Markmiller, M.; Lenich, A.; Mayr, E.; Rüter, A. Tourniquets May Increase Postoperative Swelling and Pain after Internal Fixation of Ankle Fractures. Clin. Orthop. Relat. Res. 2005, 433, 189–194. [Google Scholar] [CrossRef]
- Pedowitz, R.A.R.A. Tourniquet-Induced Neuromuscular Injury: A Recent Review of Rabbit and Clinical Experiments. Acta Orthop. Scand. 1991, 62, 1–33. [Google Scholar] [CrossRef]
- Blaisdell, F.W. The Pathophysiology of Skeletal Muscle Ischemia and the Reperfusion Syndrome: A Review. Cardiovasc. Surg. 2002, 10, 620–630. [Google Scholar] [CrossRef]
- Walters, T.J.; Kragh, J.F.; Kauvar, D.S.; Baer, D.G. The Combined Influence of Hemorrhage and Tourniquet Application on the Recovery of Muscle Function in Rats. J. Orthop. Trauma 2008, 22, 47–51. [Google Scholar] [CrossRef]
- Sheth, N.P.; Sennett, B.; Berns, J.S. Rhabdomyolysis and Acute Renal Failure Following Arthroscopic Knee Surgery in a College Football Player Taking Creatine Supplements. Clin. Nephrol. 2006, 65, 134–137. [Google Scholar] [CrossRef]
- Cherng, C.-H.; Wong, C.-S.; Chang, F.-L.; Ho, S.-T.; Lee, C.-H. Epidural Morphine Delays the Onset of Tourniquet Pain During Epidural Lidocaine Anesthesia. Anesth. Analg. 2002, 94, 1614–1616. [Google Scholar] [CrossRef]
- McNulty, P.A.; Macefield, V.G.; Taylor, J.L.; Hallett, M. Cortically Evoked Neural Volleys to the Human Hand Are Increased during Ischaemic Block of the Forearm. J. Physiol. 2002, 538, 279–288. [Google Scholar] [CrossRef]
- Estebe, J.P. The Pneumatic Tourniquet. In Surgical Techniques in Orthopaedics and Traumatology; Duparc, J., Ed.; Elsevier: Paris, France, 2001; ISBN 2-84299-168-0. [Google Scholar]
- Darmanis, S.; Papanikolaou, A.; Pavlakis, D. Fatal Intra-Operative Pulmonary Embolism Following Application of an Esmarch Bandage. Injury 2002, 33, 761–764. [Google Scholar] [CrossRef]
- Bharti, N.; Mahajan, S. Massive Pulmonary Embolism Leading to Cardiac Arrest after Tourniquet Deflation Following Lower Limb Surgery. Anaesth. Intensive Care 2009, 37, 867–868. [Google Scholar]
- Giachino, A.A.; Rody, K.; Turek, M.A.; Miller, D.R.; Wherrett, C.; Moreau, G.; O’Rourke, K.; Grabowski, J.; McLeish, W.; Fazekas, A. Systemic Fat and Thrombus Embolization in Patients Undergoing Total Knee Arthroplasty with Regional Heparinization. J. Arthroplast. 2001, 16, 288–292. [Google Scholar] [CrossRef]
- Östman, B.; Michaelsson, K.; Rahme, H.; Hillered, L. Tourniquet-Induced Ischemia and Reperfusion in Human Skeletal Muscle. Clin. Orthop. Relat. Res. 2004, 418, 260–265. [Google Scholar] [CrossRef]
- Clarke, M.T.; Longstaff, L.; Edwards, D.; Rushton, N. Tourniquet-Induced Wound Hypoxia after Total Knee Replacement. J. Bone Jt. Surg.-Ser. B 2001, 83, 40–44. [Google Scholar] [CrossRef][Green Version]
- Maradit Kremers, H.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Jt. Surg. 2015, 97, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, H.C. Tourniquet Use During Knee Replacement Surgery May Contribute to Muscle Atrophy in Older Adults. Exerc. Sport Sci. Rev. 2016, 44, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Turrentine, F.E.; Wang, H.; Simpson, V.B.; Jones, R.S. Surgical Risk Factors, Morbidity, and Mortality in Elderly Patients. J. Am. Coll. Surg. 2006, 203, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, C.A.; Marcantonio, E.; Goldman, L.; Rohde, L.E.P.; Orav, J.; Mangione, C.M.; Lee, T.H. Impact of Age on Perioperative Complications and Length of Stay in Patients Undergoing Noncardiac Surgery. Ann. Intern. Med. 2001, 134, 637–643. [Google Scholar] [CrossRef]
- Gupta, K.; Aggarwal, N.; Rao, M.; Verma, U.C.; Anand, R. Re-Emphasizing the Importance of Tourniquet Time: Severe Myocardial Depression Following Tourniquet Deflation. Acta Anaesthesiol. Scand. 2008, 52, 873. [Google Scholar] [CrossRef]
- Peled, E.; Keren, Y.; Halachmi, S.; Soudry, M.; Zinman, C.; Kats, Y.; Barak, M. Patients Aged 80 and Older Undergoing Orthopedic or Urologic Surgery: A Prospective Study Focusing on Perioperative Morbidity and Mortality. Gerontology 2009, 55, 517–522. [Google Scholar] [CrossRef]
- Gjorgjievski, M.; Ristevski, B. Postoperative Management Considerations of the Elderly Patient Undergoing Orthopaedic Surgery. Injury 2020, 51, S23–S27. [Google Scholar] [CrossRef]
- de Lucia, C.; Piedepalumbo, M.; Wang, L.; Carnevale Neto, F.; Raftery, D.; Gao, E.; Praticò, D.; Promislow, D.E.L.; Koch, W.J. Effects of Myocardial Ischemia/Reperfusion Injury on Plasma Metabolomic Profile during Aging. Aging Cell 2021, 20, e13284. [Google Scholar] [CrossRef]
- Hide, D.; Warren, A.; Fernández-Iglesias, A.; Maeso-Díaz, R.; Peralta, C.; Le Couteur, D.G.; Bosch, J.; Cogger, V.C.; Gracia-Sancho, J. Ischemia/Reperfusion Injury in the Aged Liver: The Importance of the Sinusoidal Endothelium in Developing Therapeutic Strategies for the Elderly. J. Gerontol. Ser. A 2020, 75, 268–277. [Google Scholar] [CrossRef]
- Lucock, M. Folic Acid: Nutritional Biochemistry, Molecular Biology, and Role in Disease Processes. Mol. Genet. Metab. 2000, 71, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.L.; Reid, J.v.; Newberne, P.M.; Burgess, B.; Marston, R.; Hift, W. Depressed Cell-Mediated Immunity in Megaloblastic Anemia Due to Folic Acid Deficiency. Am. J. Clin. Nutr. 1975, 28, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Cousens, S.; Modell, B.; Lawn, J. Folic Acid to Reduce Neonatal Mortality from Neural Tube Disorders. Int. J. Epidemiol. 2010, 39, i110–i121. [Google Scholar] [CrossRef]
- Altmäe, S.; Stavreus-Evers, A.; Ruiz, J.R.; Laanpere, M.; Syvänen, T.; Yngve, A.; Salumets, A.; Nilsson, T.K. Variations in Folate Pathway Genes Are Associated with Unexplained Female Infertility. Fertil. Steril. 2010, 94, 130–137. [Google Scholar] [CrossRef]
- Ebisch, I.M.W.; Thomas, C.M.G.; Peters, W.H.M.; Braat, D.D.M.; Steegers-Theunissen, R.P.M. The Importance of Folate, Zinc and Antioxidants in the Pathogenesis and Prevention of Subfertility. Hum. Reprod. Update 2007, 13, 163–174. [Google Scholar] [CrossRef]
- Coppen, A.; Bolander-Gouaille, C. Treatment of Depression: Time to Consider Folic Acid and Vitamin B12. J. Psychopharmacol. 2005, 19, 59–65. [Google Scholar] [CrossRef]
- Christen, W.G.; Glynn, R.J.; Chew, E.Y.; Albert, C.M.; Manson, J.E. Folic Acid, Pyridoxine, and Cyanocobalamin Combination Treatment and Age-Related Macular Degeneration in Women: The Women’s Antioxidant and Folic Acid Cardiovascular Study. Arch. Intern. Med. 2009, 169, 335–341. [Google Scholar] [CrossRef]
- Stroes, E.S.G.; van Faassen, E.E.; Yo, M.; Martasek, P.; Boer, P.; Govers, R.; Rabelink, T.J. Folic Acid Reverts Dysfunction of Endothelial Nitric Oxide Synthase. Circ. Res. 2000, 86, 1129–1134. [Google Scholar] [CrossRef]
- Guo, X.; Cui, H.; Zhang, H.; Guan, X.; Zhang, Z.; Jia, C.; Wu, J.; Yang, H.; Qiu, W.; Zhang, C.; et al. Protective Effect of Folic Acid on Oxidative DNA Damage: A Randomized, Double-Blind, and Placebo Controlled Clinical Trial. Medicine 2015, 94, e1872. [Google Scholar] [CrossRef]
- Portugal, V.; García-Alonso, I.; Méndez, J. Hepatotrophic Effect of Folinic Acid in Rats. J. Surg. Res. 1996, 61, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Moat, S.J.; Clarke, Z.L.; Madhavan, A.K.; Lewis, M.J.; Lang, D. Folic Acid Reverses Endothelial Dysfunction Induced by Inhibition of Tetrahydrobiopterin Biosynthesis. Eur. J. Pharmacol. 2006, 530, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Shirodaria, C.; Antoniades, C.; Lee, J.; Jackson, C.E.; Robson, M.D.; Francis, J.M.; Moat, S.J.; Ratnatunga, C.; Pillai, R.; Refsum, H.; et al. Global Improvement of Vascular Function and Redox State with Low-Dose Folic Acid: Implications for Folate Therapy in Patients with Coronary Artery Disease. Circulation 2007, 115, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Adhikari, S.; Patro, B.S.; Chattopadhyay, S.; Mukherjee, T. Free Radical Scavenging Behavior of Folic Acid: Evidence for Possible Antioxidant Activity. Free Radic. Biol. Med. 2001, 30, 1390–1399. [Google Scholar] [CrossRef]
- San Cristóbal Epalza, J. Modulación Farmacológica Del Síndrome de Isquemia-Reperfusión Retiniana En La Rata. Available online: https://addi.ehu.es/bitstream/handle/10810/23865/TESIS_SANCRISTOBAL_EPALZA_JUAN.pdf?sequence=1&isAllowed=y (accessed on 3 May 2021).
- Portugal, V.; Garcia-Alonso, I.; Bilbao, J.E.; Barceló, P.; Méndez, J. Effect of Antioxidative Therapy on Regeneration of Ischemic Liver. Rev. Española Enferm. Dig. 1993, 83, 434–438. [Google Scholar]
- Ulibarrena, M.Á.; García-Alonso Montoya, I.; Portugal Porras, V.; García Redondo, B.; Méndez Martín, J.J. Hemodynamic Monitoring in a Model of Shock Induced by Intestinal Reperfusion in the Rat. Rev. Española Enferm. Dig. 1998, 90, 100–104. [Google Scholar]
- Bilbao, J.E.; Garcia-Alonso, I.; Portugal, V.; Barceló, P.; Ortiz Lacorzana, J.; Méndez, J. Therapeutic Usefulness of Antioxidant Drugs in Experimental Intestinal Reperfusion Syndrome. Rev. Española Enferm. Dig. 1991, 80, 237–241. [Google Scholar]
- Cearra, I.; Herrero de la Parte, B.; Moreno-Franco, D.I.; García-Alonso, I. A Reproducible Method for Biochemical, Histological and Functional Assessment of the Effects of Ischaemia–Reperfusion Syndrome in the Lower Limbs. Sci. Rep. 2021, 11, 19325. [Google Scholar] [CrossRef]
- Cearra, I.; Herrero de la Parte, B.; Ruiz Montesinos, I.; Alonso-Varona, A.; Moreno-Franco, D.I.; García-Alonso, I. Effects of Folinic Acid Administration on Lower Limb Ischemia/Reperfusion Injury in Rats. Antioxidants 2021, 10, 1887. [Google Scholar] [CrossRef]
- European Union. Ageing Europe: Looking at the Lives of Older People in the EU; Corselli-Nordblad, L., Strandell, H., Eds.; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-76-21520-2. [Google Scholar]
- Zueras, P.; Rentería, E. Trends in Disease-Free Life Expectancy at Age 65 in Spain: Diverging Patterns by Sex, Region and Disease. PLoS ONE 2020, 15, e0240923. [Google Scholar] [CrossRef]
- Deandrea, S.; Lucenteforte, E.; Bravi, F.; Foschi, R.; La Vecchia, C.; Negri, E. Risk Factors for Falls in Community-Dwelling Older People: A Systematic Review and Meta-Analysis. Epidemiology 2010, 21, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Leveille, S.G.; Jones, R.N.; Kiely, D.K.; Hausdorff, J.M.; Shmerling, R.H.; Guralnik, J.M.; Kiel, D.P.; Lipsitz, L.A.; Bean, J.F. Chronic Musculoskeletal Pain and the Occurrence of Falls in an Older Population. JAMA 2009, 302, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.K.; Voaklander, D.; Perry, D.; Jones, C.A. Falls and Fear of Falling in Older Adults with Total Joint Arthroplasty: A Scoping Review. BMC Musculoskelet. Disord. 2019, 20, 599. [Google Scholar] [CrossRef]
- Smith, T.O.; Hing, C.B. Is a Tourniquet Beneficial in Total Knee Replacement Surgery? A Meta-Analysis and Systematic Review. Knee 2010, 17, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.O.; Hing, C.B. The Efficacy of the Tourniquet in Foot and Ankle Surgery? A Systematic Review and Meta-Analysis. Foot Ankle Surg. 2010, 16, 3–8. [Google Scholar] [CrossRef]
- Greene, E.C. Anatomy of the Rat. Trans. Am. Philos. Soc. 1935, 27, 1–370. [Google Scholar] [CrossRef]
- Park, Y.; Hirose, R.; Coatney, J.L.; Ferrell, L.; Behrends, M.; Roberts, J.P.; Serkova, N.J.; Niemann, C.U. Ischemia-Reperfusion Injury Is More Severe in Older Versus Young Rat Livers. J. Surg. Res. 2007, 137, 96–102. [Google Scholar] [CrossRef]
- Trocha, M.; Merwid-Ląd, A.; Pieśniewska, M.; Kwiatkowska, J.; Fereniec-Gołębiewska, L.; Kowalski, P.; Szeląg, A.; Sozański, T. Age-Related Differences in Function and Structure of Rat Livers Subjected to Ischemia/Reperfusion. Arch. Med. Sci. 2018, 14, 388–395. [Google Scholar] [CrossRef]
- Huang, X.; Gao, Y.; Qin, J.; Lu, S. The Role of MiR-34a in the Hepatoprotective Effect of Hydrogen Sulfide on Ischemia/Reperfusion Injury in Young and Old Rats. PLoS ONE 2014, 9, e113305. [Google Scholar] [CrossRef]
- Walter, G.L.; Smith, G.S.; Walker, R.M. Interpretation of Clinical Pathology Results in Non-Clinical Toxicology Testing. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Haschek-Hock, W.M., Rousseaux, C.G., Wallig, M.A., Eds.; Academic Press: London, UK, 2013; pp. 853–892. ISBN 978-0-12-415759-0. [Google Scholar]
- Naregal, G.V.; Devaranavadagi, B.B.; Patil, S.G.; Aski, B.S. Elevation of Oxidative Stress and Decline in Endogenous Antioxidant Defense in Elderly Individuals with Hypertension. J. Clin. Diagn. Res. 2017, 11, BC09–BC12. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Pevzner, I.B.; Andrianova, N.v.; Zorova, L.D.; Popkov, V.A.; Silachev, D.N.; Kolosova, N.G.; Plotnikov, E.Y.; Zorov, D.B. The Age-Associated Loss of Ischemic Preconditioning in the Kidney Is Accompanied by Mitochondrial Dysfunction, Increased Protein Acetylation and Decreased Autophagy. Sci. Rep. 2017, 7, 44430. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.C.; Kwon, E.; Eberlin, K.R.; Randolph, M.; Friend, D.S.; Thomas, A.C.; Watkins, M.T.; Austen, W.G. Development of Reproducible Histologic Injury Severity Scores: Skeletal Muscle Reperfusion Injury. Surgery 2008, 143, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Deune, E.G.; Koopman, R.; Smith, M.E.; Hong, S.P.; Ozbek, M.R.; Khouri, R.K. Prevention of Ischemia-Reperfusion Injury with a Synthetic Metalloprotein Superoxide Dismutase Mimic, SC52608. Plast. Reconstr. Surg. 1996, 98, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; He, Z.; Jiang, X.; Hou, M.; Tang, Z.; Zhen, X.; Liang, Y.; Ma, J. Folic Acid Represses Hypoxia-Induced Inflammation in THP-1 Cells through Inhibition of the PI3K/Akt/HIF-1α Pathway. PLoS ONE 2016, 11, e0151553. [Google Scholar] [CrossRef] [PubMed]
- Tommy, T.; Islam, A.A.; Hatta, M.; Bukhari, A.; Nasrullah; Adhimarta, W.; Aminuddin; Zainuddin, A.A. Effect of Folinic Acid on Serum Homocysteine, TNFα, IL-10, and HMGB1 Gene Expression in Head Injury Model. Ann. Med. Surg. 2021, 65, 102273. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhou, Y.; Xia, M.; Ma, J. Folic Acid Inhibits Lipopolysaccharide-Induced Inflammatory Response in RAW264.7 Macrophages by Suppressing MAPKs and NF-ΚB Activation. Inflamm. Res. 2011, 60, 817–822. [Google Scholar] [CrossRef]
- Bonheur, J.A.; Albadawi, H.; Patton, G.M.; Watkins, M.T. A Noninvasive Murine Model of Hind Limb Ischemia-Reperfusion Injury. J. Surg. Res. 2004, 116, 55–63. [Google Scholar] [CrossRef]
- Dillon, J.P.; Laing, A.J.; Chandler, J.R.S.; Wang, J.H.; McGuinness, A.; Redmond, H.P. Pravastatin Attenuates Tourniquet-Induced Skeletal Muscle Ischemia Reperfusion Injury. Acta Orthop. 2006, 77, 27–32. [Google Scholar] [CrossRef]
- Gersoff, W.K.; Ruwe, P.; Jokl, P.; Panjabi, M. The Effect of Tourniquet Pressure on Muscle Function. Am. J. Sports Med. 1989, 17, 123–127. [Google Scholar] [CrossRef]
- Crawford, R.S.; Hashmi, F.F.; Jones, J.E.; Albadawi, H.; McCormack, M.; Eberlin, K.; Entabi, F.; Atkins, M.D.; Conrad, M.F.; Austen, W.G.; et al. A Novel Model of Acute Murine Hindlimb Ischemia. Am. J. Physiol.-Heart Circ. Physiol. 2007, 292, H830–H837. [Google Scholar] [CrossRef]
- Orban, J.-C.; Levraut, J.; Gindre, S.; Deroche, D.; Schlatterer, B.; Ichai, C.; Grimaud, D. Effects of Acetylcysteine and Ischaemic Preconditioning on Muscular Function and Postoperative Pain after Orthopaedic Surgery Using a Pneumatic Tourniquet. Eur. J. Anaesthesiol. 2006, 23, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Kamat, P.K.; Ahmad, A.S.; Doré, S. Distinctive Effect of Anesthetics on the Effect of Limb Remote Ischemic Postconditioning Following Ischemic Stroke. PLoS ONE 2020, 15, e0227624. [Google Scholar] [CrossRef] [PubMed]


| Group | Control | Vehicle 3 h | FA 3 h | Vehicle 14 Days | FA 14 Days |
|---|---|---|---|---|---|
| Creatinine (mg/dL) | 0.36 ± 0.06 | 1.4 ± 0.22 **** | 1.1 ± 0.29 **** | 0.65 ± 0.15 * | 0.32 ± 0.02 ns |
| Urea (mg/dL) | 28.1 ± 3.8 | 63.4 ± 5.1 *** | 62.2 ± 8.6 *** | 50.2 ± 8.5 *** | 25 ± 2.6 ns |
| AST (IU/L) | 62.6 ± 8.6 | 372.8 ± 48.3 *** | 324 ± 28.6 *** | 210 ± 61.1 *** | 83.2 ± 16.3 ns |
| ALT (IU/L) | 41.4 ± 5.9 | 10.8 ± 8.1 **** | 22.3 ± 16.4 ** | 28 ± 10.9 ns | 32.4 ± 4.4 ns |
| CK (IU/L) | 101.5 ± 10.7 | 8512 ± 3199 **** | 5777 ± 2255 **** | 1476 ± 415.7 *** | 371.8 ± 275.9 ns |
| LDH (IU/L) | 66 ± 26.6 | 1878 ± 388.8 **** | 1336 ± 231.4 **** | 922 ± 314.1 **** | 351 ± 213 ns |
| Na+ (mEq/L) | 146.7 ± 0.9 | 138.1 ± 3.3 *** | 137.3 ± 2.3 *** | 139.8 ± 1.9 *** | 141.6 ± 1.1 *** |
| K+ (mEq/L) | 3.99 ± 0.45 | 4.4 ± 0.57 ns | 4.3 ± 0.39 ns | 4.38 ± 0.43 ns | 4.24 ± 0.19 ns |
| Cl− (mEq/L) | 103.7 ± 1.5 | 88.6 ± 1.9 *** | 90.7 ± 2.8 *** | 94.7 ± 1.6 *** | 101.4 ± 1.1 ns |
| Day | Vehicle (s) | FA (s) | p Value |
|---|---|---|---|
| −1 | 158.5 ± 5.32 | 159.6 ± 8.26 | ns |
| 1 | 12.67 ± 4.04 | 21.4 ± 3.44 | ns |
| 2 | 12.17 ± 4.95 | 21 ± 3.08 | ns |
| 3 | 19.5 ± 5.28 | 33 ± 2.73 | p < 0.01 |
| 4 | 25.17 ± 5.30 | 47 ± 7.03 | p < 0.0001 |
| 5 | 46.83 ± 5.84 | 57 ± 6.36 | p < 0.05 |
| 6 | 69.17 ± 4.96 | 82.4 ± 10.69 | p < 0.01 |
| 7 | 84.83 ± 10.52 | 100.2 ± 10.52 | p < 0.01 |
| 10 | 113 ± 15.59 | 123 ± 4.85 | p < 0.05 |
| 14 | 119.17 ± 9.75 | 157.4 ± 11.24 | p < 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero de la Parte, B.; Roa-Esparza, J.; Cearra, I.; Ruiz Montesinos, I.; Alonso-Alconada, D.; Alonso-Varona, A.; Mar Medina, C.; Iturrizaga Correcher, S.; García-Alonso, I. The Prevention of Ischemia-Reperfusion Injury in Elderly Rats after Lower Limb Tourniquet Use. Antioxidants 2022, 11, 1936. https://doi.org/10.3390/antiox11101936
Herrero de la Parte B, Roa-Esparza J, Cearra I, Ruiz Montesinos I, Alonso-Alconada D, Alonso-Varona A, Mar Medina C, Iturrizaga Correcher S, García-Alonso I. The Prevention of Ischemia-Reperfusion Injury in Elderly Rats after Lower Limb Tourniquet Use. Antioxidants. 2022; 11(10):1936. https://doi.org/10.3390/antiox11101936
Chicago/Turabian StyleHerrero de la Parte, Borja, Javier Roa-Esparza, Iñigo Cearra, Inmaculada Ruiz Montesinos, Daniel Alonso-Alconada, Ana Alonso-Varona, Carmen Mar Medina, Sira Iturrizaga Correcher, and Ignacio García-Alonso. 2022. "The Prevention of Ischemia-Reperfusion Injury in Elderly Rats after Lower Limb Tourniquet Use" Antioxidants 11, no. 10: 1936. https://doi.org/10.3390/antiox11101936
APA StyleHerrero de la Parte, B., Roa-Esparza, J., Cearra, I., Ruiz Montesinos, I., Alonso-Alconada, D., Alonso-Varona, A., Mar Medina, C., Iturrizaga Correcher, S., & García-Alonso, I. (2022). The Prevention of Ischemia-Reperfusion Injury in Elderly Rats after Lower Limb Tourniquet Use. Antioxidants, 11(10), 1936. https://doi.org/10.3390/antiox11101936









