Antioxidant Molecular Brain Changes Parallel Adaptive Cardiovascular Response to Forced Running in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Cardiovascular Function
2.3. Behavioral Testing
2.4. Brain Tissue Analysis
2.5. Statistics
3. Results
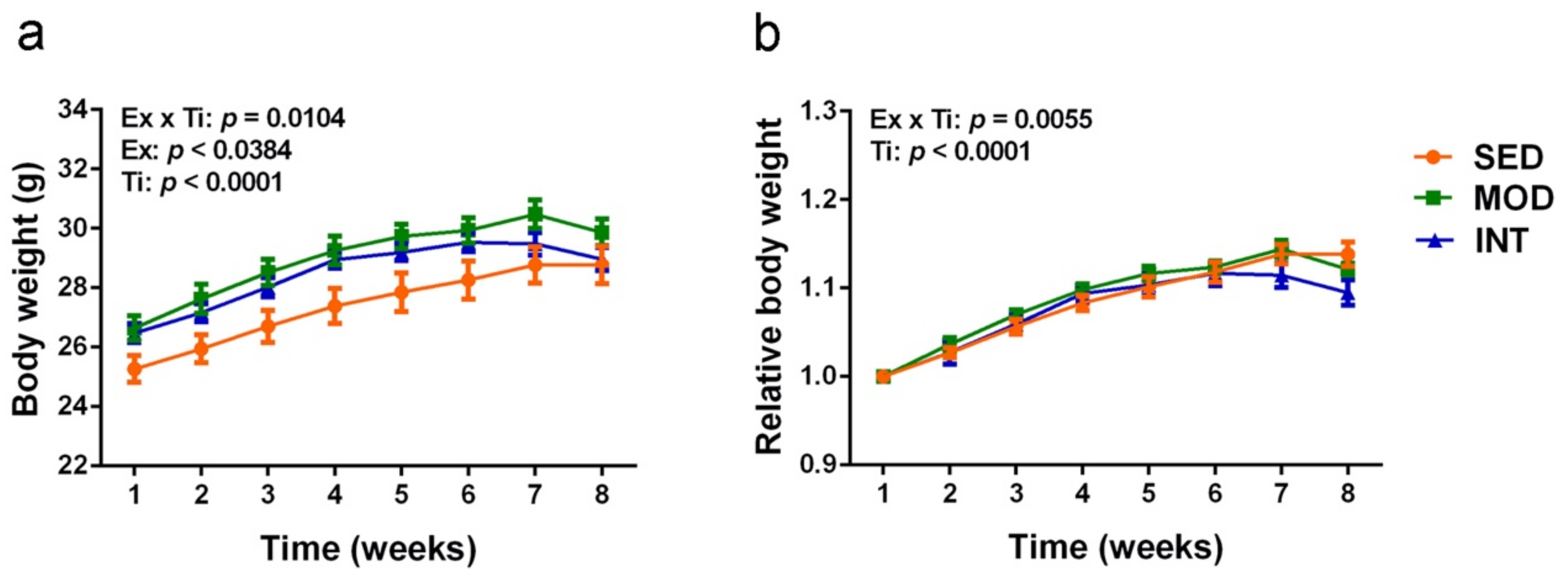
3.1. Mice Exercise Training and Body Weight Evolution
3.2. Electrocardiography Changes Induced by Exercise
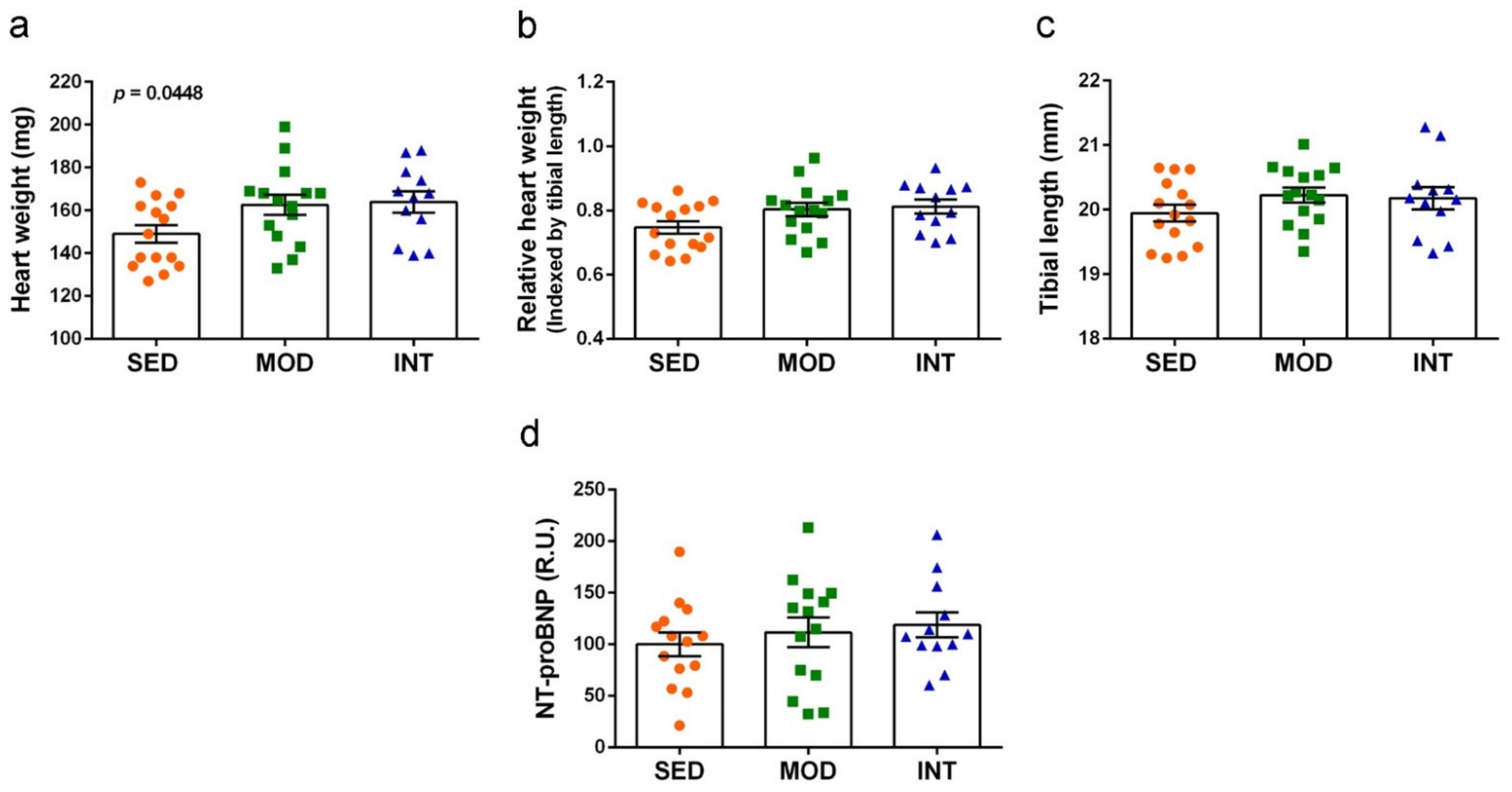
3.3. Heart Hypertrophy Induced by Exercise
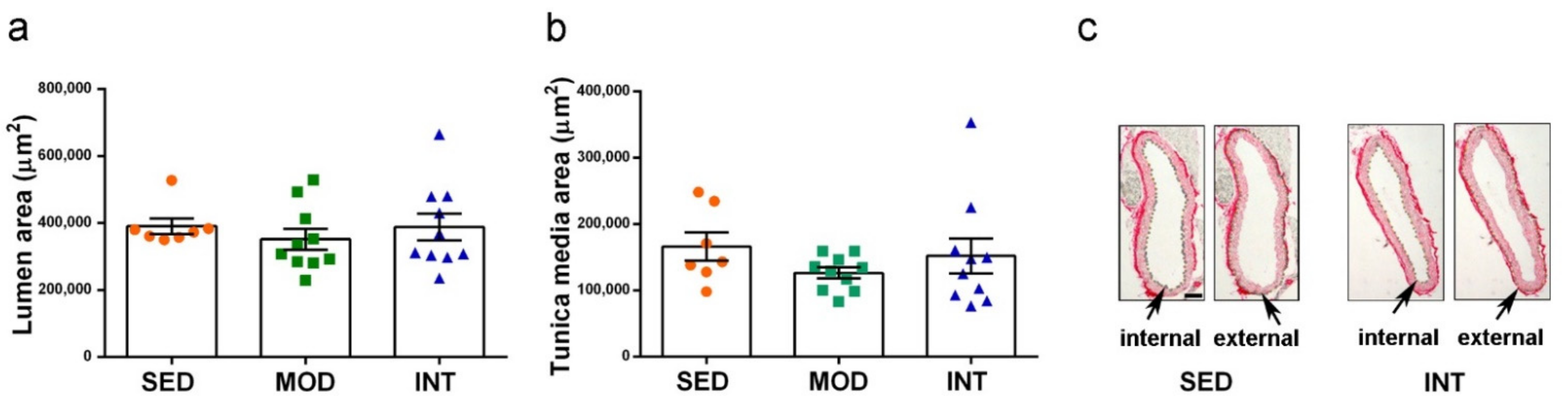
3.4. Absence of Gross Aortic Changes
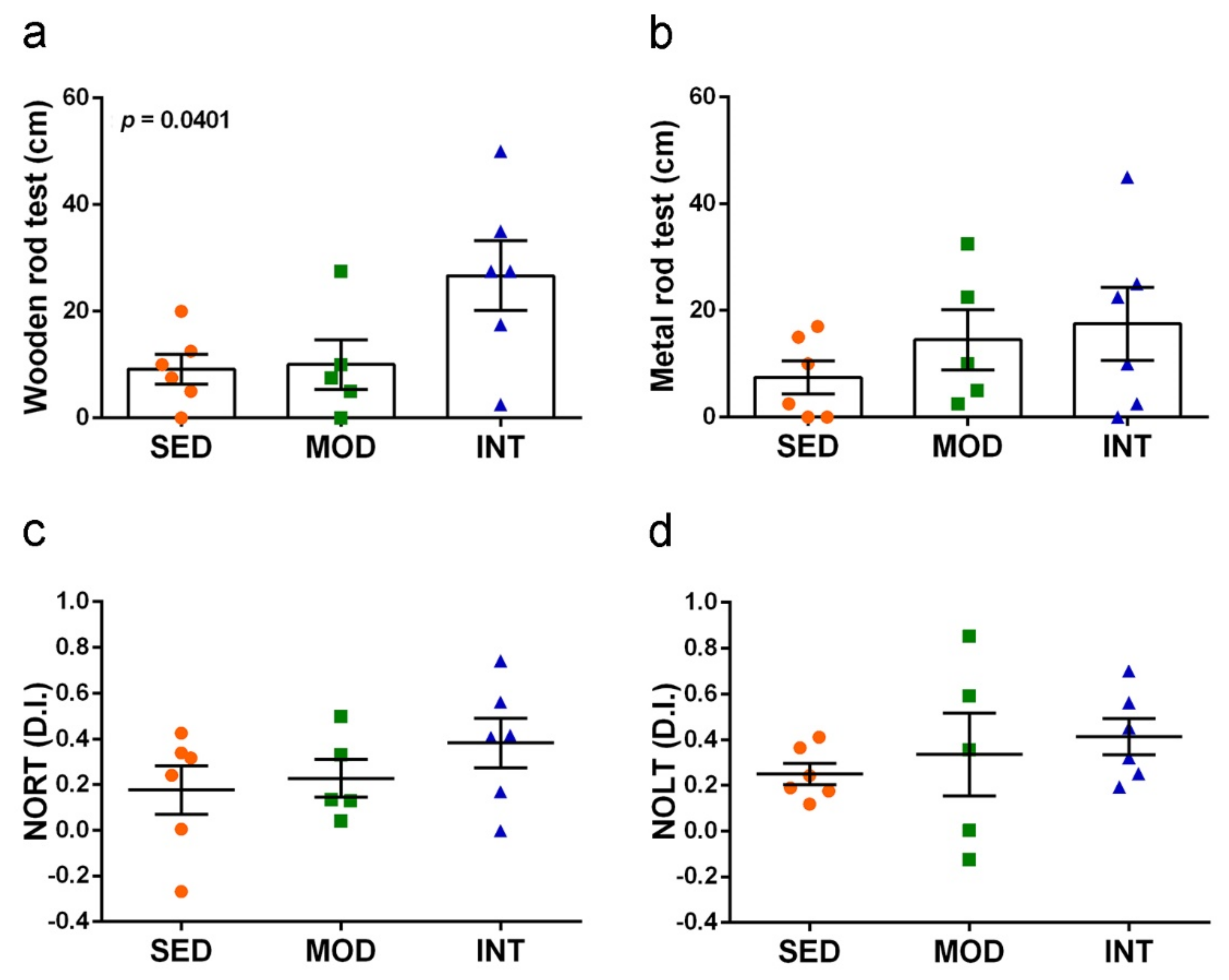
3.5. Exercise Improved Motor Coordination and Did Not Interfere with Cognitive Abilities
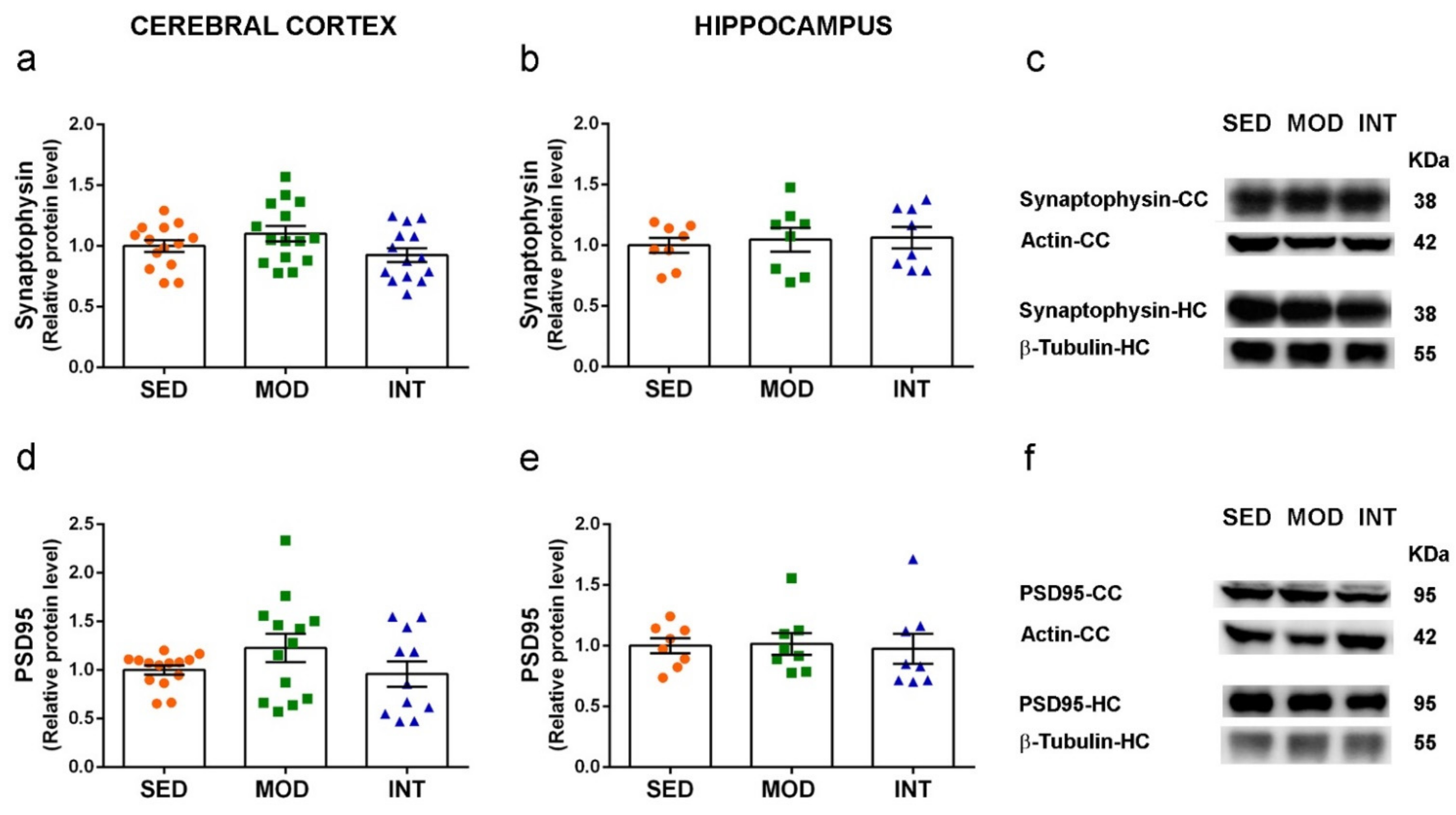
3.6. Absence of Changes in Markers of Neuronal Function
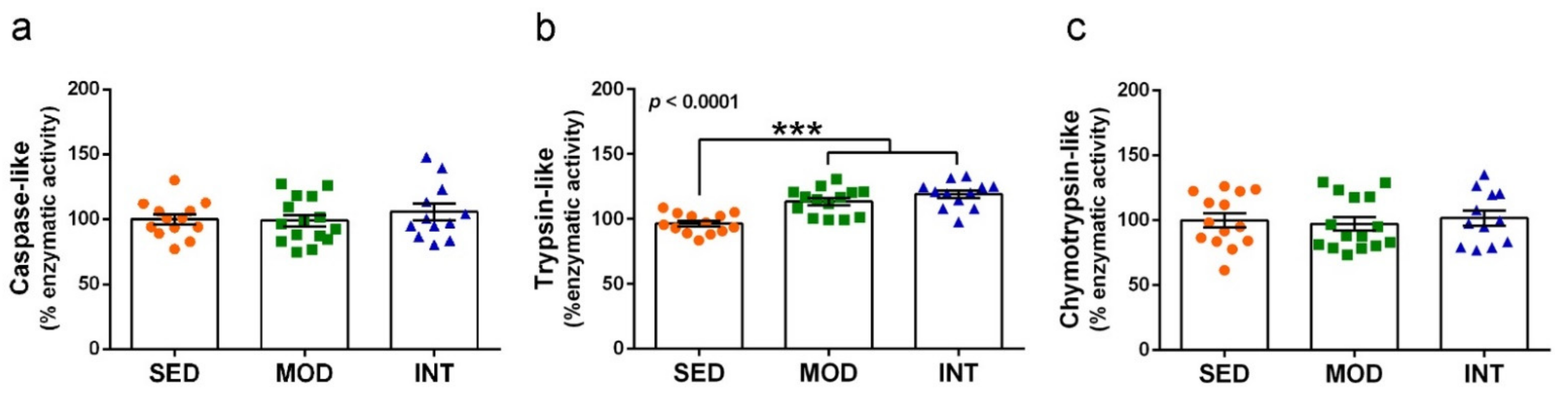
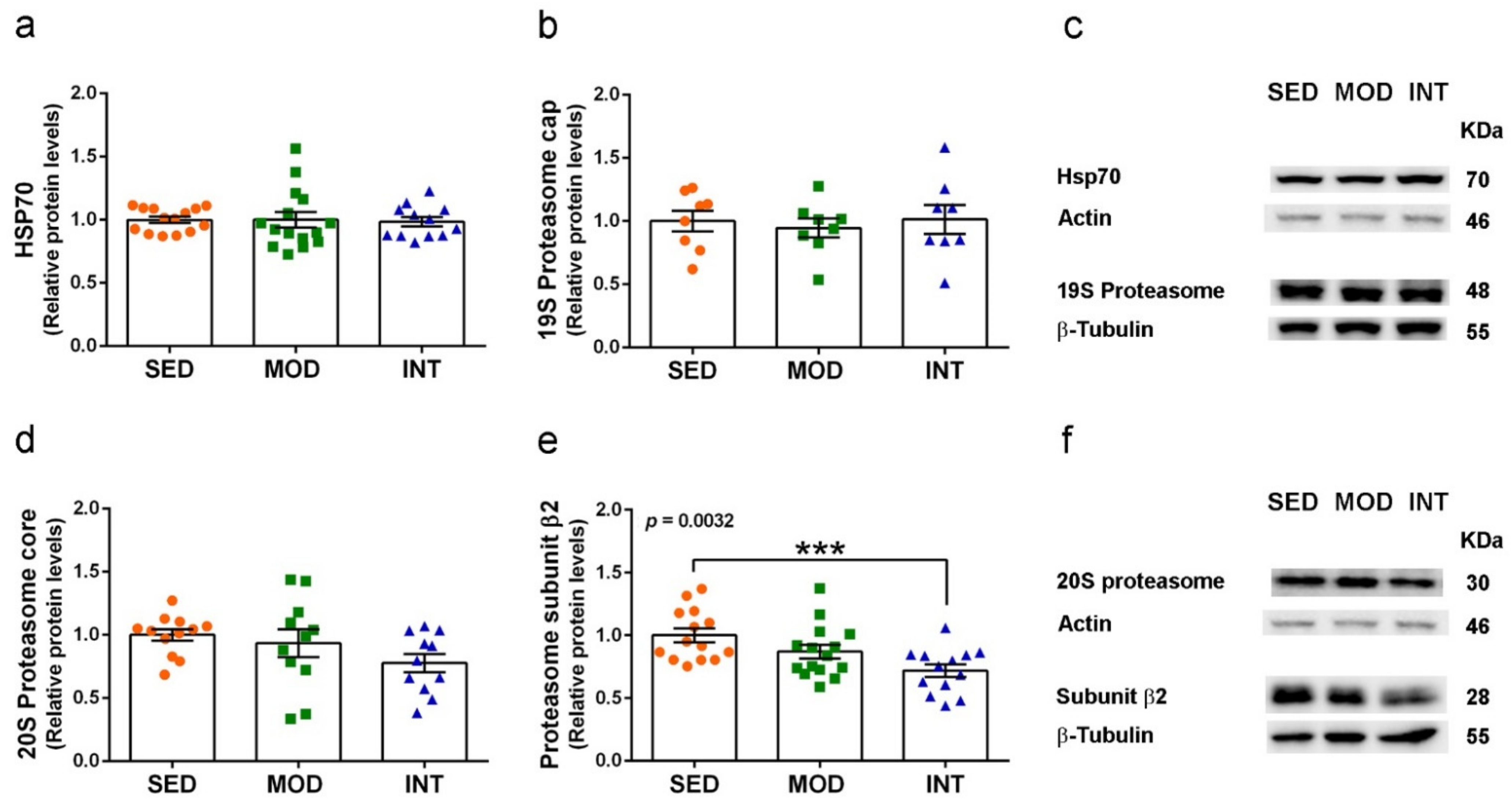
3.7. Proteasome Activation by Exercise
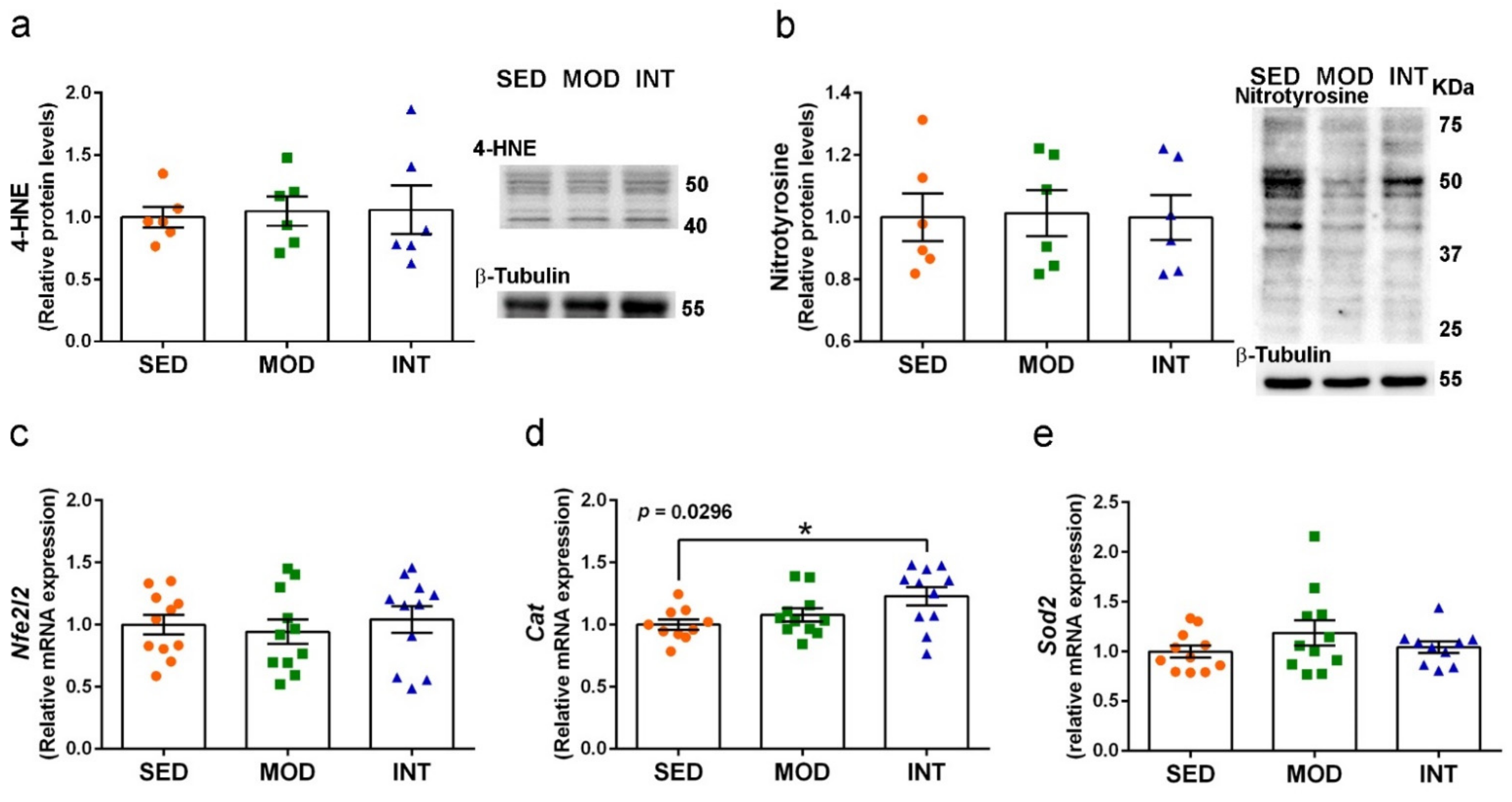
3.8. Maintenance of Redox Homeostasis under Exercise Training
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.S.; Saliasi, E.; van den Berg, V.; Uijtdewilligen, L.; de Groot, R.H.M.; Jolles, J.; Andersen, L.B.; Bailey, R.; Chang, Y.K.; Diamond, A.; et al. Effects of physical activity interventions on cognitive and academic performance in children and adolescents: A novel combination of a systematic review and recommendations from an expert panel. Br. J. Sports Med. 2019, 53, 640–647. [Google Scholar] [CrossRef]
- Doré, I.; Sabiston, C.M.; Sylvestre, M.P.; Brunet, J.; O’Loughlin, J.; Nader, P.A.; Gallant, F.; Bélanger, M. Years Participating in Sports During Childhood Predicts Mental Health in Adolescence: A 5-Year Longitudinal Study. J. Adolesc. Health 2019, 64, 790–796. [Google Scholar] [CrossRef]
- Buchman, A.S.; Yu, L.; Wilson, R.S.; Lim, A.; Dawe, R.J.; Gaiteri, C.; Leurgans, S.E.; Schneider, J.A.; Bennett, D.A. Physical activity, common brain pathologies, and cognition in community-dwelling older adults. Neurology 2019, 92, e811–e822. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Donofry, S.D.; Sewell, K.R.; Brown, B.M.; Stillman, C.M. Cognitive Aging and the Promise of Physical Activity. Annu. Rev. Clin. Psychol. 2022, 8, 417–442. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cao, C.; Kantor, E.D.; Nguyen, L.H.; Zheng, X.; Park, Y.; Giovannucci, E.L.; Matthews, C.E.; Colditz, G.A.; Cao, Y. Trends in Sedentary Behavior Among the US Population, 2001–2016. JAMA 2019, 321, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- López-Valenciano, A.; Mayo, X.; Liguori, G.; Copeland, R.J.; Lamb, M.; Jimenez, A. Changes in sedentary behaviour in European Union adults between 2002 and 2017. BMC Public Health 2020, 20, 1206. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Revilla, S.; Suñol, C.; García-Mesa, Y.; Giménez-Llort, L.; Sanfeliu, C.; Cristòfol, R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology 2014, 81, 55–63. [Google Scholar] [CrossRef]
- García-Mesa, Y.; Colie, S.; Corpas, R.; Cristòfol, R.; Comellas, F.; Nebreda, A.R.; Giménez-Llort, L.; Sanfeliu, C. Oxidative Stress Is a Central Target for Physical Exercise Neuroprotection Against Pathological Brain Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 40–49. [Google Scholar] [CrossRef]
- Melo, K.P.; Silva, C.M.; Almeida, M.F.; Chaves, R.S.; Marcourakis, T.; Cardoso, S.M.; Demasi, M.; Netto, L.E.S.; Ferrari, M.F.R. Mild Exercise Differently Affects Proteostasis and Oxidative Stress on Motor Areas During Neurodegeneration: A Comparative Study of Three Treadmill Running Protocols. Neurotox. Res. 2019, 35, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, H.; Zeng, Y.; Qu, Y.; Liu, Q.; Zhao, F.; Duan, J.; Jiang, Y.; Li, S.; Ying, J.; et al. Physical exercise promotes brain remodeling by regulating epigenetics, neuroplasticity and neurotrophins. Rev. Neurosci. 2021, 32, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Felisatti, F.; Gonneaud, J.; Palix, C.; Garnier-Crussard, A.; Mézenge, F.; Landeau, B.; Chocat, A.; Quillard, A.; Ferrand-Devouge, E.; de La Sayette, V.; et al. Role of Cardiovascular Risk Factors on the Association Between Physical Activity and Brain Integrity Markers in Older Adults. Neurology 2022, 98, e2023–e2035. [Google Scholar] [CrossRef]
- Reigal, R.E.; Hernández-Mendo, A.; Juárez-Ruiz de Mier, R.; Morales-Sánchez, V. Physical Exercise and Fitness Level Are Related to Cognitive and Psychosocial Functioning in Adolescents. Front. Psychol. 2020, 11, 777. [Google Scholar] [CrossRef]
- Buchman, A.S.; Boyle, P.A.; Yu, L.; Shah, R.C.; Wilson, R.S.; Bennett, D.A. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 2012, 78, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef]
- Carlson, S.A.; Adams, E.K.; Yang, Z.; Fulton, J.E. Percentage of Deaths Associated With Inadequate Physical Activity in the United States. Prev. Chronic Dis. 2018, 15, E38. [Google Scholar] [CrossRef]
- Guasch, E.; Mont, L. Diagnosis, pathophysiology, and management of exercise-induced arrhythmias. Rev. Cardiol. 2017, 14, 88–101. [Google Scholar] [CrossRef]
- La Gerche, A.; Claessen, G.; Dymarkowski, S.; Voigt, J.U.; De Buck, F.; Vanhees, L.; Droogne, W.; Van Cleemput, J.; Claus, P.; Heidbuchel, H. Exercise-induced right ventricular dysfunction is associated with ventricular arrhythmias in endurance athletes. Eur. Heart J. 2015, 36, 1998–2010. [Google Scholar] [CrossRef]
- Goodman, J.M.; Thomas, S.G.; Burr, J. Evidence-based risk assessment and recommendations for exercise testing and physical activity clearance in apparently healthy individuals. Appl. Physiol. Nutr. Metab. 2011, 36 (Suppl. S1), S14–S32. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.C.; Moineddin, R.; Morra, A.; Manson, J.; Blake, J. Intensity of recreational physical activity throughout life and later life cognitive functioning in women. J. Alzheimers Dis. 2010, 22, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Gradari, S.; Pallé, A.; McGreevy, K.R.; Fontán-Lozano, Á.; Trejo, J.L. Can Exercise Make You Smarter, Happier, and Have More Neurons? A Hormetic Perspective. Front. Neurosci. 2016, 10, 93. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Redox basis of exercise physiology. Redox Biol. 2020, 35, 101499. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Domenech, E.; Viña, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Koltai, E.; Taylor, A.W.; Goto, S. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 2008, 7, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Morales-González, Á.; Madrigal-Santillán, E.O.; Madrigal-Bujaidar, E.; Álvarez-González, I.; García-Melo, L.F.; Anguiano-Robledo, L.; Fregoso-Aguilar, T.; Morales-Gonzalez, J.A. Antioxidant and Adaptative Response Mediated by Nrf2 during Physical Exercise. Antioxidants 2019, 8, 196. [Google Scholar] [CrossRef]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef]
- Sutkowy, P.; Woźniak, A.; Mila-Kierzenkowska, C.; Szewczyk-Golec, K.; Wesołowski, R.; Pawłowska, M.; Nuszkiewicz, J. Physical Activity vs. Redox Balance in the Brain: Brain Health, Aging and Diseases. Antioxidants 2021, 11, 95. [Google Scholar] [CrossRef]
- Muthusamy, V.R.; Kannan, S.; Sadhaasivam, K.; Gounder, S.S.; Davidson, C.J.; Boeheme, C.; Hoidal, J.R.; Wang, L.; Rajasekaran, N.S. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic. Biol. Med. 2012, 52, 366–376. [Google Scholar] [CrossRef]
- Massett, M.P.; Matejka, C.; Kim, H. Systematic Review and Meta-Analysis of Endurance Exercise Training Protocols for Mice. Front. Physiol. 2021, 12, 782695. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Copp, S.W.; Colburn, T.D.; Craig, J.C.; Allen, D.L.; Sturek, M.; O’Leary, D.S.; Zucker, I.H.; Musch, T.I. Guidelines for animal exercise and training protocols for cardiovascular studies. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1100–H1138. [Google Scholar] [CrossRef] [PubMed]
- García-Mesa, Y.; López-Ramos, J.C.; Giménez-Llort, L.; Revilla, S.; Guerra, R.; Gruart, A.; Laferla, F.M.; Cristòfol, R.; Delgado-García, J.M.; Sanfeliu, C. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J. Alzheimer’s Dis. 2011, 24, 421–454. [Google Scholar] [CrossRef]
- García-Lara, E.; Aguirre, S.; Clotet, N.; Sawkulycz, X.; Bartra, C.; Almenara-Fuentes, L.; Suñol, C.; Corpas, R.; Olah, P.; Tripon, F.; et al. Antibody Protection against Long-Term Memory Loss Induced by Monomeric C-Reactive Protein in a Mouse Model of Dementia. Biomedicines 2021, 9, 828. [Google Scholar] [CrossRef] [PubMed]
- Corpas, R.; Griñán-Ferré, C.; Rodríguez-Farré, E.; Pallàs, M.; Sanfeliu, C. Resveratrol Induces Brain Resilience Against Alzheimer Neurodegeneration Through Proteostasis Enhancement. Mol. Neurobiol. 2019, 56, 1502–1516. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, H.J.; Lee, W.J.; Seong, J.K. A comparison of the metabolic effects of treadmill and wheel running exercise in mouse model. Lab. Anim. Res. 2020, 36, 3. [Google Scholar] [CrossRef]
- Röchner, F.; Schmitt, A.; Brändle, A.L.; Fragasso, A.; Munz, B. The ROS scavenger PDTC affects adaptation to treadmill running in mice: Distinct effects on murine body mass, resting heart rate and skeletal muscle fiber type composition. J. Exp. Biol. 2021, 224, jeb234237. [Google Scholar] [CrossRef]
- Al-Jarrah, M.; Pothakos, K.; Novikova, L.; Smirnova, I.V.; Kurz, M.J.; Stehno-Bittel, L.; Lau, Y.S. Endurance exercise promotes cardiorespiratory rehabilitation without neurorestoration in the chronic mouse model of parkinsonism with severe neurodegeneration. Neuroscience 2007, 149, 28–37. [Google Scholar] [CrossRef]
- Zanuto, E.F.; Ritti-Dias, R.M.; Tebar, W.R.; Scarabottolo, C.C.; Delfino, L.D.; Casonatto, J.; Vanderlei, L.C.M.; Christofaro, D.G.D. Is physical activity associated with resting heart rate in boys and girls? A representative study controlled for confounders. J. Pediatr. 2020, 96, 247–254. [Google Scholar] [CrossRef]
- Batty, G.D.; Shipley, M.J.; Kivimaki, M.; Marmot, M.; Davey Smith, G. Walking pace, leisure time physical activity, and resting heart rate in relation to disease-specific mortality in London: 40 years follow-up of the original Whitehall study. An update of our work with professor Jerry N. Morris (1910–2009). Ann. Epidemiol. 2010, 20, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.A.S.; de Lima, T.R.; Tremblay, M.S. Association between Resting Heart Rate and Health-Related Physical Fitness in Brazilian Adolescents. Biomed. Res. Int. 2018, 2018, 3812197. [Google Scholar] [CrossRef] [PubMed]
- Kemi, O.J.; Loennechen, J.P.; Wisløff, U.; Ellingsen, Ø. Intensity-controlled treadmill running in mice: Cardiac and skeletal muscle hypertrophy. J. Appl. Physiol. 2002, 93, 1301–1309. [Google Scholar] [CrossRef]
- Perrino, C.; Gargiulo, G.; Pironti, G.; Franzone, A.; Scudiero, L.; De Laurentis, M.; Magliulo, F.; Ilardi, F.; Carotenuto, G.; Schiattarella, G.G.; et al. Cardiovascular effects of treadmill exercise in physiological and pathological preclinical settings. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1983–H1989. [Google Scholar] [CrossRef]
- Pieles, G.E.; Stuart, A.G. The adolescent athlete’s heart; A miniature adult or grown-up child? Clin. Cardiol. 2020, 43, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Kusy, K.; Błażejewski, J.; Gilewski, W.; Karasek, D.; Banach, J.; Bujak, R.; Zieliński, J.; Sinkiewicz, W.; Grześk, G. Aging Athlete’s Heart: An Echocardiographic Evaluation of Competitive Sprint-versus Endurance-Trained Master Athletes. J. Am. Soc. Echocardiogr. 2021, 34, 1160–1169. [Google Scholar] [CrossRef]
- Basu, J.; Malhotra, A.; Papadakis, M. Exercise and hypertrophic cardiomyopathy: Two incompatible entities? Clin. Cardiol. 2020, 43, 889–896. [Google Scholar] [CrossRef]
- Vittorini, S.; Clerico, A. Cardiovascular biomarkers: Increasing impact of laboratory medicine in cardiology practice. Clin. Chem. Lab. Med. 2008, 46, 748–763. [Google Scholar] [CrossRef]
- Tang, W.H.; Francis, G.S.; Morrow, D.A.; Newby, L.K.; Cannon, C.P.; Jesse, R.L.; Storrow, A.B.; Christenson, R.H.; NACB Committee. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical utilization of cardiac biomarker testing in heart failure. Clin. Biochem. 2008, 41, 210–221. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Omland, T.; Wolf, P.A.; Vasan, R.S. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N. Engl. J. Med. 2004, 350, 655–663. [Google Scholar] [CrossRef]
- Green, D.J.; Spence, A.; Rowley, N.; Thijssen, D.H.; Naylor, L.H. Vascular adaptation in athletes: Is there an ‘athlete’s artery’? Exp. Physiol. 2012, 97, 295–304. [Google Scholar] [CrossRef]
- Cheedipudi, S.M.; Hu, J.; Fan, S.; Yuan, P.; Karmouch, J.; Czernuszewicz, G.; Robertson, M.J.; Coarfa, C.; Hong, K.; Yao, Y.; et al. Exercise restores dysregulated gene expression in a mouse model of arrhythmogenic cardiomyopathy. Cardiovasc. Res. 2020, 116, 1199–1213. [Google Scholar] [CrossRef] [PubMed]
- Mas-Stachurska, A.; Siegert, A.M.; Batlle, M.; Gorbenko Del Blanco, D.; Meirelles, T.; Rubies, C.; Bonorino, F.; Serra-Peinado, C.; Bijnens, B.; Baudin, J.; et al. Cardiovascular Benefits of Moderate Exercise Training in Marfan Syndrome: Insights From an Animal Model. J. Am. Heart Assoc. 2017, 6, e006438. [Google Scholar] [CrossRef] [PubMed]
- Rubies, C.; Batlle, M.; Sanz-de la Garza, M.; Dantas, A.-P.; Jorba, I.; Fernandez, G.; Sangüesa, G.; Abuli, M.; Brugada, J.; Sitges, M.; et al. Long-term strenuous exercise promotes vascular injury by selectively damaging the tunica media: Experimental evidence. Basic Transl. Sci. 2022, 7, 681–693. [Google Scholar] [CrossRef]
- De la Rosa, A.; Solana, E.; Corpas, R.; Bartrés-Faz, D.; Pallàs, M.; Vina, J.; Sanfeliu, C.; Gomez-Cabrera, M.C. Long-term exercise training improves memory in middle-aged men and modulates peripheral levels of BDNF and Cathepsin B. Sci. Rep. 2019, 9, 3337. [Google Scholar] [CrossRef]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. When does age-related cognitive decline begin? Neurobiol. Aging 2009, 30, 507–514. [Google Scholar] [CrossRef]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H.; Strauman, T.A.; Welsh-Bohmer, K.; Browndyke, J.N.; Sherwood, A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom. Med. 2010, 72, 239–252. [Google Scholar] [CrossRef]
- Ferrer-Uris, B.; Ramos, M.A.; Busquets, A.; Angulo-Barroso, R. Can exercise shape your brain? A review of aerobic exercise effects on cognitive function and neuro-physiological underpinning mechanisms. AIMS Neurosci. 2022, 9, 150–174. [Google Scholar] [CrossRef]
- Popovic, L.M.; Mitic, N.R.; Radic, I.; Miric, D.; Kisic, B.; Krdzic, B.; Djokic, T. The effect of exhaustive exercise on oxidative stress generation and antioxidant defense in guinea pigs. Adv. Clin. Exp. Med. 2012, 21, 313–320. [Google Scholar]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Chung, H.Y.; Goto, S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic. Biol. Med. 2008, 44, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Irving, B.A.; Lanza, I.R.; Vendelbo, M.H.; Konopka, A.R.; Robinson, M.M.; Henderson, G.C.; Klaus, K.A.; Morse, D.M.; Heppelmann, C.; et al. Differential Effect of Endurance Training on Mitochondrial Protein Damage, Degradation, and Acetylation in the Context of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gong, W.; Wu, S.; Perrett, S. Hsp70 in Redox Homeostasis. Cells 2022, 11, 829. [Google Scholar] [CrossRef]
- Niu, X.; Zhao, Y.; Yang, N.; Zhao, X.; Zhang, W.; Bai, X.; Li, A.; Yang, W.; Lu, L. Proteasome activation by insulin-like growth factor-1/nuclear factor erythroid 2-related factor 2 signaling promotes exercise-induced neurogenesis. Stem Cells 2020, 38, 246–260. [Google Scholar] [CrossRef]
- Kodydková, J.; Vávrová, L.; Kocík, M.; Žák, A. Human catalase, its polymorphisms, regulation and changes of its activity in different diseases. Folia Biol. 2014, 60, 153–167. [Google Scholar]
- Corpas, R.; Solana, E.; De la Rosa, A.; Sarroca, S.; Griñán-Ferré, C.; Oriol, M.; Corbella, E.; Rodríguez-Farré, E.; Vina, J.; Pallàs, M.; et al. Peripheral Maintenance of the Axis SIRT1-SIRT3 at Youth Level May Contribute to Brain Resilience in Middle-Aged Amateur Rugby Players. Front. Aging Neurosci. 2019, 11, 352. [Google Scholar] [CrossRef]
- Feter, N.; Spanevello, R.M.; Soares, M.S.P.; Spohr, L.; Pedra, N.S.; Bona, N.P.; Freitas, M.P.; Gonzales, N.G.; Ito, L.G.M.S.; Stefanello, F.M.; et al. How does physical activity and different models of exercise training affect oxidative parameters and memory? Physiol. Behav. 2019, 201, 42–52. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef]
- Opoku-Nsiah, K.A.; Gestwicki, J.E. Aim for the core: Suitability of the ubiquitin-independent 20S proteasome as a drug target in neurodegeneration. Transl. Res. 2018, 198, 48–57. [Google Scholar] [CrossRef]
- Aczel, D.; Gyorgy, B.; Bakonyi, P.; BukhAri, R.; Pinho, R.; Boldogh, I.; Yaodong, G.; Radak, Z. The Systemic Effects of Exercise on the Systemic Effects of Alzheimer’s Disease. Antioxidants 2022, 11, 1028. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, A.H.; McKenzie, M.J.; Bloomer, R.J. Gender comparisons of exercise-induced oxidative stress: Influence of antioxidant supplementation. Appl. Physiol. Nutr. Metab. 2007, 32, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Wooten, S.V.; Moestl, S.; Chilibeck, P.; Alvero Cruz, J.R.; Mittag, U.; Tank, J.; Tanaka, H.; Rittweger, J.; Hoffmann, F. Age- and Sex-Differences in Cardiac Characteristics Determined by Echocardiography in Masters Athletes. Front. Physiol. 2021, 11, 630148. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartra, C.; Jager, L.A.; Alcarraz, A.; Meza-Ramos, A.; Sangüesa, G.; Corpas, R.; Guasch, E.; Batlle, M.; Sanfeliu, C. Antioxidant Molecular Brain Changes Parallel Adaptive Cardiovascular Response to Forced Running in Mice. Antioxidants 2022, 11, 1891. https://doi.org/10.3390/antiox11101891
Bartra C, Jager LA, Alcarraz A, Meza-Ramos A, Sangüesa G, Corpas R, Guasch E, Batlle M, Sanfeliu C. Antioxidant Molecular Brain Changes Parallel Adaptive Cardiovascular Response to Forced Running in Mice. Antioxidants. 2022; 11(10):1891. https://doi.org/10.3390/antiox11101891
Chicago/Turabian StyleBartra, Clara, Lars Andre Jager, Anna Alcarraz, Aline Meza-Ramos, Gemma Sangüesa, Rubén Corpas, Eduard Guasch, Montserrat Batlle, and Coral Sanfeliu. 2022. "Antioxidant Molecular Brain Changes Parallel Adaptive Cardiovascular Response to Forced Running in Mice" Antioxidants 11, no. 10: 1891. https://doi.org/10.3390/antiox11101891
APA StyleBartra, C., Jager, L. A., Alcarraz, A., Meza-Ramos, A., Sangüesa, G., Corpas, R., Guasch, E., Batlle, M., & Sanfeliu, C. (2022). Antioxidant Molecular Brain Changes Parallel Adaptive Cardiovascular Response to Forced Running in Mice. Antioxidants, 11(10), 1891. https://doi.org/10.3390/antiox11101891







