Oxidative Stress and Emergence of Psychosis
Abstract
1. Introduction
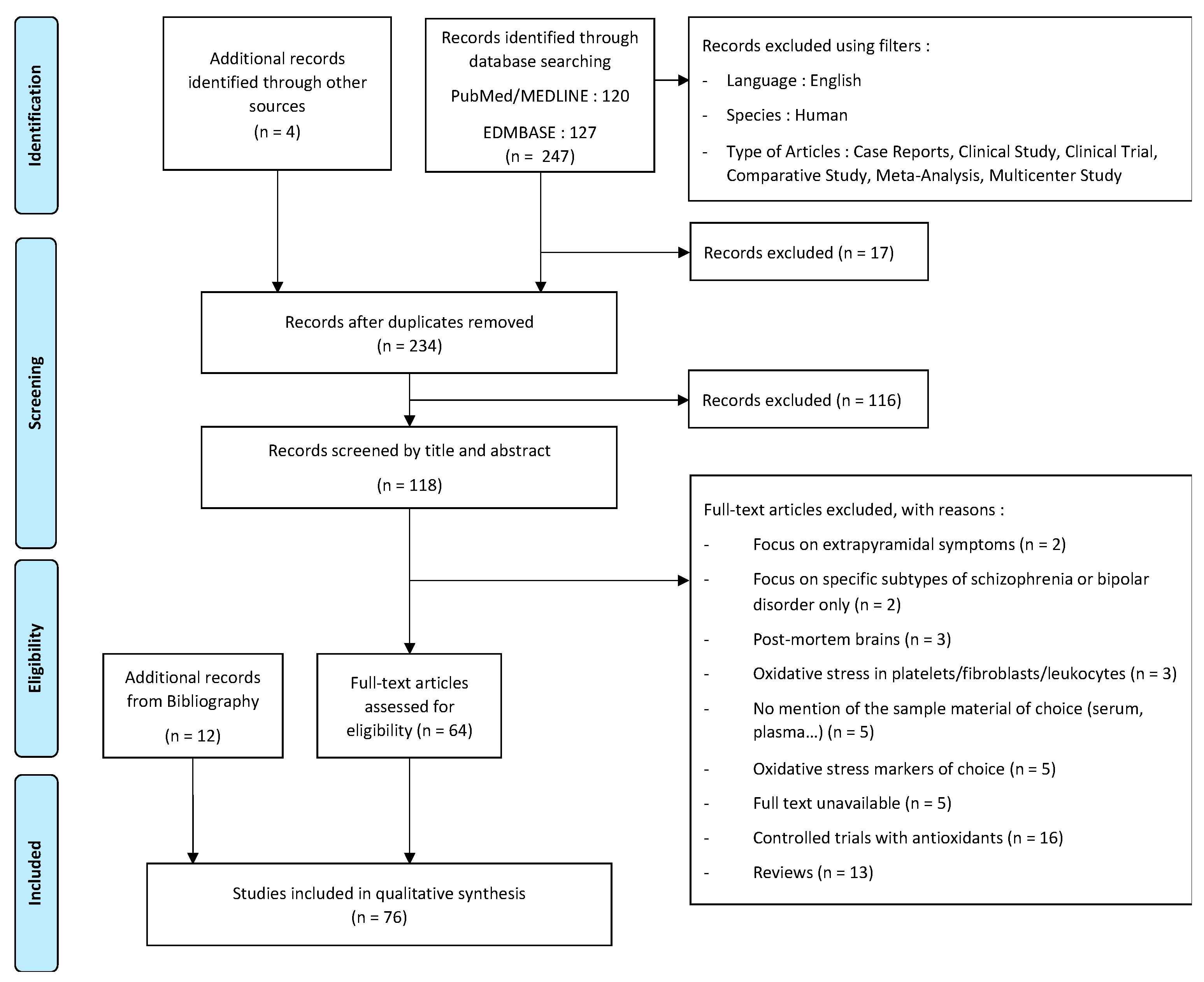
2. Materials and Methods
- (i)
- articles published in peer-review journal
- (ii)
- articles published in English language
- (iii)
- patients diagnosed with SZ using standard diagnostic methods according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) or the International Classification of Disease (ICD) systems
- (iv)
- studies including both patients and healthy controls cohorts
3. Results
| Variables | Schizophrenia | Sources | Status |
|---|---|---|---|
| Antioxidant Defense Peripheral Biomarkers | |||
| GPx | ↑ | Erythrocytes [28] | Unaffected FDR [28] |
| ↓ | Serum [29] | UHR subjects [29] | |
| Catalase | ↓ | Erythrocytes [28] | Unaffected FDR [28] |
| SOD | ↓ | Erythrocytes [28], Serum [29] | Unaffected FDR [28], UHR subjects [29] |
| TAS | ↓ | Serum [30], Plasma [31] | Unaffected FDR [30,31] |
| Variables | Schizophrenia | Sources | Status |
|---|---|---|---|
| Antioxidant Defense Peripheral Biomarkers | |||
| GPx | ↑ | Erythrocytes [32,33], Serum [34] | Antipsychotic-naïve [32,33,34] and antipsychotic-treated [32,34] FEP |
| ↔ | Erythrocytes [23], Serum [35] | Antipsychotic-naïve FEP [23,35] | |
| ↓ | Erythrocytes [36], Serum [37], Plasma [38], Whole Blood [39] | Antipsychotic-naïve FEP [36,37,38,39] | |
| GR | ↔ | Erythrocytes [23] | Antipsychotic-naïve FEP [23] |
| Catalase | ↑ | Plasma [38] | Antipsychotic-naïve FEP [38] |
| ↓ | Erythrocytes [33,36,40] | Antipsychotic-naïve [33,36] and antipsychotic-treated FEP [40] | |
| GSH | ↓ | Erythrocytes [41], Serum [34,42], Plasma [32,33,43] | Antipsychotic-naïve [32,33,34], antipsychotic-free [42,43] and antipsychotic-treated [32,34,41] FEP |
| SOD | ↑ | Erythrocytes [39], Serum [44], Plasma [38,45] | Antipsychotic-naïve FEP [38,39,44,45] |
| ↔ | Erythrocytes [33,40,46], Serum [35], Plasma [36,47] | Antipsychotic-naïve [33,35,36] and antipsychotic-treated FEP [40], Antipsychotic-treated patients with SZ [46,47] | |
| TAS | ↑ | Serum [34], Plasma [38] | Antipsychotic-naïve [38] and antipsychotic-treated [34,38] FEP |
| ↔ | Serum [37,48] | Antipsychotic-naïve FEP [37,48] | |
| ↓ | Serum [49], Plasma [32,36,41,50,51] | Antipsychotic-naïve [32,36,49,50,51] and antipsychotic-treated [32,41] FEP | |
| Oxidative Damage Products | |||
| AGEs | ↑ | Serum [34] | Antipsychotic-naïve and antipsychotic-treated FEP [34] |
| Kynurenine | ↓ | Serum [34] | Antipsychotic-naïve and antipsychotic-treated FEP [34] |
| MDA/TBARS (Lipid Peroxidation) | ↑ | Plasma [39,52,53] | Antipsychotic-naïve FEP [39,52,53] |
| ↔ | Plasma [23,36,40,44] | Antipsychotic-naïve [23,36,44], and antipsychotic-treated FEP [40] | |
| ↓ | Plasma [38] | Antipsychotic-naïve FEP [38] | |
| LOOH (Lipid Peroxidation) | ↑ | Plasma [32] | Antipsychotic-naïve and antipsychotic-treated FEP [32] |
| NO | ↑ | Serum [42] | Antipsychotic-free FEP [42] |
| Variables | Schizophrenia | Sources | Status |
|---|---|---|---|
| Antioxidant Defense Biomarkers in the CNS | |||
| GSH | ↓ | CSF [54], mPFC [54] | Antipsychotic-naïve patients with SZ [54] |
| SOD | ↓ | CSF [55] | Antipsychotic-naïve and antipsychotic-treated patients with SZ [55] |
| Antioxidant Defense Peripheral Biomarkers | |||
| GPx | ↑ | Erythrocytes [56], Serum [34], Plasma [43] | Antipsychotic-naïve and antipsychotic-treated patients with SZ [34,56], Antipsychotic-free patients with SZ [43] |
| ↔ | Erythrocytes [46,57,58], Serum [37], Plasma [59], Whole Blood [60] | Antipsychotic-naïve [60], antipsychotic-free [46,60] and antipsychotic-treated [37,46,57,58,59,60] patients with SZ | |
| ↓ | Erythrocytes [28,61,62,63,64,65,66], Plasma [24,25,26,67] | Antipsychotic-naïve [63,64], antipsychotic-free [66] and antipsychotic-treated [24,25,26,28,61,62,63,65,67] patients with SZ | |
| Catalase | ↑ | Erythrocytes [61,62,68], Serum [69] | Antipsychotic-treated patients with SZ [61,62,68,69] |
| ↔ | Erythrocytes [46,58,65], Plasma [24,25,26,43] | Antipsychotic-free [43,46] and antipsychotic-treated [24,25,26,46,58,65] patients with SZ | |
| ↓ | Erythrocytes [28,63,66] | Antipsychotic-naïve [63], antipsychotic-free [66] and antipsychotic-treated [28,63] patients with SZ | |
| GSH | ↓ | Erythrocytes [61,64,65], Serum [34,70], Plasma [71,72], Whole Blood [73,74] | Antipsychotic-naïve [34,64] and antipsychotic-treated [34,61,65,70,71,72,73,74] patients with SZ |
| ↔ | Erythrocytes [58] | Antipsychotic-treated patients with SZ [58] | |
| GSSG | ↑ | Whole Blood [74] | Antipsychotic-treated patients with SZ [74] |
| SOD | ↑ | Erythrocytes [46,56,61,65,75], Serum [69,76,77], Plasma [45,59] | Antipsychotic-naïve [75,76], antipsychotic-free [46] and antipsychotic-treated [45,56,59,61,65,69,77] patients with SZ |
| ↔ | Erythrocytes [46], Plasma [47] | Antipsychotic-treated patients with SZ [46,47] | |
| ↓ | Erythrocytes [28,58,60,63,64,66,78], Serum [73], Plasma [24,25,26,59,67] | Antipsychotic-naïve [60,63,64,78], antipsychotic-free [60,66] and antipsychotic-treated [24,25,26,28,58,59,60,63,67,73,78] patients with SZ | |
| Ascorbic Acid | ↓ | Plasma [76,79] | Antipsychotic-naïve [76] and antipsychotic-treated [79] patients with SZ |
| TAS | ↑ | Serum [34] | Antipsychotic-naïve and antipsychotic-treated patients with SZ [34] |
| ↔ | Serum [37,60] | Antipsychotic-naïve [60], antipsychotic-free [60] and antipsychotic-treated patients with SZ [37,60] | |
| ↓ | Plasma [27,80,81] | Antipsychotic-free [81] and antipsychotic-treated [27,80,81] patients with SZ | |
| ROS-producing enzymes | |||
| XO | ↑ | Plasma [59] | Antipsychotic-treated patients with SZ [59] |
| Oxidative Damage Products | |||
| AGEs | ↑ | Serum [34] | Antipsychotic-naïve and antipsychotic-treated patients with SZ [34] |
| Kynurenine | ↓ | Serum [34] | Antipsychotic-naïve and antipsychotic-treated patients with SZ [34] |
| MDA/TBARS (Lipid Peroxidation) | ↑ | Erythrocytes [61,62,64,65], Serum [49,69,70,76,77], Plasma [24,25,26,43,52,56,58,59,60,67,73], | Antipsychotic-naïve [49,60,64,76], antipsychotic-free [43,60] and antipsychotic-treated [24,25,26,52,56,58,59,60,61,62,65,67,69,70,73,77] patients with SZ |
| ↔ | Erythrocytes [60,68], Serum [47] | Antipsychotic-treated patients with SZ [47,60,68] | |
| LOOH (Lipid Peroxidation) | ↑ | Plasma [66] | Antipsychotic-free patients with SZ [66] |
| NO | ↑ | Plasma [59,66], Serum [73] | Antipsychotic-free [66] and antipsychotic-treated [59,73] patients with SZ |
4. Discussion
4.1. Evidence of the Involvement of Oxidative Stress in SZ
4.2. Oxidative Stress Biomarkers and Clinical Course of SZ
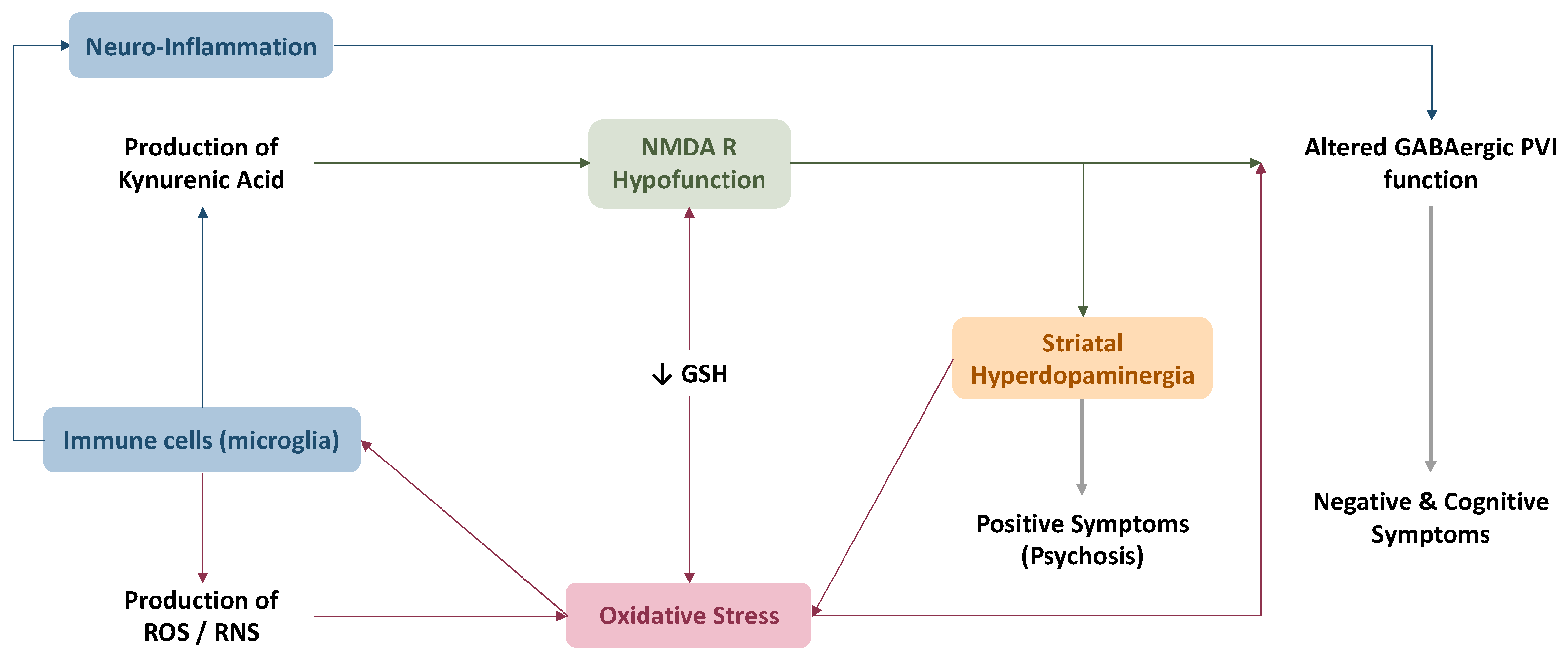
4.3. Link between Oxidative Stress and Current Physiopathological Hypotheses
4.4. Re-Establishing Redox Balance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Charlson, F.J.; Ferrari, A.J.; Santomauro, D.F.; Diminic, S.; Stockings, E.; Scott, J.G.; McGrath, J.J.; Whiteford, H.A. Global epidemiology and burden of schizophrenia: Findings from the global burden of disease study 2016. Schizophr. Bull. 2018, 44, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Yung, A.R.; Nelson, B.; Stanford, C.; Simmons, M.B.; Cosgrave, E.M.; Killackey, E.; Phillips, L.J.; Bechdolf, A.; Buckby, J.; McGorry, P.D. Validation of “prodromal” criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophr. Res. 2008, 105, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Cannon, T.D.; Cadenhead, K.; Cornblatt, B.; Woods, S.W.; Addington, J.; Walker, E.; Seidman, L.J.; Perkins, D.; Tsuang, M.; McGlashan, T. Prediction of psychosis in youth at high clinical risk: A multisite longitudinal study in North America. Arch. Gen. Psychiatry 2008, 65, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Remington, G.; Foussias, G.; Fervaha, G.; Agid, O.; Takeuchi, H.; Lee, J.; Hahn, M. Treating Negative Symptoms in Schizophrenia: An Update. Curr. Treat. Options Psychiatry 2016, 3, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.F.; Mors, O.; Secher, R.G.; Hjorthøj, C.R.; Albert, N.; Bertelsen, M.; Jensen, H.; Jeppesen, P.; Petersen, L.; Randers, L. Predictors of recovery in first episode psychosis: The OPUS cohort at 10 year follow-up. Schizophr. Res. 2013, 150, 163–168. [Google Scholar] [CrossRef]
- Meyer, E.C.; Carrión, R.E.; Cornblatt, B.A.; Addington, J.; Cadenhead, K.S.; Cannon, T.D.; McGlashan, T.H.; Perkins, D.O.; Tsuang, M.T.; Walker, E.F. The relationship of neurocognition and negative symptoms to social and role functioning over time in individuals at clinical high risk in the first phase of the North American Prodrome Longitudinal Study. Schizophr. Bull. 2014, 40, 1452–1461. [Google Scholar] [CrossRef]
- Harvey, R.C.; James, A.C.; Shields, G.E. A systematic review and network meta-analysis to assess the relative efficacy of antipsychotics for the treatment of positive and negative symptoms in early-onset schizophrenia. CNS Drugs 2016, 30, 27–39. [Google Scholar] [CrossRef]
- Shoja Shafti, S.; Fallah Jahromi, P. A comparative study between olanzapine and risperidone regarding drug-induced electrocardiographic changes. Cardiovasc. Psychiatry Neurol. 2014, 2014, 37016. [Google Scholar] [CrossRef]
- Raballo, A.; Poletti, M.; Preti, A. Negative Prognostic Effect of Baseline Antipsychotic Exposure in Clinical High Risk for Psychosis (CHR-P): Is Pre-Test Risk Enrichment the Hidden Culprit? Int. J. Neuropsychopharmacol. 2021, 24, 710–720. [Google Scholar] [CrossRef]
- Millan, M.J.; Andrieux, A.; Bartzokis, G.; Cadenhead, K.; Dazzan, P.; Fusar-Poli, P.; Gallinat, J.; Giedd, J.; Grayson, D.R.; Heinrichs, M.; et al. Altering the course of schizophrenia: Progress and perspectives. Nat. Rev. Drug Discov. 2016, 15, 485–515. [Google Scholar] [CrossRef]
- Do, K.Q.; Cabungcal, J.H.; Frank, A.; Steullet, P.; Cuenod, M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr. Opin. Neurobiol. 2009, 19, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.O.; Jeffries, C.D.; Addington, J.; Bearden, C.E.; Cadenhead, K.S.; Cannon, T.D.; Cornblatt, B.A.; Mathalon, D.H.; McGlashan, T.H.; Seidman, L.J. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: Preliminary results from the NAPLS project. Schizophr. Bull. 2015, 41, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Flatow, J.; Buckley, P.; Miller, B.J. Meta-analysis of oxidative stress in schizophrenia. Biol. Psychiatry 2013, 74, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Zarkovic, N. Antioxidants and Second Messengers of Free Radicals. Antioxidants 2018, 7, 158. [Google Scholar] [CrossRef]
- Murphy, M.P.; Holmgren, A.; Larsson, N.-G.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011, 13, 361–366. [Google Scholar] [CrossRef]
- Lewis, D.A.; Curley, A.A.; Glausier, J.R.; Volk, D.W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012, 35, 57–67. [Google Scholar] [CrossRef]
- Irshad, M.; Chaudhuri, P. Oxidant-Antioxidant System: Role and Significance in Human Body; NISCAIR-CSIR: New Delhi, India, 2002. [Google Scholar]
- Gravina, P.; Spoletini, I.; Masini, S.; Valentini, A.; Vanni, D.; Paladini, E.; Bossù, P.; Caltagirone, C.; Federici, G.; Spalletta, G. Genetic polymorphisms of glutathione S-transferases GSTM1, GSTT1, GSTP1 and GSTA1 as risk factors for schizophrenia. Psychiatry Res. 2011, 187, 454–456. [Google Scholar] [CrossRef]
- Gysin, R.; Kraftsik, R.; Sandell, J.; Bovet, P.; Chappuis, C.; Conus, P.; Deppen, P.; Preisig, M.; Ruiz, V.; Steullet, P. Impaired glutathione synthesis in schizophrenia: Convergent genetic and functional evidence. Proc. Natl. Acad. Sci. USA 2007, 104, 16621–16626. [Google Scholar] [CrossRef]
- Rodríguez-Santiago, B.; Brunet, A.; Sobrino, B.; Serra-Juhe, C.; Flores, R.; Armengol, L.; Vilella, E.; Gabau, E.; Guitart, M.; Guillamat, R. Association of common copy number variants at the glutathione S-transferase genes and rare novel genomic changes with schizophrenia. Mol. Psychiatry 2010, 15, 1023–1033. [Google Scholar] [CrossRef]
- Tosic, M.; Ott, J.; Barral, S.; Bovet, P.; Deppen, P.; Gheorghita, F.; Matthey, M.L.; Parnas, J.; Preisig, M.; Saraga, M.; et al. Schizophrenia and oxidative stress: Glutamate cysteine ligase modifier as a susceptibility gene. Am. J. Hum. Genet. 2006, 79, 586–592. [Google Scholar] [CrossRef]
- Lavoie, S.; Berger, M.; Schlögelhofer, M.; Schäfer, M.; Rice, S.; Kim, S.; Hesse, J.; McGorry, P.; Smesny, S.; Amminger, G. Erythrocyte glutathione levels as long-term predictor of transition to psychosis. Transl. Psychiatry 2017, 7, e1064. [Google Scholar] [CrossRef] [PubMed]
- Langbein, K.; Hesse, J.; Gussew, A.; Milleit, B.; Lavoie, S.; Amminger, G.P.; Gaser, C.; Wagner, G.; Reichenbach, J.R.; Hipler, U.C.; et al. Disturbed glutathione antioxidative defense is associated with structural brain changes in neuroleptic-naïve first-episode psychosis patients. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Sun, Y.; Chen, N.; Chen, S.; Xiu, M.; Zhang, X. Interaction of oxidative stress and BDNF on executive dysfunction in patients with chronic schizophrenia. Psychoneuroendocrinology 2020, 111, 104473. [Google Scholar] [CrossRef]
- Wu, Z.W.; Yu, H.H.; Wang, X.; Guan, H.Y.; Xiu, M.H.; Zhang, X.Y. Interrelationships between Oxidative Stress, Cytokines, and Psychotic Symptoms and Executive Functions in Patients with Chronic Schizophrenia. Psychosom. Med. 2021, 83, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Chen, D.C.; Tan, Y.L.; Tan, S.P.; Wang, Z.R.; Yang, F.D.; Okusaga, O.O.; Zunta-Soares, G.B.; Soares, J.C. The interplay between BDNF and oxidative stress in chronic schizophrenia. Psychoneuroendocrinology 2015, 51, 201–208. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, D.C.; Xiu, M.H.; Tang, W.; Zhang, F.; Liu, L.; Chen, Y.; Liu, J.; Yao, J.K.; Kosten, T.A.; et al. Plasma total antioxidant status and cognitive impairments in schizophrenia. Schizophr. Res. 2012, 139, 66–72. [Google Scholar] [CrossRef]
- Ben Othmen, L.; Mechri, A.; Fendri, C.; Bost, M.; Chazot, G.; Gaha, L.; Kerkeni, A. Altered antioxidant defense system in clinically stable patients with schizophrenia and their unaffected siblings. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 155–159. [Google Scholar] [CrossRef]
- Zeni-Graiff, M.; Rios, A.C.; Maurya, P.K.; Rizzo, L.B.; Sethi, S.; Yamagata, A.S.; Mansur, R.B.; Pan, P.M.; Asevedo, E.; Cunha, G.R.; et al. Peripheral levels of superoxide dismutase and glutathione peroxidase in youths in ultra-high risk for psychosis: A pilot study. CNS Spectr. 2019, 24, 333–337. [Google Scholar] [CrossRef]
- Guler, E.M.; Kurtulmus, A.; Gul, A.Z.; Kocyigit, A.; Kirpinar, I. Oxidative stress and schizophrenia: A comparative cross-sectional study of multiple oxidative markers in patients and their first-degree relatives. Int. J. Clin. Pract. 2021, 75, e14711. [Google Scholar] [CrossRef]
- Gonzalez-Pinto, A.; Martinez-Cengotitabengoa, M.; Arango, C.; Baeza, I.; Otero-Cuesta, S.; Graell-Berna, M.; Soutullo, C.; Leza, J.C.; Mico, J.A. Antioxidant defense system and family environment in adolescents with family history of psychosis. BMC Psychiatry 2012, 12, 200. [Google Scholar] [CrossRef]
- Micó, J.A.; Rojas-Corrales, M.O.; Gibert-Rahola, J.; Parellada, M.; Moreno, D.; Fraguas, D.; Graell, M.; Gil, J.; Irazusta, J.; Castro-Fornieles, J.; et al. Reduced antioxidant defense in early onset first-episode psychosis: A case-control study. BMC Psychiatry 2011, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Raffa, M.; Atig, F.; Mhalla, A.; Kerkeni, A.; Mechri, A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry 2011, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Juchnowicz, D.; Dzikowski, M.; Rog, J.; Waszkiewicz, N.; Zalewska, A.; Maciejczyk, M.; Karakuła-Juchnowicz, H. Oxidative Stress Biomarkers as a Predictor of Stage Illness and Clinical Course of Schizophrenia. Front. Psychiatry 2021, 12, 728986. [Google Scholar] [CrossRef] [PubMed]
- Simsek, S.; Gencoglan, S.; Yuksel, T.; Kaplan, I.; Alaca, R.; Aktas, H. Oxidative stress and DNA damage in untreated first-episode psychosis in adolescents. Neuropsychobiology 2016, 73, 92–97. [Google Scholar] [CrossRef]
- Martínez-Cengotitabengoa, M.; Mac-Dowell, K.S.; Leza, J.C.; Micó, J.A.; Fernandez, M.; Echevarría, E.; Sanjuan, J.; Elorza, J.; González-Pinto, A. Cognitive impairment is related to oxidative stress and chemokine levels in first psychotic episodes. Schizophr. Res. 2012, 137, 66–72. [Google Scholar] [CrossRef]
- Bai, Z.L.; Li, X.S.; Chen, G.Y.; Du, Y.; Wei, Z.X.; Chen, X.; Zheng, G.E.; Deng, W.; Cheng, Y. Serum Oxidative Stress Marker Levels in Unmedicated and Medicated Patients with Schizophrenia. J. Mol. Neurosci. 2018, 66, 428–436. [Google Scholar] [CrossRef]
- Li, X.R.; Xiu, M.H.; Guan, X.N.; Wang, Y.C.; Wang, J.; Leung, E.; Zhang, X.Y. Altered Antioxidant Defenses in Drug-Naive First Episode Patients with Schizophrenia Are Associated with Poor Treatment Response to Risperidone: 12-Week Results from a Prospective Longitudinal Study. Neurotherapeutics 2021, 18, 1316–1324. [Google Scholar] [CrossRef]
- Sarandol, A.; Sarandol, E.; Acikgoz, H.E.; Eker, S.S.; Akkaya, C.; Dirican, M. First-episode psychosis is associated with oxidative stress: Effects of short-term antipsychotic treatment. Psychiatry Clin. Neurosci. 2015, 69, 699–707. [Google Scholar] [CrossRef]
- Piatoikina, A.S.; Lyakhova, A.A.; Semennov, I.V.; Zhilyaeva, T.V.; Kostina, O.V.; Zhukova, E.S.; Shcherbatyuk, T.G.; Kasyanov, E.D.; Blagonravova, A.S.; Mazo, G.E. Association of antioxidant deficiency and the level of products of protein and lipid peroxidation in patients with the first episode of schizophrenia. J. Mol. Neurosci. 2022, 72, 217–225. [Google Scholar] [CrossRef]
- Martínez-Cengotitabengoa, M.; Micó, J.A.; Arango, C.; Castro-Fornieles, J.; Graell, M.; Payá, B.; Leza, J.C.; Zorrilla, I.; Parellada, M.; López, M.P. Basal low antioxidant capacity correlates with cognitive deficits in early onset psychosis. A 2-year follow-up study. Schizophr. Res. 2014, 156, 23–29. [Google Scholar] [CrossRef]
- Zhai, X.; Kang, Y.; Yuan, X.; Wang, Y.; Lu, S.; Lu, Z. Dysfunctional oxidative stress response in first-episode of schizophrenia. Trop. J. Pharm. Res. 2021, 20, 1251–1259. [Google Scholar] [CrossRef]
- Guidara, W.; Messedi, M.; Naifar, M.; Grayaa, S.; Omri, S.; Ben Thabet, J.; Maalej, M.; Charfi, N.; Ayadi, F. Predictive value of oxidative stress biomarkers in drug-free patients with schizophrenia and schizo-affective disorder. Psychiatry Res. 2020, 293, 113467. [Google Scholar] [CrossRef] [PubMed]
- Jordan, W.; Dobrowolny, H.; Bahn, S.; Bernstein, H.G.; Brigadski, T.; Frodl, T.; Isermann, B.; Lessmann, V.; Pilz, J.; Rodenbeck, A.; et al. Oxidative stress in drug-naive first episode patients with schizophrenia and major depression: Effects of disease acuity and potential confounders. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, X.Y.; Wang, H.; Tang, W.; Xia, Y.; Zhang, F.; Liu, J.; Fu, Y.; Hu, J.; Chen, Y.; et al. Elevated plasma superoxide dismutase in first-episode and drug naive patients with schizophrenia: Inverse association with positive symptoms. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 36, 34–38. [Google Scholar] [CrossRef]
- Yao, J.K.; Reddy, R.; McElhinny, L.G.; Van Kammen, D.P. Effects of haloperidol on antioxidant defense system enzymes in schizophrenia. J. Psychiatr. Res. 1998, 32, 385–391. [Google Scholar] [CrossRef]
- Tuncel, O.K.; Sarisoy, G.; Bilgici, B.; Pazvantoglu, O.; Cetin, E.; Unverdi, E.; Avci, B.; Boke, T. Oxidative stress in bipolar and schizophrenia patients. Psychiatry Res. 2015, 228, 688–694. [Google Scholar] [CrossRef]
- Kriisa, K.; Haring, L.; Vasar, E.; Koido, K.; Janno, S.; Vasar, V.; Zilmer, K.; Zilmer, M. Antipsychotic Treatment Reduces Indices of Oxidative Stress in First-Episode Psychosis Patients. Oxidative Med. Cell. Longev. 2016, 2016, 9616593. [Google Scholar] [CrossRef]
- Devanarayanan, S.; Nandeesha, H.; Kattimani, S.; Sarkar, S. Relationship between matrix metalloproteinase-9 and oxidative stress in drug-free male schizophrenia: A case control study. Clin. Chem. Lab. Med. 2016, 54, 447–452. [Google Scholar] [CrossRef]
- Li, X.F.; Zheng, Y.L.; Xiu, M.H.; Chen, D.C.; Kosten, T.R.; Zhang, X.Y. Reduced plasma total antioxidant status in first-episode drug-naive patients with schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1064–1067. [Google Scholar] [CrossRef]
- Xie, T.; Li, Q.; Luo, X.; Tian, L.; Wang, Z.; Tan, S.; Chen, S.; Yang, G.; An, H.; Yang, F.; et al. Plasma total antioxidant status and cognitive impairments in first-episode drug-naive patients with schizophrenia. Cogn. Neurodynamics 2019, 13, 357–365. [Google Scholar] [CrossRef]
- Khan, M.M.; Evans, D.R.; Gunna, V.; Scheffer, R.E.; Parikh, V.V.; Mahadik, S.P. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr. Res. 2002, 58, 1–10. [Google Scholar] [CrossRef]
- Mahadik, S.P.; Mukherjee, S.; Scheffer, R.; Correnti, E.E.; Mahadik, J.S. Elevated plasma lipid peroxides at the onset of nonaffective psychosis. Biol. Psychiatry 1998, 43, 674–679. [Google Scholar] [CrossRef]
- Do, K.Q.; Trabesinger, A.H.; Kirsten-Krüger, M.; Lauer, C.J.; Dydak, U.; Hell, D.; Holsboer, F.; Boesiger, P.; Cuénod, M. Schizophrenia: Glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur. J. Neurosci. 2000, 12, 3721–3728. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, J.M.; Hayes, L.N.; Tanaka, T.; Xiao, M.; Yolken, R.H.; Worley, P.; Leweke, F.M.; Sawa, A. Reduced superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with early psychosis in association with clinical features. Schizophr. Res. 2017, 183, 64–69. [Google Scholar] [CrossRef]
- Kuloglu, M.; Ustundag, B.; Atmaca, M.; Canatan, H.; Tezcan, A.E.; Cinkilinc, N. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem. Funct. 2002, 20, 171–175. [Google Scholar] [CrossRef]
- Reddy, R.; Sahebarao, M.P.; Mukherjee, S.; Murthy, J.N. Enzymes of the antioxidant defense system in chronic schizophrenic patients. Biol. Psychiatry 1991, 30, 409–412. [Google Scholar] [CrossRef]
- Buosi, P.; Borghi, F.A.; Lopes, A.M.; Facincani, I.D.S.; Fernandes-Ferreira, R.; Oliveira-Brancati, C.I.F.; do Carmo, T.S.; Souza, D.R.S.; da Silva, D.G.H.; de Almeida, E.G.H.; et al. Oxidative stress biomarkers in treatment-responsive and treatment-resistant schizophrenia patients. Trends Psychiatry Psychother. 2021, 43, 278–285. [Google Scholar] [CrossRef]
- Akyol, Ö.; Herken, H.; Uz, E.; Fadıllıoǧlu, E.; Ünal, S.; Söǧüt, S.; Özyurt, H.; Savaş, H.A. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients: The possible role of oxidant/antioxidant imbalance. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2002, 26, 995–1005. [Google Scholar] [CrossRef]
- Sarandol, A.; Kirli, S.; Akkaya, C.; Altin, A.; Demirci, M.; Sarandol, E. Oxidative-antioxidative systems and their relation with serum S100 B levels in patients with schizophrenia: Effects of short term antipsychotic treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 1164–1169. [Google Scholar] [CrossRef]
- Altuntas, I.; Aksoy, H.; Coskun, I.; Çayköylü, A.; Akçay, F. Erythrocyte Superoxide Dismutase and Glutathione Peroxidase Activities, and Malondialdehyde and Reduced Glutathione Levels in Schizophrenic Patients. Clin. Chem. Lab. Med. (CCLM) 2000, 38, 1277–1281. [Google Scholar] [CrossRef]
- Herken, H.; Uz, E.; Özyurt, H.; Söğüt, S.; Virit, O.; Akyol, Ö. Evidence that the activities of erythrocyte free radical scavenging enzymes and the products of lipid peroxidation are increased in different forms of schizophrenia. Mol. Psychiatry 2001, 6, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ranjekar, P.K.; Hinge, A.; Hegde, M.V.; Ghate, M.; Kale, A.; Sitasawad, S.; Wagh, U.V.; Debsikdar, V.B.; Mahadik, S.P. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003, 121, 109–122. [Google Scholar] [CrossRef]
- Dadheech, G.; Mishra, S.; Gautam, S.; Sharma, P. Evaluation of antioxidant deficit in schizophrenia. Indian J. Psychiatry 2008, 50, 16. [Google Scholar] [PubMed]
- Pavlović, D.; Tamburić, V.; Stojanović, I.; Kocić, G.; Jevtović, T.; Đorđević, V. Oxidative stress as marker of positive symptoms in schizophrenia. Facta Univ. 2002, 9, 157–161. [Google Scholar]
- Li, H.C.; Chen, Q.Z.; Ma, Y.; Zhou, J.F. Imbalanced free radicals and antioxidant defense systems in schizophrenia: A comparative study. J. Zhejiang Univ. Sci. B 2006, 7, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Tan, Y.L.; Cao, L.Y.; Wu, G.Y.; Xu, Q.; Shen, Y.; Zhou, D.F. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr. Res. 2006, 81, 291–300. [Google Scholar] [CrossRef] [PubMed]
- González-Blanco, L.; García-Portilla, M.P.; García-Álvarez, L.; de la Fuente-Tomás, L.; Iglesias García, C.; Sáiz, P.A.; Rodríguez-González, S.; Coto-Montes, A.; Bobes, J. Oxidative stress biomarkers and clinical dimensions in first 10 years of schizophrenia. Rev. Psiquiatr. Salud Ment. (Engl. Ed.) 2018, 11, 130–140. [Google Scholar] [CrossRef]
- Hursitoglu, O.; Orhan, F.O.; Kurutas, E.B.; Doganer, A.; Durmus, H.T.; Kopar, H. Diagnostic performance of increased malondialdehyde level and oxidative stress in patients with schizophrenia. Noropsikiyatri Ars. 2021, 58, 184–188. [Google Scholar] [CrossRef]
- Cruz, B.F.; de Campos-Carli, S.M.; de Oliveira, A.M.; de Brito, C.B.; Garcia, Z.M.; Duque do Arifa, R.; de Souza, D.D.G.; Teixeira, A.L.; Salgado, J.V. Investigating potential associations between neurocognition/social cognition and oxidative stress in schizophrenia. Psychiatry Res. 2021, 298, 113832. [Google Scholar] [CrossRef]
- Nucifora, L.G.; Tanaka, T.; Hayes, L.N.; Kim, M.; Lee, B.J.; Matsuda, T.; Nucifora, F.C., Jr.; Sedlak, T.; Mojtabai, R.; Eaton, W.; et al. Reduction of plasma glutathione in psychosis associated with schizophrenia and bipolar disorder in translational psychiatry. Transl. Psychiatry 2017, 7, e1215. [Google Scholar] [CrossRef]
- Coughlin, J.M.; Yang, K.; Marsman, A.; Pradhan, S.; Wang, M.; Ward, R.E.; Bonekamp, S.; Ambinder, E.B.; Higgs, C.P.; Kim, P.K.; et al. A multimodal approach to studying the relationship between peripheral glutathione, brain glutamate, and cognition in health and in schizophrenia. Mol. Psychiatry 2021, 26, 3502–3511. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Liencres, C.; Tas, C.; Brown, E.C.; Erdin, S.; Onur, E.; Cubukcoglu, Z.; Aydemir, O.; Esen-Danaci, A.; Brüne, M. Oxidative stress in schizophrenia: A case-control study on the effects on social cognition and neurocognition. BMC Psychiatry 2014, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, A.; Summerfelt, A.; Du, X.; Jiang, P.; Chiappelli, J.; Tagamets, M.; O’Donnell, P.; Kochunov, P.; Hong, L.E. Electrophysiological intermediate biomarkers for oxidative stress in schizophrenia. Clin. Neurophysiol. 2013, 124, 2209–2215. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.S.; Das, I. Oxidative stress and superoxide dismutase in schizophrenia. Biochem. Soc. Trans. 1997, 25, 418S. [Google Scholar] [CrossRef]
- Dakhale, G.; Khanzode, S.; Saoji, A.; Khobragade, L.; Turankar, A. Oxidative damage and schizophrenia: The potential benefit by atypical antipsychotics. Neuropsychobiology 2004, 49, 205–209. [Google Scholar] [CrossRef]
- Gama, C.S.; Salvador, M.; Andreazza, A.C.; Kapczinski, F.; Silva Belmonte-de-Abreu, P. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in schizophrenia: A study of patients treated with haloperidol or clozapine. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 512–515. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mahadik, S.P.; Scheffer, R.; Correnti, E.E.; Kelkar, H. Impaired antioxidant defense at the onset of psychosis. Schizophr. Res. 1996, 19, 19–26. [Google Scholar] [CrossRef]
- Subotičanec, K.; Folnegović-Šmalc, V.; Korbar, M.; Meštrović, B.; Buzina, R. Vitamin C status in chronic schizophrenia. Biol. Psychiatry 1990, 28, 959–966. [Google Scholar] [CrossRef]
- Virit, O.; Altindag, A.; Yumru, M.; Dalkilic, A.; Savas, H.A.; Selek, S.; Erel, O.; Herken, H. A defect in the antioxidant defense system in schizophrenia. Neuropsychobiology 2009, 60, 87–93. [Google Scholar] [CrossRef]
- Yao, J.K.; Reddy, R.; McElhinny, L.G.; Van Kammen, D.P. Reduced status of plasma total antioxidant capacity in schizophrenia. Schizophr. Res. 1998, 32, 1–8. [Google Scholar] [CrossRef]
- Goh, X.X.; Tang, P.Y.; Tee, S.F. Blood-based oxidation markers in medicated and unmedicated schizophrenia patients: A meta-analysis. Asian J. Psychiatr. 2022, 67, 102932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, Z.; He, L.; Wan, C. A meta-analysis of oxidative stress markers in schizophrenia. Sci. China Life Sci. 2010, 53, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Alameda, L.; Fournier, M.; Khadimallah, I.; Griffa, A.; Cleusix, M.; Jenni, R.; Ferrari, C.; Klauser, P.; Baumann, P.S.; Cuenod, M.; et al. Redox dysregulation as a link between childhood trauma and psychopathological and neurocognitive profile in patients with early psychosis. Proc. Natl. Acad. Sci. USA 2018, 115, 12495–12500. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Javadzadeh, A.; Dey, A.; Sabesan, P.; Théberge, J.; Radua, J.; Palaniyappan, L. Antioxidant defense in schizophrenia and bipolar disorder: A meta-analysis of MRS studies of anterior cingulate glutathione. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 91, 94–102. [Google Scholar] [CrossRef]
- Limongi, R.; Jeon, P.; Théberge, J.; Palaniyappan, L. Counteracting Effects of Glutathione on the Glutamate-Driven Excitation/Inhibition Imbalance in First-Episode Schizophrenia: A 7T MRS and Dynamic Causal Modeling Study. Antioxidants 2021, 10, 75. [Google Scholar] [CrossRef]
- Wang, A.M.; Pradhan, S.; Coughlin, J.M.; Trivedi, A.; DuBois, S.L.; Crawford, J.L.; Sedlak, T.W.; Nucifora, F.C., Jr.; Nestadt, G.; Nucifora, L.G.; et al. Assessing Brain Metabolism with 7-T Proton Magnetic Resonance Spectroscopy in Patients with First-Episode Psychosis. JAMA Psychiatry 2019, 76, 314–323. [Google Scholar] [CrossRef]
- Kumar, J.; Liddle, E.B.; Fernandes, C.C.; Palaniyappan, L.; Hall, E.L.; Robson, S.E.; Simmonite, M.; Fiesal, J.; Katshu, M.Z.; Qureshi, A.; et al. Glutathione and glutamate in schizophrenia: A 7T MRS study. Mol. Psychiatry 2020, 25, 873–882. [Google Scholar] [CrossRef]
- Yang, Y.S.; Maddock, R.J.; Lee, J.; Zhang, H.; Hellemann, G.; Narr, K.L.; Marder, S.R.; Green, M.F. Brain glutathione levels and age at onset of illness in chronic schizophrenia. Acta Neuropsychiatr. 2019, 31, 343–347. [Google Scholar] [CrossRef]
- Xin, L.; Mekle, R.; Fournier, M.; Baumann, P.S.; Ferrari, C.; Alameda, L.; Jenni, R.; Lu, H.; Schaller, B.; Cuenod, M.; et al. Genetic Polymorphism Associated Prefrontal Glutathione and Its Coupling with Brain Glutamate and Peripheral Redox Status in Early Psychosis. Schizophr. Bull. 2016, 42, 1185–1196. [Google Scholar] [CrossRef]
- Iwata, Y.; Nakajima, S.; Plitman, E.; Truong, P.; Bani-Fatemi, A.; Caravaggio, F.; Kim, J.; Shah, P.; Mar, W.; Chavez, S.; et al. Glutathione Levels and Glutathione-Glutamate Correlation in Patients with Treatment-Resistant Schizophrenia. Schizophr. Bull. Open 2021, 2, sgab006. [Google Scholar] [CrossRef]
- Kim, S.K.; Kang, S.W.; Chung, J.-H.; Park, H.J.; Cho, K.B.; Park, M.-S. Genetic polymorphisms of glutathione-related enzymes (GSTM1, GSTT1, and GSTP1) and schizophrenia risk: A meta-analysis. Int. J. Mol. Sci. 2015, 16, 19602–19611. [Google Scholar] [CrossRef] [PubMed]
- Clay, H.B.; Sillivan, S.; Konradi, C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int. J. Dev. Neurosci. 2011, 29, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.U.; Jeong, J.; Lee, H.; Mun, J.Y.; Kim, J.H.; Lee, J.S.; Nguyen, M.D.; Han, S.S.; Suh, P.G.; Park, S.K. Disrupted-in-schizophrenia 1 (DISC1) plays essential roles in mitochondria in collaboration with Mitofilin. Proc. Natl. Acad. Sci. USA 2010, 107, 17785–17790. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.W.; Jaaro-Peled, H.; Shahani, N.; Sedlak, T.W.; Zoubovsky, S.; Burruss, D.; Emiliani, F.; Sawa, A.; Gallagher, M. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc. Natl. Acad. Sci. USA 2013, 110, 12462–12467. [Google Scholar] [CrossRef]
- Goldshmit, Y.; Erlich, S.; Pinkas-Kramarski, R. Neuregulin rescues PC12-ErbB4 cells from cell death induced by H(2)O(2). Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. J. Biol. Chem. 2001, 276, 46379–46385. [Google Scholar] [CrossRef]
- Palaniyappan, L.; Park, M.T.M.; Jeon, P.; Limongi, R.; Yang, K.; Sawa, A.; Théberge, J. Is there a glutathione centered redox dysregulation subtype of schizophrenia? Antioxidants 2021, 10, 1703. [Google Scholar] [CrossRef]
- Prabakaran, S.; Swatton, J.E.; Ryan, M.M.; Huffaker, S.J.; Huang, J.-J.; Griffin, J.L.; Wayland, M.; Freeman, T.; Dudbridge, F.; Lilley, K.S.; et al. Mitochondrial dysfunction in schizophrenia: Evidence for compromised brain metabolism and oxidative stress. Mol. Psychiatry 2004, 9, 684–697. [Google Scholar] [CrossRef]
- Ustundag, B.; Atmaca, M.; Kirtas, O.; Selek, S.; Metin, K.; Tezcan, E. Total antioxidant response in patients with schizophrenia. Psychiatry Clin. Neurosci. 2006, 60, 458–464. [Google Scholar] [CrossRef]
- Noto, C.; Ota, V.K.; Gadelha, A.; Noto, M.N.; Barbosa, D.S.; Bonifacio, K.L.; Nunes, S.O.; Cordeiro, Q.; Belangero, S.I.; Bressan, R.A.; et al. Oxidative stress in drug naive first episode psychosis and antioxidant effects of risperidone. J. Psychiatr. Res. 2015, 68, 210–216. [Google Scholar] [CrossRef]
- Ruiz-Litago, F.; Seco, J.; Echevarria, E.; Martinez-Cengotitabengoa, M.; Gil, J.; Irazusta, J.; Gonzalez-Pinto, A.M. Adaptive response in the antioxidant defence system in the course and outcome in first-episode schizophrenia patients: A 12-months follow-up study. Psychiatry Res. 2012, 200, 218–222. [Google Scholar] [CrossRef]
- Tsugawa, S.; Noda, Y.; Tarumi, R.; Mimura, Y.; Yoshida, K.; Iwata, Y.; Elsalhy, M.; Kuromiya, M.; Kurose, S.; Masuda, F.; et al. Glutathione levels and activities of glutathione metabolism enzymes in patients with schizophrenia: A systematic review and meta-analysis. J. Psychopharmacol. 2019, 33, 1199–1214. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.R. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry 1987, 44, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Horrobin, D.F. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr. Res. 1998, 30, 193–208. [Google Scholar] [CrossRef]
- Weng, M.; Xie, X.; Liu, C.; Lim, K.-L.; Zhang, C.-W.; Li, L. The sources of reactive oxygen species and its possible role in the pathogenesis of Parkinson’s disease. Parkinson’s Dis. 2018, 2018, 9163040. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Davis, K.L.; Kahn, R.S.; Ko, G.; Davidson, M. Dopamine in schizophrenia: A review and reconceptualization. Am. J. Psychiatry 1991, 148, 1474–1486. [Google Scholar]
- Lewis, D.A.; Hashimoto, T.; Volk, D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005, 6, 312–324. [Google Scholar] [CrossRef]
- Back, S.A.; Gan, X.; Li, Y.; Rosenberg, P.A.; Volpe, J.J. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J. Neurosci. 1998, 18, 6241–6253. [Google Scholar] [CrossRef]
- Baumann, N.; Pham-Dinh, D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001, 81. [Google Scholar] [CrossRef]
- Bartos, M.; Vida, I.; Jonas, P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 2007, 8, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Pettersson-Yeo, W.; Allen, P.; Benetti, S.; McGuire, P.; Mechelli, A. Dysconnectivity in schizophrenia: Where are we now? Neurosci. Biobehav. Rev. 2011, 35, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Reynolds, G.P. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr. Res. 2002, 55, 1–10. [Google Scholar] [CrossRef]
- Gao, R.; Penzes, P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr. Mol. Med. 2015, 15, 146–167. [Google Scholar] [CrossRef] [PubMed]
- Uno, Y.; Coyle, J.T. Glutamate hypothesis in schizophrenia. Psychiatry Clin. Neurosci. 2019, 73, 204–215. [Google Scholar] [CrossRef]
- Koga, M.; Serritella, A.V.; Messmer, M.M.; Hayashi-Takagi, A.; Hester, L.D.; Snyder, S.H.; Sawa, A.; Sedlak, T.W. Glutathione is a physiologic reservoir of neuronal glutamate. Biochem. Biophys. Res. Commun. 2011, 409, 596–602. [Google Scholar] [CrossRef]
- Steullet, P.; Cabungcal, J.H.; Monin, A.; Dwir, D.; O’Donnell, P.; Cuenod, M.; Do, K.Q. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: A “central hub” in schizophrenia pathophysiology? Schizophr. Res. 2016, 176, 41–51. [Google Scholar] [CrossRef]
- Dietrich-Muszalska, A.; Kolińska-Łukaszuk, J. Comparative effects of aripiprazole and selected antipsychotic drugs on lipid peroxidation in plasma. Psychiatry Clin. Neurosci. 2018, 72, 329–336. [Google Scholar] [CrossRef]
- Dakhale, G.N.; Khanzode, S.D.; Khanzode, S.S.; Saoji, A. Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology 2005, 182, 494–498. [Google Scholar] [CrossRef]
- Balijepalli, S.; Kenchappa, R.S.; Boyd, M.R.; Ravindranath, V. Protein thiol oxidation by haloperidol results in inhibition of mitochondrial complex I in brain regions: Comparison with atypical antipsychotics. Neurochem. Int. 2001, 38, 425–435. [Google Scholar] [CrossRef]
- Miljević, Č.; Nikolić-Kokić, A.; Nikolić, M.; Niketić, V.; Spasić, M.B.; Lečić-Toševski, D.; Blagojević, D. Effect of atypical antipsychotics on antioxidant enzyme activities in human erythrocytes (in vitro study). Hum. Psychopharmacol. Clin. Exp. 2013, 28, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schillevoort, I.; de Boer, A.; Herings, R.M.; Roos, R.A.; Jansen, P.A.; Leufkens, H.G. Risk of extrapyramidal syndromes with haloperidol, risperidone, or olanzapine. Ann. Pharmacother. 2001, 35, 1517–1522. [Google Scholar] [CrossRef]
- Tollefson, G.D.; Beasley, C.M., Jr.; Tamura, R.N.; Tran, P.V.; Potvin, J.H. Blind, controlled, long-term study of the comparative incidence of treatment-emergent tardive dyskinesia with olanzapine or halperidol. Am. J. Psychiatry 1997, 154, 1248–1254. [Google Scholar]
- Yolland, C.O.; Hanratty, D.; Neill, E.; Rossell, S.L.; Berk, M.; Dean, O.M.; Castle, D.J.; Tan, E.J.; Phillipou, A.; Harris, A.W.; et al. Meta-analysis of randomised controlled trials with N-acetylcysteine in the treatment of schizophrenia. Aust. N. Z. J. Psychiatry 2020, 54, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Pyatoykina, A.S.; Zhilyaeva, T.V.; Semennov, I.V.; Mishanov, G.A.; Blagonravova, A.S.; Mazo, G.E. The double-blind randomized placebo-controlled trial of N-acetylcysteine use in schizophrenia: Preliminary results. Zhurnal Nevrol. Psikhiatrii Im. SS Korsakova 2020, 120, 66–71. [Google Scholar] [CrossRef]
- Cotton, S.M.; Berk, M.; Watson, A.; Wood, S.; Allott, K.; Bartholomeusz, C.F.; Bortolasci, C.C.; Walder, K.; O’Donoghue, B.; Dean, O.M.; et al. ENACT: A protocol for a randomised placebo-controlled trial investigating the efficacy and mechanisms of action of adjunctive N-acetylcysteine for first-episode psychosis. Trials 2019, 20, 658. [Google Scholar] [CrossRef]
- Smesny, S.; Milleit, B.; Schaefer, M.R.; Hipler, U.C.; Milleit, C.; Wiegand, C.; Hesse, J.; Klier, C.M.; Holub, M.; Holzer, I.; et al. Effects of omega-3 PUFA on the vitamin E and glutathione antioxidant defense system in individuals at ultra-high risk of psychosis. Prostaglandins Leukot. Essent. Fat. Acids 2015, 101, 15–21. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rambaud, V.; Marzo, A.; Chaumette, B. Oxidative Stress and Emergence of Psychosis. Antioxidants 2022, 11, 1870. https://doi.org/10.3390/antiox11101870
Rambaud V, Marzo A, Chaumette B. Oxidative Stress and Emergence of Psychosis. Antioxidants. 2022; 11(10):1870. https://doi.org/10.3390/antiox11101870
Chicago/Turabian StyleRambaud, Victoria, Aude Marzo, and Boris Chaumette. 2022. "Oxidative Stress and Emergence of Psychosis" Antioxidants 11, no. 10: 1870. https://doi.org/10.3390/antiox11101870
APA StyleRambaud, V., Marzo, A., & Chaumette, B. (2022). Oxidative Stress and Emergence of Psychosis. Antioxidants, 11(10), 1870. https://doi.org/10.3390/antiox11101870






