Protection against Neurological Symptoms by Consuming Corn Silk Water Extract in Artery-Occluded Gerbils with Reducing Oxidative Stress, Inflammation, and Post-Stroke Hyperglycemia through the Gut-Brain Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Extract Preparation of Corn Silk
2.2. Animals and Diets
2.3. Induction of Transient Forebrain Ischemia and Reperfusion (I/R) in Gerbils
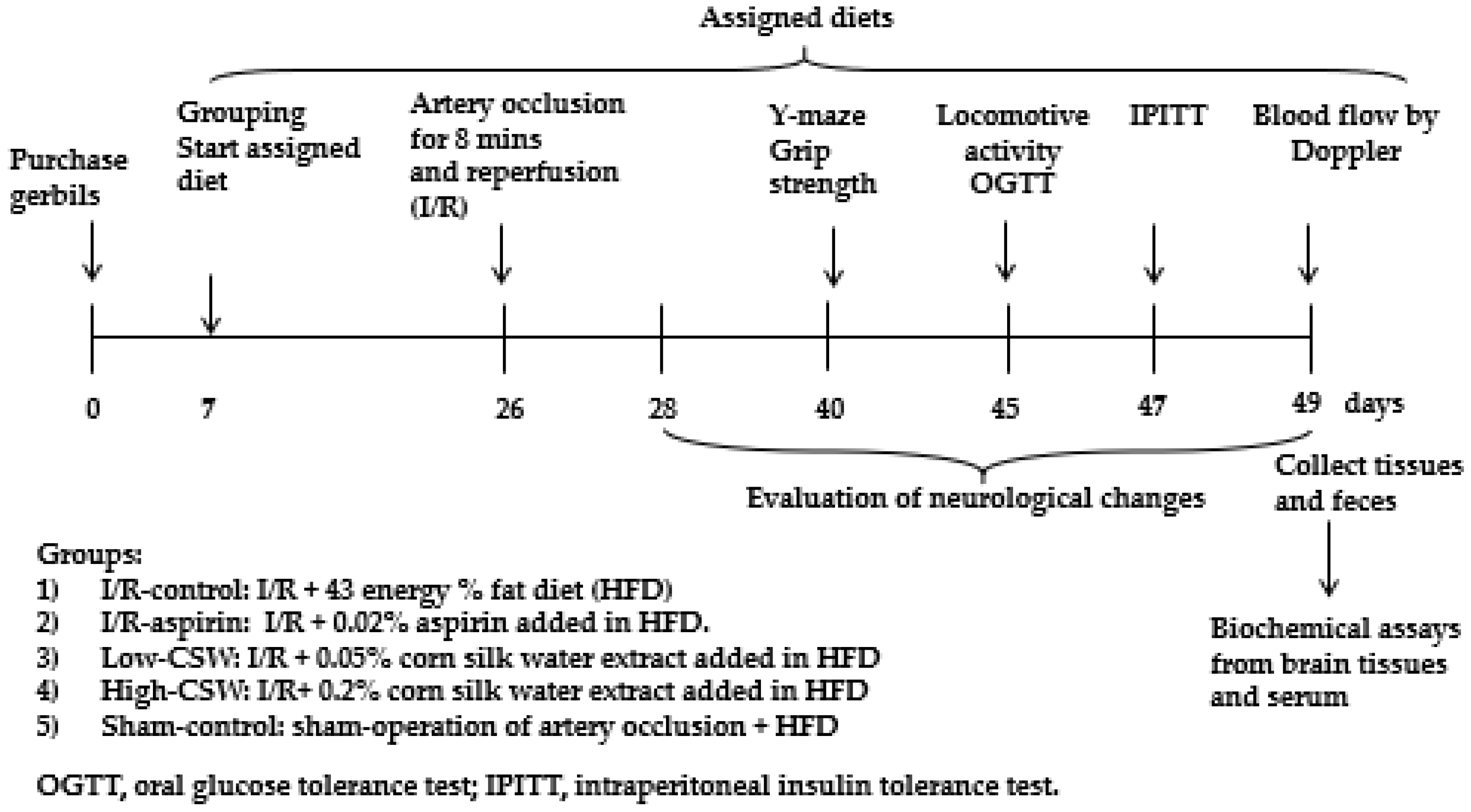
2.4. Experimental Design and Metabolic Analysis
2.5. Neurological Severity Score and Grip Strength
2.6. Memory Impairment Assessment Using Y Maze Tests
2.7. Blood Flow Determination
2.8. Determination of Lipid Peroxidation, Superoxide Anion Contents, and Superoxide Dismutase Activity in the Hippocampus
2.9. Real-Time Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) in the Hippocampus and Liver
2.10. Cresyl Violet Staining
2.11. Islet Morphometry by Immunohistochemistry
2.12. Measurement of the Serum Short-Chain Fatty Acid (SCFA) Concentrations
2.13. Next-Generation Sequencing (NGS) of Gut Microbiomes
2.14. Gut Microbiota Metabolism Predicted by PICRUSt2 Pipeline Analysis
2.15. Statistical Analysis
3. Results
3.1. Total Phenols and Flavonoids in the Corn Silk Water Extract
3.2. Energy Metabolism
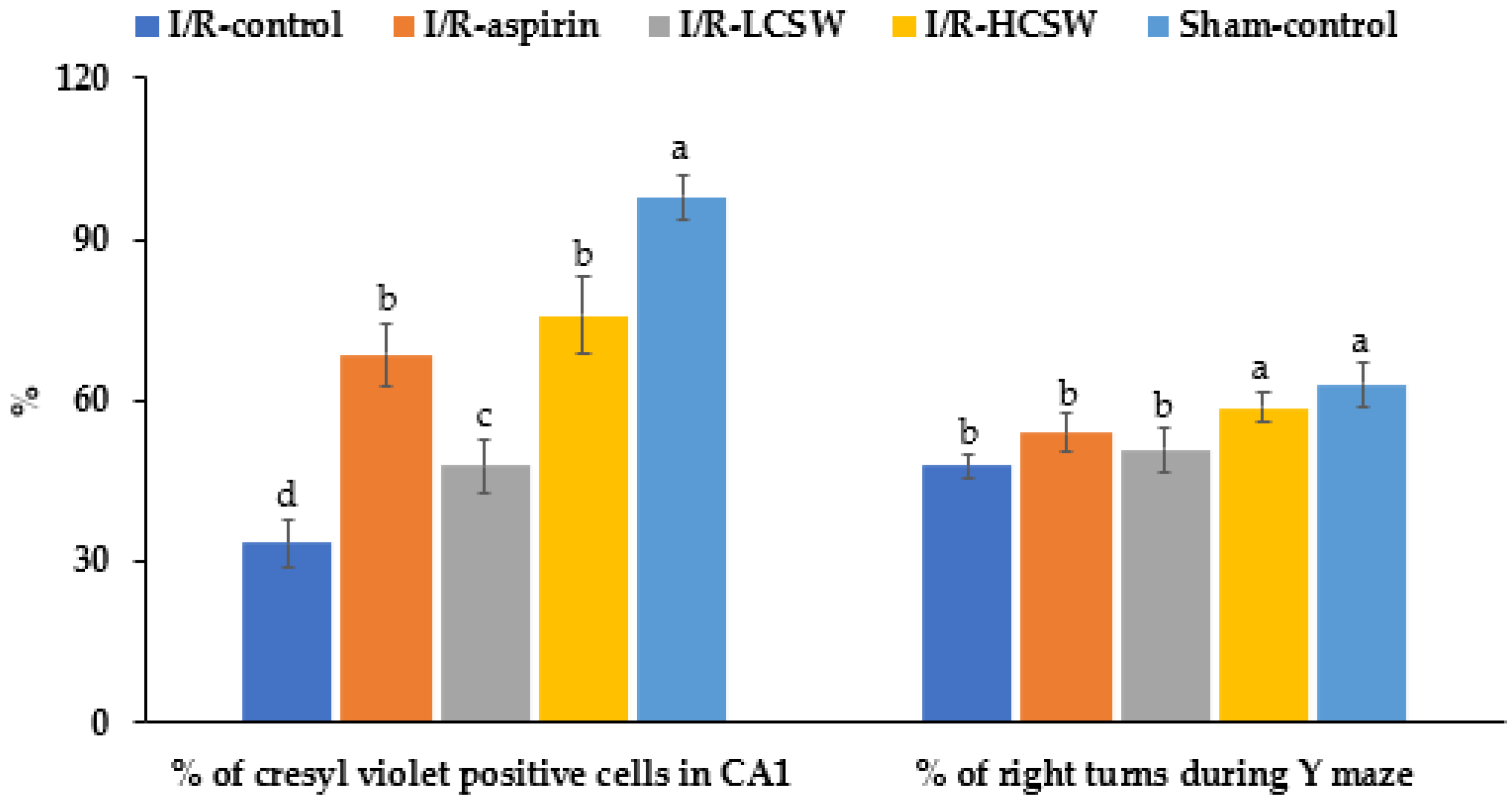
3.3. Neuronal Cell Death and Memory-Related Brain Function
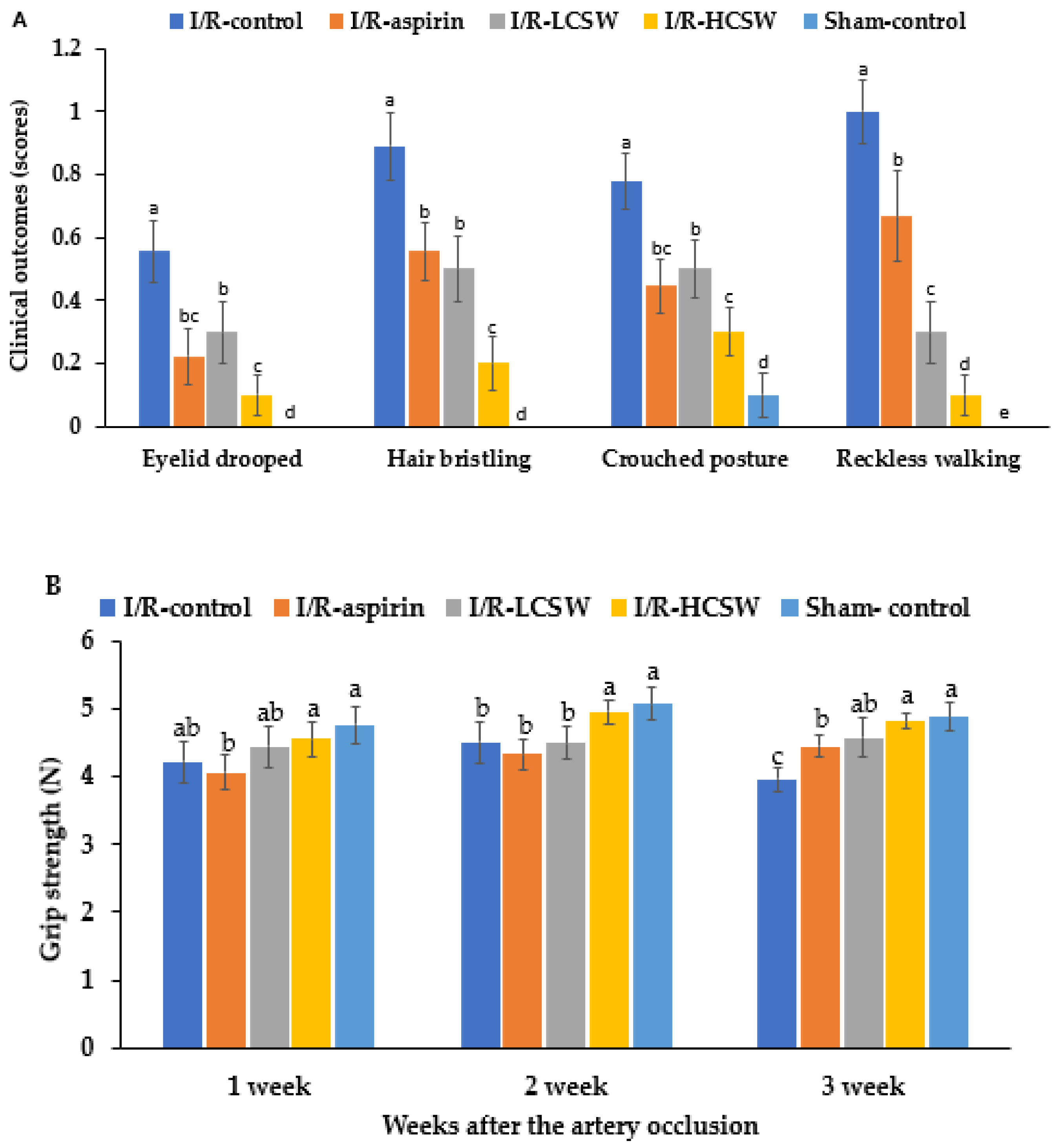
3.4. Assessment of Neurological Symptoms
3.5. Blood Flow and Lipid Profiles
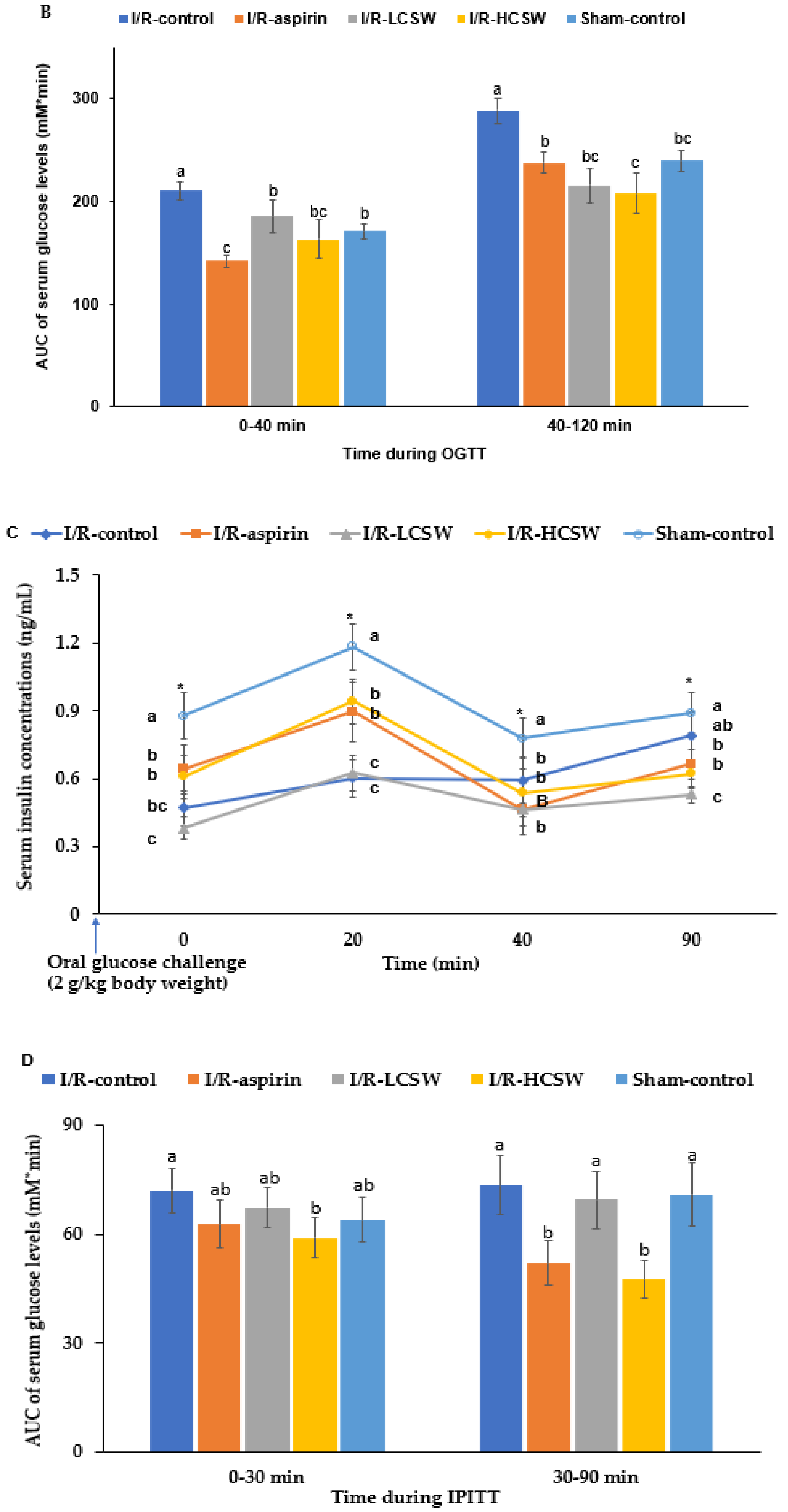
3.6. Glucose Metabolism
3.7. Pancreatic β-Cell Mass
3.8. Oxidative Stress and Inflammation in the Hippocampus
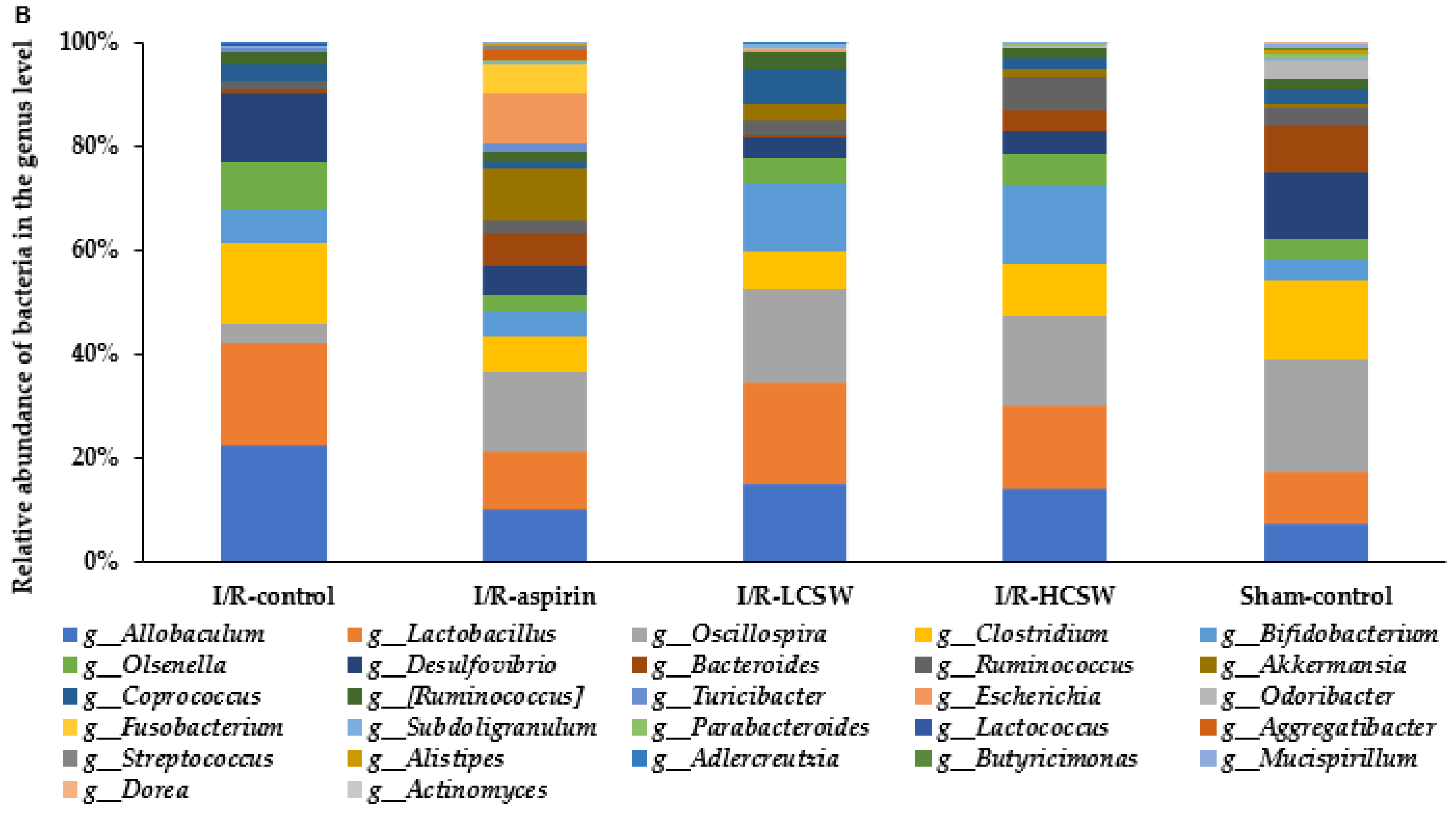
3.9. Gut Microbiome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef]
- Singh, R.J.; Chen, S.; Ganesh, A.; Hill, M.D. Long-term neurological, vascular, and mortality outcomes after stroke. Int. J. Stroke 2018, 13, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Kagansky, N.; Levy, S.; Knobler, H. The Role of Hyperglycemia in Acute Stroke. Arch. Neurol. 2001, 58, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, S.; Kim, D.S.; Shin, B.K.; Moon, N.R.; Daily, J.W., 3rd. Ebselen pretreatment attenuates ischemia/reperfusion injury and prevents hyperglycemia by improving hepatic insulin signaling and beta-cell survival in gerbils. Free Radic. Res. 2014, 48, 864–874. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Jeong, S.Y.; Zhang, T.; Wu, X.; Qiu, J.Y.; Park, S. Chungkookjang, a soy food, fermented with Bacillus amyloliquefaciens protects gerbils against ishcmeic stroke injury, and post-stroke hyperglycemia. Food Res. Int. 2020, 128, 108769. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.N. Peroxisome proliferator-activated receptor gamma agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack. Cochrane Database Syst. Rev. 2017, 12, Cd010693. [Google Scholar] [CrossRef]
- Lee, Y.T.; Mohd Ismail, N.I.; Wei, L.K. Microbiome and ischemic stroke: A systematic review. PLoS ONE 2021, 16, e0245038. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, J.; Shen, J. Herbal medicines for ischemic stroke: Combating inflammation as therapeutic targets. J. Neuroimmune Pharmacol. 2014, 9, 313–339. [Google Scholar] [CrossRef]
- Colarusso, L.; Serafini, M.; Lagerros, Y.T.; Nyren, O.; La Vecchia, C.; Rossi, M.; Ye, W.; Tavani, A.; Adami, H.O.; Grotta, A.; et al. Dietary antioxidant capacity and risk for stroke in a prospective cohort study of Swedish men and women. Nutrition 2017, 33, 234–239. [Google Scholar] [CrossRef]
- Hasanudin, K.; Hashim, P.; Mustafa, S. Cornsilk (Stigma maydis) in healthcare: A phytochemical and pharmacological review. Molecules 2012, 17, 9697–9715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Applová, L.; Karlíčková, J.; Warncke, P.; Macáková, K.; Hrubša, M.; Macháček, M.; Tvrdý, V.; Fischer, D.; Mladěnka, P. 4-Methylcatechol, a Flavonoid Metabolite with Potent Antiplatelet Effects. Mol. Nutr. Food Res. 2019, 63, 1900261. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Kang, S.; Park, S. Protection against Alzheimer’s disease by luteolin: Role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. Biofactors 2021, 47, 218–231. [Google Scholar] [CrossRef]
- Shi, S.; Li, S.; Li, W.; Xu, H. Corn Silk Tea for Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Alternat. Med. 2019, 2019, 2915498. [Google Scholar] [CrossRef]
- Shi, S.; Yu, B.; Li, W.; Shan, J.; Ma, T. Corn silk decoction for blood lipid in patients with angina pectoris: A systematic review and meta-analysis. Phytother. Res. 2019, 33, 2862–2869. [Google Scholar] [CrossRef]
- Ko, B.S.; Lee, H.W.; Kim, D.S.; Kang, S.; Ryuk, J.A.; Park, S. Supplementing with Opuntia ficus-indica Mill and Dioscorea nipponica Makino extracts synergistically attenuates menopausal symptoms in estrogen-deficient rats. J. Ethnopharmacol. 2014, 155, 267–276. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis. In Method Association of Official Analytical Communities, 19th ed.; AOAC International: Arlington, TX, USA, 2012. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Kang, S.; Kwon, D.Y. Ischemic hippocampal cell death induces glucose dysregulation by attenuating glucose-stimulated insulin secretion, which is exacerbated by a high-fat diet. Life Sci. 2011, 88, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.S.; Kang, E.S.; Kim, D.B.; Kang, S. Low-dose brain estrogen prevents menopausal syndrome while maintaining the diversity of the gut microbiomes in estrogen-deficient rats. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E99–E109. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, H.; Yamamoto, K.; Nozato, S.; Inagaki, T.; Tsuchimochi, H.; Shirai, M.; Yamamoto, R.; Imaizumi, Y.; Hongyo, K.; Yokoyama, S.; et al. Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice. Sci. Rep. 2017, 7, 42323. [Google Scholar] [CrossRef]
- Ko, B.-S.; Ryuk, J.A.; Hwang, J.T.; Zhang, T.; Wu, X.; Park, S. Ojayeonjonghwan, an oriental medicine composed of five seeds, protect against vasomotor and neurological disorders in estrogen-deficient rats. Exp. Biol. Med. 2019, 244, 193–206. [Google Scholar] [CrossRef]
- Kim, M.J.; Yang, H.J.; Moon, B.R.; Kim, J.E.; Kim, K.S.; Park, S. Gastrodia elata Blume Rhizome Aqueous Extract Improves Arterial Thrombosis, Dyslipidemia, and Insulin Response in Testosterone-Deficient Rats. Evid. Based Complem. Altern. Med. 2017, 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Rauš Balind, S.; Selaković, V.; Radenović, L.; Prolić, Z.; Janać, B. Extremely Low Frequency Magnetic Field (50 Hz, 0.5 mT) Reduces Oxidative Stress in the Brain of Gerbils Submitted to Global Cerebral Ischemia. PLoS ONE 2014, 9, e88921. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Kim, D.S.; Kang, S. Exercise training attenuates cerebral ischemic hyperglycemia by improving hepatic insulin signaling and beta-cell survival. Life Sci. 2013, 93, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Wu, X.; Yi, Q.J. Mulberry and dandelion water extracts prevent alcohol-induced steatosis with alleviating gut microbiome dysbiosis. Exp. Biol. Med. 2018, 243, 882–894. [Google Scholar] [CrossRef]
- Ryuk, J.A.; Kang, S.; Daily, J.W.; Ko, B.S.; Park, S. Moderate intake of aspartame and sucralose with meals, but not fructose, does not exacerbate energy and glucose metabolism in estrogen-deficient rats. J. Clin. Biochem. Nutr. 2019, 65, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Unno, T.; Kang, S.; Park, S. A Korean-Style Balanced Diet Has a Potential Connection with Ruminococcaceae Enterotype and Reduction of Metabolic Syndrome Incidence in Korean Adults. Nutrients 2021, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.; Lim, C. Anticancer activity of flavonoids accompanied by redox state modulation and the potential for a chemotherapeutic strategy. Food Sci. Biotechnol. 2021, 30, 321–340. [Google Scholar] [CrossRef]
- Choi, D.J.; Kim, S.L.; Choi, J.W.; Park, Y.I. Neuroprotective effects of corn silk maysin via inhibition of H2O2-induced apoptotic cell death in SK-N-MC cells. Life Sci. 2014, 109, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, T.; Han, L.; Liu, Y. The effects of corn silk on glycaemic metabolism. Nutr. Metab. 2009, 6, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayasaka, N.; Shimizu, N.; Komoda, T.; Mohri, S.; Tsushida, T.; Eitsuka, T.; Miyazawa, T.; Nakagawa, K. Absorption and Metabolism of Luteolin in Rats and Humans in Relation to in Vitro Anti-inflammatory Effects. J. Agric. Food Chem. 2018, 66, 11320–11329. [Google Scholar] [CrossRef] [PubMed]
- Del Zoppo, G.J. The role of platelets in ischemic stroke. Neurology 1998, 51, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Meschia, J.F.; Brott, T. Ischaemic stroke. Eur. J. Neurol. 2018, 25, 35–40. [Google Scholar] [CrossRef]
- Yang, M.; Pan, Y.; Li, Z.; Yan, H.; Zhao, X.; Liu, L.; Jing, J.; Meng, X.; Wang, Y.; Wang, Y. Platelet Count Predicts Adverse Clinical Outcomes After Ischemic Stroke or TIA: Subgroup Analysis of CNSR II. Front. Neurol. 2019, 10, 370. [Google Scholar] [CrossRef] [Green Version]
- Worthley, M.I.; Holmes, A.S.; Willoughby, S.R.; Kucia, A.M.; Heresztyn, T.; Stewart, S.; Chirkov, Y.Y.; Zeitz, C.J.; Horowitz, J.D. The Deleterious Effects of Hyperglycemia on Platelet Function in Diabetic Patients with Acute Coronary Syndromes: Mediation by Superoxide Production, Resolution with Intensive Insulin Administration. J. Am. Coll. Cardiol. 2007, 49, 304–310. [Google Scholar] [CrossRef]
- Gray, C.S.; Hildreth, A.J.; Alberti, G.K.M.M.; O’Connell, J.E. Poststroke Hyperglycemia: Natural history and immediate management. Stroke 2004, 35, 122–126. [Google Scholar] [CrossRef] [Green Version]
- Vallance, T.M.; Ravishankar, D.; Albadawi, D.A.I.; Osborn, H.M.I.; Vaiyapuri, S. Synthetic Flavonoids as Novel Modulators of Platelet Function and Thrombosis. Int. J. Mol. Sci. 2019, 20, 3106. [Google Scholar] [CrossRef] [Green Version]
- Faggio, C.; Sureda, A.; Morabito, S.; Sanches-Silva, A.; Mocan, A.; Nabavi, S.F.; Nabavi, S.M. Flavonoids and platelet aggregation: A brief review. Eur. J. Pharmacol. 2017, 807, 91–101. [Google Scholar] [CrossRef]
- Arthur, J.F.; Gardiner, E.E.; Kenny, D.; Andrews, R.K.; Berndt, M.C. Platelet receptor redox regulation. Platelets 2008, 19, 1–8. [Google Scholar] [CrossRef]
- Ho, T.-Y.; Li, C.-C.; Lo, H.-Y.; Chen, F.-Y.; Hsiang, C.-Y. Corn Silk Extract and Its Bioactive Peptide Ameliorated Lipopolysaccharide-Induced Inflammation in Mice via the Nuclear Factor-κB Signaling Pathway. J. Agric. Food Chem. 2017, 65, 759–768. [Google Scholar] [CrossRef]
- Wajngarten, M.; Silva, G.S. Hypertension and Stroke: Update on Treatment. Eur. Cardiol. 2019, 14, 111–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, J.H.; Kim, S.R.; Kang, H.J.; Kim, M.H.; Ha, A.W.; Kim, W.K. Corn silk extract improves cholesterol metabolism in C57BL/6J mouse fed high-fat diets. Nutr. Res. Prac. 2016, 10, 501–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winek, K.; Dirnagl, U.; Meisel, A. The Gut Microbiome as Therapeutic Target in Central Nervous System Diseases: Implications for Stroke. Neurotherapeutics 2016, 13, 762–774. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Xiong, Q.; Stary, C.M.; Mahgoub, O.K.; Ye, Y.; Gu, L.; Xiong, X.; Zhu, S. Bidirectional gut-brain-microbiota axis as a potential link between inflammatory bowel disease and ischemic stroke. J. Neuroinflamm. 2018, 15, 339. [Google Scholar] [CrossRef] [PubMed]
- Winek, K.; Meisel, A.; Dirnagl, U. Gut microbiota impact on stroke outcome: Fad or fact? J. Cereb. Blood Flow Metab. 2016, 36, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef] [Green Version]
- Takahama, U.; Hirota, S. Interactions of flavonoids with α-amylase and starch slowing down its digestion. Food Funct. 2018, 9, 677–687. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Daily, J.W.; Lee, G.H.; Ryu, M.S.; Yang, H.J.; Jeong, S.Y.; Qiu, J.Y.; Zhang, T.; Park, S. Short-Term Fermented Soybeans with Bacillus amyloliquefaciens Potentiated Insulin Secretion Capacity and Improved Gut Microbiome Diversity and Intestinal Integrity to Alleviate Asian Type 2 Diabetic Symptoms. J. Agric. Food Chem. 2020, 68, 13168–13178. [Google Scholar] [CrossRef]
- Tan, B.Y.Q.; Paliwal, P.R.; Sharma, V.K. Gut Microbiota and Stroke. Ann. Indian Acad. Neurol. 2020, 23, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, K.; Tanaka, R.; Urabe, T.; Ueno, Y.; Yamashiro, Y.; Nomoto, K.; Takahashi, T.; Tsuji, H.; Asahara, T.; Hattori, N. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS ONE 2017, 12, e0171521. [Google Scholar]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef]


| I/R-Control (n = 10) | I/R-Aspirin (n = 10) | I/R-LCSW (n = 10) | I/R-HCSW (n = 10) | Sham-Control (n = 10) | |
|---|---|---|---|---|---|
| Body weight gain during 1–3 weeks (g) | 3.18 ± 0.54 a | 3.39 ± 0.62 b | 1.8 ± 0.58 c | 2.02 ± 0.64 c | 3.55 ± 0.81 a |
| Body weight gain during 3–5 weeks (g) | −3.94 ± 0.72 d | 1.02 ± 0.61 b | −1.20 ± 0.58 c | 1.05 ± 0.65 b | 1.85 ± 0.41 a |
| Food intake during 1–3 weeks (g/day) | 3.9 ± 0.49 a | 3.0 ± 0.42 b | 2.7 ± 0.38 b | 2.8 ± 0.37 b | 4.2 ± 0.53 a |
| Food intake during 3–5 weeks (g/day) | 2.92 ± 0.53 | 3.32 ± 0.57 | 3.14 ± 0.58 | 2.83 ± 0.56 | 3.40 ± 0.55 |
| Corn silk water extract (mg/kg bw) | 0 d | 4.1 ± 0.3 c | 19.1 ± 1.2 b | 73.2 ± 3.6 a | 0 d |
| I/R-Control (n = 10) | I/R-Aspirin (n = 10) | I/R-LCSW (n = 10) | I/R-HCSW (n = 10) | Sham-Control (n = 10) | |
|---|---|---|---|---|---|
| Peak blood pressure (BPU) | 22.6 ± 3.24 c | 23.4 ± 3.41 b,c | 26.4 ± 3.26 b | 30.4 ± 3.42 a | 28.7 ± 3.11 a |
| Periods to remove the clog (min) | 5.44 ± 0.75 a | 3.42 ± 0.43 c | 4.67 ± 0.55 b | 3.76 ± 0.58 c | 4.48 ± 0.57 b |
| Serum total cholesterol (mg/dL) | 157.4 ± 12.8 a | 153.5 ± 14.9 a | 150.4 ± 9.44 a | 135.0 ± 14.4 b | 121.2 ± 16.5 c |
| Serum HDL (mg/dL) | 48.6 ± 5.36 | 45.2 ± 3.93 | 44.0 ± 5.19 | 42.9 ± 4.85 | 48.2 ± 6.10 |
| Serum LDL (mg/dL) | 75.6 ± 6.81 a | 80.1 ± 8.60 a | 73.0 ± 7.00 a | 63.6 ± 7.13 b | 46.4 ± 6.26 c |
| Serum triglyceride (mg/dL) | 166.2 ± 14.0 a | 140.9 ± 14.8 b | 167.2 ± 10.7 a | 142.2 ± 16.0 b | 148.9 ± 16.5 b |
| Serum glucose levels (mg/dL) | 94.3 ± 1.68 a | 84.7 ± 2.17 b | 83.8 ± 4.04 b | 76.4 ± 3.28 c | 78.3 ± 4.08 c |
| Serum insulin levels (ng/mL) | 0.47 ± 0.07 b | 0.64 ± 0.09 a | 0.43 ± 0.05 b | 0.61 ± 0.09 a | 0.65 ± 0.10 a |
| Hepatic expression of SREBP1c (AU) | 1.0 ± 0.0 a | 0.98 ± 0.11 a | 0.94 ± 0.10 a | 0.84 ± 0.09 b | 0.81 ± 0.09 b |
| Hepatic expression of FAS (AU) | 1.0 ± 0.0 a | 0.78 ± 0.09 b | 0.98 ± 0.11 a | 0.83 ± 0.09 b | 0.85 ± 0.09 b |
| Variables | I/R-Control (n = 10) | I/R-Aspirin (n = 10) | I/R-LCSW (n = 10) | I/R-HCSW (n = 10) | Sham-Control (n = 10) |
|---|---|---|---|---|---|
| Individual β-cell size (μm2) | 7.97 ± 0.98 a | 6.76 ± 0.70 b | 6.94 ± 0.77 b | 6.28 ± 0.73 b,c | 6.05 ± 0.71 c |
| β-cell area (%) | 20.3 ± 3.15 c | 24.9 ± 3.34 b | 23.9 ± 3.04 b | 27.7 ± 3.46 a | 27.9 ± 3.28 a |
| Total β-cell mass (mg) | 0.94 ± 0.12 c | 1.12 ± 0.14 b | 1.13 ± 0.14 b | 1.28 ± 0.15 a | 1.24 ± 0.14 a |
| BrdU+ cells (% BrdU+ cells of islets) | 5.11 ± 0.59 b | 5.61 ± 0.66 a,b | 5.58 ± 0.71 a,b | 6.11 ± 0.67 a | 5.97 ± 0.65 a |
| Apoptosis (% apoptotic bodies of islets) | 22.1 ± 2.54 a | 16.2 ± 2.02 c | 18.5 ± 2.14 b | 15.7 ± 1.74 c | 14.6 ± 1.59 c |
| I/R-Control (n = 10) | I/R-Aspirin (n = 10) | I/R-LCSW (n = 10) | I/R-HCSW (n = 10) | Sham-Control (n = 10) | |
|---|---|---|---|---|---|
| Hippocampal lipid peroxides (MDA μmol/g tissue) | 0.43 ± 0.06 a | 0.30 ± 0.05 b | 0.33 ± 0.05 b | 0.25 ± 0.04 c | 0.21 ± 0.04 c |
| Superoxide contents in the hippocampus (μM NBT/mg protein) | 6.83 ± 0.49 a | 5.85 ± 0.52 b | 5.91 ± 0.46 b | 5.14 ± 0.43 c | 5.02 ± 0.41 c |
| SOD in the hippocampus (U/mg protein) | 503 ± 44 a | 581 ± 39 b | 573 ± 42 b | 625 ± 45 c | 638 ± 42 c |
| Relative mRNA expression of hippocampal TNF-α (AU) | 1.0 ± 0 a | 0.71 ± 0.09 b,c | 0.79 ± 0.09 b | 0.66 ± 0.08 c | 0.60 ± 0.07 c |
| Relative mRNA expression of hippocampal IL-1β (AU) | 1.0 ± 0 a | 0.76 ± 0.08 b | 0.78 ± 0.09 b | 0.72 ± 0.09 b,c | 0.66 ± 0.08 c |
| Serum interleukin-1β levels (pg/mL) | 10.7 ± 1.17 a | 7.84 ± 0.88 c | 8.78 ± 0.91 b | 7.94 ± 0.84 c | 7.76 ± 0.79 c |
| Serum TNF-α levels (pg/mL) | 24.8 ± 2.94 a | 18.4 ± 2.17 c | 21.4 ± 2.51 b | 18.9 ± 2.14 c | 17.5 ± 1.94 c |
| I/R-Control (n = 10) | I/R-Aspirin (n = 10) | I/R-LCSW (n = 10) | I/R-HCSW (n = 10) | Sham-Control (n = 10) | |
|---|---|---|---|---|---|
| SCFA by gas chromatography | |||||
| Serum acetate | 0.782 ± 0.003 b | 0.837 ± 0.011 b | 1.036 ± 0.003 a | 0.958 ± 0.006 a | 0.838 ± 0.004 b |
| Serum propionate | 0.403 ± 0.003 b | 0.411 ± 0.011 a,b | 0.404 ± 0.003 b | 0.414 ± 0.006 a | 0.408 ± 0.004 a,b |
| Serum butyrate | 0.368 ± 0.003 b | 0.368 ± 0.011 b | 0.375 ± 0.003 b | 0.384 ± 0.006 a | 0.370 ± 0.004 b |
| α-diversity | |||||
| Shannon index | 5.0 ± 0.4 c | 5.6 ± 0.6 b | 5.4 ± 0.8 b,c | 5.8 ± 0.5 b | 6.1 ± 0.6 a |
| Chao index | 9763 ± 924 b | 9562 ± 849 b | 11,467 ± 1045 a | 12,042 ± 1175 a | 12,093 ± 1345 a |
| Metagenome analysis by Picrust2 | |||||
| Propionate metabolism | 1.05 ± 0.09 b | 1.14 ± 0.04 a | 1.09 ± 0.07 a,b | 1.07 ± 0.07 b | 1.08 ± 0.06 b |
| Butanoate metabolism | 0.96 ± 0.07 b | 1.16 ± 0.10 a | 1.11 ± 0.14 a | 1.12 ± 0.06 a | 1.14 ± 0.11 a |
| LPS biosynthesis | 0.29 ± 0.04 a | 0.26 ± 0.12 a | 0.18 ± 0.06 b | 0.11 ± 0.08 b | 0.12 ± 0.09 b |
| Starch metabolism | 1.75 ± 0.16 c | 2.28 ± 0.04 b | 2.35 ± 0.45 a,b | 2.30 ± 0.05 b | 2.76 ± 0.11 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryuk, J.A.; Ko, B.S.; Moon, N.R.; Park, S. Protection against Neurological Symptoms by Consuming Corn Silk Water Extract in Artery-Occluded Gerbils with Reducing Oxidative Stress, Inflammation, and Post-Stroke Hyperglycemia through the Gut-Brain Axis. Antioxidants 2022, 11, 168. https://doi.org/10.3390/antiox11010168
Ryuk JA, Ko BS, Moon NR, Park S. Protection against Neurological Symptoms by Consuming Corn Silk Water Extract in Artery-Occluded Gerbils with Reducing Oxidative Stress, Inflammation, and Post-Stroke Hyperglycemia through the Gut-Brain Axis. Antioxidants. 2022; 11(1):168. https://doi.org/10.3390/antiox11010168
Chicago/Turabian StyleRyuk, Jin Ah, Byoung Seob Ko, Na Rang Moon, and Sunmin Park. 2022. "Protection against Neurological Symptoms by Consuming Corn Silk Water Extract in Artery-Occluded Gerbils with Reducing Oxidative Stress, Inflammation, and Post-Stroke Hyperglycemia through the Gut-Brain Axis" Antioxidants 11, no. 1: 168. https://doi.org/10.3390/antiox11010168
APA StyleRyuk, J. A., Ko, B. S., Moon, N. R., & Park, S. (2022). Protection against Neurological Symptoms by Consuming Corn Silk Water Extract in Artery-Occluded Gerbils with Reducing Oxidative Stress, Inflammation, and Post-Stroke Hyperglycemia through the Gut-Brain Axis. Antioxidants, 11(1), 168. https://doi.org/10.3390/antiox11010168







