Effect of 5-Azacitidine Treatment on Redox Status and Inflammatory Condition in MDS Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Blood Samples
2.3. Measurement of GSH and GSSG Levels
2.4. Measurement of GPx, GRd, CAT, and SOD Activities
2.5. Measurement of LPO, AOPP, and NOx Levels
2.6. Assessment of Cytokine Levels
2.7. Determination of CoQ10 Concentration in Plasma
2.8. Statistical Analysis
3. Results
3.1. An Improvement of Oxidative Status WasObserved in Untreated MDS Patients
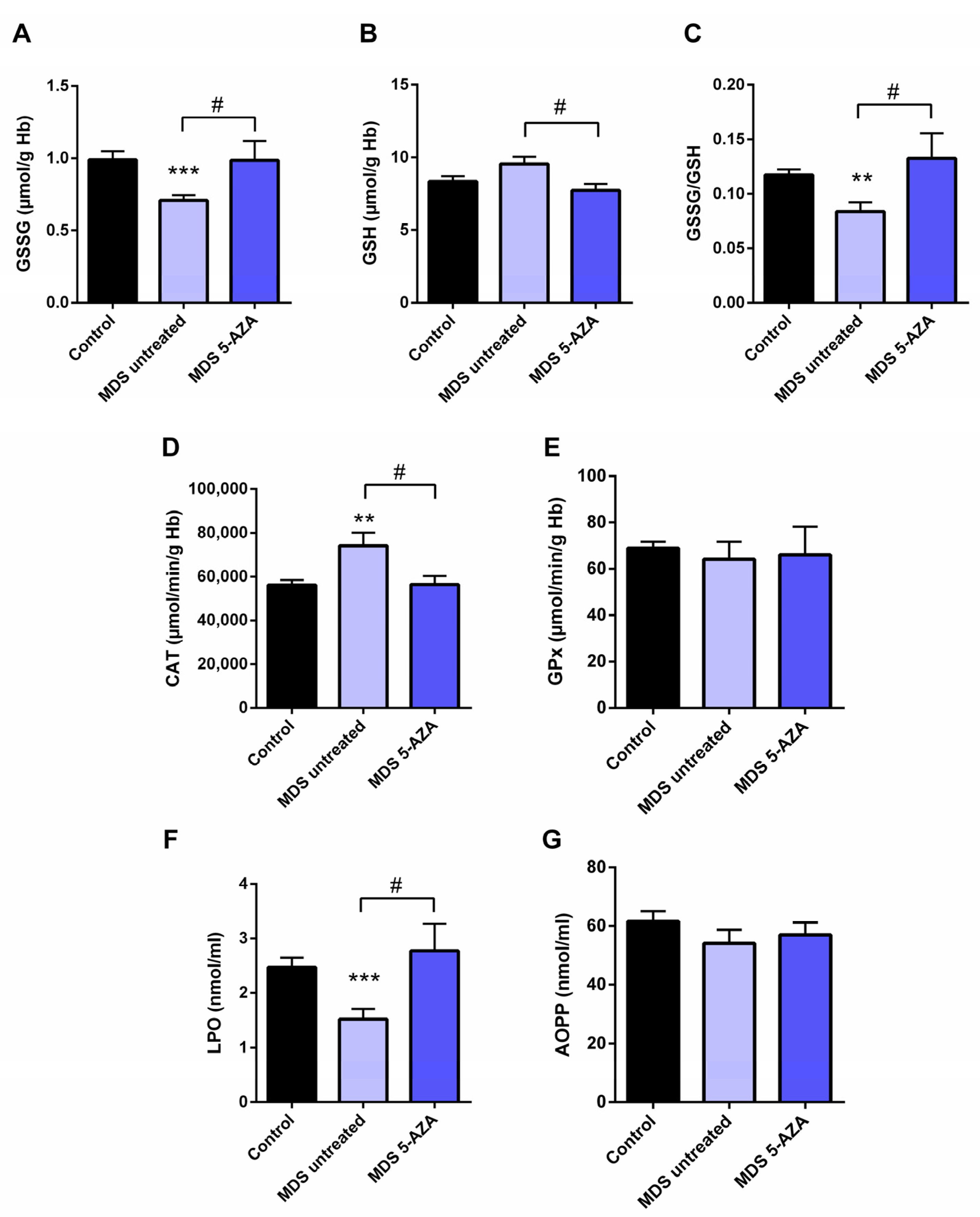
3.2. Azacitidine Increased Oxidative Stress in MDS Patients
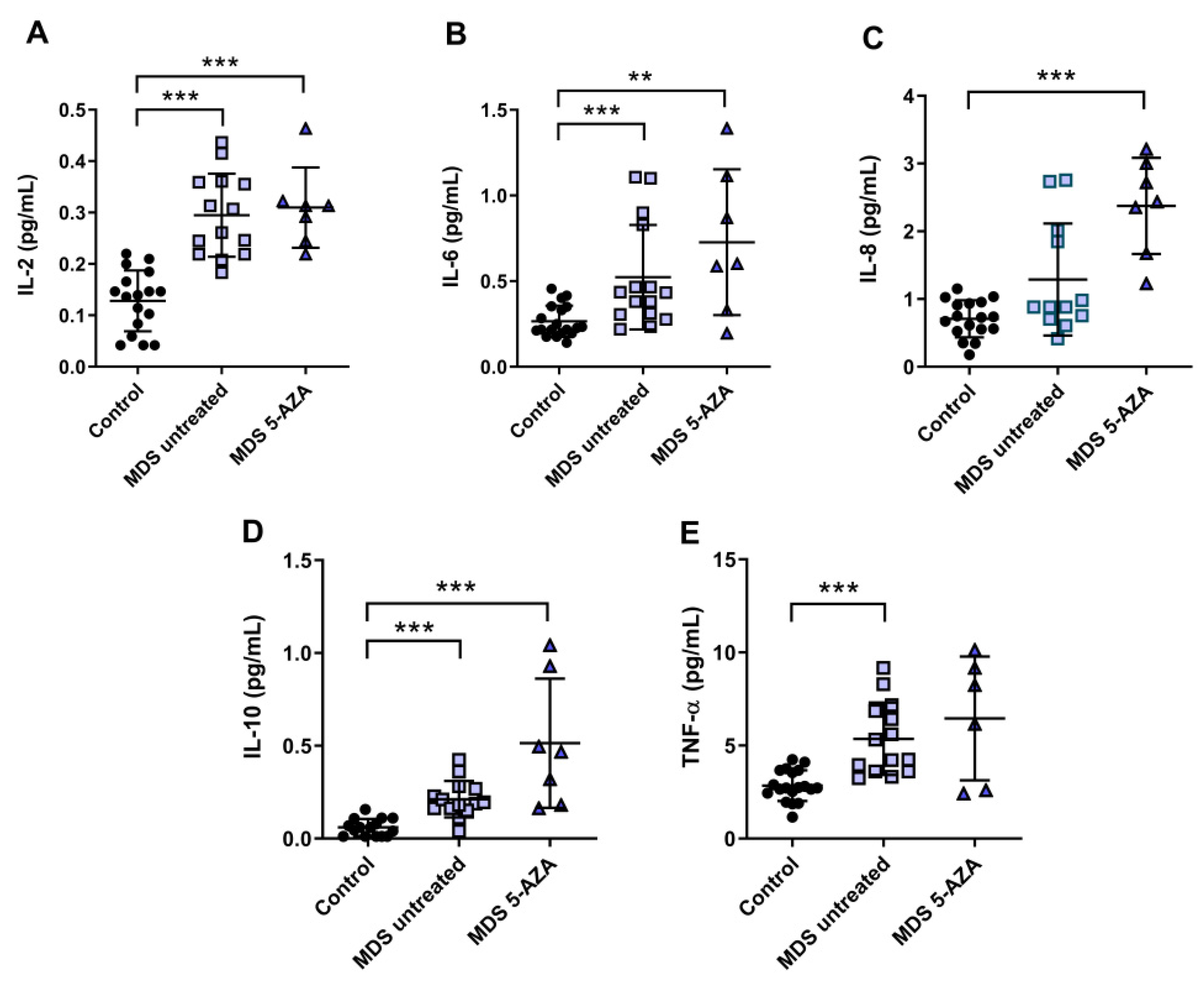
3.3. An Inflammatory Cytokine Microenvironment Was Observed in MDS Patients
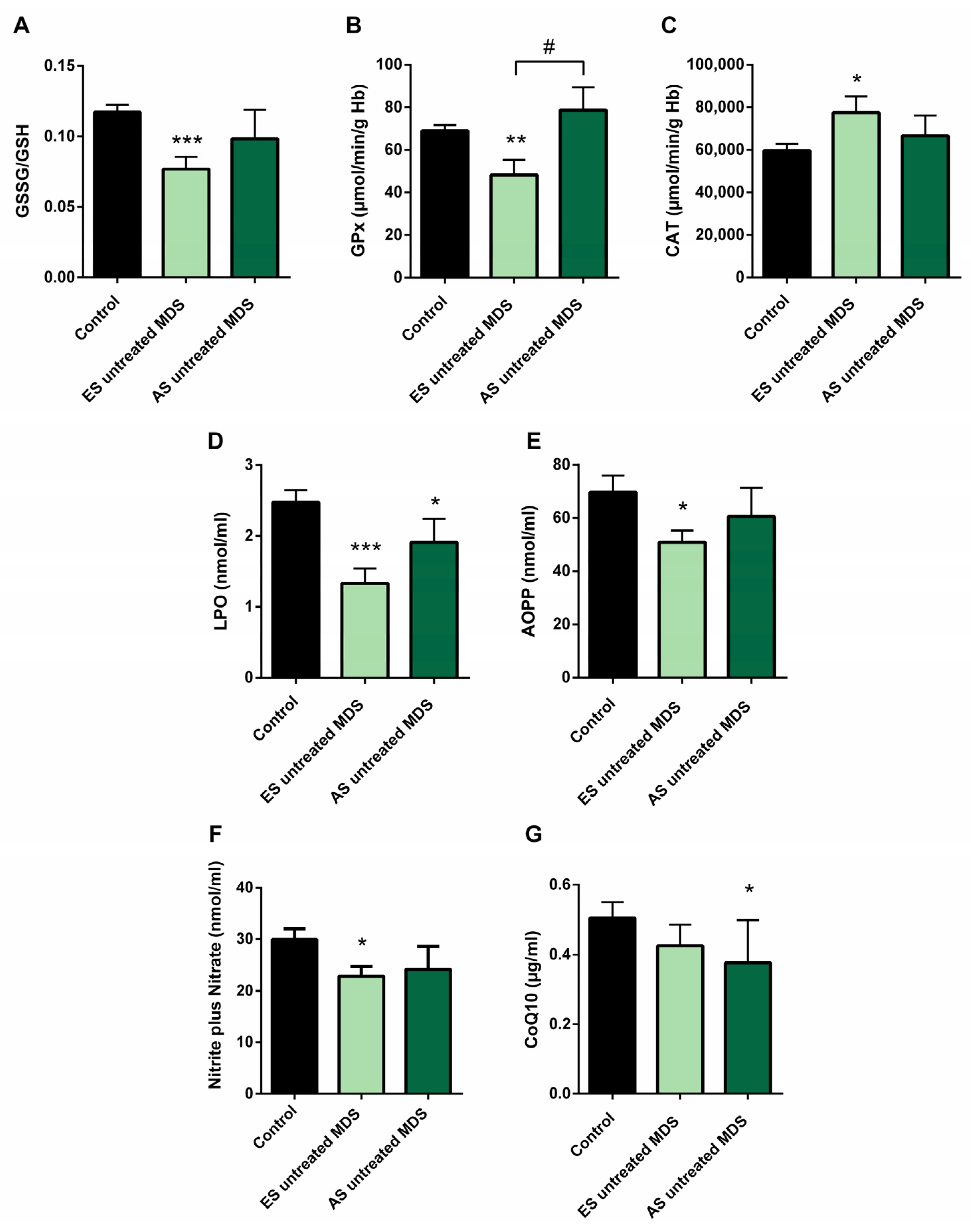
3.4. Redox Balance in MDS Patients during Disease Progression
3.5. Cytokine Profile Expression in MDS Patients Was Maintained during Disease Progression
3.6. Oxidative Stress and Risk Prognostic Factors in MDS Patients
3.7. Leukocyte Populations in Peripheral Blood of MDS Patients
4. Discussion
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Garcia-Manero, G.; Chien, K.S.; Montalban-Bravo, G. Myelodysplastic syndromes: 2021 update on diagnosis, risk stratification and management. Am. J. Hematol. 2020, 95, 1399–1420. [Google Scholar] [CrossRef]
- Ma, X. Epidemiology of myelodysplastic syndromes. Am. J. Med. 2012, 125, S2. [Google Scholar] [CrossRef] [Green Version]
- Chiereghin, C.; Travaglino, E.; Zampini, M.; Saba, E.; Saitta, C.; Riva, E.; Bersanelli, M.; Della Porta, M.G. The genetics of myelodysplastic syndromes: Clinical relevance. Genes 2021, 12, 1144. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.F.; Malik, U.A.; Sohail, M.; Hassan, I.N.; Ali, S.; Shaukat, M.H.S. Cytogenetic abnormalities in myelodysplastic syndromes: An overview. Int. J. Hematol. Stem Cell Res. 2017, 11, 232–240. [Google Scholar]
- Bejar, R.; Steensma, D.P. Recent developments in myelodysplastic syndromes. Blood 2014, 124, 2793–2803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montes, P.; Kerick, M.; Bernal, M.; Hernández, F.; Jiménez, P.; Garrido, P.; Márquez, A.; Jurado, M.; Martin, J.; Garrido, F.; et al. Genomic loss of HLA alleles may affect the clinical outcome in low-risk myelodysplastic syndrome patients. Oncotarget 2018, 9, 36929–36944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montes, P.; Bernal, M.; Campo, L.N.; González-Ramírez, A.R.; Jiménez, P.; Garrido, P.; Jurado, M.; Garrido, F.; Ruiz-Cabello, F.; Hernández, F. Tumor genetic alterations and features of the immune microenvironment drive myelodysplastic syndrome escape and progression. Cancer Immunol. Immunother. 2019, 68, 2015–2027. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Fenaux, P.; Ades, L. Review of azacitidine trials in Intermediate-2-and High-risk myelodysplastic syndromes. Leuk. Res. 2009, 33, S7–S11. [Google Scholar] [CrossRef]
- Kuhn, V.; Diederich, L.; Keller, T.C.S.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef] [PubMed]
- Peddie, C.M.; Wolf, C.R.; Mclellan, L.I.; Collins, A.R.; Bowen, D.T. Oxidative DNA damage in CD34+ myelodysplastic cells is associated with intracellular redox changes and elevated plasma tumour necrosis factor-α concentration. Br. J. Haematol. 1997, 99, 625–631. [Google Scholar] [CrossRef]
- Basiorka, A.A.; McGraw, K.L.; Eksioglu, E.A.; Chen, X.; Johnson, J.; Zhang, L.; Zhang, Q.; Irvine, B.A.; Cluzeau, T.; Sallman, D.A.; et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood 2016, 128, 2960–2975. [Google Scholar] [CrossRef]
- Jamaluddin, M.; Wang, S.; Boldogh, I.; Tian, B.; Brasier, A.R. TNF-α-induced NF-κB/RelA Ser276 phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway. Cell. Signal. 2007, 19, 1419–1433. [Google Scholar] [CrossRef]
- Chung, Y.J.; Robert, C.; Gough, S.M.; Rassool, F.V.; Aplan, P.D. Oxidative stress leads to increased mutation frequency in a murine model of myelodysplastic syndrome. Leuk. Res. 2014, 38, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivars, D.; Orero, M.T.; Javier, K.; Díaz-Vico, L.; García-Giménez, J.L.; Mena, S.; Tormos, C.; Egea, M.; Pérez, P.L.; Arrizabalaga, B.; et al. Oxidative imbalance in low/intermediate-1-risk myelodysplastic syndrome patients: The influence of iron overload. Clin. Biochem. 2017, 50, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Pimková, K.; Chrastinová, L.; Suttnar, J.; Štikarová, J.; Kotlín, R.; Čermák, J.; Dyr, J.E. Plasma levels of aminothiols, nitrite, nitrate, and malondialdehyde in myelodysplastic syndromes in the context of clinical outcomes and as a consequence of iron overload. Oxid. Med. Cell. Longev. 2014, 2014, 416028. [Google Scholar] [CrossRef] [PubMed]
- Picou, F.; Vignon, C.; Debeissat, C.; Lachot, S.; Kosmider, O.; Gallay, N.; Foucault, A.; Estienne, M.H.; Ravalet, N.; Bene, M.C.; et al. Bone marrow oxidative stress and specific antioxidant signatures in myelodysplastic syndromes. Blood Adv. 2019, 3, 4271–4279. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.M. Changes in the Updated 2016: WHO Classification of the Myelodysplastic Syndromes and Related Myeloid Neoplasms. Clin. Lymphoma, Myeloma Leuk. 2016, 16, 607–609. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Jaskot, R.H.; Charlet, E.G.; Grose, E.C.; Grady, M.A.; Roycroft, J.H.; Roycroft, J.H. An Automated Analysis of Glutathione Peroxidase, S-Transferase, and Reductase Activity in Animal Tissue. J. Anal. Toxicol. 1983, 7, 86–88. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Balasubramaniam, P.; Malathi, A. Comparative study of hemoglobin estimated by Drabkin’s and Sahli’s methods. J. Postgrad. Med. 1992, 38, 8–9. [Google Scholar]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Green, L.C.; Ruiz de Luzuriaga, K.; Wagner, D.A.; Rand, W.; Istfan, N.; Young, V.R.; Tannenbaum, S.R. Nitrate biosynthesis in man. Proc. Natl. Acad. Sci. USA 1981, 78, 7764–7768. [Google Scholar] [CrossRef] [Green Version]
- López, L.C.; Schuelke, M.; Quinzii, C.M.; Kanki, T.; Rodenburg, R.J.T.; Naini, A.; Dimauro, S.; Hirano, M. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am. J. Hum. Genet. 2006, 79, 1125–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, S.; Kini, A.; Srivastava, V.M.; Marie Therese, M.; Nair, S.C.; Abraham, A.; Mathews, V.; George, B.; Kumar, S.; Venkatraman, A.; et al. Coexistence of aberrant hematopoietic and stromal elements in myelodysplastic syndromes. Blood Cells Mol. Dis. 2017, 66, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hole, P.S.; Zabkiewicz, J.; Munje, C.; Newton, Z.; Pearn, L.; White, P.; Marquez, N.; Hills, R.K.; Burnett, A.K.; Tonks, A.; et al. Overproduction of NOX-derived ROS in AML promotes proliferation and is associated with defective oxidative stress signaling. Blood 2013, 122, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Nedelec, M.; Csillag, M.J.G.; Boussetta, T.; Belambri, S.A.; Fay, M.; Cassinat, B.; Gougerot, M.A.P.; Dang, P.M.C.; El-Benna, J. Increased reactive oxygen species production and p47phox phosphorylation in neutrophils from myeloproliferative disorders patients with JAK2 (V617F) mutation. Haematologica 2013, 98, 1517–1524. [Google Scholar] [CrossRef]
- Bowen, D.; Wang, L.; Frew, M.; Kerr, R.; Groves, M. Antioxidant enzyme expression in myelodysplastic and acute myeloid leukemia bone marrow: Further evidence of a pathogenetic role for oxidative stress? Haematologica 2003, 88, 1070–1072. [Google Scholar]
- Jones, D.P. Redox potential of GSH/GSSG couple: Assay and biological significance. Methods Enzymol. 2002, 348, 93–112. [Google Scholar] [CrossRef]
- de Souza, G.F.; Barbosa, M.C.; de Jesus Santos, T.E.; de Jesus Ponte Carvalho, T.M.; de Freitas, R.M.; Martins, M.R.A.; Gonçalves, R.P.; Pinheiro, R.F.; Magalhães, S.M.M. Increased parameters of oxidative stress and its relation to transfusion iron overload in patients with myelodysplastic syndromes. J. Clin. Pathol. 2013, 66, 996–998. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.; Macías, M.; Escames, G.; León, J.; Acuña-Castroviejo, D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J. 2000, 14, 1677–1679. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Cortesão, E.; Oliveiros, B.; Alves, V.; Espadana, A.I.; Rito, L.; Magalhães, E.; Lobão, M.J.; Pereira, A.; Nascimento Costa, J.M.; et al. Oxidative stress and mitochondrial dysfunction play a role in myelodysplastic syndrome development, diagnosis, and prognosis: A pilot study. Free Radic. Res. 2015, 49, 1081–1094. [Google Scholar] [CrossRef]
- Jankowska, A.M.; Gondek, L.P.; Szpurka, H.; Nearman, Z.P.; Tiu, R.V.; Maciejewski, J.P. Base excision repair dysfunction in a subgroup of patients with myelodysplastic syndrome. Leukemia 2008, 22, 551–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fracchiolla, N.S.; Bamonti Catena, F.; Novembrino, C.; Ippolito, S.; Maisonneuve, P.; Cortelezzi, A. Possible association between reactive oxygen metabolites and karyotypic abnormalities in myelodysplastic syndromes. Haematologica 2003, 88, 594–597. [Google Scholar]
- Kornblau, S.M.; McCue, D.; Singh, N.; Chen, W.; Estrov, Z.; Coombes, K.R. Recurrent expression signatures of cytokines and chemokines are present and are independently prognostic in acute myelogenous leukemia and myelodysplasia. Blood 2010, 116, 4251–4261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, H.C.; Lee, Y.M.; Tsai, W.H.; Jiang, M.L.; Ho, C.H.; Ho, C.K.; Wang, S.Y. Circulating levels of thrombopoietic and inflammatory cytokines in patients with acute myeloblastic leukemia and myelodysplastic syndrome. Oncology 2002, 63, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Shetty, V.; Mundle, S.; Alvi, S.; Showel, M.; Broady-Robinson, L.T.; Dar, S.; Borok, R.; Showel, J.; Gregory, S.; Rifkin, S.; et al. Measurement of apoptosis, proliferation and three cytokines in 46 patients with myelodysplastic syndromes. Leuk. Res. 1996, 20, 891–900. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Estey, E.; Wen, S.; Pierce, S.; Kantarjian, H.; Albitar, M.; Kurzrock, R. The prognostic significance of cytokine levels in newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndromes. Cancer 2008, 113, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Kordasti, S.Y.; Afzali, B.; Lim, Z.; Ingram, W.; Hayden, J.; Barber, L.; Matthews, K.; Chelliah, R.; Guinn, B.; Lombardi, G.; et al. IL-17-producing CD4+ T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br. J. Haematol. 2009, 145, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Saito, I.; Kuwata, T.; Yoshida, S.; Yamaguchi, S.; Takahashi, M.; Tanizawa, T.; Kamiyama, R.; Hirokawa, K. Overexpression of tumor necrosis factor (TNF)-α and interferon (IFN)-γ by bone marrow cells from patients with myelodysplastic syndromes. Leukemia 1997, 11, 2049–2054. [Google Scholar] [CrossRef] [Green Version]
- Von Hoff, D.D.; Slavik, M.; Muggia, F.M. 5-Azacytidine. A new anticancer drug with effectiveness in acute myelogenous leukemia. Ann. Intern. Med. 1976, 85, 237–245. [Google Scholar] [CrossRef]
- Wang, L.; Guo, X.; Guo, X.; Zhang, X.; Ren, J. Decitabine promotes apoptosis in mesenchymal stromal cells isolated from patients with myelodysplastic syndromes by inducing reactive oxygen species generation. Eur. J. Pharmacol. 2019, 863, 172676. [Google Scholar] [CrossRef] [PubMed]
- Hasunuma, H.; Shimizu, N.; Yokota, H.; Tatsuno, I. Azacitidine decreases reactive oxygen species production in peripheral white blood cells: A case report. World J. Clin. Cases 2020, 8, 5657–5662. [Google Scholar] [CrossRef]
- Hartung, E.E.; Mukhtar, S.Z.; Shah, S.M.; Niles, L.P. 5-Azacytidine upregulates melatonin MT(1) receptor expression in rat C6 glioma cells: Oncostatic implications. Mol. Biol. Rep. 2020, 47, 4867–4873. [Google Scholar] [CrossRef] [PubMed]


| MDS Patients | |
|---|---|
| No. | 24 |
| Mean age (years) | 70 (42–88) |
| Sex | 12 male/12 female |
| WHO-2016 classification | MDS-SLD (8.3%) |
| MDS-RS-SLD (4.2%) | |
| MDS-MLD (29.2%) | |
| MDS-RS-MLD (8.3%) | |
| MDS-RS (4.2%) | |
| MDS del(5q) (8.3%) | |
| MDS EB-1 (20,8%) | |
| MDS EB-2 (16.7%) | |
| IPSS-R risk groups | Low risk (50%) (very low (VL)/low (LR)) |
| High risk (50%) (intermediate (INT)/high (HR)/very high (VHR)) | |
| Cytogenetic | Favorable (75%) |
| Intermediate (8.3%) | |
| Poor (16.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes, P.; Guerra-Librero, A.; García, P.; Cornejo-Calvo, M.E.; López, M.d.S.; Haro, T.d.; Martínez-Ruiz, L.; Escames, G.; Acuña-Castroviejo, D. Effect of 5-Azacitidine Treatment on Redox Status and Inflammatory Condition in MDS Patients. Antioxidants 2022, 11, 139. https://doi.org/10.3390/antiox11010139
Montes P, Guerra-Librero A, García P, Cornejo-Calvo ME, López MdS, Haro Td, Martínez-Ruiz L, Escames G, Acuña-Castroviejo D. Effect of 5-Azacitidine Treatment on Redox Status and Inflammatory Condition in MDS Patients. Antioxidants. 2022; 11(1):139. https://doi.org/10.3390/antiox11010139
Chicago/Turabian StyleMontes, Paola, Ana Guerra-Librero, Paloma García, María Elena Cornejo-Calvo, María del Señor López, Tomás de Haro, Laura Martínez-Ruiz, Germaine Escames, and Darío Acuña-Castroviejo. 2022. "Effect of 5-Azacitidine Treatment on Redox Status and Inflammatory Condition in MDS Patients" Antioxidants 11, no. 1: 139. https://doi.org/10.3390/antiox11010139
APA StyleMontes, P., Guerra-Librero, A., García, P., Cornejo-Calvo, M. E., López, M. d. S., Haro, T. d., Martínez-Ruiz, L., Escames, G., & Acuña-Castroviejo, D. (2022). Effect of 5-Azacitidine Treatment on Redox Status and Inflammatory Condition in MDS Patients. Antioxidants, 11(1), 139. https://doi.org/10.3390/antiox11010139








