The Effects of Dietary Protein Supplementation on Exercise-Induced Inflammation and Oxidative Stress: A Systematic Review of Human Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
3. Results
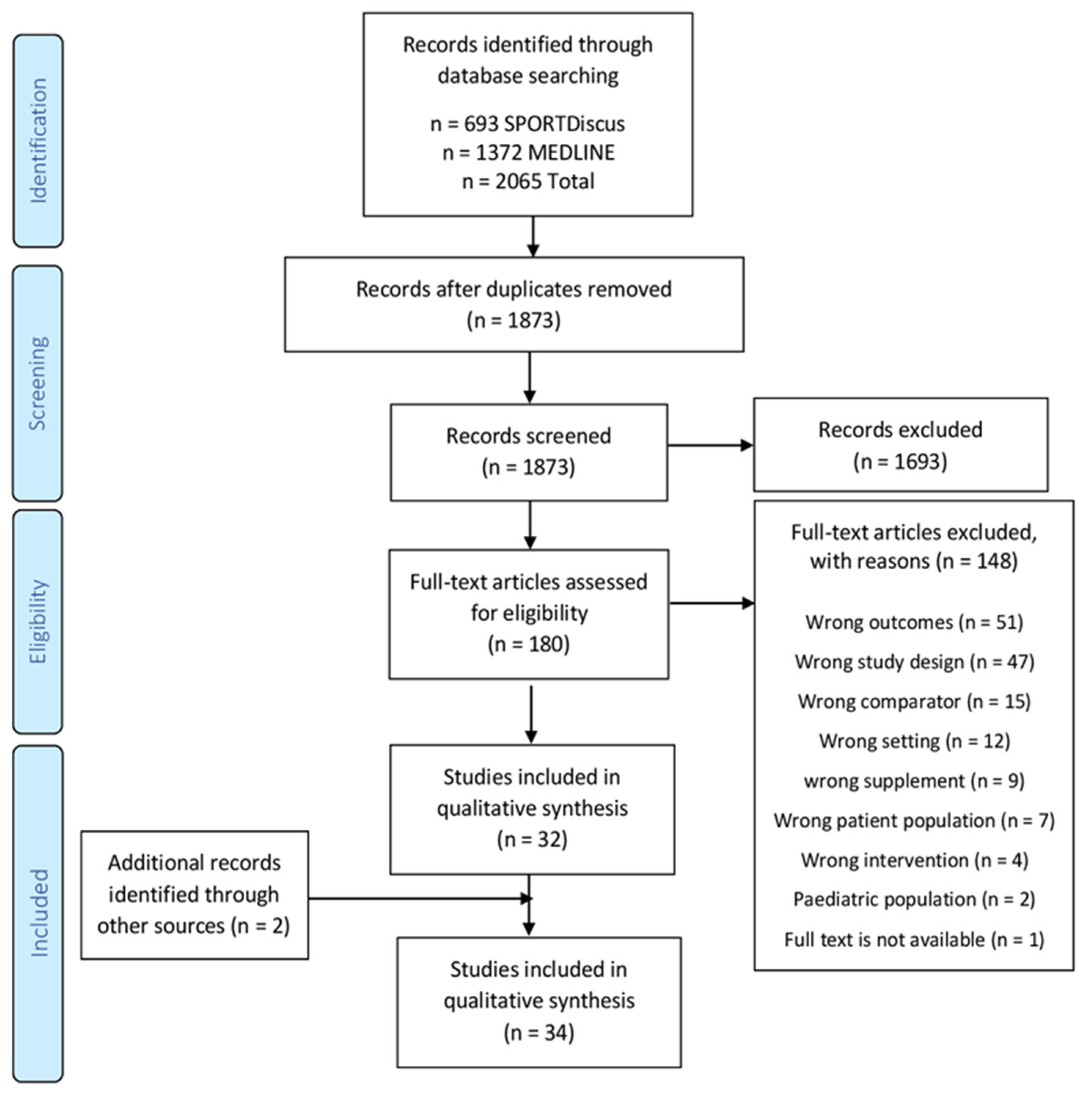
3.1. Search Results
3.2. Study Characteristics
3.3. Whole Protein Interventions
3.4. Amino Acid Interventions
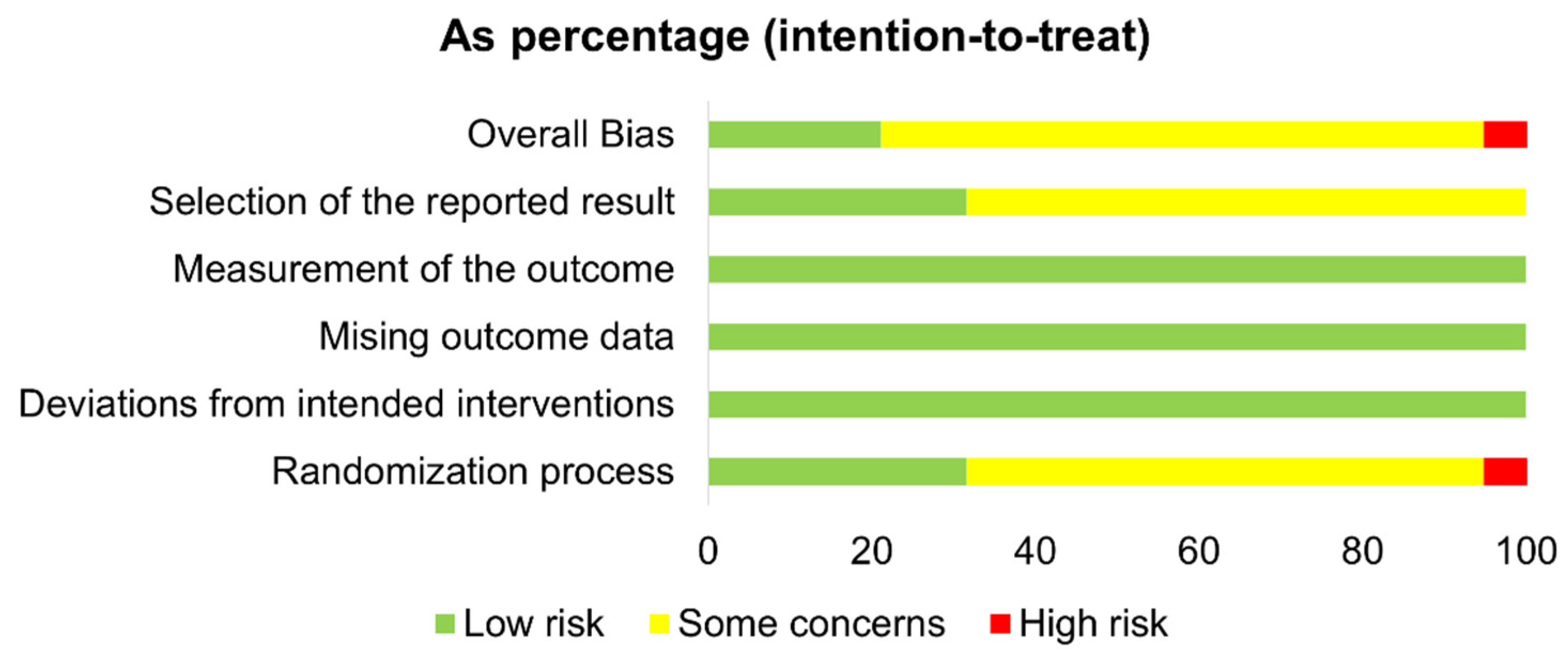
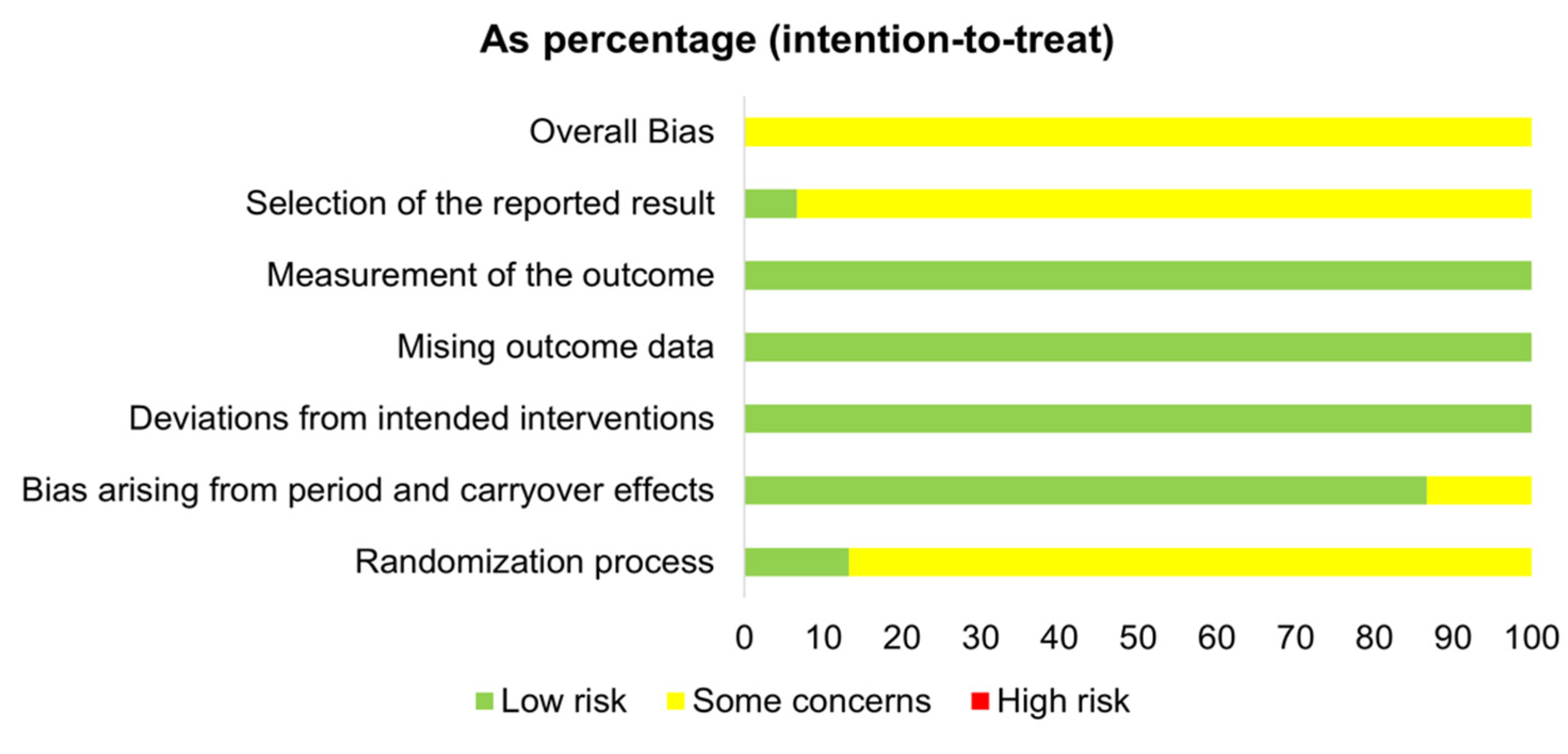
3.5. Risk of Bias
4. Discussion
5. Future Research
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hyldahl, R.D.; Hubal, M.J. Lengthening Our Perspective: Morphological, Cellular, and Molecular Responses to Eccentric Exercise, Muscle and Nerve. Muscle Nerve 2014, 49, 155–170. Available online: https://pubmed.ncbi.nlm.nih.gov/24030935/ (accessed on 6 August 2021). [CrossRef] [PubMed]
- Warren, G.; Ingalls, C.; Lowe, D.; Armstrong, R. What Mechanisms Contribute to the Strength Loss That Occurs during and in the Recovery From Skeletal Muscle Injury? J. Orthop. Sports Phys. Ther. 2002, 32, 58–64. Available online: https://pubmed.ncbi.nlm.nih.gov/11838581/ (accessed on 4 August 2021). [CrossRef] [PubMed]
- Paulsen, G.; Mikkelsen, U.R.; Raastad, T.; Peake, J.M. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar]
- Warren, G.; Lowe, D.; Armstrong, R. Measurement Tools Used in the Study of Eccentric Contraction-Induced Injury. Sports Med. 1999, 27, 43–59. Available online: https://pubmed.ncbi.nlm.nih.gov/10028132/ (accessed on 6 August 2021). [CrossRef]
- Bel, L.P.; Walshe, I.; Davison, G.; Stevenson, E.; Howatson, G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 2014, 6, 829–843. Available online: https://pubmed.ncbi.nlm.nih.gov/24566440/ (accessed on 4 August 2021). [CrossRef] [PubMed] [Green Version]
- Buford, T.; Cooke, M.; Willoughby, D. Resistance Exercise-Induced Changes of Inflammatory Gene Expression within Human Skeletal Muscle. Eur. J. Appl. Physiol. 2009, 107, 463–471. Available online: https://pubmed.ncbi.nlm.nih.gov/19669788/ (accessed on 4 August 2021). [CrossRef]
- Vella, L.; Markworth, J.F.; Peake, J.M.; Snow, R.J.; Cameron-Smith, D.; Russell, A.P. Ibuprofen supplementation and its effects on NF-κB activation in skeletal muscle following resistance exercise. Physiol. Rep. 2014, 2, e12172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draganidis, D.; Chondrogianni, N.; Chatzinikolaou, A.; Terzis, G.; Karagounis, L.G.; Sovatzidis, A.; Avloniti, A.; Lefaki, M.; Protopapa, M.; Deli, C.K.; et al. Protein ingestion preserves proteasome activity during intense aseptic inflammation and facilitates skeletal muscle recovery in humans. Br. J. Nutr. 2017, 118, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Calvo, J.S.; Martínez, A.C.; García, A.C.; Fernandez-Lazaro, C.I. Modulation of exercise-induced muscle damage, inflammation, and oxidative markers by curcumin supplementation in a physically active population: A systematic review. Nutrients 2020, 12, 501. [Google Scholar] [CrossRef] [Green Version]
- Nikolaidis, M.; Jamurtas, A.; Paschalis, V.; Fatouros, I.; Koutedakis, Y.; Kouretas, D. The Effect of Muscle-Damaging Exercise on Blood and Skeletal Muscle Oxidative Stress: Magnitude and Time-Course Considerations. Sports Med. 2008, 38, 579–606. Available online: https://pubmed.ncbi.nlm.nih.gov/18557660/ (accessed on 4 August 2021). [CrossRef]
- Pizza, F.; Koh, T.; McGregor, S.; Brooks, S. Muscle Inflammatory Cells after Passive Stretches, Isometric Contractions, and Lengthening Contractions. J. Appl. Physiol. 2002, 92, 1873–1878. Available online: https://pubmed.ncbi.nlm.nih.gov/11960936/ (accessed on 4 August 2021). [CrossRef] [Green Version]
- Suzuki, K.; Sato, H.; Kikuchi, T.; Abe, T.; Nakaji, S.; Sugawara, K.; Totsuka, M.; Sato, K.; Yamaya, K. Capacity of Circulating Neutrophils to Produce Reactive Oxygen Species after Exhaustive Exercise. J. Appl. Physiol. 1996, 81, 1213–1222. Available online: https://pubmed.ncbi.nlm.nih.gov/8889756/ (accessed on 1 October 2021). [CrossRef]
- Yamada, M.; Suzuki, K.; Kudo, S.; Totsuka, M.; Simoyama, T.; Nakaji, S.; Sugawara, K. Effect of Exhaustive Exercise on Human Neutrophils in Athletes. Luminescence 2000, 15, 15–20. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/epdf/10.1002/%28SICI%291522-7243%28200001/02%2915%3A1%3C15%3A%3AAID-BIO570%3E3.0.CO%3B2-O (accessed on 1 October 2021). [CrossRef]
- Methenitis, S.; Stergiou, I.; Antonopoulou, S.; Nomikos, T. Can exercise-induced muscle damage be a good model for the investigation of the anti-inflammatory properties of diet in humans? Biomedicines 2021, 9, 36. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited review: Whey proteins as antioxidants and promoters of cellular antioxidant pathways. J. Dairy Sci. 2018, 101, 4747–4761. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? J. Sport Health Sci. 2020, 9, 415–425. Available online: https://pubmed.ncbi.nlm.nih.gov/32380253/ (accessed on 1 October 2021). [CrossRef]
- Toumi, H.; F’guyer, S.; Best, T.M. The Role of Neutrophils in Injury and Repair Following Muscle Stretch. J. Anat. 2006, 208, 459–470. Available online: https://pmc/articles/PMC2100203/ (accessed on 4 August 2021). [CrossRef]
- Place, N.; Ivarsson, N.; Venckunas, T.; Neyroud, D.; Brazaitis, M.; Cheng, A.J.; Ochala, J.; Kamandulis, S.; Girard, S.; Volungevičius, G.; et al. Ryanodine Receptor Fragmentation and Sarcoplasmic Reticulum Ca2+ Leak after One Session of High-Intensity Interval Exercise. Proc. Natl. Acad. Sci. USA 2015, 112, 15492–15497. Available online: https://pubmed.ncbi.nlm.nih.gov/26575622/ (accessed on 1 October 2021). [CrossRef] [PubMed] [Green Version]
- Cheng, A.J.; Jude, B.; Lanner, J.T. Intramuscular Mechanisms of Overtraining. Redox Biol. 2020, 35, 101480. Available online: https://pubmed.ncbi.nlm.nih.gov/32179050/ (accessed on 1 October 2021). [CrossRef] [PubMed]
- Peake, J.M.; Markworth, J.F.; Nosaka, K.; Raastad, T.; Wadley, G.D.; Coffey, V.G. Modulating Exercise-Induced Hormesis: Does Less Equal More? J. Appl. Physiol. 2015, 119, 172–189. Available online: https://pubmed.ncbi.nlm.nih.gov/25977451/ (accessed on 1 October 2021). [CrossRef] [PubMed] [Green Version]
- Pizza, F.X.; Peterson, J.M.; Baas, J.H.; Koh, T.J. Neutrophils Contribute to Muscle Injury and Impair Its Resolution after Lengthening Contractions in Mice. J. Physiol. 2005, 562, 899–913. Available online: https://pubmed.ncbi.nlm.nih.gov/15550464/ (accessed on 1 October 2021). [CrossRef]
- Libby, P. Inflammatory Mechanisms: The Molecular Basis of Inflammation and Disease. Nutr. Rev. 2007, 65, S140–S146. Available online: https://pubmed.ncbi.nlm.nih.gov/18240538/ (accessed on 1 October 2021). [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. Available online: https://pubmed.ncbi.nlm.nih.gov/29731617/ (accessed on 4 August 2021). [CrossRef] [PubMed] [Green Version]
- Sies, H. Oxidative eustress and oxidative distress: Introductory remarks. In Oxidative Stress: Eustress and Distress; Academic Press: Cambridge, MA, USA, 2019; pp. 3–12. [Google Scholar]
- Nikolaidis, M.G.; Kyparos, A.; Dipla, K.; Zafeiridis, A.; Sambanis, M.; Grivas, G.V.; Paschalis, V.; Theodorou, A.A.; Papadopoulos, S.; Spanou, C.; et al. Exercise as A Model to Study Redox Homeostasis in Blood: The Effect of Protocol and Sampling Point. Biomarkers 2012, 17, 28–35. Available online: https://www.tandfonline.com/doi/abs/10.3109/1354750X.2011.635805 (accessed on 4 August 2021). [CrossRef] [PubMed]
- Bloomer, R.J. The Role of Nutritional Supplements in the Prevention and Treatment of Resistance Exercise-Induced Skeletal Muscle Injury. Sports Med. 2007, 37, 519–532. Available online: https://pubmed.ncbi.nlm.nih.gov/17503877/ (accessed on 1 October 2021). [CrossRef]
- Cermak, N.M.; Res, P.T.; De Groot, L.C.P.G.M.; Saris, W.H.M.; Van Loon, L.J.C. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef] [Green Version]
- Lunn, W.R.; Pasiakos, S.M.; Colletto, M.R.; Karfonta, K.E.; Carbone, J.W.; Anderson, J.M.; Rodriguez, N.R. Chocolate milk and endurance exercise recovery: Protein balance, glycogen, and performance. Med. Sci. Sports Exerc. 2012, 44, 682–691. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of Whey Hydrolysate, Casein, or Soy Protein Isolate: Effects on Mixed Muscle Protein Synthesis at Rest and Following Resistance Exercise in Young Men. J. Appl. Physiol. 2009, 107, 987–992. Available online: https://journals.physiology.org/doi/abs/10.1152/japplphysiol.00076.2009 (accessed on 1 October 2021). [CrossRef] [PubMed]
- Kerasioti, E.; Stagos, D.; Jamurtas, A.; Kiskini, A.; Koutedakis, Y.; Goutzourelas, N.; Pournaras, S.; Tsatsakis, A.M.; Kouretas, D. Anti-Inflammatory Effects of a Special Carbohydrate-Whey Protein Cake after Exhaustive Cycling in Humans. Food Chem. Toxicol. 2013, 61, 42–46. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=23360676&site=ehost-liveNS (accessed on 30 June 2021). [CrossRef]
- Draganidis, D.; Karagounis, L.G.; Athanailidis, I.; Chatzinikolaou, A.; Jamurtas, A.Z.; Fatouros, I.G. Inflammaging and Skeletal Muscle: Can Protein Intake Make a Difference? J. Nutr. 2016, 146, 1940–1952. [Google Scholar] [CrossRef]
- Athira, S.; Mann, B.; Sharma, R.; Kumar, R. Ameliorative potential of whey protein hydrolysate against paracetamol-induced oxidative stress. J. Dairy Sci. 2013, 96, 1431–1437. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Paik, H.D.; Yoon, Y.C.; Park, E. Whey protein inhibits iron overload-induced oxidative stress in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2013, 59, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebaid, H.; Salem, A.; Sayed, A.; Metwalli, A. Whey protein enhances normal inflammatory responses during cutaneous wound healing in diabetic rats. Lipids Health Dis. 2011, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Liu, N.; Xu, X.; Kong, B. Antioxidative effects of whey protein on peroxide-induced cytotoxicity. J. Dairy Sci. 2011, 94, 3739–3746. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.M.; Zhang, L.; Yu, H.X.; Meng, J.; Sun, Z.; Lu, R.R. Protective effect of whey protein hydrolysates on H2O 2-induced PC12 cells oxidative stress via a mitochondria-mediated pathway. Food Chem. 2013, 141, 847–852. [Google Scholar] [CrossRef]
- Kerasioti, E.; Stagos, D.; Priftis, A.; Aivazidis, S.; Tsatsakis, A.M.; Hayes, A.W.; Kouretas, D. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014, 155, 271–278. [Google Scholar] [CrossRef]
- Burris, R.L.; Ng, H.P.; Nagarajan, S. Soy protein inhibits inflammation-induced VCAM-1 and inflammatory cytokine induction by inhibiting the NF-κB and AKT signaling pathway in apolipoprotein E-deficient mice. Eur J. Nutr. 2014, 53, 135–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreeja, S.; Geetha, R.; Priyadarshini, E.; Bhavani, K.; Anuradha, C.V. Substitution of Soy Protein for Casein Prevents Oxidative Modification and Inflammatory Response Induced in Rats Fed High Fructose Diet. ISRN Inflamm. Int. Sch. Res. Not. 2014, 2014, 641096. [Google Scholar]
- Wheeler, M.D.; Ikejema, K.; Enomoto, N.; Stacklewitz, R.F.; Seabra, V.; Zhong, Z.; Yin, M.; Schemmer, P.; Rose, M.L.; Rusyn, I.; et al. Glycine: A new anti-inflammatory immunonutrient. Cell. Mol. Life Sci. 1999, 56, 843–856. [Google Scholar] [CrossRef]
- Raizel, R.; Leite, J.S.M.; Hypólito, T.M.; Coqueiro, A.Y.; Newsholme, P.; Cruzat, V.F.; Tirapegui, J. Determination of the Anti-Inflammatory and Cytoprotective Effects of L-Glutamine and L-Alanine, or Dipeptide, Supplementation in Rats Submitted to Resistance Exercise. Br. J. Nutr. 2016, 116, 470–479. Available online: https://pubmed.ncbi.nlm.nih.gov/27215379/ (accessed on 1 October 2021). [CrossRef] [PubMed] [Green Version]
- Wischmeyer, P.E.; Riehm, J.; Singleton, K.D.; Ren, H.; Musch, M.W.; Kahana, M.; Chang, E.B. Glutamine attenuates tumor necrosis factor-α release and enhances heat shock protein 72 in human peripheral blood mononuclear cells. Nutrition 2003, 19, 1–6. [Google Scholar] [CrossRef]
- Liboni, K.C.; Li, N.; Scumpia, P.O.; Neu, J. Glutamine modulates LPS-induced IL-8 production through IκB/NF-κB in human fetal and adult intestinal epithelium. J. Nutr. 2005, 135, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicastro, H.; Da Luz, C.R.; Chaves, D.F.S.; Bechara, L.R.G.; Voltarelli, V.A.; Rogero, M.M.E.; Lancha, A.H. Does branched-chain amino acids supplementation modulate skeletal muscle remodeling through inflammation modulation? Possible mechanisms of action. J. Nutr. Metab. 2012, 2012, 136937. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, M.; Stachlewitz, R.F.; Yamashina, S.; Ikejima, K.; Morrow, A.L.; Thurman, R.G. Glycine-gated chloride channels in neutrophils attenuate calcium influx and superoxide production. FASEB J. 2000, 14, 476–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volek, J.S.; Judelson, D.A.; Silvestre, R.; Yamamoto, L.M.; Spiering, B.A.; Hatfield, D.L.; Vingren, J.L.; Quann, E.E.; Anderson, J.M.; Maresh, C.M.; et al. Effects of carnitine supplementation on flow-mediated dilation and vascular inflammatory responses to a high-fat meal in healthy young adults. Am. J. Cardiol. 2008, 102, 1413–1417. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=18993165&site=ehost-live (accessed on 5 August 2021). [CrossRef]
- Gomez-Cabrera, M.C.; Close, G.L.; Kayani, A.; McArdle, A.; Viña, J.; Jackson, M.J. Effect of xanthine oxidase-generated extracellular superoxide on skeletal muscle force generation. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 298, R2–R8. [Google Scholar] [CrossRef] [Green Version]
- Nakhostin-Roohi, B.; Javanamani, R.; Zardoost, N.; Ramazanzadeh, R. Influence of glutamine supplementation on muscle damage and oxidative stress indices following 14km running. Hormozgan Med. J. 2016, 20, 323–331. [Google Scholar]
- Parandak, K.; Arazi, H.; Khoshkhahesh, F.; Nakhostin-Roohi, B. The Effect of Two-Week L-Carnitine Supplementation on Exercise -Induced Oxidative Stress and Muscle Damage. Asian J. Sports Med. 2014, 5, 123. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4374610/ (accessed on 5 August 2021).
- Rowlands, D.S.; Nelson, A.R.; Raymond, F.; Metairon, S.; Mansourian, R.; Clarke, J.; Stellingwerf, T.; Phillips, S.M. Protein-Leucine Ingestion Activates a Regenerative Inflammo-Myogenic Transcriptome in Skeletal Muscle Following Intense Endurance Exercise. Physiol. Genom. 2016, 48, 21–32. Available online: https://journals.physiology.org/doi/abs/10.1152/physiolgenomics.00068.2015 (accessed on 5 August 2021). [CrossRef] [Green Version]
- Hilkens, L.; De Bock, J.; Kretzers, J.; Kardinaal, A.F.M.; Floris-Vollenbroek, E.G.; Scholtens, P.A.M.J.; Horstman, A.M.H.; van Loon, L.J.C.; van Dijk, J.-W. Whey Protein Supplementation Does Not Accelerate Recovery from A Single Bout of Eccentric Exercise. J. Sports Sci. 2021, 39, 322–331. Available online: https://www.tandfonline.com/doi/full/10.1080/02640414.2020.1820184 (accessed on 4 August 2021). [CrossRef]
- Wojcik, J.R.; Walberg-Rankin, J.; Smith, L.L.; Gwazdauskas, F.C. Comparison of carbohydrate and milk-based beverages on muscle damage and glycogen following exercise. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Ventress, M.; Allerton, D.M.; Stansfield, S.; Tang, J.C.Y.Y.; Fraser, W.D.; Vanhoecke, B.; Prawitt, J.; Stevenson, E. The Effects of Collagen Peptides on Muscle Damage, Inflammation and Bone Turnover Following Exercise: A Randomized, Controlled Trial. Amino. Acids 2019, 51, 691–704. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=30783776&site=ehost-liveNS (accessed on 30 June 2021). [CrossRef] [Green Version]
- Nieman, D.C.; Zwetsloot, K.A.; Simonson, A.J.; Hoyle, A.T.; Wang, X.; Nelson, H.K.; Lefranc-Millot, C.; Guérin-Deremaux, L. Effects of Whey and Pea Protein Supplementation on Post-Eccentric Exercise Muscle Damage: A Randomized Trial. Nutrients 2020, 12, 2382. Available online: http://pmc/articles/PMC7468723/ (accessed on 5 August 2021). [CrossRef]
- Wilson, J.M.; Lowery, R.P.; Joy, J.M.; Walters, J.A.; Baier, S.M.; Fuller, J.C.; Stout, J.R.; Norton, L.E.; Sikorski, E.M.; Wilson, S.M.C.; et al. Β-Hydroxy-Β-Methylbutyrate Free Acid Reduces Markers of Exercise-Induced Muscle Damage and Improves Recovery in Resistance-Trained Men. Br. J. Nutr. 2013, 110, 538–544. Available online: https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/hydroxymethylbutyrate-free-acid-reduces-markers-of-exerciseinduced-muscle-damage-and-improves-recovery-in-resistancetrained-men/65C5A7619B0F0E857960170011B1FFD2 (accessed on 5 August 2021). [CrossRef] [Green Version]
- Rankin, P.; Lawlor, M.J.; Hills, F.A.; Bell, P.G.; Stevenson, E.J.; Cockburn, E. The effect of milk on recovery from repeat-sprint cycling in female team-sport athletes. Appl. Physiol. Nutr. Metab. 2018, 43, 113–122. Available online: https://cdnsciencepub.com/doi/abs/10.1139/apnm-2017-0275 (accessed on 5 August 2021). [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. Available online: https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000097 (accessed on 3 August 2021). [CrossRef] [Green Version]
- Northeast, B.; Clifford, T. The effect of creatine supplementation on markers of exercise-induced muscle damage: A systematic review and meta-analysis of human intervention trials. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 276–291. [Google Scholar] [CrossRef]
- Cowan, S.F.; Leeming, E.R.; Sinclair, A.; Dordevic, A.L.; Truby, H.; Gibson, S.J. Effect of whole foods and dietary patterns on markers of subclinical inflammation in weight-stable overweight and obese adults: A systematic review. Nutr. Rev. 2020, 78, 19–38. Available online: https://academic.oup.com/nutritionreviews/article/78/1/19/5552126. (accessed on 3 September 2021). [CrossRef]
- Powers, S.K.; Smuder, A.J.; Kavazis, A.N.; Hudson, M.B. Experimental Guidelines for Studies Designed to Investigate the Impact of Antioxidant Supplementation on Exercise Performance. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 2–14. Available online: https://pubmed.ncbi.nlm.nih.gov/20190346/ (accessed on 1 October 2021). [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, D5928. Available online: https://www.bmj.com/content/343/bmj.d5928 (accessed on 1 October 2021). [CrossRef] [Green Version]
- Harty, P.S.; Cottet, M.L.; Malloy, J.K.; Kerksick, C.M. Nutritional and Supplementation Strategies to Prevent and Attenuate Exercise-Induced Muscle Damage: A Brief Review. Sports Med. Open 2019, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.D.; Thomson, R.L.; Coates, A.M.; Howe, P.R.C.; DeNichilo, M.O.; Rowney, M.K. Supplementation with a whey protein hydrolysate enhances recovery of muscle force-generating capacity following eccentric exercise. J. Sci. Med. Sport 2010, 13, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.A.; Tromm, C.B.; Bom, K.F.; Mariano, I.; Pozzi, B.; da Rosa, G.L.; Tuon, T.; da Luz, G.; Vuolo, F.; Petronilho, F.; et al. Effects of Taurine Supplementation Following Eccentric Exercise in Young Adults. Appl. Physiol. Nutr. Metab. 2014, 39, 38–46. Available online: https://cdnsciencepub.com/doi/abs/10.1139/apnm-2012-0229 (accessed on 17 August 2021). [CrossRef]
- Jackman, S.R.; Witard, O.C.; Jeukendrup, A.E.; Tipton, K.D. Branched-Chain Amino Acid Ingestion Can Ameliorate Soreness from Eccentric Exercise. Med. Sci. Sports Exerc. 2010, 42, 962–970. Available online: https://journals.lww.com/acsm-msse/Fulltext/2010/05000/Branched_Chain_Amino_Acid_Ingestion_Can_Ameliorate.16.aspx (accessed on 16 August 2021). [CrossRef]
- Karakuş, M.; Akkurt, S. The Effect of Protein Supplements on Blood Parameters after Heavy Exercises. Kinesiol. Slov. 2020, 26, 16–24. Available online: http://ezproxy.uwe.ac.uk/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=jlh&AN=145352630&site=ehost-live (accessed on 5 August 2021).
- Nemati, A.; Alipanah-Moghadam, R.; Molazadeh, L.; Baghi, A.N. The Effect of Glutamine Supplementation on Oxidative Stress and Matrix Metalloproteinase 2 and 9 after Exhaustive Exercise. Drug Des. Devel. Ther. 2019, 13, 4215. Available online: https://doi.org/10.2147/DDDT.S218606 (accessed on 17 August 2021). [CrossRef] [Green Version]
- Ra, S.G.; Miyazaki, T.; Ishikura, K.; Nagayama, H.; Komine, S.; Nakata, Y.; Maeda, S.; Matsuzaki, Y.; Ohmori, H. Combined effect of branched-chain amino acids and taurine supplementation on delayed onset muscle soreness and muscle damage in high-intensity eccentric exercise. J. Int. Soc. Sports Nutr. 2013, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ra, S.G.; Choi, Y.; Akazawa, N.; Ohmori, H.; Maeda, S. Taurine supplementation attenuates delayed increase in exercise-induced arterial stiffness. Appl. Physiol. Nutr. Metab. 2016, 41, 618–623. [Google Scholar] [CrossRef]
- Shenoy, S.; Dhawan, M.; Sandhu, J.S. Four Weeks of Supplementation with Isolated Soy Protein Attenuates Exercise-Induced Muscle Damage and Enhances Muscle Recovery in Well Trained Athletes: A Randomized Trial. Asian J. Sports Med. 2016, 7, 1–11. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=s3h&AN=119606703&site=ehost-liveNS (accessed on 30 June 2021). [CrossRef] [Green Version]
- Sureda, A.; Cordova, A.; Ferrer, M.D.; Tauler, P.; Perez, G.; Tur, J.A.; Pons, A.; Córdova, A.; Ferrer, M.D.; Tauler, P.; et al. Effects of L-Citrulline Oral Supplementation on Polymorphonuclear Neutrophils Oxidative Burst and Nitric Oxide Production after Exercise. Free Radic. Res. 2009, 43, 828–835. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=19585317&site=ehost-liveNS (accessed on 21 July 2021). [CrossRef] [PubMed]
- Takegaki, J.; Sase, K.; Yasuda, J.; Shindo, D.; Kato, H.; Toyoda, S.; Yamada, T.; Shinohara, Y.; Fujita, S. The Effect of Leucine-Enriched Essential Amino Acid Supplementation on Anabolic and Catabolic Signaling in Human Skeletal Muscle after Acute Resistance Exercise: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial. Nutrients 2020, 12, 2421. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=32806711&site=ehost-liveNS (accessed on 30 June 2021). [CrossRef] [PubMed]
- Waskiw-Ford, M.; Hannaian, S.; Duncan, J.; Kato, H.; Sawan, S.A.; Locke, M.; Kumbhare, D.; Moore, D. Leucine-enriched essential amino acids improve recovery from post-exercise muscle damage independent of increases in integrated myofibrillar protein synthesis in young men. Nutrients 2020, 14, 1061. [Google Scholar] [CrossRef] [PubMed]
- Zembron-Lacny, A.; Szyszka, K.; Szygula, Z. Effect of Cysteine Derivatives Administration in Healthy Men Exposed to Intense Resistance Exercise by Evaluation of Pro-Antioxidant Ratio. J. Physiol. Sci. 2007, 57, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Baba, S.; Ebihara, S.; Sakano, K.; Natsume, M. Whey Protein-Containing Product Reduces Muscle Damage Induced by Running in Male Adults. Sport Sci. Health 2014, 10, 85–95. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=s3h&AN=97163454&site=ehost-liveNS (accessed on 30 June 2021). [CrossRef]
- Betts, J.A.; Toone, R.J.; Stokes, K.A.; Thompson, D. Systemic indices of skeletal muscle damage and recovery of muscle function after exercise: Effect of combined carbohydrate-protein ingestion. Appl. Physiol. Nutr. Metab. 2009, 34, 773–784. [Google Scholar] [CrossRef]
- Cury-Boaventura, M.F.; Levada-Pires, A.C.; Folador, A.; Gorjão, R.; Alba-Loureiro, T.C.; Hirabara, S.M.; Peres, F.P.; Silva, P.R.S.; Curi, R.; Pithon-Curi, T.C. Effects of Exercise on Leukocyte Death: Prevention by Hydrolyzed Whey Protein Enriched with Glutamine Dipeptide. Eur. J. Appl. Physiol. 2008, 103, 289–294. Available online: https://link.springer.com/article/10.1007/s00421-008-0702-1 (accessed on 10 August 2021). [CrossRef]
- Grubic, T.J.; Sowinski, R.J.; Nevares, B.E.; Jenkins, V.M.; Williamson, S.L.; Reyes, A.G.; Rasmussen, C.; Greenwood, M.; Murano, P.S.; Earnest, C.P.; et al. Comparison of Ingesting a Food Bar Containing Whey Protein and Isomalto-Oligosaccharides to Carbohydrate on Performance and Recovery from an Acute Bout of Resistance-Exercise and Sprint Conditioning: An Open Label, Randomized, Counterbalanced, Crossover P. J. Int. Soc. Sports Nutr. 2019, 16, 1–17. Available online: https://pubmed.ncbi.nlm.nih.gov/31409363/ (accessed on 5 August 2021). [CrossRef] [Green Version]
- Hall, A.H.; Leveritt, M.D.; Ahuja, K.D.K.; Shing, C.M. Coingestion of carbohydrate and protein during training reduces training stress and enhances subsequent exercise performance. Appl. Physiol. Nutr. Metab. 2013, 38, 597–604. [Google Scholar] [CrossRef]
- Kritikos, S.; Papanikolaou, K.; Draganidis, D.; Poulios, A.; Georgakouli, K.; Tsimeas, P.; Tzatzakis, T.; Batsilas, D.; Batrakoulis, A.; Deli, C.K.; et al. Effect of Whey vs. Soy Protein Supplementation on Recovery Kinetics Following Speed Endurance Training in Competitive Male Soccer Players: A Randomized Controlled Trial. J. Int. Soc. Sports Nutr. 2021, 18, 1–15. Available online: https://doi.org/10.1186/s12970-021-00420-w (accessed on 6 August 2021). [CrossRef]
- Naclerio, F.; Larumbe-Zabala, E.; Cooper, R.; Jimenez, A.; Goss-Sampson, M. Effect of A Carbohydrate-Protein Multi-Ingredient Supplement on Intermittent Sprint Performance and Muscle Damage in Recreational Athletes. Appl. Physiol. Nutr. Metab. 2014, 39, 1151–1158. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=25029675&site=ehost-liveNS (accessed on 30 June 2021). [CrossRef]
- Rothschild, J.A.; Kilding, A.E.; Broome, S.C.; Stewart, T.; Cronin, J.B.; Plews, D.J. Pre-Exercise Carbohydrate or Protein Ingestion Influences Substrate Oxidation but Not Performance or Hunger Compared with Cycling in the Fasted State. Nutrients 2021, 13, 1291. Available online: https://www.mdpi.com/2072-6643/13/4/1291 (accessed on 4 August 2021). [CrossRef] [PubMed]
- Volek, J.S.; Kraemer, W.J.; Rubin, M.R.; Gómez, A.L.; Ratamess, N.A.; Gaynor, P. L-carnitine L-tartrate supplementation favorably affects markers of recovery from exercise stress. Am. J. Physiol.-Endocrinol. Metab. 2002, 282, E474–E482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, A.J.; Hoffman, J.R.; Jajtner, A.R.; Varanoske, A.N.; Church, D.D.; Gonzalez, A.M.; Townsend, J.R.; Boone, C.H.; Baker, K.M.; Beyer, K.S. The effect of post-resistance exercise amino acids on plasma MCP-1 and CCR2 expression. Nutrients 2016, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.J.; Jajtner, A.R.; Varanoske, A.N.; Church, D.D.; Gonzalez, A.M.; Townsend, J.R.; Boone, C.H.; Baker, K.M.; Beyer, K.S.; Mangine, G.T.; et al. Post-Resistance Exercise Ingestion of Milk Protein Attenuates Plasma Tnfα and Tnfr1 Expression on Monocyte Subpopulations. Amin. Acids 2017, 49, 1415–1426. Available online: https://link.springer.com/article/10.1007/s00726-017-2443-0 (accessed on 6 August 2021). [CrossRef] [PubMed]
- Pasiakos, S.M.; Lieberman, H.R.; McLellan, T.M. Effects of protein supplements on muscle damage, soreness and recovery of muscle function and physical performance: A systematic review. Sport Med. 2014, 44, 655–670. [Google Scholar] [CrossRef]
- Davies, R.; Carson, B.; Jakeman, P. The Effect of Whey Protein Supplementation on the Temporal Recovery of Muscle Function Following Resistance Training: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 221. Available online: https://www.mdpi.com/2072-6643/10/2/221 (accessed on 4 August 2021). [CrossRef] [Green Version]
- Bordoni, A.; Danesi, F.; Dardevet, D.; Dupont, D.; Fernandez, A.S.; Gille, D.; Nunes dos Santos, C.; Pinto, P.; Re, R.; Rémond, D. Dairy Products and Inflammation: A Review of The Clinical Evidence. Crit. Rev. Food Sci. Nutr. 2017, 57, 2497–2525. Available online: https://www.tandfonline.com/doi/abs/10.1080/10408398.2014.967385 (accessed on 20 October 2021). [CrossRef]
- Ulven, S.M.; Holven, K.B.; Gil, A.; Rangel-Huerta, O.D. Milk and Dairy Product Consumption and Inflammatory Biomarkers: An Updated Systematic Review of Randomized Clinical Trials. Adv. Nutr. 2019, 10, S239. Available online: https://doi.org/10.1093/ADVANCES/NMY072 (accessed on 21 October 2021). [CrossRef]
- Jones, L.; Bailey, S.J.; Rowland, S.N.; Alsharif, N.; Shannon, O.M.; Clifford, T. The Effect of Nitrate-Rich Beetroot Juice on Markers of Exercise-Induced Muscle Damage: A Systematic Review and Meta-Analysis of Human Intervention Trials. J. Diet. Suppl. 2021, 1–23. Available online: https://www.tandfonline.com/doi/abs/10.1080/19390211.2021.1939472 (accessed on 21 October 2021). [CrossRef]
- Schulz, K.F. Assessing allocation concealment and blinding in randomised controlled trials: Why bother? Evid.-Based Nurs. 2001, 4, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Kahan, B.C.; Forbes, G.; Cro, S. How to Design a Pre-Specified Statistical Analysis Approach to Limit P-Hacking in Clinical Trials: The Pre-SPEC Framework. BMC Med. 2020, 18, 1–7. Available online: https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-020-01706-7 (accessed on 21 October 2021). [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample Size, Power and Effect Size Revisited: Simplified and Practical Approachin Pre-Clinical, Clinical and Laboratory Studies. Biochem. Medica 2021, 31, 1–27. Available online: https://pubmed.ncbi.nlm.nih.gov/33380887/ (accessed on 21 October 2021). [CrossRef] [PubMed]
- Button, K.S.; Ioannidis, J.P.A.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.J.; Munafò, M.R. Power Failure: Why Small Sample Size Undermines the Reliability of Neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376. Available online: https://pubmed.ncbi.nlm.nih.gov/23571845/v (accessed on 21 October 2021). [CrossRef] [PubMed] [Green Version]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Vina, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, G.; Hamarsland, H.; Cumming, K.T.; Johansen, R.E.; Hulmi, J.J.; Børsheim, E.; Wiig, H.; Garthe, I.; Raastad, T. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J. Physiol. 2014, 592, 5391–5408. [Google Scholar] [CrossRef]
- Clifford, T.; Jeffries, O.; Stevenson, E.J.; Davies, K.A.B. The effects of vitamin C and E on exercise-induced physiological adaptations: A systematic review and Meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 3669–3679. [Google Scholar] [CrossRef]
- Jordan, A.C.; Perry, C.G.R.; Cheng, A.J. Promoting a pro-oxidant state in skeletal muscle: Potential dietary, environmental, and exercise interventions for enhancing endurance-training adaptations. Free Radic. Biol. Med. 2021, 176, 189–202. Available online: https://doi.org/10.1016/j.freeradbiomed.2021.09.014 (accessed on 8 December 2021). [CrossRef] [PubMed]
- Margolis, L.M.; Pasiakos, S.M. Optimizing intramuscular adaptations to aerobic exercise: Effects of carbohydrate restriction and protein supplementation on mitochondrial biogenesis. Adv. Nutr. 2013, 4, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Wynne, J.L.; Ehlert, A.M.; Wilson, P.B. Effects of High-Carbohydrate versus Mixed-Macronutrient Meals on Female Soccer Physiology and Performance. Eur. J. Appl. Physiol. 2021, 121, 1125–1134. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=33484335&site=ehost-live (accessed on 8 December 2021). [CrossRef] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Albers, R.; Bosco, N.; Bourdet-Sicard, R.; Haller, D.; Holgate, S.T.; Johnsson, L.S.; Latulippe, M.E.; Marcos, A.; et al. A Consideration of Biomarkers to be Used for Evaluation of Inflammation in Human Nutritional Studies. Br. J. Nutr. 2013, 109, S1–S34. Available online: https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/consideration-of-biomarkers-to-be-used-for-evaluation-of-inflammation-in-human-nutritional-studies/9BCFFC03ABE69F78E20BE05846C1D502 (accessed on 25 October 2021). [CrossRef] [Green Version]
- Cobley, J.N.; Close, G.L.; Bailey, D.M.; Davison, G.W. Exercise redox biochemistry: Conceptual, methodological and technical recommendations. Redox Biol. 2017, 12, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Cobley, J.N.; Paschalis, V.; Veskoukis, A.S.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Going retro: Oxidative stress biomarkers in modern redox biology. Free Radic. Biol. Med. 2016, 98, 2–12. [Google Scholar] [CrossRef]
- Ralser, M.; Wamelink, M.M.; Kowald, A.; Gerisch, B.; Heeren, G.; Struys, E.A.; Klipp, E.; Jakobs, C.; Breitenbach, M.; Lehrach, H.; et al. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J. Biol. 2007, 6, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Ah Morano, A.E.; Dorneles, G.P.; Peres, A.; Lira, F.S. The role of glucose homeostasis on immune function in response to exercise: The impact of low or higher energetic conditions. J. Cell Physiol. 2020, 235, 3169–3188. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Chatzinikolaoum, P.N.; Bousiou, F.V.; Malliou, V.J.; Papadopoulou, S.K.; Potsaki, P.; Theodorous, A.A.; Kyparos, A.; Geldas, N.D.; Nikolaidis, M.G.; et al. Dietary Cysteine Intake is Associated with Blood Glutathione Levels and Isometric Strength. Int. J. Sports Med. 2021, 42, 441–447. [Google Scholar] [CrossRef]
| Study | Participants | Design | Intervention | Comparator | Timing of Supplement | Exercise | Outcomes | Difference |
|---|---|---|---|---|---|---|---|---|
| Baba et al. (2014) | 14 M 31 ± 6 | Crossover | Drink (22.8-g WPI, 4.4-g CHO) | Drink (5.2 g of the same minus WPI | Pre, during, and post-exercise | O2 max) | Plasma IL-6 | ↔ |
| Betts et al. (2009) | 17 M 26 ± 5 | SB, Crossover | Drink (0.4-g.kg−1 BM·h−1 WPI, 1.2-g·kg−1 BM·h−1 CHO) | Drink (1.2-g·kg−1 BM·h−1 CHO) | During and ≤4 h post-exercise | 90 min LIST | Serum IL-6 | ↔ |
| Serum IL-10 | ↔ | |||||||
| Serum CRP | ↔ | |||||||
| Serum IL-1 ra | ↔ | |||||||
| Buckley et al. (2010) | 28 M 24 ± 4 | DB, 3-arm Parallel | Drink (1) (25-g WPI) (2) (25-g WPI-HD) | Drink (flavored water) | 0, 6 and 22 h post-exercise | 100 eccentric contractions of knee extensors | Plasma TNF-α | ↔ |
| Draganidis et al. (2017) | 11 M 22 ± 1 | DB, Crossover | Drink (20-g milk PRO) | Drink (20 g of maltodextrin) | 9 h post and 20 g/d for 8 days post-exercise | 300 eccentric contractions of knee extensors | Muscle NF-κB | ↔ |
| Muscle HSP70 | ↔ | |||||||
| Serum PC | ↓ | |||||||
| Leukocytes | ↔ | |||||||
| Grubic et al. (2019) | 12 M 22 ± 2 | Open label, Crossover | Food bar (20-g WP, 25-g IMO, 7-g fat) | Gel (25-g dextrose) | 30-min pre-, mid-way, and post-exercise | RT (3 × 10 reps at 70% of 1 RM) + agility and sprint drills | Serum IFNy | ↔ |
| Serum IL-13 | ↔ | |||||||
| Serum IL-1 β | ↔ | |||||||
| Serum IL-4 | ↔ | |||||||
| Serum IL-6 | ↔ | |||||||
| Serum IL-8 | ↔ | |||||||
| Serum TNF-α | ↔ | |||||||
| Hall et al. (2013) | 10 M 30 ± 8 | DB, Crossover | Drink (0.23-g·kg−1·h−1 casein + 0.1-g·kg−1·h−1 leucine + 0.87-g·kg−1·h−1 CHO) | CHO beverage (1.2 g·kg−1·h−1) | Every 15 min during exercise | 2.5-h intermittent cycling (30–90% Wmax) | Neutrophils | ↓ |
| Lymphocytes | ↓ | |||||||
| Hilkens et al. (2020) | INT: 20 M 24 ± 4 CON: 19 M, 23 ± 4 | DB, Parallel | Drink (58.5-g WP + 5.0-g CHO) | Drink (72-g CHO) | 2/day for 9 days pre- and post-exercise | 10 × 10 DJs with 5 kg vest | Plasma CRP | ↔ |
| Karakuş et al. (2020) | 22 ± 2 M INT: 15 CON: 9 | Parallel | 35-g WP drink | NR | 3 meals, 3 days post-exercise | RT (10 exercises for different body parts) | Neutrophils | ↔ |
| Platelets | ↔ | |||||||
| Leukocytes | ↔ | |||||||
| Lymphocytes | ↔ | |||||||
| Kerasioti et al. (2013) | 9 M 28 ± 2 | DB, crossover | Cake (0.9-g·kg−1·h−1 CHO + 0.26-g·kg−1·h−1 WP) | Isocaloric cake (1.1-g·kg−1·h−1 CHO + 0.1-g·kg−1·h−1 WP) | 3 h Post-exercise | O2 max | Plasma IL-6 | ↓ 4 h post |
| Plasma IL-10 | ↔ | |||||||
| Plasma CRP | ↓ 4 h post | |||||||
| Kritikos et al. (2021) | 10 M 21 ± 2 | DB, 3-arm crossover | Drink (WPI or SOY)—enough to reach 1.5 g/kg/day | Drink (maltodextrin) | 1/day for 3 days | 60 min speed-endurance training | Glutathione | ↔ |
| Serum TAC | ↔ | |||||||
| Plasma PC | ↓ SOY vs. PL, 48 h | |||||||
| Naclerio et al. (2014) | 10 M 25 ± 4 | DB, 3-arm crossover | Drink (53-g CHO,14.5-g WP, 5-g glutamine, 1.5-g L-carnitine-L-tartrate) | Drink 1: (69.5-g CHO) 2: low calorie water | 1 dose during exercise and 1 post-exercise | 90-min intermittent exercise | Plasma IL-6 | ↔ |
| Nieman et al. (2020) | Pea: 31 M 37 ± 2 WP: 31 M 40 ± 2 CON: 30 M 38 ± 2 | DB, 3-arm parallel | Drink (1) Pea PRO (0.3 g·kg−1) (2) WPI (0.3 g·kg−1) | Drink (water) | 13 doses over 5 days on day of and post-exercise | 90-min eccentric exercise | Serum CRP | ↔ |
| Rankin et al. (2017) | 10 F 22 ± 2 | Crossover | Drink (17-g milk PRO; 25.5-g CHO) | Drink (52.6-g CHO) | <30 min post-exercise | Intermittent sprint cycling (~60 min) | Serum hsCRP | ↔ |
| Serum GSH/GSSG | ↔ | |||||||
| Serum PC | ↔ | |||||||
| Rothschild et al. (2021) | 17 M 31 ± 12 | Crossover | PRO-rich meal (0.45-g·kg−1 WPI + 0.24-g·kg−1 fat) | CHO-rich meal (1-g·kg−1 CHO) | 30 pre-exercises | HI cycling (~45 min) | Urinary F2-Isoprostanes | ↔ |
| Rowlands et al. (2016) | 12 M cyclists 30 ± 7 | SB, 3-arm crossover | Drink 1: WP (70 g, 15-g LEU, 180-g CHO, 30-g FAT) 2: WP (23.5 g, 5-g LEU, 180-g CHO, 30-g FAT) | Isocaloric drink (0-g WP, 274-g CHO, 30-g FAT). | 90 min post-exercise (in 4 doses) | HI cycling (70–90% Wmax,100 min) | * Muscle inflammatory-myogenic regenerative processes | ↑ |
| Shenoy et al. (2016) | 40 M 20 ± 2 | DB, Parallel | Drink (21-g SOY, 21-mg isoflavones) | Drink (sweetened water) | 2/day for 4 weeks | 100 DJs | Plasma hsCRP | ↔ |
| Plasma MPO | ↔ | |||||||
| Wells et al. (2017) | 10 M 25 ± 3 | Crossover | Drink (Milk PRO 20-g AAs, 6-g CHO) | Drink (Flavored water, 2.5-g CHO) | Post-exercise | Lower body RT (10–12 reps at 70% 1 RM) | Plasma TNF-α | ↔ |
| TNFr1 expression on monocytes | ↔ | |||||||
| Wojcik et al. (2001) | 27 M 24 ± 1 | 3-arm parallel | Drink (1) Milk (0.9-g·kg−1 CHO, 0.4-g·kg−1 PRO) (2) CHO (1.25 g·kg−1) | Drink (sweetened water) | 2 × post-exercise | 100 eccentric contractions of knee flexors | Serum IL-6 | ↔ |
| Serum IL-1 | ↔ | |||||||
| Serum TNF | ↔ |
| Study | Participants | Design | Intervention | Comparator | Timing of supplement | Exercise | Outcomes | Difference |
|---|---|---|---|---|---|---|---|---|
| Mixed Amino Acids | ||||||||
| Jackman et al. (2010) | INT: 12 M CON:12 M | SB, Parallel | Drink (BCAA: 3.5-g LEU, 2 g of isoleucine, + 1.7 g of valine) | Drink (sweetened water) | 4/day on the day of and 3 days post-exercise | 120 eccentric knee extensions | Serum IL-6 | ↔ |
| Ra et al. (2013) | 36 M 9 per group 22 ± 4 | DB, 4-arm parallel | Drink: 1: BCAA (3.2 g) + taurine (2 g) 2: BCAA (3.2 g) + PL 3: Taurine (2 g) + PL | Starch (to match treatment volumes) | 3/day pre- and post- exercise for 18 days | Eccentric elbow flexor exercises (6 × 5 reps at 90% MVC) | Serum 8-OHdG | ↓ BCAA + taurine vs. BCAA + PL and PL |
| Takegaki et al. (2020) | INT: 10 M 21 ± 1 CON:10 M 22 ± 2 | DB, parallel | Drink (5-g Leucine-enriched Aas) | Drink (water) | 2.5 g × 2, pre- and post-exercise | Lower body RT (3 × 10 reps at 70% of 1 RM) | IL-6 muscle mRNA | ↔ |
| IL-1β muscle mRNA | ↔ | |||||||
| Waskiw-Ford et al. (2020) | INT: 10 M 24 ± 4 CON:10 M 23 ± 5 | DB, parallel | Drink (4 g of essential Aas) | Isocaloric CHO PL | 3/day, for 4 days post-exercise | Lower-body RT (5 × 9–12 reps at 75% of 1 RM) | Muscle HSP25 | ↔ |
| Plasma IL-6 | ↔ | |||||||
| Muscle HSP72 | ↓ | |||||||
| Wells et al. (2016) | 10 M 25 ± 3 | Crossover | Drink (20 g of milk PRO, 6 g of CHO) | Drink (flavored water, 2.5-g CHO) | Post-exercise | Lower-body RT (6 × 10–12 reps at 70% of 1 RM) | Plasma MCP-1 | ↔ |
| CCR2 | ↔ | |||||||
| CD11 b | ↔ | |||||||
| CD14+ MON | ↔ | |||||||
| CD14 ++ CD16- MON | ↔ | |||||||
| β-hydroxy-β-methylbutyrate | ||||||||
| Wilson et al. (2013) | INT: 11 M 20 ± 5 CON: 9 M 22 ± 2 | Parallel | Drink (3-g·day−1 HMB-FA) | Drink (sweetened water) | Pre- and post-exercise | Full body RT (3 × 12 reps) | Plasma CRP | ↔ |
| L-carnitine | ||||||||
| Parandak et al. (2014) | INT: 10 M 22 ± 3 CON:11 M 22 ± 3 | DB, Parallel | Capsules (2-g L-carnitine) | Capsules (2-g lactose) | Daily for 2-wk pre-exercise | O2 max | Serum TAC | ↑ 24 h post |
| Serum TBARS | ↓ 24 h post | |||||||
| Volek et al. (2002) | 10 M 24 ± 2 | Crossover | Capsules (2-g·day−1 L-carnitine 944-mg·day−1 L-tartrate) | Capsules (cellulose) | Daily for 3-wk, pre and post-exercise | Lower body RT (5 × 15–20 reps at 50% of 1 RM) | Plasma MDA | ↓ at 15 min post |
| Plasma XO | ↓ at 0, 15, 180 min post | |||||||
| Citrulline | ||||||||
| Sureda et al. (2009) | 22 ± 4 INT: 8 M CON: 9 M | DB, Parallel | Drink (6-g citrulline-malate) | Drink (lemon juice) | 2 h pre-exercise | 137.1 km cycling | PMN-ROS | ↑ post |
| PMN-MDA | ↔ | |||||||
| DNA damage | ↔ | |||||||
| Collagen peptides | ||||||||
| Clifford et al. (2019) | INT: 12 M 24 ± 4 CON: 12 M 25 ± 5 | DB, Parallel | Drink (20-g·day−1 CP) | Drink (20-g maltodextrin) | 10 g × 2, 7 days pre and 2 days post-exercise | 150 DJs | Leukocytes | ↔ |
| Neutrophils | ↔ | |||||||
| Monocytes | ↔ | |||||||
| Lymphocytes | ↔ | |||||||
| Serum IL-6 | ↔ | |||||||
| Glutamine | ||||||||
| Cury-Boaventura et al. (2008) | 9 M 25 ± 4 | DB, Crossover | Drink (2.8-g WP,+ 175-mg glutamine, 50-g maltodextrin) | Drink (50-g maltodextrin) | 30 min pre-exercise | Treadmill running to exhaustion | DNA damage in leukocytes | ↔ |
| Neutrophil O2− | ↔ | |||||||
| Nakhostin-Roohi et al. (2017) | INT: 9 M 25 ± 1 CON:10 M 22 ± 1 | DB, Parallel | Drink (1.5-g·kg−1 BM·day−1 glutamine) | Drink (sweetened water) | 1/day for 7 days pre-exercise | O2 max | Plasma TAC | ↔ |
| plasma Glutathione | ↔ | |||||||
| Plasma MDA | ↔ | |||||||
| Nemati et al. (2019) | INT: 15 M 19.7 ± 2 CON: 15 M 19 ± 1 | Parallel | Drink (0.3-g·kg−1 BM·day−1 glutamine + 25-g sugar) | Drink (25-g sugar) | 1/day for 14 days pre-exercise | O2 max | Serum TAC | ↑ |
| Serum MDA | ↓ | |||||||
| Serum hsCRP | ↓ | |||||||
| Glutathione | ↑ | |||||||
| Taurine | ||||||||
| Da Silva et al. (2014) | 21 ± 6 INT: 11 M CON: 10 M | DB, Parallel | Capsules (Taurine 50-mg·kg BM−1·day−1) | Capsules (Starch 50-mg·kg BM−1·day−1) | 1/day, 14 days pre and 7 days post-exercise | Eccentric elbow flexion and extension exercise (3 × 11–15 reps at 80% of 1 RM) | Xylenol | ↓ |
| Plasma PC | ↓ | |||||||
| Plasma TT | ↑ | |||||||
| Erythrocyte-derived SOD | ↔ | |||||||
| Erythrocyte-derived CAT | ↔ | |||||||
| Erythrocyte-derived GPX | ↔ | |||||||
| Plasma TNF-α | ↔ | |||||||
| Plasma IL-1ß | ↔ | |||||||
| Plasma IL-10 | ↔ | |||||||
| Ra et al. (2016) | INT: 15 M 25 ± 1 CON: 14 M 25 ± 1 | DB, Parallel | Powder (6-g taurine) | Powder (6-g lactose) | Daily for 14 days pre-, on, & 3 days post-exercise | Eccentric contractions of the elbow flexors (2 × 20 reps) | Serum MDA | ↓ 3 d, 4 d post |
| Zembron-Lacny et al. (2007) | INT: TAU: 15 M, 22 ± 1 CON: (1) PL: 15 M 22 ± 1 (2) NAC: 15 M, 22 ± 2 (3) LIP: 10 M, 23 ± 1 | 4-arm parallel | Drink TAU (3 g·day−1) | Drink (1) PL (0.35-g·day−1 saccharum lactis) (2) NAC (1.8 g·day−1) (3) LIP (1.2 g·day−1) | 3/day for 3 days pre-exercise | Full body RT (3 exercises in a circuit until exhaustion) | SOD | ↑ TAU vs. PL 24 h post |
| GPX | ↑ TAU vs. PL 24 h post | |||||||
| CAT | ↔ | |||||||
| Plasma Protein thiols | ↔ | |||||||
| TBARS | ↔ | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhebshi, A.; Alsharif, N.; Thorley, J.; James, L.J.; Clifford, T. The Effects of Dietary Protein Supplementation on Exercise-Induced Inflammation and Oxidative Stress: A Systematic Review of Human Trials. Antioxidants 2022, 11, 13. https://doi.org/10.3390/antiox11010013
Alhebshi A, Alsharif N, Thorley J, James LJ, Clifford T. The Effects of Dietary Protein Supplementation on Exercise-Induced Inflammation and Oxidative Stress: A Systematic Review of Human Trials. Antioxidants. 2022; 11(1):13. https://doi.org/10.3390/antiox11010013
Chicago/Turabian StyleAlhebshi, Abrar, Nehal Alsharif, Josh Thorley, Lewis J. James, and Tom Clifford. 2022. "The Effects of Dietary Protein Supplementation on Exercise-Induced Inflammation and Oxidative Stress: A Systematic Review of Human Trials" Antioxidants 11, no. 1: 13. https://doi.org/10.3390/antiox11010013
APA StyleAlhebshi, A., Alsharif, N., Thorley, J., James, L. J., & Clifford, T. (2022). The Effects of Dietary Protein Supplementation on Exercise-Induced Inflammation and Oxidative Stress: A Systematic Review of Human Trials. Antioxidants, 11(1), 13. https://doi.org/10.3390/antiox11010013






