Molecular Mechanistic Pathways Targeted by Natural Antioxidants in the Prevention and Treatment of Chronic Kidney Disease
Abstract
1. Introduction
2. Signaling Pathways That Predispose to the Progression of CKD
2.1. NF-κB Pathway
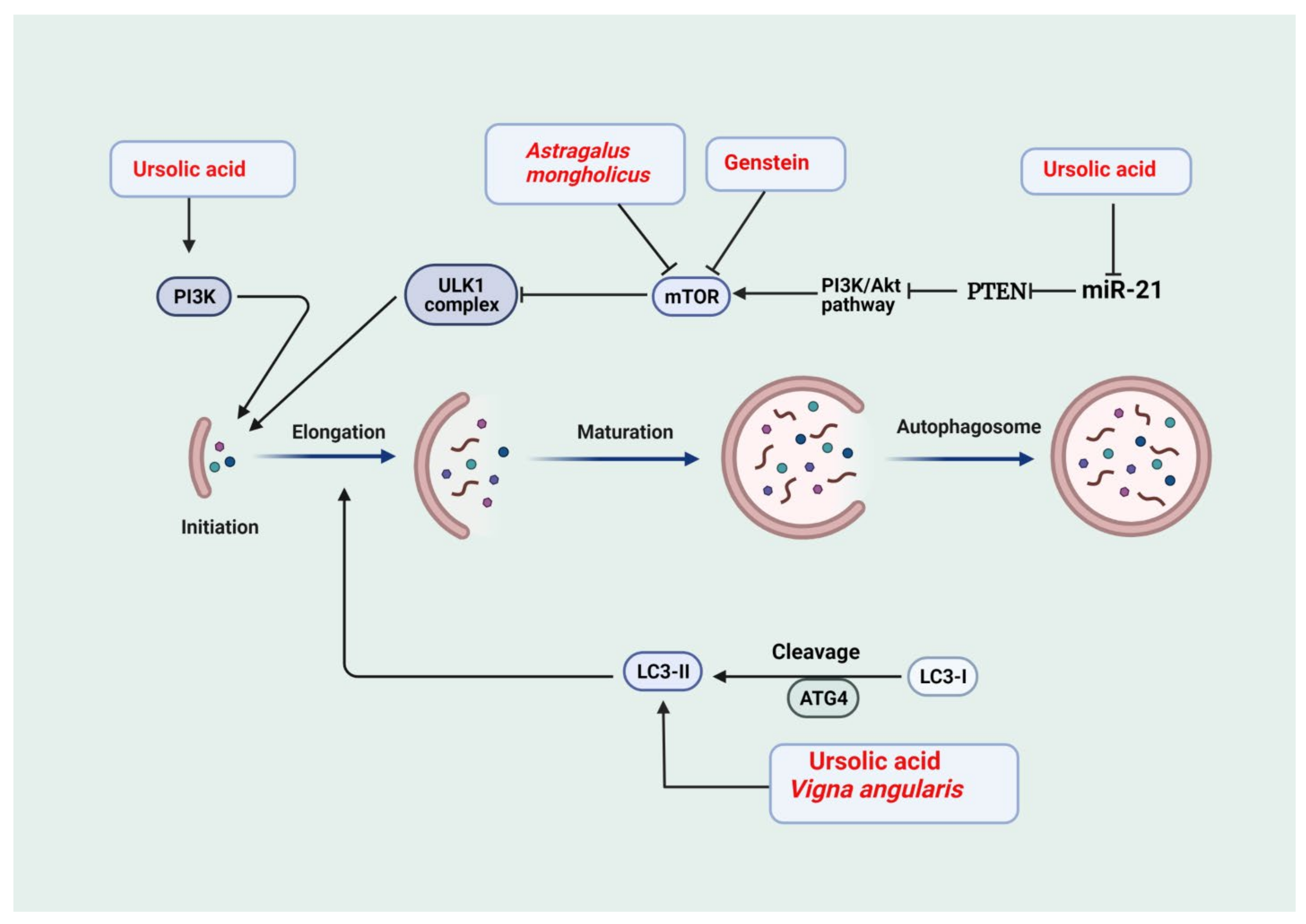
2.2. Autophagy
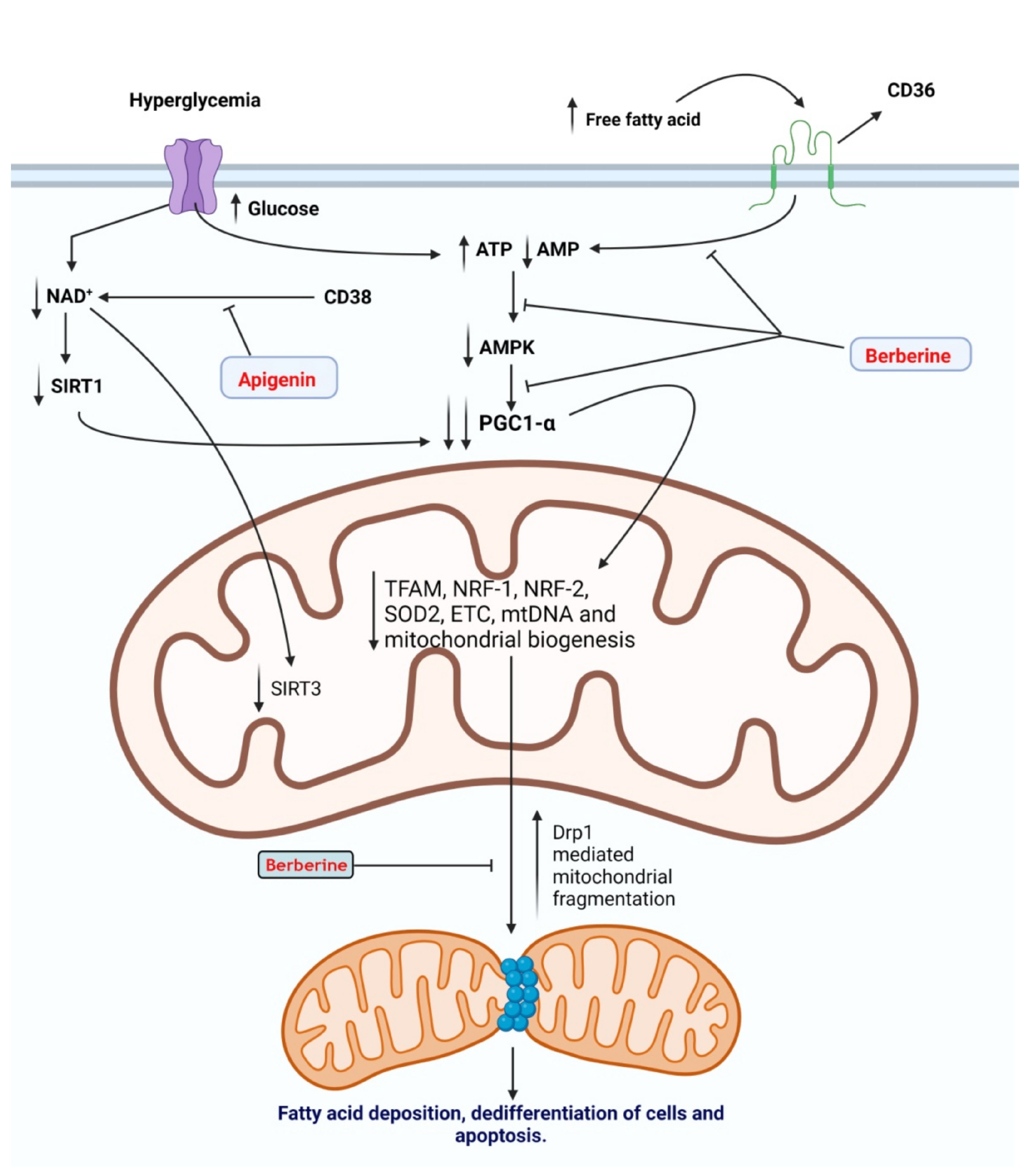
2.3. Mitochondrial Dysfunction
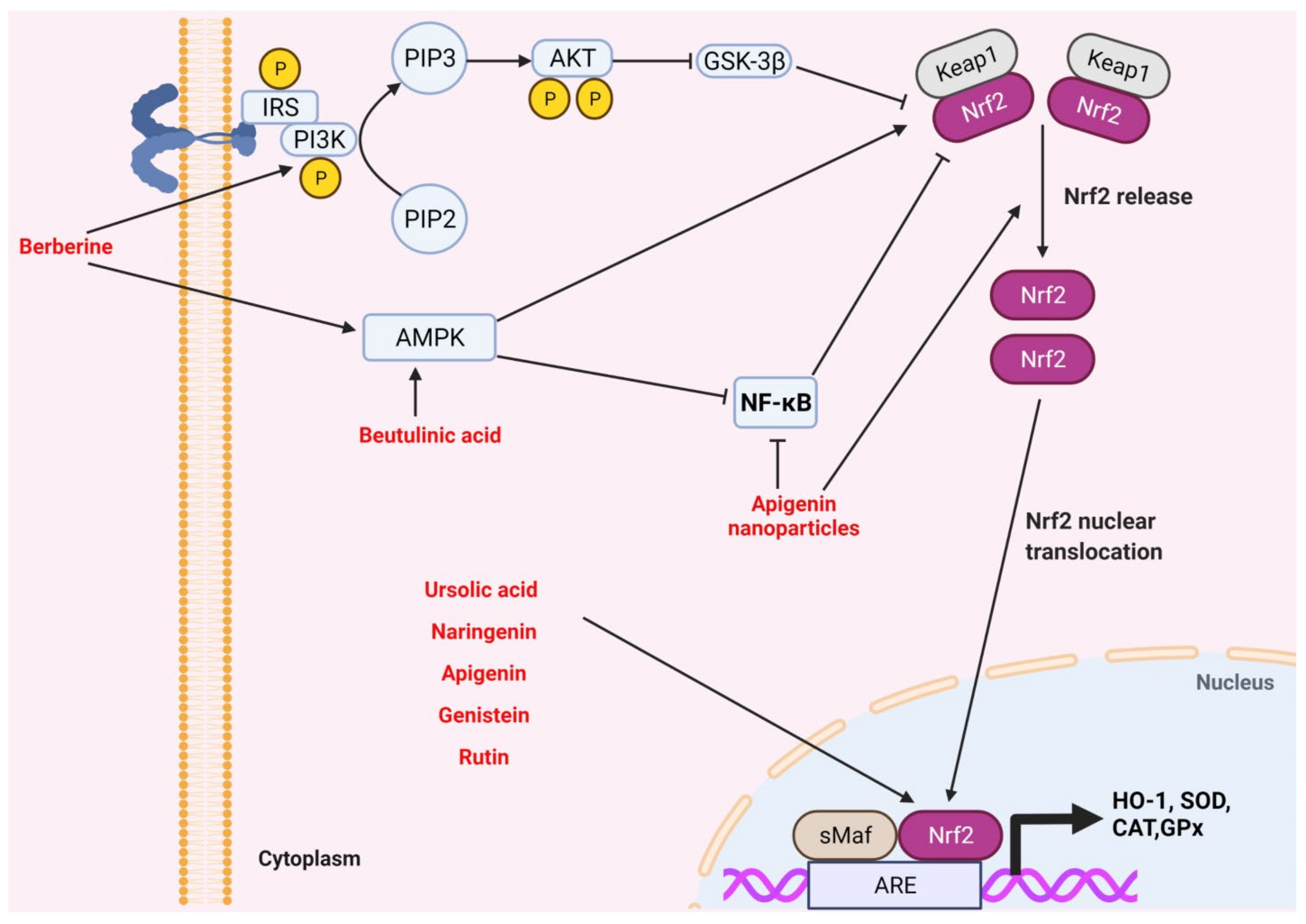
2.4. Nrf-2 Signaling Pathway
2.5. TGF-β Signaling Pathway
3. Role of Antioxidants in the Prevention of CKD
3.1. Medicinal Plants and Natural Compounds against CKD
3.2. Small Bioactive Compounds against CKD
3.2.1. Berberine
3.2.2. Ursolic Acid
3.2.3. Naringenin
3.2.4. Apigenin
3.2.5. Genistein
3.2.6. Rutin
3.2.7. Proanthocyanin
3.2.8. Betulinic Acid
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.R. Global prevalence of chronic kidney disease—A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative stress in the pathophysiology of kidney disease: Implications for noninvasive monitoring and identification of biomarkers. Oxidative Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, C.; Noreddin, A.; Nunes, A. Inflammation in Nonimmune-Mediated Chronic Kidney. In Chronic Kidney Disease: From Pathophysiology to Clinical Improvements; Intechopen: London, UK, 2018; 153p. [Google Scholar]
- Vaidya, S.R.; Aeddula, N.R. Chronic Renal Failure; StatPearls Publishing: Treasure Islands, FL, USA, 2019. [Google Scholar]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Samadi, K.; Naghibi, M.; Shabestari, M.; Sharifipour, F.; Khajeh Dalooee, M.; Raeesi, V.; Nik, S.M.; Samadi, M. Evaluation the Effects of Alpha-tocopherol in Comparison with N-acetylcystein for Prevention of Contrast Induced Nephropathy (CIN) in CKD Patients. Iran. J. Kidney Dis. 2020, 14, 26–30. [Google Scholar] [PubMed]
- Lai, S.; Petramala, L.; Muscaritoli, M.; Cianci, R.; Mazzaferro, S.; Mitterhofer, A.P.; Pasquali, M.; D’Ambrosio, V.; Carta, M.; Ansuini, M.; et al. α-lipoic acid in patients with autosomal dominant polycystic kidney disease. Nutrition 2020, 71, 110594. [Google Scholar] [CrossRef]
- Andrade-Oliveira, V.; Foresto-Neto, O.; Watanabe, I.K.M.; Zatz, R.; Câmara, N.O.S. Inflammation in renal diseases: New and old players. Front. Pharmacol. 2019, 10, 1192. [Google Scholar] [CrossRef]
- Jiang, M.; Bai, M.; Lei, J.; Xie, Y.; Xu, S.; Jia, Z.; Zhang, A. Mitochondrial dysfunction and the AKI-to-CKD transition. Am. J. Physiol. Ren. Physiol. 2020, 319, F1105–F1116. [Google Scholar] [CrossRef]
- Uddin, M.J.; Kim, E.H.; Hannan, M.; Ha, H. Pharmacotherapy against Oxidative stress in chronic kidney disease: Promising small molecule natural products targeting Nrf2-HO-1 signaling. Antioxidants 2021, 10, 258. [Google Scholar] [CrossRef]
- Chen, D.-Q.; Hu, H.-H.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Zhao, Y.-Y. Natural products for the prevention and treatment of kidney disease. Phytomedicine 2018, 50, 50–60. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Dietary antioxidant supplements and uric acid in chronic kidney disease: A review. Nutrients 2019, 11, 1911. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and oxidative stress in chronic kidney disease—Potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci 2020, 21, 263. [Google Scholar] [CrossRef]
- Souza, A.C.; Tsuji, T.; Baranova, I.N.; Bocharov, A.V.; Wilkins, K.J.; Street, J.M.; Alvarez-Prats, A.; Hu, X.; Eggerman, T.; Yuen, P.S. TLR 4 mutant mice are protected from renal fibrosis and chronic kidney disease progression. Physiol. Rep. 2015, 3, e12558. [Google Scholar] [CrossRef]
- Leemans, J.C.; Kors, L.; Anders, H.-J.; Florquin, S. Pattern recognition receptors and the inflammasome in kidney disease. Nat. Rev. Nephrol. 2014, 10, 398–414. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Ma, J.; Chadban, S.J.; Zhao, C.Y.; Chen, X.; Kwan, T.; Panchapakesan, U.; Pollock, C.A.; Wu, H. TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS ONE 2014, 9, e97985. [Google Scholar] [CrossRef]
- Yu, M.; Wang, H.; Ding, A.; Golenbock, D.T.; Latz, E.; Czura, C.J.; Fenton, M.J.; Tracey, K.J.; Yang, H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 2006, 26, 174–179. [Google Scholar] [CrossRef]
- Schaefer, L.; Babelova, A.; Kiss, E.; Hausser, H.-J.; Baliova, M.; Krzyzankova, M.; Marsche, G.; Young, M.F.; Mihalik, D.; Götte, M. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J. Clin. Investig. 2005, 115, 2223–2233. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Dhillon, B.; Aleithan, F.; Abdul-Sater, Z.; Abdul-Sater, A.A. The evolving role of TRAFs in mediating inflammatory responses. Front. Immunol. 2019, 10, 104. [Google Scholar] [CrossRef]
- Rangan, G.; Wang, Y.; Harris, D. NF-kappaB signalling in chronic kidney disease. Front. Biosci. 2009, 14, 3496–3522. [Google Scholar] [CrossRef]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the control of the life and death balance of macrophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Chandrashekar, K.; Juncos, L.A.; Shah, S.V. Autophagy function and regulation in kidney disease. Biomolecules 2020, 10, 100. [Google Scholar] [CrossRef]
- Yun, H.R.; Jo, Y.H.; Kim, J.; Shin, Y.; Kim, S.S.; Choi, T.G. Roles of autophagy in oxidative stress. Int. J. Mol. Sci. 2020, 21, 3289. [Google Scholar] [CrossRef]
- Birgisdottir, Å.B.; Mouilleron, S.; Bhujabal, Z.; Wirth, M.; Sjøttem, E.; Evjen, G.; Zhang, W.; Lee, R.; O’Reilly, N.; Tooze, S.A. Members of the autophagy class III phosphatidylinositol 3-kinase complex I interact with GABARAP and GABARAPL1 via LIR motifs. Autophagy 2019, 15, 1333–1355. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Peters, L.J.; Floege, J.; Biessen, E.A.; Jankowski, J.; van der Vorst, E.P. MicroRNAs in chronic kidney disease: Four candidates for clinical application. Int. J. Mol. Sci. 2020, 21, 6547. [Google Scholar] [CrossRef]
- Sun, J.; Li, Z.P.; Zhang, R.Q.; Zhang, H.M. Repression of miR-217 protects against high glucose-induced podocyte injury and insulin resistance by restoring PTEN-mediated autophagy pathway. Biochem. Biophys. Res. Commun. 2017, 483, 318–324. [Google Scholar] [CrossRef]
- Ueno, T.; Komatsu, M. Monitoring autophagy flux and activity: Principles and applications. Bioessays 2020, 42, 2000122. [Google Scholar] [CrossRef]
- Galvan, D.L.; Green, N.H.; Danesh, F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017, 92, 1051–1057. [Google Scholar] [CrossRef]
- Kim, Y.; Park, C.W. Adenosine monophosphate–activated protein kinase in diabetic nephropathy. Kidney Res. Clin. Pract. 2016, 35, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Liu, X.; Dagda, R.K.; Zhang, Y. How AMPK and PKA interplay to regulate mitochondrial function and survival in models of ischemia and diabetes. Oxidative Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Puchałowicz, K.; Rać, M.E. The Multifunctionality of CD36 in Diabetes Mellitus and Its Complications—Update in Pathogenesis. Treat. Monit. Cells. 2020, 9, 1877. [Google Scholar]
- Wu, J.; Jin, Z.; Zheng, H.; Yan, L.-J. Sources and implications of NADH/NAD+ redox imbalance in diabetes and its complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2016, 9, 145. [Google Scholar]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 1–37. [Google Scholar] [CrossRef]
- Guo, K.; Lu, J.; Huang, Y.; Wu, M.; Zhang, L.; Yu, H.; Zhang, M.; Bao, Y.; He, J.C.; Chen, H. Protective role of PGC-1α in diabetic nephropathy is associated with the inhibition of ROS through mitochondrial dynamic remodeling. PLoS ONE 2015, 10, e0125176. [Google Scholar] [CrossRef]
- Ayanga, B.A.; Badal, S.S.; Wang, Y.; Galvan, D.L.; Chang, B.H.; Schumacker, P.T.; Danesh, F.R. Dynamin–Related Protein 1 Deficiency Improves Mitochondrial Fitness and Protects against Progression of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2016, 27, 2733–2747. [Google Scholar] [CrossRef]
- Aon, M.A.; Bhatt, N.; Cortassa, S.C. Mitochondrial and cellular mechanisms for managing lipid excess. Front. Physiol. 2014, 5, 282. [Google Scholar] [CrossRef]
- Zhan, M.; Usman, I.M.; Sun, L.; Kanwar, Y.S. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J. Am. Soc. Nephrol. 2015, 26, 1304–1321. [Google Scholar] [CrossRef]
- Rajpathak, S.N.; Gunter, M.J.; Wylie-Rosett, J.; Ho, G.Y.; Kaplan, R.C.; Muzumdar, R.; Rohan, T.E.; Strickler, H.D. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab. Res. Rev. 2009, 25, 3–12. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483. [Google Scholar] [CrossRef]
- Lu, M.; Wang, P.; Qiao, Y.; Jiang, C.; Ge, Y.; Flickinger, B.; Malhotra, D.K.; Dworkin, L.D.; Liu, Z.; Gong, R. GSK3β-mediated Keap1-independent regulation of Nrf2 antioxidant response: A molecular rheostat of acute kidney injury to chronic kidney disease transition. Redox Biol. 2019, 26, 101275. [Google Scholar] [CrossRef]
- Adelusi, T.I.; Du, L.; Hao, M.; Zhou, X.; Xuan, Q.; Apu, C.; Sun, Y.; Lu, Q.; Yin, X. Keap1/Nrf2/ARE signaling unfolds therapeutic targets for redox imbalanced-mediated diseases and diabetic nephropathy. Biomed. Pharmacother. 2020, 123, 109732. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Chertow, G.M.; Devarajan, P.; Levin, A.; Andreoli, S.P.; Bangalore, S.; Warady, B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021, 6, 1775–1787. [Google Scholar] [CrossRef]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Muhsin, S.A.; Choi, M.E. TGF-β signaling in the kidney: Profibrotic and protective effects. Am. J. Physiol. Ren. Physiol. 2016, 310, F596–F606. [Google Scholar] [CrossRef]
- Gu, Y.-Y.; Liu, X.-S.; Huang, X.-R.; Yu, X.-Q.; Lan, H.-Y. Diverse role of TGF-β in kidney disease. Front. Cell Dev. Biol. 2020, 8, 123. [Google Scholar] [CrossRef]
- Meng, X.-M.; Tang, P.M.-K.; Li, J.; Lan, H.Y. TGF-β/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef]
- Murayama, K.; Kato-Murayama, M.; Itoh, Y.; Miyazono, K.; Miyazawa, K.; Shirouzu, M. Structural basis for inhibitory effects of Smad7 on TGF-β family signaling. J. Struct. Biol. 2020, 212, 107661. [Google Scholar] [CrossRef]
- Lin, L.; Gan, H.; Zhang, H.; Tang, W.; Sun, Y.; Tang, X.; Kong, D.; Zhou, J.; Wang, Y.; Zhu, Y. MicroRNA-21 inhibits SMAD7 expression through a target sequence in the 3'untranslated region and inhibits proliferation of renal tubular epithelial cells. Mol. Med. Rep. 2014, 10, 707–712. [Google Scholar] [CrossRef]
- Li, J.H.; Huang, X.R.; Zhu, H.J.; Oldfield, M.; Cooper, M.; Truong, L.D.; Johnson, R.J.; Lan, H.Y. Advanced glycation end products activate Smad signaling via TGF-β-dependent and-independent mechanisms: Implications for diabetic renal and vascular disease. FASEB J. 2004, 18, 176–178. [Google Scholar] [CrossRef]
- Rodríguez-Vita, J.; Sánchez-López, E.; Esteban, V.; Rupérez, M.; Egido, J.; Ruiz-Ortega, M. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-β–independent mechanism. Circulation 2005, 111, 2509–2517. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kim, S.I.; Choi, M.E. Therapeutic targets for treating fibrotic kidney diseases. Transl. Res. 2015, 165, 512–530. [Google Scholar] [CrossRef]
- Chauveau, P.; Koppe, L.; Combe, C.; Lasseur, C.; Trolonge, S.; Aparicio, M. Vegetarian diets and chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 199–207. [Google Scholar] [CrossRef]
- Baracho, N.C.; Monteiro, N.F.; Borges, M.G.; Arguelho, R.R. Effect of aqueous extract of the Vigna angularis in rats subjected to an experimental model of moderate chronic kidney disease. Acta Cir. Bras. 2016, 31, 527–532. [Google Scholar] [CrossRef]
- Zhang, H.W.; Lin, Z.X.; Xu, C.; Leung, C.; Chan, L.S. Astragalus (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane Database Syst. Rev. 2014, 10, Cd008369. [Google Scholar] [CrossRef]
- Parveen, A.; Jin, M.; Kim, S.Y. Bioactive phytochemicals that regulate the cellular processes involved in diabetic nephropathy. Phytomedicine 2018, 39, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Giribabu, N.; Karim, K.; Kilari, E.K.; Salleh, N. Phyllanthus niruri leaves aqueous extract improves kidney functions, ameliorates kidney oxidative stress, inflammation, fibrosis and apoptosis and enhances kidney cell proliferation in adult male rats with diabetes mellitus. J. Ethnopharmacol. 2017, 205, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ding, X.; Ding, Z.; Jia, P. Total Flavonoids from Leaves of Carya Cathayensis Ameliorate Renal Fibrosis via the miR-21/Smad7 Signaling Pathway. Cell Physiol. Biochem. 2018, 49, 1551–1563. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, G.; Sun, X.; Chen, X. Treatment of chronic kidney disease using a traditional Chinese medicine, Flos Abelmoschus manihot (Linnaeus) Medicus (Malvaceae). Clin Exp Pharm. Physiol 2016, 43, 145–148. [Google Scholar] [CrossRef]
- Yu, J.Y.; Xiong, N.N. Pathogenic factor (Dampness-heat) of glomerulopathy. Zhongguo Zhong Xi Yi Jie He Za Zhi 1992, 12, 458–460. [Google Scholar]
- Zhou, L.; An, X.F.; Teng, S.C.; Liu, J.S.; Shang, W.B.; Zhang, A.H.; Yuan, Y.G.; Yu, J.Y. Pretreatment with the total flavone glycosides of Flos Abelmoschus manihot and hyperoside prevents glomerular podocyte apoptosis in streptozotocin-induced diabetic nephropathy. J. Med. Food 2012, 15, 461–468. [Google Scholar] [CrossRef]
- Mao, Z.M.; Shen, S.M.; Wan, Y.G.; Sun, W.; Chen, H.L.; Huang, M.M.; Yang, J.J.; Wu, W.; Tang, H.T.; Tang, R.M. Huangkui capsule attenuates renal fibrosis in diabetic nephropathy rats through regulating oxidative stress and p38MAPK/Akt pathways, compared to α-lipoic acid. J. Ethnopharmacol. 2015, 173, 256–265. [Google Scholar] [CrossRef]
- Zhao, Q.; Wan, Y.G.; Sun, W.; Wang, C.J.; Wei, Q.X.; Chen, H.L.; Meng, X.J. Effects of huangkui capsule on renal inflammatory injury by intervening p38MAPK signaling pathway in rats with adriamycin-induced nephropathy. Zhongguo Zhong Yao Za Zhi 2012, 37, 2926–2934. [Google Scholar]
- Tu, Y.; Sun, W.; Wan, Y.G.; Che, X.Y.; Pu, H.P.; Yin, X.J.; Chen, H.L.; Meng, X.J.; Huang, Y.R.; Shi, X.M. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, ameliorates adriamycin-induced renal inflammation and glomerular injury via inhibiting p38MAPK signaling pathway activity in rats. J. Ethnopharmacol. 2013, 147, 311–320. [Google Scholar] [CrossRef]
- Carney, E.F. Glomerular disease. Antiproteinuric efficacy of A. manihot superior to losartan. Nat. Rev. Nephrol. 2014, 10, 300. [Google Scholar] [CrossRef]
- Huang, H.; Luo, S.h.; Huang, D.c.; Cheng, S.j.; Cao, C.j.; Chen, G.t. Immunomodulatory activities of proteins from Astragalus membranaceus waste. J. Sci. Food Agric. 2019, 99, 4174–4181. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Zengin, G. Polyphenol profile and biological activity comparisons of different parts of Astragalus macrocephalus subsp. finitimus from Turkey. Biology 2020, 9, 231. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, D.B.; Song, B.N.; Park, B.R.; Lee, S.H.; Choi, J.H.; Park, S.Y. Physicochemical Properties and Anti-inflammatory Effects of Astragalus membranaceus (Fisch.) Bunge Fermented by Aspergillus awamori. Korean J. Med. Crop. Sci. 2020, 28, 347–353. [Google Scholar] [CrossRef]
- Hui, D.; Rui-Zhi, T.; Jian-Chun, L.; Xia, Z.; Dan, W.; Jun-Ming, F.; Li, W. Astragalus propinquus Schischkin and Panax notoginseng (A&P) compound relieved cisplatin-induced acute kidney injury through inhibiting the mincle maintained macrophage inflammation. J. Ethnopharmacol. 2020, 252, 112637. [Google Scholar]
- Shahzad, M.; Small, D.M.; Morais, C.; Wojcikowski, K.; Shabbir, A.; Gobe, G.C. Protection against oxidative stress-induced apoptosis in kidney epithelium by Angelica and Astragalus. J. Ethnopharmacol. 2016, 179, 412–419. [Google Scholar] [CrossRef]
- Meng, L.; Qu, L.; Tang, J.; Cai, S.-Q.; Wang, H.; Li, X. A combination of Chinese herbs, Astragalus membranaceus var. mongholicus and Angelica sinensis, enhanced nitric oxide production in obstructed rat kidney. Vasc. Pharmacol. 2007, 47, 174–183. [Google Scholar] [CrossRef]
- Alshinnawy, A.; Elsayed, W.; Taha, A.; Sayed, A.; Salem, A. Astragalus membranaceus and Punica granatum alleviate infertility and kidney dysfunction induced by aging in male rats. Turk. J. Biol. 2020, 44, 166–175. [Google Scholar] [CrossRef]
- Okuda, M.; Horikoshi, S.; Matsumoto, M.; Tanimoto, M.; Yasui, H.; Tomino, Y. Beneficial effect of Astragalus membranaceus on estimated glomerular filtration rate in patients with progressive chronic kidney disease. Hong Kong J. Nephrol. 2012, 14, 17–23. [Google Scholar] [CrossRef][Green Version]
- Li, M.; Wang, W.; Xue, J.; Gu, Y.; Lin, S. Meta-analysis of the clinical value of Astragalus membranaceus in diabetic nephropathy. J. Ethnopharmacol. 2011, 133, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Moon, E.; Kwon, S. Effect of Astragalus membranaceus extract on diabetic nephropathy. Endocrinol. Diabetes Metab. Case Rep. 2014, 2014, 14–0063. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Tan, R.-Z.; Zhao, C.-Y.; Li, J.-C.; Zhong, X.; Diao, H.; Lin, X.; Duan, D.D.; Fan, J.-M.; Xie, X.-S. Astragalus mongholicus Bunge and Panax notoginseng (Burkill) FH Chen Formula for Renal Injury in Diabetic Nephropathy—In Vivo and In Vitro Evidence for Autophagy Regulation. Front. Pharmacol. 2020, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Zuo, C.; Xie, X.S.; Qiu, H.Y.; Deng, Y.; Zhu, D.; Fan, J.M. Astragalus mongholicus ameliorates renal fibrosis by modulating HGF and TGF-beta in rats with unilateral ureteral obstruction. J. Zhejiang Univ. Sci. B 2009, 10, 380–390. [Google Scholar] [CrossRef]
- Zuo, C.; Xie, X.; Deng, Y.; Fan, J. Effect of Astragalus mongholicus on expression of transforming growth factor- beta1 in SD rats with unilateral ureteral occlusion. Zhongguo Zhong Yao Za Zhi 2009, 34, 193–198. [Google Scholar]
- Wang, H.; Li, J.; Yu, L.; Zhao, Y.; Ding, W. Antifibrotic effect of the Chinese herbs, Astragalus mongholicus and Angelica sinensis, in a rat model of chronic puromycin aminonucleoside nephrosis. Life Sci 2004, 74, 1645–1658. [Google Scholar] [CrossRef]
- Hong, X.P.; Zhang, X.Z.; He, X.L.; Huang, X.Z.; Chen, H.R.; Wang, Y.Z. Effect of Astragalus mongholicus on renal gene expression profile in mice with diabetic nephropathy. Zhongguo Zhong Yao Za Zhi 2008, 33, 676–680. [Google Scholar]
- Sato, S.; Hori, Y.; Yamate, J.; Saito, T.; Kurasaki, M.; Hatai, A. Protective effect of dietary azuki bean (Vigna angularis) seed coats against renal interstitial fibrosis of rats induced by cisplatin. Nutrition 2005, 21, 504–511. [Google Scholar] [CrossRef]
- Sato, S.; Kataoka, S.; Kimura, A.; Mukai, Y. Azuki bean (Vigna angularis) extract reduces oxidative stress and stimulates autophagy in the kidneys of streptozotocin-induced early diabetic rats. Can. J. Physiol. Pharm. 2016, 94, 1298–1303. [Google Scholar] [CrossRef]
- Sato, S.; Yamate, J.; Hori, Y.; Hatai, A.; Nozawa, M.; Sagai, M. Protective effect of polyphenol-containing azuki bean (Vigna angularis) seed coats on the renal cortex in streptozotocin-induced diabetic rats. J. Nutr. Biochem. 2005, 16, 547–553. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee Products in Dermatology and Skin Care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef]
- Premratanachai, P.; Chanchao, C. Review of the anticancer activities of bee products. Asian Pac. J. Trop. Biomed. 2014, 4, 337–344. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.; Yan, Y.; Mi, J.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. Antioxidant and immunomodulatory activities in vitro of polysaccharides from bee collected pollen of Chinese wolfberry. Int. J. Biol. Macromol. 2020, 163, 190–199. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharm. 2017, 8, 412. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; Teles, F.; Berretta, A.A.; Sanches, T.R.; Rodrigues, C.E.; Seguro, A.C.; Andrade, L. Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: A randomized, double-blind, placebo-controlled trial. BMC Nephrol. 2019, 20, 140. [Google Scholar] [CrossRef]
- Chang, J.F.; Hsieh, C.Y.; Lu, K.C.; Chen, Y.W.; Liang, S.S.; Lin, C.C.; Hung, C.F.; Liou, J.C.; Wu, M.S. Therapeutic Targeting of Aristolochic Acid Induced Uremic Toxin Retention, SMAD 2/3 and JNK/ERK Pathways in Tubulointerstitial Fibrosis: Nephroprotective Role of Propolis in Chronic Kidney Disease. Toxins 2020, 12, 364. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxidative Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of Beijing propolis extracts. Evid.-Based Complementary Altern. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of bioactive compounds potential and antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Chen, J.; Jiang, X.; Hu, F. Identification of free radical scavengers from Brazilian green propolis using off-line HPLC-DPPH assay and LC-MS. J. Food Sci. 2017, 82, 1602–1607. [Google Scholar] [CrossRef]
- Teles, F.; da Silva, T.M.; da Cruz Júnior, F.P.; Honorato, V.H.; de Oliveira Costa, H.; Barbosa, A.P.; de Oliveira, S.G.; Porfírio, Z.; Libório, A.B.; Borges, R.L.; et al. Brazilian red propolis attenuates hypertension and renal damage in 5/6 renal ablation model. PLoS ONE 2015, 10, e0116535. [Google Scholar] [CrossRef]
- Agung, S.; Bambang, P.; Ambar, M.; Suroto, S. Nephro-Protective Effect of the Indonesian Propolis Extract on Unilateral Renal Ureter Obstructive Damage. In Proceedings of the Mid-International Conference on Public Health 2018, Surakarta, Indonesia, 18–19 April 2018; p. 240. [Google Scholar]
- Sameni, H.R.; Ramhormozi, P.; Bandegi, A.R.; Taherian, A.A.; Mirmohammadkhani, M.; Safari, M. Effects of ethanol extract of propolis on histopathological changes and anti-oxidant defense of kidney in a rat model for type 1 diabetes mellitus. J. Diabetes Investig. 2016, 7, 506–513. [Google Scholar] [CrossRef]
- Silveira, M.; Teles, F.; Melo, E.; Borges, V.; Miranda, F.; Dutra, F.; Berretta, A.; Cezar, R.; Silva, J.; Santos, H. P1574 Effects of Brazilian Green Propolis Extract (EPP-AF) on Inflammation in Hemodialysis Patients. Nephrol. Dial. Transplant. 2020, 35 (Suppl. S3), gfaa142. [Google Scholar] [CrossRef]
- Lee, E.-J.; Kang, M.-K.; Kim, D.Y.; Kim, Y.-H.; Oh, H.; Kang, Y.-H. Chrysin inhibits advanced glycation end products-induced kidney fibrosis in renal mesangial cells and diabetic kidneys. Nutrients 2018, 10, 882. [Google Scholar] [CrossRef]
- Ali, B.H.; Adham, S.A.; Al Za’abi, M.; Waly, M.I.; Yasin, J.; Nemmar, A.; Schupp, N. Ameliorative effect of chrysin on adenine-induced chronic kidney disease in rats. PLoS ONE 2015, 10, e0125285. [Google Scholar] [CrossRef]
- Oktem, F.; Ozguner, F.; Sulak, O.; Olgar, Ş.; Akturk, O.; Yilmaz, H.R.; Altuntas, I. Lithium-induced renal toxicity in rats: Protection by a novel antioxidant caffeic acid phenethyl ester. Mol. Cell. Biochem. 2005, 277, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.y.; Mong, M.c.; Chan, K.c.; Yin, M.c. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Granados-Pineda, J.; Uribe-Uribe, N.; García-López, P.; Ramos-Godinez, M.D.P.; Rivero-Cruz, J.F.; Pérez-Rojas, J.M. Effect of Pinocembrin Isolated from Mexican Brown Propolis on Diabetic Nephropathy. Molecules 2018, 23, 852. [Google Scholar] [CrossRef] [PubMed]
- Duffy, C.; Sorolla, A.; Wang, E.; Golden, E.; Woodward, E.; Davern, K.; Ho, D.; Johnstone, E.; Pfleger, K.; Redfern, A. Honeybee venom and melittin suppress growth factor receptor activation in HER2-enriched and triple-negative breast cancer. NPJ Precis. Oncol. 2020, 4, 1–16. [Google Scholar] [CrossRef]
- An, H.J.; Kim, K.H.; Lee, W.R.; Kim, J.Y.; Lee, S.J.; Pak, S.C.; Han, S.M.; Park, K.K. Anti-fibrotic effect of natural toxin bee venom on animal model of unilateral ureteral obstruction. Toxins 2015, 7, 1917–1928. [Google Scholar] [CrossRef]
- Mafra, D.; Borges, N.; Alvarenga, L.; Esgalhado, M.; Cardozo, L.; Lindholm, B.; Stenvinkel, P. Dietary components that may influence the disturbed gut microbiota in chronic kidney disease. Nutrients 2019, 11, 496. [Google Scholar] [CrossRef]
- Mafra, D.; Gidlund, E.K.; Borges, N.A.; Magliano, D.A.C.; Lindholm, B.; Stenvinkel, P.; von Walden, F. Bioactive food and exercise in chronic kidney disease: Targeting the mitochondria. Eur. J. Clin. Investig. 2018, 48, e13020. [Google Scholar] [CrossRef]
- Tao, J.-h.; Zhao, M.; Wang, D.-g.; Yang, C.; Chen, G.-t.; Zhao, X.; Pu, X.-l.; Jiang, S. UPLC-Q-TOF/MS-based screening and identification of two major bioactive components and their metabolites in normal and CKD rat plasma, urine and feces after oral administration of Rehmannia glutinosa Libosch extract. J. Chromatogr. B 2015, 1001, 98–106. [Google Scholar] [CrossRef]
- Alvarenga, L.; Cardozo, L.F.; Borges, N.A.; Lindholm, B.; Stenvinkel, P.; Shiels, P.G.; Fouque, D.; Mafra, D. Can nutritional interventions modulate the activation of the NLRP3 inflammasome in chronic kidney disease? Food Res. Int. 2020, 136, 109306. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Serrano, A.; Ros, G.; Nieto, G. Bioactive compounds and extracts from traditional herbs and their potential anti-inflammatory health effects. Medicines 2018, 5, 76. [Google Scholar] [CrossRef]
- Torrens, F.; Castellano, G. Immunomodulatory molecules from Himalayan medicinal plants. In Chemistry and Chemical Engineering for Sustainable Development; Apple Academic Press: Oakville, ON, Canada, 2020; p. 267. [Google Scholar]
- Xu, J.; Long, Y.; Ni, L.; Yuan, X.; Yu, N.; Wu, R.; Tao, J.; Zhang, Y. Anticancer effect of berberine based on experimental animal models of various cancers: A systematic review and meta-analysis. BMC Cancer 2019, 19, 589. [Google Scholar] [CrossRef]
- Li, T.; Wang, P.; Guo, W.; Huang, X.; Tian, X.; Wu, G.; Xu, B.; Li, F.; Yan, C.; Liang, X.J.; et al. Natural Berberine-Based Chinese Herb Medicine Assembled Nanostructures with Modified Antibacterial Application. ACS Nano. 2019, 13, 6770–6781. [Google Scholar] [CrossRef]
- Deng, Y.X.; Zhang, X.J.; Shi, Q.Z.; Chen, Y.S.; Qiu, X.M.; Chen, B. Anti-hyperglycemic effects and mechanism of traditional Chinese medicine Huanglian Wan in streptozocin-induced diabetic rats. J. Ethnopharmacol. 2012, 144, 425–432. [Google Scholar] [CrossRef]
- Qing, Y.; Dong, X.; Hongli, L.; Yanhui, L. Berberine promoted myocardial protection of postoperative patients through regulating myocardial autophagy. Biomed Pharm. 2018, 105, 1050–1053. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.K.; Nandi, M.K.; Mishra, G.; Maurya, A.; Rai, A.; Rai, G.K.; Awasthi, R.; Sharma, B.; Kulkarni, G.T. Berberine: A Plant-derived Alkaloid with Therapeutic Potential to Combat Alzheimer's disease. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 154–170. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, D.; Zhu, H.; Zhu, J.; Weng, S.; Dong, L.; Liu, T.; Hu, Y.; Shen, X. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe(-/-) mice. Atherosclerosis 2018, 268, 117–126. [Google Scholar] [CrossRef]
- Ehteshamfar, S.M.; Akhbari, M.; Afshari, J.T.; Seyedi, M.; Nikfar, B.; Shapouri-Moghaddam, A.; Ghanbarzadeh, E.; Momtazi-Borojeni, A.A. Anti-inflammatory and immune-modulatory impacts of berberine on activation of autoreactive T cells in autoimmune inflammation. J. Cell Mol. Med. 2020, 24, 13573–13588. [Google Scholar] [CrossRef]
- Hsu, Y.-Y.; Chen, C.-S.; Wu, S.-N.; Jong, Y.-J.; Lo, Y.-C. Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent mechanism in NSC34 motor neuron-like cells. Eur. J. Pharm. Sci. 2012, 46, 415–425. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, P.; Wu, X.; Liu, W.; Shen, X.; Lan, T.; Xu, S.; Peng, J.; Xie, X.; Huang, H. Berberine attenuates lipopolysaccharide-induced extracelluar matrix accumulation and inflammation in rat mesangial cells: Involvement of NF-κB signaling pathway. Mol. Cell. Endocrinol. 2011, 331, 34–40. [Google Scholar] [CrossRef]
- Jia, L.; Liu, J.; Song, Z.; Pan, X.; Chen, L.; Cui, X.; Wang, M. Berberine suppresses amyloid-beta-induced inflammatory response in microglia by inhibiting nuclear factor-kappaB and mitogen-activated protein kinase signalling pathways. J. Pharm. Pharmacol. 2012, 64, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Han, J.; Yuan, R.; Xue, L.; Pang, W. Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB pathway. Biol. Res. 2018, 51, 9. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Jiang, M.; Zhao, Y.; Gong, J.; Su, H.; Yuan, F.; Fang, K.; Yuan, X.; Yu, X.; Dong, H. Berberine protects against diabetic kidney disease via promoting PGC-1α-regulated mitochondrial energy homeostasis. Br. J. Pharmacol. 2020, 177, 3646–3661. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhao, Y.; Gong, J.; Huang, W.; Su, H.; Yuan, F.; Fang, K.; Wang, D.; Li, J.; Zou, X.; et al. Berberine Protects Glomerular Podocytes via Inhibiting Drp1-Mediated Mitochondrial Fission and Dysfunction. Theranostics 2019, 9, 1698–1713. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Liu, B.; Zhuang, X.J.; Shen, Y.D.; Tian, H.R.; Ji, Y.; Li, L.X.; Liu, F. Effects of berberine on the serum cystatin C levels and urine albumin/creatine ratio in patients with type 2 diabetes mellitus. Zhonghua Yi Xue Za Zhi 2018, 98, 3756–3761. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants–rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Lee, S.R.; Heo, J.-W.; No, M.-H.; Rhee, B.D.; Ko, K.S.; Kwak, H.-B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Chen, R.; Wang, Z. Oral supplementation with ursolic acid ameliorates sepsis-induced acute kidney injury in a mouse model by inhibiting oxidative stress and inflammatory responses. Mol. Med. Rep. 2018, 17, 7142–7148. [Google Scholar] [CrossRef]
- Lu, X.; Fan, Q.; Xu, L.; Li, L.; Yue, Y.; Xu, Y.; Su, Y.; Zhang, D.; Wang, L. Ursolic acid attenuates diabetic mesangial cell injury through the up-regulation of autophagy via miRNA-21/PTEN/Akt/mTOR suppression. PLoS ONE 2015, 10, e0117400. [Google Scholar] [CrossRef]
- Jia, Z.; Li, W.; Bian, P.; Yang, L.; Liu, H.; Pan, D.; Dou, Z. Ursolic acid treats renal tubular epithelial cell damage induced by calcium oxalate monohydrate via inhibiting oxidative stress and inflammation. Bioengineered 2021, 12, 5450–5461. [Google Scholar] [CrossRef]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef]
- Do Nascimento, P.G.; Lemos, T.L.; Bizerra, A.; Arriaga, Â.; Ferreira, D.A.; Santiago, G.M.; Braz-Filho, R.; Costa, J.G.M. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef]
- Ou, K.; Xu, X.; Guan, S.; Zhang, R.; Zhang, X.; Kang, Y.; Wu, J. Nanodrug Carrier Based on Poly (Ursolic Acid) with Self-Anticancer Activity against Colorectal Cancer. Adv. Funct. Mater. 2020, 30, 1907857. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Zhang, Z.; Chen, C.; Chi, Q.; Xu, K.; Yang, L. Ursolic acid protects chondrocytes, exhibits anti-inflammatory properties via regulation of the NF-κB/NLRP3 inflammasome pathway and ameliorates osteoarthritis. Biomed. Pharmacother. 2020, 130, 110568. [Google Scholar] [CrossRef]
- Zheng, H.; Feng, H.; Zhang, W.; Han, Y.; Zhao, W. Targeting autophagy by natural product Ursolic acid for prevention and treatment of osteoporosis. Toxicol. Appl. Pharmacol. 2020, 409, 115271. [Google Scholar] [CrossRef]
- Thakur, R.; Sharma, A.; Lingaraju, M.C.; Begum, J.; Kumar, D.; Mathesh, K.; Kumar, P.; Singh, T.U.; Kumar, D. Ameliorative effect of ursolic acid on renal fibrosis in adenine-induced chronic kidney disease in rats. Biomed. Pharmacother. 2018, 101, 972–980. [Google Scholar] [CrossRef]
- Xu, C.G.; Zhu, X.L.; Wang, W.; Zhou, X.J. Ursolic acid inhibits epithelial-mesenchymal transition in vitro and in vivo. Pharm Biol 2019, 57, 169–175. [Google Scholar] [CrossRef]
- Yu, R.; Chen, J.A.; Xu, J.; Cao, J.; Wang, Y.; Thomas, S.S.; Hu, Z. Suppression of muscle wasting by the plant-derived compound ursolic acid in a model of chronic kidney disease. J. Cachexia Sarcopenia Muscle 2017, 8, 327–341. [Google Scholar] [CrossRef]
- Ma, T.K.; Xu, L.; Lu, L.X.; Cao, X.; Li, X.; Li, L.L.; Wang, X.; Fan, Q.L. Ursolic Acid Treatment Alleviates Diabetic Kidney Injury By Regulating The ARAP1/AT1R Signaling Pathway. Diabetes Metab. Syndr. Obes. 2019, 12, 2597–2608. [Google Scholar] [CrossRef]
- Bacanli, M.; Aydin, S.; Anlar, H.G.; Çal, T.; Ündeğer Bucurgat, Ü.; Ari, N.; Başaran, A.A.; Başaran, N. Protective Effects of Ursolic Acid in the Kidneys of Diabetic Rats. Turk. J. Pharm. Sci. 2018, 15, 166–170. [Google Scholar] [CrossRef]
- Qi, M.Y.; Yang, J.J.; Zhou, B.; Pan, D.Y.; Sun, X. Study on the protective effect of ursolic acid on alloxan-induced diabetic renal injury and its underlying mechanisms. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2014, 30, 445–448. [Google Scholar] [PubMed]
- Ding, Y.; Choi, M.E. Autophagy in diabetic nephropathy. J. Endocrinol. 2015, 224, R15–R30. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Fan, Q.; Wang, X.; Li, L.; Lu, X.; Yue, Y.; Cao, X.; Liu, J.; Zhao, X.; Wang, L. Ursolic acid improves podocyte injury caused by high glucose. Nephrol. Dial. Transpl. 2017, 32, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Zobeiri, M.; Belwal, T.; Parvizi, F.; Naseri, R.; Farzaei, M.H.; Nabavi, S.F.; Sureda, A.; Nabavi, S.M. Naringenin and its Nano-formulations for Fatty Liver: Cellular Modes of Action and Clinical Perspective. Curr. Pharm. Biotechnol. 2018, 19, 196–205. [Google Scholar] [CrossRef]
- Wilcox, L.J.; Borradaile, N.M.; Huff, M.W. Antiatherogenic properties of naringenin, a citrus flavonoid. Cardiovasc. Drug Rev. 1999, 17, 160–178. [Google Scholar] [CrossRef]
- Jeandet, P.; Sobarzo-Sánchez, E.; Clément, C.; Nabavi, S.F.; Habtemariam, S.; Nabavi, S.M.; Cordelier, S. Engineering stilbene metabolic pathways in microbial cells. Biotechnol. Adv. 2018, 36, 2264–2283. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, T.; Du, G.; Zhou, J.; Chen, J. Modular optimization of heterologous pathways for de novo synthesis of (2S)-naringenin in Escherichia coli. PLoS ONE 2014, 9, e101492. [Google Scholar] [CrossRef]
- Kanaze, F.; Bounartzi, M.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Polyviou, T.; Ludwig, I.A.; Nastase, A.-M.; Moreno-Rojas, J.M.; Garcia, A.L.; Malkova, D.; Crozier, A. Bioavailability of orange juice (poly) phenols: The impact of short-term cessation of training by male endurance athletes. Am. J. Clin. Nutr. 2017, 106, 791–800. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic Properties of Naringenin: A Citrus Fruit Polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chang, Y.M.; Wang, K.Y.; Chen, P.N.; Hseu, Y.C.; Chen, K.M.; Yeh, K.T.; Chen, C.J.; Hsu, L.S. Naringenin inhibited migration and invasion of glioblastoma cells through multiple mechanisms. Env. Toxicol. 2019, 34, 233–239. [Google Scholar] [CrossRef]
- Nouri, Z.; Fakhri, S.; El-Senduny, F.F.; Sanadgol, N.; Abd-ElGhani, G.E.; Farzaei, M.H.; Chen, J.T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules 2019, 9, 690. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Naringenin induces mitochondria-mediated apoptosis and endoplasmic reticulum stress by regulating MAPK and AKT signal transduction pathways in endometriosis cells. Mol. Hum. Reprod. 2017, 23, 842–854. [Google Scholar] [CrossRef]
- Heidary Moghaddam, R.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur. J. Pharm. 2020, 887, 173535. [Google Scholar] [CrossRef]
- Liu, Y.; An, W.; Gao, A. Protective effects of naringenin in cardiorenal syndrome. J. Surg. Res. 2016, 203, 416–423. [Google Scholar] [CrossRef]
- Manchope, M.F.; Calixto-Campos, C.; Coelho-Silva, L.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Georgetti, S.R.; Baracat, M.M.; Casagrande, R.; Verri Jr, W.A. Naringenin inhibits superoxide anion-induced inflammatory pain: Role of oxidative stress, cytokines, Nrf-2 and the NO− cGMP− PKG− KATPChannel signaling pathway. PLoS ONE 2016, 11, e0153015. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Fattori, V.; Manchope, M.F.; Mizokami, S.S.; Casagrande, R.; Verri, W.A., Jr. Naringenin reduces inflammatory pain in mice. Neuropharmacology 2016, 105, 508–519. [Google Scholar] [CrossRef]
- Tsai, S.J.; Huang, C.S.; Mong, M.C.; Kam, W.Y.; Huang, H.Y.; Yin, M.C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J. Agric. Food Chem. 2012, 60, 514–521. [Google Scholar] [CrossRef]
- Chen, L.; Yang, T.; Lu, D.W.; Zhao, H.; Feng, Y.L.; Chen, H.; Chen, D.Q.; Vaziri, N.D.; Zhao, Y.Y. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed Pharm. 2018, 101, 670–681. [Google Scholar] [CrossRef]
- Meng, X.-m.; Zhang, Y.; Huang, X.-R.; Ren, G.-l.; Li, J.; Lan, H.Y. Treatment of renal fibrosis by rebalancing TGF-β/Smad signaling with the combination of asiatic acid and naringenin. Oncotarget 2015, 6, 36984–36997. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Oh, S.-H.; Ahn, J.-S.; Yook, J.-M.; Kim, C.-D.; Park, S.-H.; Cho, J.-H.; Kim, Y.-L. The Crucial Role of Xanthine Oxidase in CKD Progression Associated with Hypercholesterolemia. Int. J. Mol. Sci. 2020, 21, 7444. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tall, A.R. Cholesterol in platelet biogenesis and activation. Blood 2016, 127, 1949–1953. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, Y.; Kamoun, Z.; Zarrouk, W.; Kebieche, M.; Kallel, C.; Gdoura, R.; Fetoui, H. Naringenin ameliorates renal and platelet purinergic signalling alterations in high-cholesterol fed rats through the suppression of ROS and NF-κB signaling pathways. Food Funct. 2016, 7, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ahmed, F.; Banerjee, S.; Saha, U. Naringenin ameliorates streptozotocin-induced diabetic rat renal impairment by downregulation of TGF-β1 and IL-1 via modulation of oxidative stress correlates with decreased apoptotic events. Pharm. Biol. 2016, 54, 1616–1627. [Google Scholar] [CrossRef]
- Ding, S.; Qiu, H.; Huang, J.; Chen, R.; Zhang, J.; Huang, B.; Zou, X.; Cheng, O.; Jiang, Q. Activation of 20-HETE/PPARs involved in reno-therapeutic effect of naringenin on diabetic nephropathy. Chem. Biol. Interact. 2019, 307, 116–124. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Sciubba, F.; Serafini, M.; Bianco, A.; Cianfaglione, K.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Maggi, F. Volatile components, polar constituents and biological activity of tansy daisy (Tanacetum macrophyllum (Waldst. et Kit.) Schultz Bip.). Ind. Crop. Prod. 2018, 118, 225–235. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; del Mar Contreras, M. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef]
- Venditti, A.; Guarcini, L.; Bianco, A.; Rosselli, S.; Bruno, M.; Senatore, F. Phytochemical analysis of Achillea ligustica all. from Lipari Island (Aeolian islands). Nat. Prod. Res. 2016, 30, 912–919. [Google Scholar] [CrossRef]
- Ornano, L.; Venditti, A.; Donno, Y.; Sanna, C.; Ballero, M.; Bianco, A. Phytochemical analysis of non-volatile fraction of Artemisia caerulescens subsp. densiflora (Viv.)(Asteraceae), an endemic species of La Maddalena Archipelago (Sardinia–Italy). Nat. Prod. Res. 2016, 30, 920–925. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.G.; Kim, M.; Ahn, J.H. Biosynthesis of Two Flavones, Apigenin and Genkwanin, in Escherichia coli. J. Microbiol. Biotechnol. 2015, 25, 1442–1448. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Molecular targets for apigenin-induced cell cycle arrest and apoptosis in prostate cancer cell xenograft. Mol. Cancer Ther. 2006, 5, 843–852. [Google Scholar] [CrossRef]
- Suh, K.S.; Oh, S.; Woo, J.-T.; Kim, S.-W.; Kim, J.-W.; Kim, Y.S.; Chon, S. Apigenin attenuates 2-deoxy-D-ribose-induced oxidative cell damage in HIT-T15 pancreatic β-cells. Biol. Pharm. Bull. 2012, 35, 121–126. [Google Scholar] [CrossRef]
- Kang, C.H.; Molagoda, I.M.N.; Choi, Y.H.; Park, C.; Moon, D.O.; Kim, G.Y. Apigenin promotes TRAIL-mediated apoptosis regardless of ROS generation. Food Chem. Toxicol. 2018, 111, 623–630. [Google Scholar] [CrossRef]
- Lee, J.-H.; Zhou, H.Y.; Cho, S.Y.; Kim, Y.S.; Lee, Y.S.; Jeong, C.S. Anti-inflammatory mechanisms of apigenin: Inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch. Pharmacal. Res. 2007, 30, 1318–1327. [Google Scholar] [CrossRef]
- Huang, C.-S.; Lii, C.-K.; Lin, A.-H.; Yeh, Y.-W.; Yao, H.-T.; Li, C.-C.; Wang, T.-S.; Chen, H.-W. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch. Toxicol. 2013, 87, 167–178. [Google Scholar] [CrossRef]
- Paredes-Gonzalez, X.; Fuentes, F.; Jeffery, S.; Saw, C.L.L.; Shu, L.; Su, Z.Y.; Kong, A.N.T. Induction of NRF2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm. Drug Dispos. 2015, 36, 440–451. [Google Scholar] [CrossRef]
- Wei, X.; Gao, P.; Pu, Y.; Li, Q.; Yang, T.; Zhang, H.; Xiong, S.; Cui, Y.; Li, L.; Ma, X. Activation of TRPV4 by dietary apigenin antagonizes renal fibrosis in deoxycorticosterone acetate (DOCA)–salt-induced hypertension. Clin. Sci. 2017, 131, 567–581. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Sun, T.; Lei, Y.; Liu, X.; Li, Z. Apigenin Alleviates Renal Fibroblast Activation through AMPK and ERK Signaling Pathways In Vitro. Curr. Pharm. Biotechnol. 2020, 21, 1107–1118. [Google Scholar] [CrossRef]
- Vera, M.; Torramade-Moix, S.; Martin-Rodriguez, S.; Cases, A.; Cruzado, J.M.; Rivera, J.; Escolar, G.; Palomo, M.; Diaz-Ricart, M. Antioxidant and anti-inflammatory strategies based on the potentiation of glutathione peroxidase activity prevent endothelial dysfunction in chronic kidney disease. Cell Physiol. Biochem. 2018, 51, 1287–1300. [Google Scholar] [CrossRef]
- Morigi, M.; Perico, L.; Benigni, A. Sirtuins in Renal Health and Disease. J. Am. Soc. Nephrol. 2018, 29, 1799–1809. [Google Scholar] [CrossRef]
- Cantó, C.; Menzies, K.J.; Auwerx, J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef]
- Ogura, Y.; Kitada, M.; Xu, J.; Monno, I.; Koya, D. CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats. Aging 2020, 12, 11325. [Google Scholar] [CrossRef]
- Li, P.; Bukhari, S.N.A.; Khan, T.; Chitti, R.; Bevoor, D.B.; Hiremath, A.R.; SreeHarsha, N.; Singh, Y.; Gubbiyappa, K.S. Apigenin-Loaded Solid Lipid Nanoparticle Attenuates Diabetic Nephropathy Induced by Streptozotocin Nicotinamide Through Nrf2/HO-1/NF-kB Signalling Pathway. Int. J. Nanomed. 2020, 15, 9115–9124. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Ye, L.; Chan, M.Y.; Leung, L.K. The soy isoflavone genistein induces estrogen synthesis in an extragonadal pathway. Mol. Cell. Endocrinol. 2009, 302, 73–80. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, W.; Gao, S.; Xu, H.; Wu, B.; Kulkarni, K.; Singh, R.; Tang, L.; Hu, M. Simultaneous determination of genistein and its four phase II metabolites in blood by a sensitive and robust UPLC–MS/MS method: Application to an oral bioavailability study of genistein in mice. J. Pharm. Biomed. Anal. 2010, 53, 81–89. [Google Scholar] [CrossRef]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit Rev Oncol. Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef]
- Thangavel, P.; Puga-Olguín, A.; Rodríguez-Landa, J.F.; Zepeda, R.C. Genistein as Potential Therapeutic Candidate for Menopausal Symptoms and Other Related Diseases. Molecules 2019, 24, 3892. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Mao, J.; Li, H.; Wang, M.; Zhang, H.; Li, H.; Chen, W. Genistein Ameliorates Non-alcoholic Fatty Liver Disease by Targeting the Thromboxane A(2) Pathway. J. Agric. Food Chem. 2018, 66, 5853–5859. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Lee, H.; Hwang-Bo, H.; Ji, S.Y.; Kim, M.Y.; Hong, S.H.; Jeong, J.W.; Han, M.H.; Choi, S.H.; et al. Induction of G2/M Cell Cycle Arrest and Apoptosis by Genistein in Human Bladder Cancer T24 Cells through Inhibition of the ROS-Dependent PI3k/Akt Signal Transduction Pathway. Antioxidants 2019, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zheng, Z.; Yin, Y.; Jiang, Z. Genistein prevents bone loss in type 2 diabetic rats induced by streptozotocin. Food Nutr. Res. 2020, 64, 10–29219. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, S.-x.; Zhou, Z.-q.; Wang, Z.; Zhang, Y.-g.; Zhang, Y.; Zhao, P. Apoptotic effect of genistein on human colon cancer cells via inhibiting the nuclear factor-kappa B (NF-κB) pathway. Tumor. Biol. 2014, 35, 11483–11488. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Saidijam, M.; Tavilani, H.; Ghasemkhani, N.; Khodadadi, I. Genistein induces apoptosis and inhibits proliferation of HT29 colon cancer cells. Int. J. Mol. Cell. Med. 2016, 5, 178. [Google Scholar] [PubMed]
- Braxas, H.; Rafraf, M.; Hasanabad, S.K.; Jafarabadi, M.A. Effectiveness of genistein supplementation on metabolic factors and antioxidant status in postmenopausal women with type 2 diabetes mellitus. Can. J. Diabetes 2019, 43, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-T.; Lee, Y.-K.; Shin, J.-I.; Parkb, O.J. Anti-inflammatory and Anticarcinogenic Effect of Genistein Alone or in Combination with Capsaicin in TPA-Treated Rat Mammary Glands or Mammary Cancer Cell Line. In Natural Compounds and Their Role in Apoptotic Cell Signaling Pathways; Blackwell Publishing on behalf of New York Academy of Sciences: Boston, MS, USA, 2009; Volume 1171, p. 415. [Google Scholar]
- Li, J.; Li, J.; Yue, Y.; Hu, Y.; Cheng, W.; Liu, R.; Pan, X.; Zhang, P. Genistein suppresses tumor necrosis factor α-induced inflammation via modulating reactive oxygen species/Akt/nuclear factor κB and adenosine monophosphate-activated protein kinase signal pathways in human synoviocyte MH7A cells. Drug Des. Dev. Ther. 2014, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Sutrisno, S.; Aprina, H.; Simanungkalit, H.M.; Andriyani, A.; Barlianto, W.; Sujuti, H.; Santoso, S.; Dwijayasa, P.M.; Wahyuni, E.S.; Mustofa, E. Genistein modulates the estrogen receptor and suppresses angiogenesis and inflammation in the murine model of peritoneal endometriosis. J. Tradit. Complementary Med. 2018, 8, 278–281. [Google Scholar] [CrossRef]
- Jia, Q.; Yang, R.; Liu, X.F.; Ma, S.F.; Wang, L. Genistein attenuates renal fibrosis in streptozotocin-induced diabetic rats. Mol. Med. Rep. 2019, 19, 423–431. [Google Scholar] [CrossRef]
- Yuan, W.J.; Jia, F.Y.; Meng, J.Z. Effects of genistein on secretion of extracellular matrix components and transforming growth factor beta in high-glucose-cultured rat mesangial cells. J. Artif. Organs. 2009, 12, 242–246. [Google Scholar] [CrossRef]
- Lin, T.-A.; Wu, V.C.-C.; Wang, C.-Y. Autophagy in Chronic Kidney Diseases. Cells 2019, 8, 61. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, T.; Chi, Y.; Liu, M.; Liu, Y. Genistein and Myd88 Activate Autophagy in High Glucose-Induced Renal Podocytes In Vitro. Med. Sci. Monit. 2018, 24, 4823–4831. [Google Scholar] [CrossRef]
- Kim, M.J.; Lim, Y. Protective effect of short-term genistein supplementation on the early stage in diabetes-induced renal damage. Mediat. Inflamm. 2013, 2013, 510212. [Google Scholar] [CrossRef]
- Asmis, R.; Stevens, J.; Begley, J.G.; Grimes, B.; Van Zant, G.; Fanti, P. The isoflavone genistein inhibits LPS-stimulated TNFalpha, but not IL-6 expression in monocytes from hemodialysis patients and healthy subjects. Clin. Nephrol. 2006, 65, 267–275. [Google Scholar] [CrossRef]
- Vázquez-Flores, L.; Casas-Grajales, S.; Hernández-Aquino, E.; Vargas-Pozada, E.; Muriel, P. Antioxidant, antiinflammatory, and antifibrotic properties of quercetin in the liver. In Liver Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 653–674. [Google Scholar]
- Singh, H.; Kaur, P.; Kaur, P.; Muthuraman, A.; Singh, G.; Kaur, M. Investigation of therapeutic potential and molecular mechanism of vitamin P and digoxin in I/R-induced myocardial infarction in rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 2015, 388, 565–574. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi. Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxidative Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Huang, C.-N.; Liao, C.-K.; Chang, H.-M.; Kuan, Y.-H.; Tseng, T.-J.; Yen, K.-J.; Yang, K.-L.; Lin, H.-C. Effects of Rutin on Wound Healing in Hyperglycemic Rats. Antioxidants 2020, 9, 1122. [Google Scholar] [CrossRef]
- Çelik, H.; Kandemir, F.M.; Caglayan, C.; Özdemir, S.; Çomaklı, S.; Kucukler, S.; Yardım, A. Neuroprotective effect of rutin against colistin-induced oxidative stress, inflammation and apoptosis in rat brain associated with the CREB/BDNF expressions. Mol. Biol. Rep. 2020, 47, 2023–2034. [Google Scholar] [CrossRef]
- Su, S.; Li, X.; Li, S.; Ming, P.; Huang, Y.; Dong, Y.; Ding, H.; Feng, S.; Li, J.; Wang, X. Rutin protects against lipopolysaccharide-induced mastitis by inhibiting the activation of the NF-κB signaling pathway and attenuating endoplasmic reticulum stress. Inflammopharmacology 2019, 27, 77–88. [Google Scholar] [CrossRef]
- Diwan, V.; Brown, L.; Gobe, G.C. The flavonoid rutin improves kidney and heart structure and function in an adenine-induced rat model of chronic kidney disease. J. Funct. Foods 2017, 33, 85–93. [Google Scholar] [CrossRef]
- Han, Y.; Lu, J.-S.; Xu, Y.; Zhang, L.; Hong, B.-F. Rutin ameliorates renal fibrosis and proteinuria in 5/6-nephrectomized rats by anti-oxidation and inhibiting activation of TGFβ1-smad signaling. Int. J. Clin. Exp. Pathol. 2015, 8, 4725–4734. [Google Scholar] [PubMed]
- Wang, B.; Liu, D.; Zhu, Q.H.; Li, M.; Chen, H.; Guo, Y.; Fan, L.P.; Yue, L.S.; Li, L.Y.; Zhao, M. Rutin ameliorates kidney interstitial fibrosis in rats with obstructive nephropathy. Int. Immunopharmacol. 2016, 35, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Al-Rejaie, S.S.; Abuohashish, H.M.; Alkhamees, O.A.; Aleisa, A.M.; Alroujayee, A.S. Gender difference following high cholesterol diet induced renal injury and the protective role of rutin and ascorbic acid combination in Wistar albino rats. Lipids Health Dis. 2012, 11, 41. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Feng, T.; Jin, G.; Li, Z. Rutin Prevents High Glucose-Induced Renal Glomerular Endothelial Hyperpermeability by Inhibiting the ROS/Rhoa/ROCK Signaling Pathway. Planta. Med. 2016, 82, 1252–1257. [Google Scholar] [CrossRef]
- Ganesan, D.; Holkar, A.; Albert, A.; Paul, E.; Mariakuttikan, J.; Sadasivam Selvam, G. Combination of ramipril and rutin alleviate alloxan induced diabetic nephropathy targeting multiple stress pathways in vivo. Biomed Pharm. 2018, 108, 1338–1346. [Google Scholar] [CrossRef]
- Ganesan, D.; Albert, A.; Paul, E.; Ananthapadmanabhan, K.; Andiappan, R.; Sadasivam, S.G. Rutin ameliorates metabolic acidosis and fibrosis in alloxan induced diabetic nephropathy and cardiomyopathy in experimental rats. Mol Cell Biochem 2020, 471, 41–50. [Google Scholar] [CrossRef]
- Hao, H.H.; Shao, Z.M.; Tang, D.Q.; Lu, Q.; Chen, X.; Yin, X.X.; Wu, J.; Chen, H. Preventive effects of rutin on the development of experimental diabetic nephropathy in rats. Life Sci. 2012, 91, 959–967. [Google Scholar] [CrossRef]
- Neto, R.T.; Santos, S.A.; Oliveira, J.; Silvestre, A.J. Biorefinery of high polymerization degree proanthocyanidins in the context of circular economy. Ind. Crop. Prod. 2020, 151, 112450. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Lin, Y.-M.; Li, Y.-Y.; Li, M.; Wei, S.-D.; Chai, W.-M.; Tam, N.F.-y. Antioxidant properties of polymeric proanthocyanidins from fruit stones and pericarps of Litchi chinensis Sonn. Food Res. Int. 2011, 44, 613–620. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, Y.I.; Kim, Y.; Choi, M.; Min, S.; Joo, Y.H.; Yim, S.-V.; Chung, N. Grape seed proanthocyanidin inhibits inflammatory responses in hepatic stellate cells by modulating the MAPK, Akt and NF-κB signaling pathways. Int. J. Mol. Med. 2017, 40, 226–234. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, J.; Huang, S.; Li, X.; Zhang, Y.; Zhang, L.; Wang, F. Analytical profiling of proanthocyanidins from Acacia mearnsii bark and in vitro assessment of antioxidant and antidiabetic potential. Molecules 2018, 23, 2891. [Google Scholar] [CrossRef]
- Zhou, Q.; Han, X.; Li, R.; Zhao, W.; Bai, B.; Yan, C.; Dong, X. Anti-atherosclerosis of oligomeric proanthocyanidins from Rhodiola rosea on rat model via hypolipemic, antioxidant, anti-inflammatory activities together with regulation of endothelial function. Phytomedicine 2018, 51, 171–180. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Bai, B.S.; Devaraj, S.N. Efficacy of grape seed proanthocyanidins on cardioprotection during isoproterenol-induced myocardial injury in rats. J. Cardiovasc. Pharmacol. 2009, 53, 109–115. [Google Scholar] [CrossRef]
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Zhan, J.; Wang, K.; Zhang, C.; Zhang, C.; Li, Y.; Zhang, Y.; Chang, X.; Zhou, Q.; Yao, Y.; Liu, Y. GSPE inhibits HMGB1 release, attenuating renal IR-induced acute renal injury and chronic renal fibrosis. Int. J. Mol. Sci. 2016, 17, 1647. [Google Scholar] [CrossRef]
- Wang, K.; Wei, H.; Zhan, J.; Liang, X.; Zhang, C.; Liu, Y.; Xu, G. GSPE alleviates renal fibrosis by inhibiting the activation of C3/HMGB1/TGF-β1 pathway. Chem. Biol. Interact. 2020, 316, 108926. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Y.; Gao, H.; Li, B.; Xu, L.; Cheng, M.; Jiang, B.; Ma, Y. Grape seed proanthocyanidins ameliorate diabetic nephropathy via modulation of levels of AGE, RAGE and CTGF. Nephron Exp. Nephrol. 2009, 111, e31–e41. [Google Scholar] [CrossRef]
- Sayed, A.A. Thymoquinone and proanthocyanidin attenuation of diabetic nephropathy in rats. Eur. Rev. Med. Pharm. Sci. 2012, 16, 808–815. [Google Scholar] [CrossRef]
- Ding, Y.; Li, H.; Li, Y.; Liu, D.; Zhang, L.; Wang, T.; Liu, T.; Ma, L. Protective Effects of Grape Seed Proanthocyanidins on the Kidneys of Diabetic Rats through the Nrf2 Signalling Pathway. Evid. Based Complementary Altern. Med. 2020, 2020, 5205903. [Google Scholar] [CrossRef]
- Bao, L.; Zhang, Z.; Dai, X.; Ding, Y.; Jiang, Y.; Li, Y.; Li, Y. Effects of grape seed proanthocyanidin extract on renal injury in type 2 diabetic rats. Mol. Med. Rep. 2015, 11, 645–652. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, G.; Hu, Z.; Shi, W.; Chen, B.; Zou, P.; Li, X. Grape seed proanthocyanidins protect against streptozotocin-induced diabetic nephropathy by attenuating endoplasmic reticulum stress-induced apoptosis. Mol. Med. Rep. 2018, 18, 1447–1454. [Google Scholar] [CrossRef]
- Izumi, T.; Terauchi, M. The Diverse Efficacy of Food-Derived Proanthocyanidins for Middle-Aged and Elderly Women. Nutrients 2020, 12, 3833. [Google Scholar] [CrossRef]
- Ivey, K.L.; Lewis, J.R.; Lim, W.H.; Lim, E.M.; Hodgson, J.M.; Prince, R.L. Associations of proanthocyanidin intake with renal function and clinical outcomes in elderly women. PLoS ONE 2013, 8, e71166. [Google Scholar]
- Odai, T.; Terauchi, M.; Kato, K.; Hirose, A.; Miyasaka, N. Effects of Grape Seed Proanthocyanidin Extract on Vascular Endothelial Function in Participants with Prehypertension: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2844. [Google Scholar] [CrossRef]
- Ríos, J.L.; Máñez, S. New Pharmacological Opportunities for Betulinic Acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef]
- Karagöz, A.; Leidenberger, M.; Hahn, F.; Hampel, F.; Friedrich, O.; Marschall, M.; Kappes, B.; Tsogoeva, S.B. Synthesis of new betulinic acid/betulin-derived dimers and hybrids with potent antimalarial and antiviral activities. Bioorg. Med. Chem. 2019, 27, 110–115. [Google Scholar] [CrossRef]
- Nicolov, M.; Ghiulai, R.M.; Voicu, M.; Mioc, M.; Duse, A.O.; Roman, R.; Ambrus, R.; Zupko, I.; Moaca, E.A.; Coricovac, D.E.; et al. Cocrystal Formation of Betulinic Acid and Ascorbic Acid: Synthesis, Physico-Chemical Assessment, Antioxidant, and Antiproliferative Activity. Front. Chem. 2019, 7, 92. [Google Scholar] [CrossRef]
- Oriakhi, K.; Uadia, P.O.; Shaheen, F.; Jahan, H.; Ibeji, C.U.; Iqbal, C.M. Isolation, characterization, and hepatoprotective properties of betulinic acid and ricinine from Tetracarpidium conophorum seeds (Euphorbiaceae). J. Food. Biochem. 2020, 45, e13288. [Google Scholar] [CrossRef]
- Lee, S.; Jung, K.; Lee, D.; Lee, S.R.; Lee, K.R.; Kang, K.S.; Kim, K.H. Protective effect and mechanism of action of lupane triterpenes from Cornus walteri in cisplatin-induced nephrotoxicity. Bioorg. Med. Chem. Lett. 2015, 25, 5613–5618. [Google Scholar] [CrossRef]
- Ekuadzi, E.; Biney, R.P.; Benneh, C.K.; Osei Amankwaa, B.; Jato, J. Antiinflammatory properties of betulinic acid and xylopic acid in the carrageenan-induced pleurisy model of lung inflammation in mice. Phytother. Res. 2018, 32, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, S.R.; Kang, K.S.; Ko, Y.; Pang, C.; Yamabe, N.; Kim, K.H. Betulinic Acid Suppresses Ovarian Cancer Cell Proliferation through Induction of Apoptosis. Biomolecules 2019, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, P.; Wang, N.; Wang, S.; Yang, B.; Li, M.; Chen, J.; Situ, H.; Xie, M.; Lin, Y.; et al. Betulinic Acid Suppresses Breast Cancer Metastasis by Targeting GRP78-Mediated Glycolysis and ER Stress Apoptotic Pathway. Oxid. Med. Cell Longev. 2019, 2019, 8781690. [Google Scholar] [CrossRef] [PubMed]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Wu, J.; Niu, Y.; Bakur, A.; Li, H.; Chen, Q. Cell-free production of pentacyclic triterpenoid compound betulinic acid from betulin by the engineered Saccharomyces cerevisiae. Molecules 2017, 22, 1075. [Google Scholar] [CrossRef]
- Fan, R.; Hu, P.-c.; Wang, Y.; Lin, H.-y.; Su, K.; Feng, X.-s.; Wei, L.; Yang, F. Betulinic acid protects mice from cadmium chloride-induced toxicity by inhibiting cadmium-induced apoptosis in kidney and liver. Toxicol. Lett. 2018, 299, 56–66. [Google Scholar] [CrossRef]
- Prakash, B.; Surendran, A.; Chandraprabha, V.R.; Pettamanna, A.; Nair, H.N.R. Betulinic acid, natural pentacyclic triterpenoid prevents arsenic-induced nephrotoxicity in male Wistar rats. Comp. Clin. Pathol. 2018, 27, 37–44. [Google Scholar] [CrossRef]
- Noushida, N.; Shenoy, P.J.; Rao, R.R.; Teerthanath, S. Nephroprotective activity of betulinic acid in gentamicin induced murine model of Renotoxicity. Res. J. Pharm. Technol. 2020, 13, 1391–1396. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, R.; Lingaraju, M.C.; Kumar, D.; Mathesh, K.; Telang, A.G.; Singh, T.U.; Kumar, D. Betulinic acid attenuates renal fibrosis in rat chronic kidney disease model. Biomed. Pharmacother. 2017, 89, 796–804. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Z.; Xiong, F.; Chen, C.; Chao, X.; Huang, J.; Huang, H. Betulinic acid ameliorates experimental diabetic-induced renal inflammation and fibrosis via inhibiting the activation of NF-κB signaling pathway. Mol. Cell. Endocrinol. 2016, 434, 135–143. [Google Scholar] [CrossRef]
- Xie, R.; Zhang, H.; Wang, X.-z.; Yang, X.-z.; Wu, S.-n.; Wang, H.-g.; Shen, P.; Ma, T.-h. The protective effect of betulinic acid (BA) diabetic nephropathy on streptozotocin (STZ)-induced diabetic rats. Food Funct. 2017, 8, 299–306. [Google Scholar] [CrossRef]
- Sutariya, B.; Taneja, N.; Saraf, M. Betulinic acid, isolated from the leaves of Syzygium cumini (L.) Skeels, ameliorates the proteinuria in experimental membranous nephropathy through regulating Nrf2/NF-κB pathways. Chem. Biol. Interact. 2017, 274, 124–137. [Google Scholar] [CrossRef]


| Natural Products | Type of Study | Therapeutic Effect | Major Findings | References |
|---|---|---|---|---|
| Phylanthus niruri | STZ-induced diabetic nephropathy (in vivo) | Antioxidant Anti-inflammatory Anti-apoptotic Anti-fibrotic | Kidney homogenate (TBARS ↓), (SOD, CAT and GPx ↑), (NF-κB-p65, Ikk-β, TNF-α, IL-1β and IL-6↓), (caspase-3, caspase-9, Bax ↓), (TGF-β1, VEGF, FGF-1 ↓), | [61] |
| Carya Cathayensis | - Mouse model of UUO (in vivo) - TGF-β1-treated mouse tubular epithelial cells (mTECs) (in vitro) | Anti-fibrotic | Collagens and α-SMA ↓ in the kidneys In vitro, fibrotic markers ↓ and miR-21 ↓ in TGF-β1-treated mouse tubular epithelial cells (mTECs). Smad7↑ | [62] |
| Flos A. manihot | -Glomerulonephritis rabbit model - Diabetic nephropathy - Adriamycin-induced nephropathy - 417 patients with glomerular disease stages 1–2 CKD | Renoprotective agent Anti-apoptotic Antioxidant | Protein levels in urine ↓, apoptosis of podocytes↓, glomerulosclerosis ↓, and mesangial proliferation ↓ (kidneys) | [64,65,66,67,68,69] |
| Astragalus membranaceus | Human kidney proximal tubular epithelial cells (in vitro) | Anti-apoptotic and anti-inflammatory | H2O2 ↓, apoptosis↓, NF-κB ↓, TNF-α↓ (proximal epithelial cells) | [74] |
| UUO rat kidney | Anti-fibrotic | Interstitial fibrosis ↓ eNOS ↑, ROS scavenging (kidney tissue) | [75] | |
| 35 CKD patients (Stage 4 and 5, dose; 2.5 g/day) | Delayed kidney replacement | Maintain eGFR | [77] | |
| 1804 CKD patients with diabetic nephropathy stage III–IV and case study (dose 30 g/day for 1 month) | Renal protective agent | Maintain serum BUN, SCr, CCr and urine protein↓, eGFR↑ | [78,79] | |
| 1323 CKD patients (all stages) | Renal protective agent | Blood hemoglobin and serum albumin ↑ | [59] | |
| Astragalus mongholicus | Diabetic nephropathy | Anti-inflammatory | Autophagy ↑, mTOR ↓, and PINK1/Parkin ↑ | [80] |
| UUO and puromycin aminonucleoside nephrosis rat model | Anti-fibrotic | mRNA TGF-β1 ↓, α-SMA ↓ | [81,82,83] | |
| Vigna angularis | STZ-induced diabetic nephropathy (in vivo) | Antioxidant Stimulate autophagy | Plasma GSH ↑, LC3B-II ↑, mRNA HO-1↓, p47phox ↓, plasma MDA↓, and mRNA MCP-1 ↓ | [86,87] |
| Propolis | Aristolochic acids-induced nephropathy (in vivo) | Anti-fibrotic | Tubulointerstitial fibrosis ↓, TGF-β/Smad pathway ↓ | [93] |
| Brazilian red propolis | 5/6 nephroctomized rats | Antioxidant Anti-inflammatory | SCr ↓, proteinuria ↓ (serum and urine), infiltration of macrophages (kidney tissue) ↓ | [98] |
| Indonesian propolis | UUO rat model | Antioxidant | Oxidative stress ↓, blood pressure ↓ | [99] |
| Iranian propolis | STZ-induced diabetic nephropathy | Antioxidant | Serum MDA ↓, SOD↑, GPx ↑, improvement in histological architecture | [100] |
| Brazilian green propolis | 148 CKD patients (type 2 diabetes) (Dose; 500 mg/day) Hemodialysis patients (250 mg/day, in capsules). | Renal protective agent Anti-inflammatory | Proteinuria ↓ (urine), inflammation ↓ | [92,101] |
| Bee venom | UUO rat model | Anti-inflammatory Anti-fibrotic | mRNA TNF-α, IL-1β ↓, TGF-β1, FN, α-SMA ↓ | [108] |
| Small Bioactive Compounds | Type of Study | Therapeutic Effect | Major Findings | References |
|---|---|---|---|---|
| Chrysin | Human mesangial cells (in vitro) Diabetic kidney disease (in vivo) | Anti-fibrotic | TGFβ1 and SMAD 2/3 ↓ | [102] |
| Adenine-induced CKD (in vivo) | Anti-inflammatory Antioxidant | Plasma TNF-α↓, SOD, CAT, GSH, TAC ↑ (renal homogenate) | [103] | |
| Caffeic acid phenethyl ester (CAPE) | Lithium-induced renal toxicity (in vivo) | Antioxidant | Oxidative stress ↓, Antioxidant enzymes↑ | [105] |
| Caffeic acid | STZ-induced diabetic nephropathy (in vivo) | Anti-inflammatory | IL-6, IL-1β, TNF-α, and MCP-1 ↓ | [106] |
| Pinocembrin | STZ-induced diabetic nephropathy (in vivo) | Antioxidant | Oxidative stress and dyslipidemia↓ | [107] |
| Berberine | - Cultured mouse podocytes (in vitro) | Anti-inflammatory | NF-κB ↓ | [125] |
| - STZ-induced diabetic nephropathy (in vivo) | Anti-inflammatory | TLR4, NF-κB ↓ | [126] | |
| - Diabetic nephropathy (in vitro) | Prevent mitochondrial dysfunction | PGC-1α ↑, mitochondrial ROS ↓ | [127] | |
| - 114 diabetic patients (type 2 diabetes) (0.4 g, 3 times a day) | Antioxidant | Urinary albumin/creatine ratio (UACR) ↓ and serum cystatin C (Cys C) ↓ | [129] | |
| Ursolic acid | - Adenine-induced kidney injury (in vivo) | Anti-fibrotic | TGF-β/Smad ↓, FN and collagen ↓ | [140] |
| - UUO mouse model (in vivo) and TGF-β1-treated HK-2 cells (in vitro) | Anti-fibrotic | Collagen 1, FN, α-SMA, snail1, slug, TGF-β1, and p-smad3 ↓ | [141] | |
| - CKD nephroctomized mouse model (in vivo) | Anti-inflammatory | TGF-β, IL-6, and TNFα ↓ | [142] | |
| Diabetic nephropathy (in vivo) | Anti-fibrotic Anti-inflammatory Antioxidant | ARAP1/AT1R ↓, renal inflammation, fibrosis, and oxidative stress↓ | [143,144,145] | |
| Cultured murine podocytes (in vitro) | Activate autophagy | LC3II and Beclin1 ↑ | [147] | |
| Naringenin | - Obstructive nephropathy mice model and cell line (NRK52E) | Anti-fibrotic | Smad3 ↓, collagen I, α-SMA ↓ (renal tissue) | [164] |
| - High cholesterol diet rat model | Antioxidant Anti-inflammatory | iNOS, TNF-α, IL-6, and NF-κB ↓ (renal tissue) | [167] | |
| - Diabetic rat model | Antioxidant Anti-fibrotic Anti-inflammatory | MDA ↓, (SOD, CAT, GSH ↑), TGF-β1 and IL-1 ↓ (renal tissue) | [168] | |
| Diabetic mice model and NRK-52E cells | Renoprotective | PPARs-CYP4A-20-HETE pathway ↑ | [169] | |
| Apigenin | Human endothelial cells (in vitro) | Antioxidant | Oxidative stress ↓, p38/MAPK pathway ↓ | [184] |
| Diabetic rat model | Activation of autophagy Anti-inflammatory | Mitochondrial dysfunction ↓, oxidative stress ↓, Sirt3 ↓, CD38↓ Nrf2/HO-1 ↑, NF-κB ↓ | [187,188] | |
| Genistein | Diabetic rat model and rat mesangial cells exposed to high glucose | Anti-fibrotic | TGF-β1, p-Smad3, collagen IV ↓ (renal tissue) | [203,204] |
| Cultured murine podocytes exposed to high glucose | Activation of autophagy | Autophagy ↑, mTOR signaling pathway ↓ | [206] | |
| Diabetic mouse model | Antioxidant Anti-inflammatory | Cox-2, MCP-1, TNF-α and NF-κB ↓, Nrf2, GPx, SOD and HO-1↑ (renal tissue) | [207] | |
| Isolated mononuclear cells from hemodialysis patients | Anti-inflammatory | TNF-α ↓ (mononuclear cells) | [208] | |
| Rutin | Adenine-induced CKD rat animal model | Anti-fibrotic Antioxidant | Tubulointerstitial fibrosis ↓, HO-1↓, PLA-2 ↓ (renal tissue) | [217] |
| 5/6 nephrectomy and UUO rat models | Anti-fibrotic | Renal fibrosis ↓, TGFβ1-Smad signaling pathway↓ | [218,219] | |
| Endothelial cells of glomeruli exposed to hyperglycemia | Antioxidant | Nrf2 ↑, RhoA/ROCK pathway ↓ | [221] | |
| Alloxan-induced diabetic nephropathy in rats | Anti-fibrotic Antioxidant | TGF- β1↓, podocin ↑, GRP78 and CHOP ↓, ketoacidosis and fibrosis ↓. (renal tissue) | [222,223] | |
| STZ-induced diabetic nephropathy in rats | Anti-fibrotic | Collagen IV, laminin, TGF-β1, p-Smad 2/3, (CTGF) ↓ (renal tissue) | [224] | |
| Proanthocyanidin | Mice subjected to ischemia/reperfusion (I/R) | Anti-fibrotic Anti-inflammatory | TGF-β, IL-6 and TNFα ↓, HMGB1/TLR4/p65/TGF-β1 signaling pathway ↓ (renal tissue) | [232] |
| UUO mice and primary renal tubular epithelial cells (PTEC), normal rat kidney fibroblast (NRK-49F) | Anti-fibrotic | C3/HMGB1//TGF-β1 ↓ (renal tissue) | [233] | |
| STZ-induced diabetic nephropathy in rats | Anti-inflammatory | AGEs/RAGE axis ↓ (renal tissue) | [234] | |
| STZ-induced diabetic nephropathy in rats | Antioxidant Anti-inflammatory Anti-apoptotic | MDA ↓, IL-6 ↓, GSH ↑, SOD, Nrf2 ↑, GRP78), p-ERK, and caspase 12 ↓ (renal tissue) | [235,236,237,238] | |
| Betulinic acid | Adenine-induced CKD rat animal model | Anti-fibrotic | TGF-β, (CTGF), FN, collagen type I, and hydroxyproline ↓ (renal tissue) | [255] |
| STZ-induced diabetic nephropathy in rats and glomerular mesangial cells treated with high glucose level | Anti-inflammatory Anti-fibrotic | IκBα, NF-κB pathway ↓, FN expression ↓ | [256] | |
| STZ-induced diabetic nephropathy in rats | Anti-inflammatory Antioxidant | IL-6, IL-1β, TNF-α ↓ (serum and kidney tissue) AMPK ↑/NF-κB↓/Nrf2 ↑ | [257] | |
| Membranous nephropathy rat model | Anti-inflammatory Antioxidant | NF-κB ↓, iNOS ↓, TNF-α ↓, Nrf2 ↑, HO-1↑, and NQO1 ↑ (renal tissue) | [258] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohany, M.; Ahmed, M.M.; Al-Rejaie, S.S. Molecular Mechanistic Pathways Targeted by Natural Antioxidants in the Prevention and Treatment of Chronic Kidney Disease. Antioxidants 2022, 11, 15. https://doi.org/10.3390/antiox11010015
Mohany M, Ahmed MM, Al-Rejaie SS. Molecular Mechanistic Pathways Targeted by Natural Antioxidants in the Prevention and Treatment of Chronic Kidney Disease. Antioxidants. 2022; 11(1):15. https://doi.org/10.3390/antiox11010015
Chicago/Turabian StyleMohany, Mohamed, Mohammed M. Ahmed, and Salim S. Al-Rejaie. 2022. "Molecular Mechanistic Pathways Targeted by Natural Antioxidants in the Prevention and Treatment of Chronic Kidney Disease" Antioxidants 11, no. 1: 15. https://doi.org/10.3390/antiox11010015
APA StyleMohany, M., Ahmed, M. M., & Al-Rejaie, S. S. (2022). Molecular Mechanistic Pathways Targeted by Natural Antioxidants in the Prevention and Treatment of Chronic Kidney Disease. Antioxidants, 11(1), 15. https://doi.org/10.3390/antiox11010015








