Mulberry (Morus alba L.) Fruit Extract Ameliorates Inflammation via Regulating MicroRNA-21/132/143 Expression and Increases the Skeletal Muscle Mitochondrial Content and AMPK/SIRT Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Mulberry Fruit Extract
2.2. Animals and Diets
2.3. Serum Biochemical Measurements
2.4. Hepatic Lipid Analysis
2.5. Hematoxylin and Eosin (H&E) Staining
2.6. Immunohistochemistry (IHC)
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Western Blot Analysis
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Transmission Electron Microscopy (TEM)
2.11. Mitochondrial DNA Content
2.12. AMPK and SIRT Activities
2.13. Statistical Analysis
3. Results
3.1. Effects of ME on Body Weight, Food Intake, Liver Weight, and Serum AST and ALT Levels
3.2. Effects of ME on Lipid Profiles of Serum and Liver
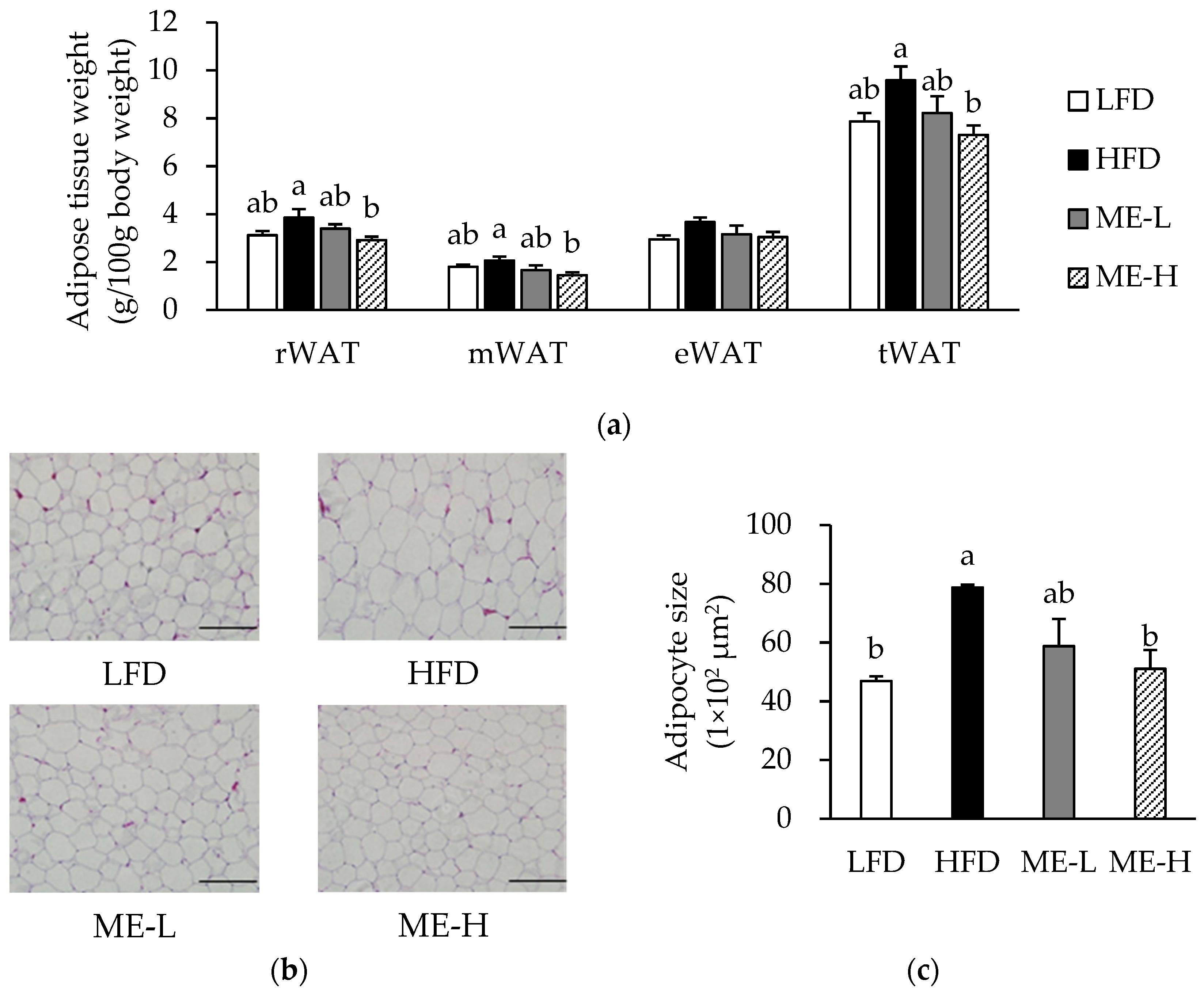
3.3. Effects of ME on Adiposity
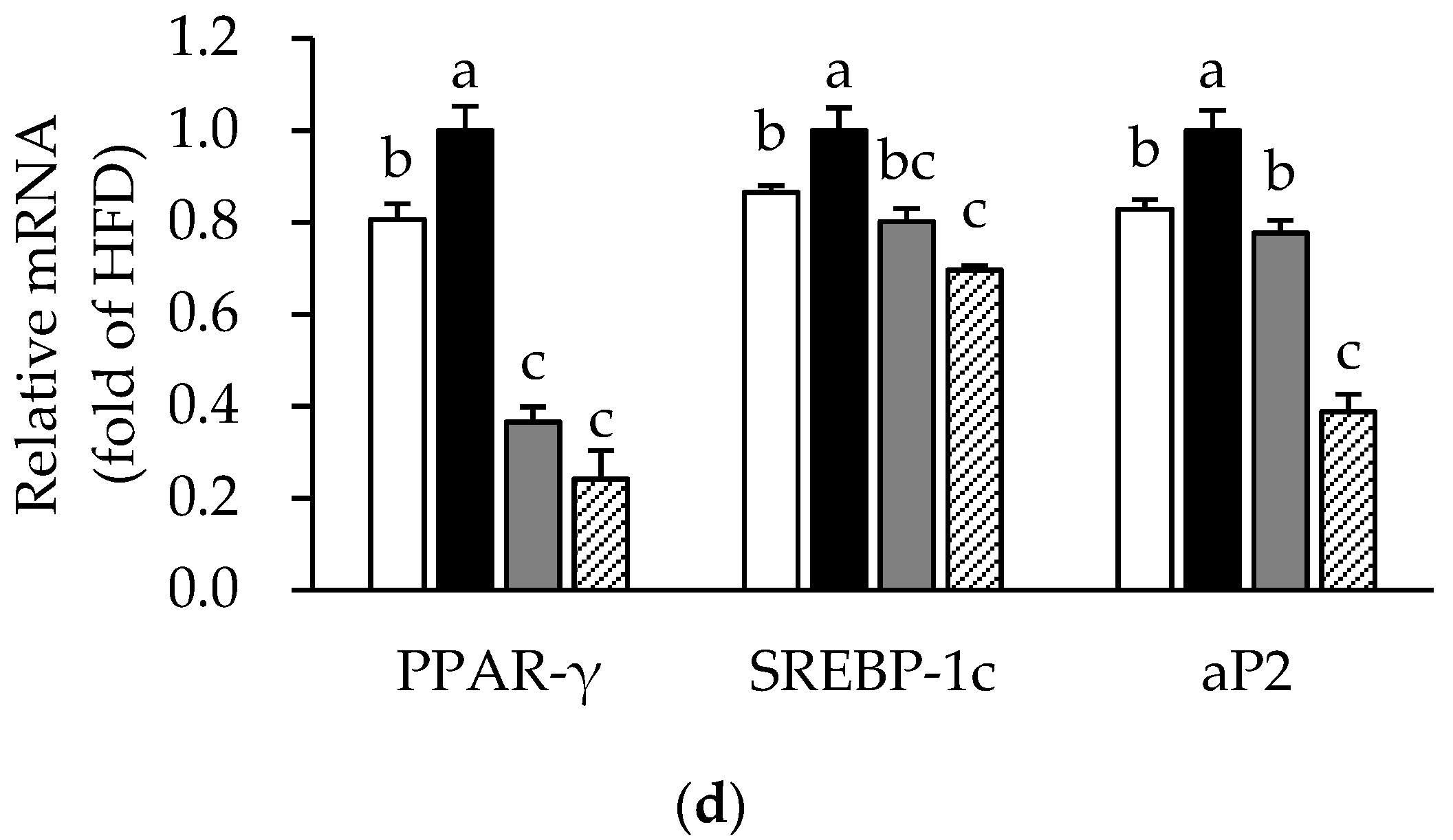
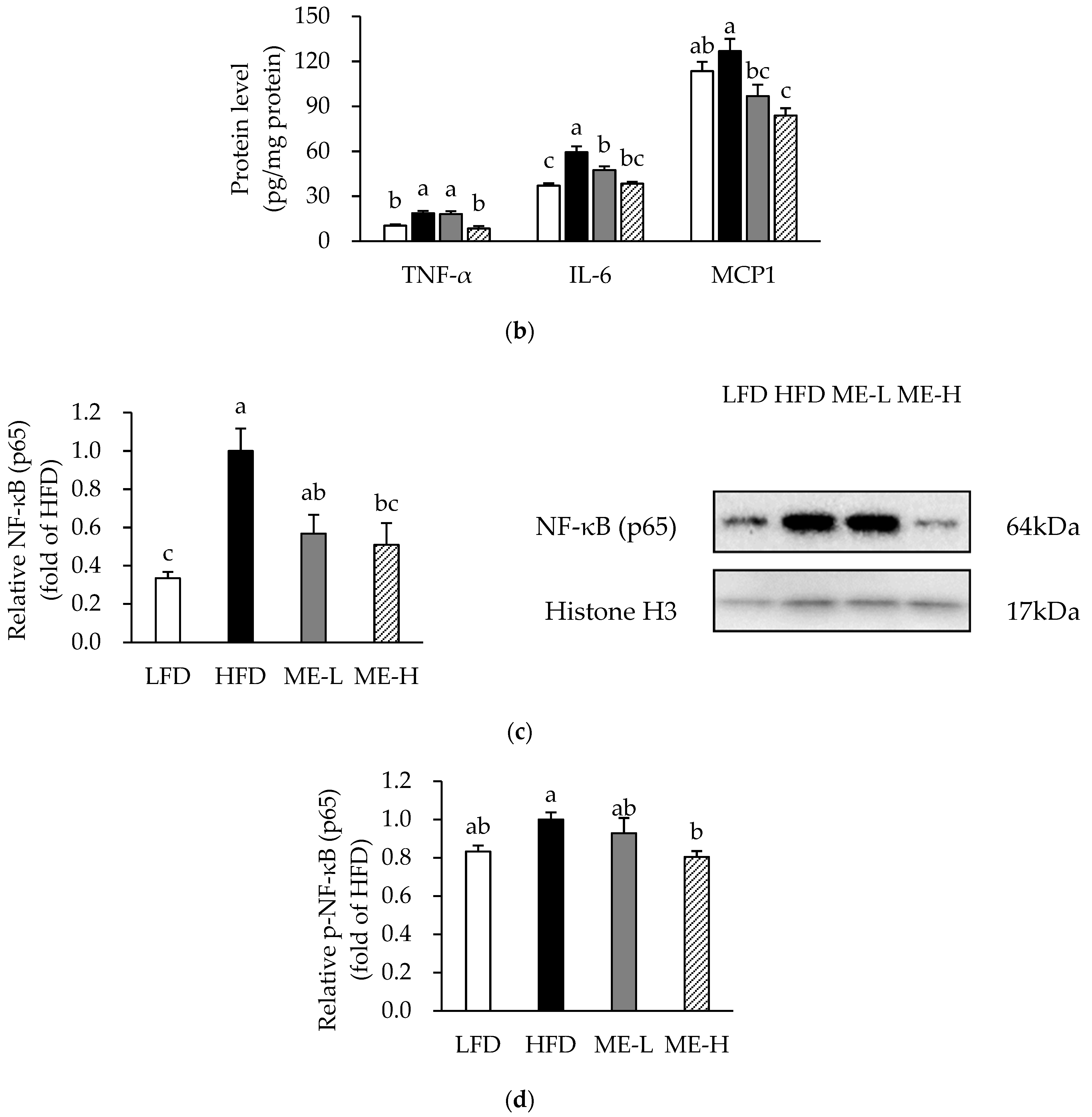
3.4. Effects of ME on Adipose Tissue Inflammation
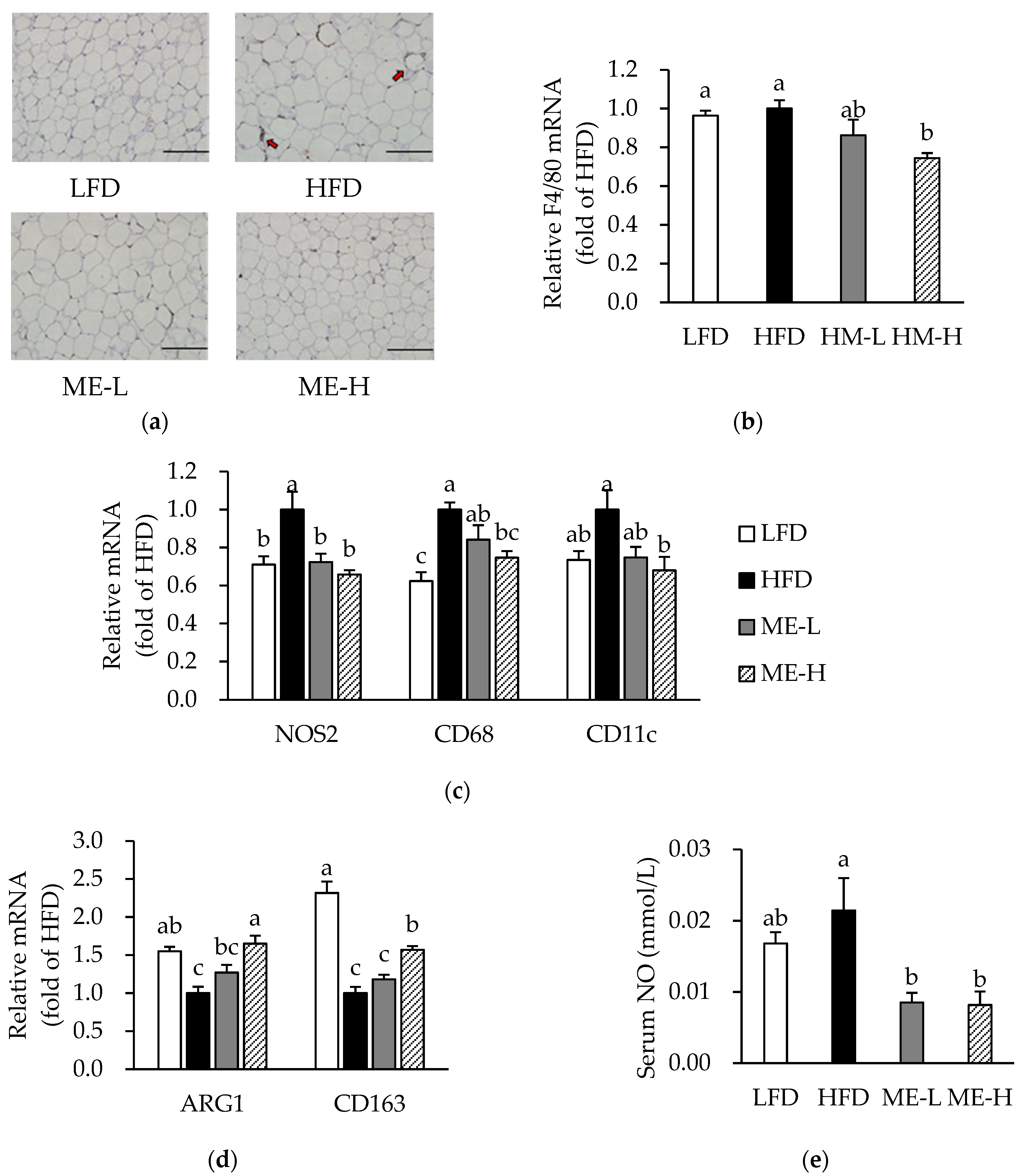
3.5. Effects of ME on Macrophage Infiltration and Phenotypic Switching
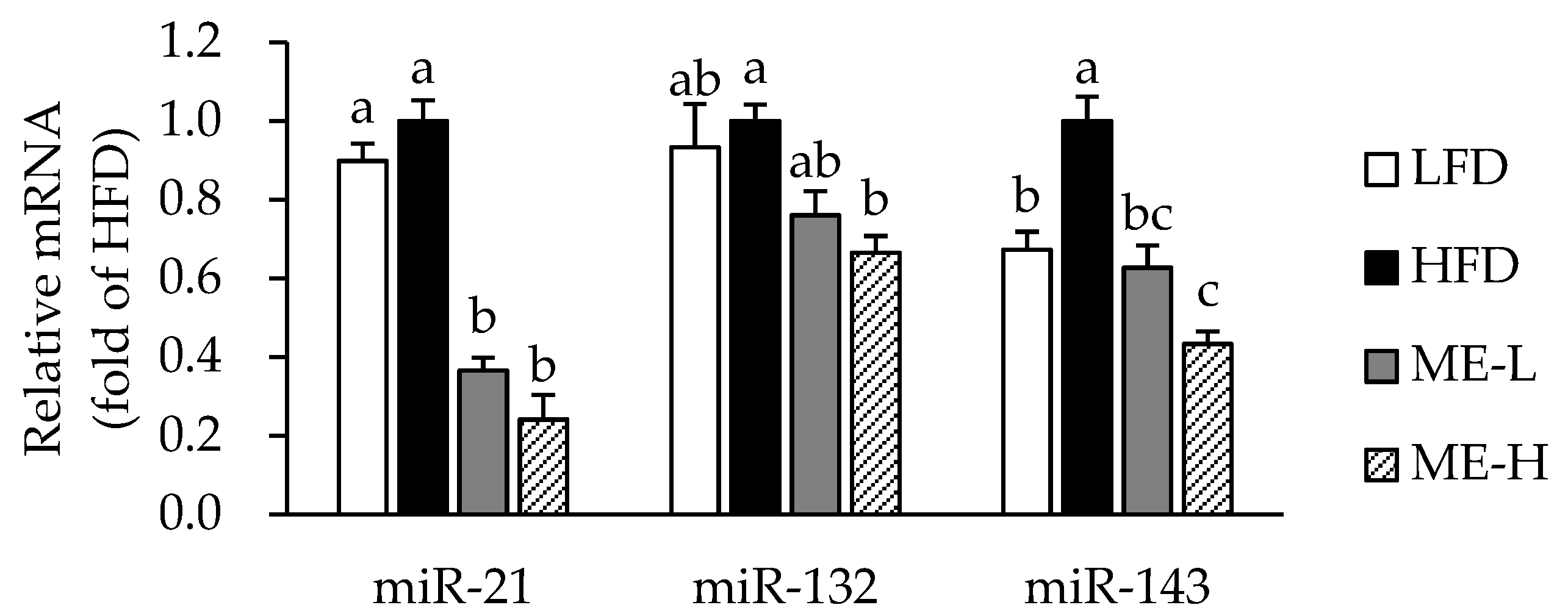
3.6. Effects of ME on MicroRNA Expression in WAT
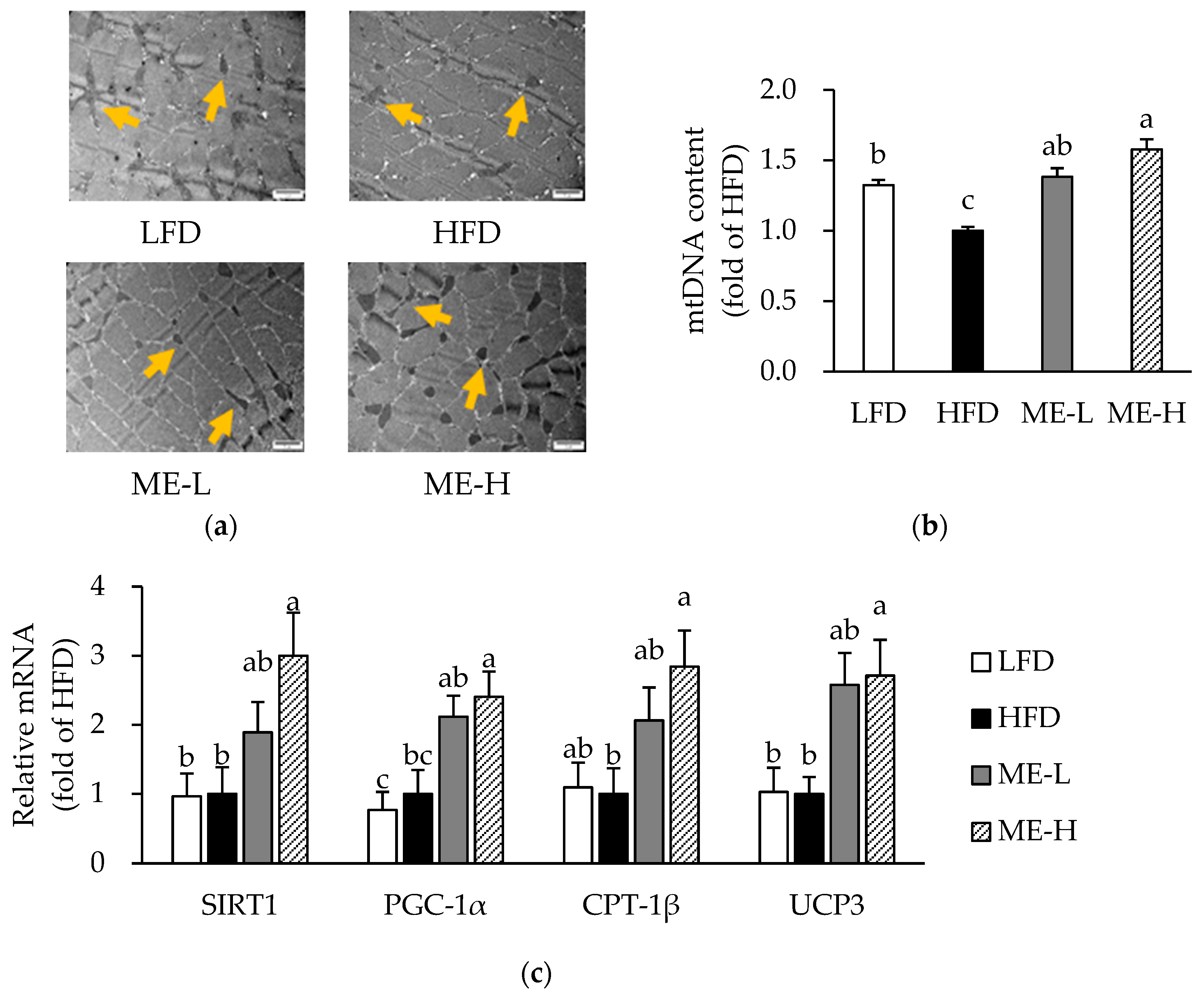
3.7. Effects of ME on Skeletal Muscle Mitochondrial Content and mRNA Expression Related to Mitochondrial Function
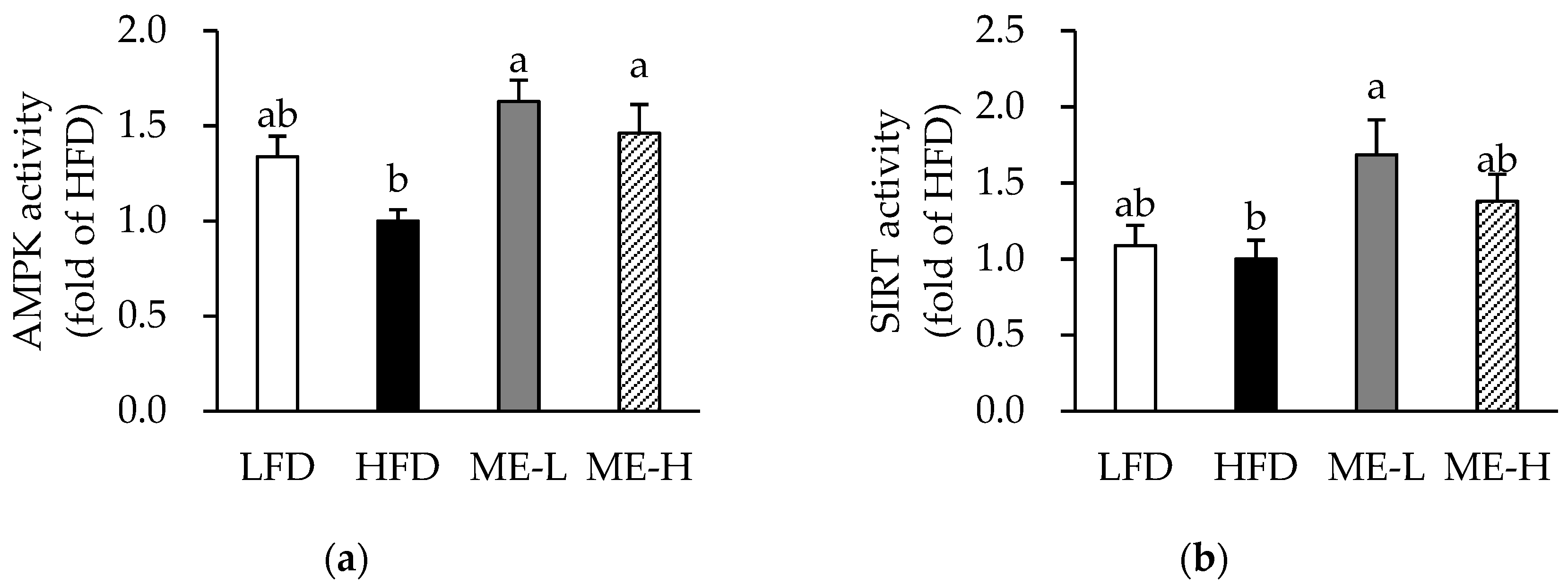
3.8. Effects of ME on Skeletal Muscle AMPK and SIRT Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Yuan, M. Inflammation and the IKKβ/IκB/NF-κB axis in obesity- and diet-induced insulin resistance. Int. J. Obes. 2003, 27, S49–S52. [Google Scholar] [CrossRef]
- Alexander, R.; Lodish, H.; Sun, L. MicroRNAs in adipogenesis and as therapeutic targets for obesity. Expert Opin. Ther. Targets 2011, 15, 623–636. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Rouault, C.; Rodriguez-Cuenca, S.; Albert, V.; Edom-Vovard, F.; Vidal-Puig, A.; Clément, K.; Butler-Browne, G.; Lacasa, D. Human Adipocytes Induce Inflammation and Atrophy in Muscle Cells During Obesity. Diabetes 2015, 64, 3121–3134. [Google Scholar] [CrossRef] [PubMed]
- Rogge, M.M. The Role of Impaired Mitochondrial Lipid Oxidation in Obesity. Biol. Res. Nurs. 2009, 10, 356–373. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; Costa, M. Nutritionally Mediated Oxidative Stress and Inflammation. Oxidative Med. Cell. Longev. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, C.S.; Thang, L.A.; Maier, A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef]
- Safdar, A.; Hamadeh, M.J.; Kaczor, J.J.; Raha, S.; Debeer, J.; Tarnopolsky, M.A. Aberrant Mitochondrial Homeostasis in the Skeletal Muscle of Sedentary Older Adults. PLoS ONE 2010, 5, e10778. [Google Scholar] [CrossRef] [PubMed]
- Price, N.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.; et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit—A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Bendokas, V.; Stanys, V.; Mažeikienė, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the Field to the Antioxidants in the Body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-K.; Chou, F.-P.; Chen, Y.-C.; Chyau, C.-C.; Ho, H.-H.; Wang, C.-J. Effects of Mulberry (Morus alba L.) Extracts on Lipid Homeostasis in Vitro and in Vivo. J. Agric. Food Chem. 2009, 57, 7605–7611. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Q.; Li, C.; Fu, X. Hypoglycemic effects of a Fructus Mori polysaccharide in vitro and in vivo. Food Funct. 2017, 8, 2523–2535. [Google Scholar] [CrossRef]
- Arfan, M.; Khan, R.; Rybarczyk, A.; Amarowicz, R. Antioxidant Activity of Mulberry Fruit Extracts. Int. J. Mol. Sci. 2012, 13, 2472–2480. [Google Scholar] [CrossRef]
- Kim, A.-J.; Park, S. Mulberry Extract Supplements Ameliorate the Inflammation-Related Hematological Parameters in Carrageenan-Induced Arthritic Rats. J. Med. Food 2006, 9, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Na, H.-N.; Park, S.; Jeon, H.-J.; Kim, H.-B.; Nam, J.-H. Reduction of adenovirus 36-induced obesity and inflammation by mulberry extract. Microbiol. Immunol. 2014, 58, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-H.; Liu, L.-K.; Huang, C.-N.; Chyau, C.-C.; Wang, C.-J. Mulberry Water Extracts Possess an Anti-obesity Effect and Ability to Inhibit Hepatic Lipogenesis and Promote Lipolysis. J. Agric. Food Chem. 2011, 59, 2663–2671. [Google Scholar] [CrossRef]
- Khan, S.A.; Aslam, R.; Makroo, H.A. High pressure extraction and its application in the extraction of bio-active compounds: A review. J. Food Process. Eng. 2018, 42, e12896. [Google Scholar] [CrossRef]
- Wang, F.; Du, B.-L.; Cui, Z.-W.; Xu, L.-P.; Li, C. Effects of high hydrostatic pressure and thermal processing on bioactive compounds, antioxidant activity, and volatile profile of mulberry juice. Food Sci. Technol. Int. 2016, 23, 119–127. [Google Scholar] [CrossRef]
- Jung, S.; Lee, M.-S.; Choi, A.-J.; Kim, C.-T.; Kim, Y. Anti-Inflammatory Effects of High Hydrostatic Pressure Extract of Mulberry (Morus alba) Fruit on LPS-Stimulated RAW264.7 Cells. Molecules 2019, 24, 1425. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Costanzini, A.; Sgarbi, G.; Maresca, A.; Del Dotto, V.; Solaini, G.; Baracca, A. Mitochondrial Mass Assessment in a Selected Cell Line under Different Metabolic Conditions. Cells 2019, 8, 1454. [Google Scholar] [CrossRef]
- Duwaerts, C.C.; Maher, J.J. Macronutrients and the Adipose-Liver Axis in Obesity and Fatty Liver. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 749–761. [Google Scholar] [CrossRef]
- Li, Y.; Bao, T.; Chen, W. Comparison of the protective effect of black and white mulberry against ethyl carbamate-induced cytotoxicity and oxidative damage. Food Chem. 2018, 243, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-E. Optimal Evaluation of the Results of Liver Function Tests. Korean J. Med. 2019, 94, 89–95. [Google Scholar] [CrossRef][Green Version]
- Song, H.; Lai, J.; Tang, Q.; Zheng, X. Mulberry ethanol extract attenuates hepatic steatosis and insulin resistance in high-fat diet–fed mice. Nutr. Res. 2016, 36, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Qi, X.; Liu, Y.; Guo, J.; Zhu, R.; Chen, W.; Zheng, X.; Yu, T. Dietary supplementation with purified mulberry (Morus australis Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chem. 2013, 141, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yin, J.; Zhang, G.; Long, H.; Zheng, X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Duplus, E.; Forest, C. Is there a single mechanism for fatty acid regulation of gene transcription? Biochem. Pharmacol. 2002, 64, 893–901. [Google Scholar] [CrossRef]
- Mancuso, P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Wu, T.; Tang, Q.; Gao, Z.; Yu, Z.; Song, H.; Zheng, X.; Chen, W. Blueberry and Mulberry Juice Prevent Obesity Development in C57BL/6 Mice. PLoS ONE 2013, 8, e77585. [Google Scholar] [CrossRef]
- Murano, I.; Barbatelli, G.; Parisani, V.; Latini, C.; Muzzonigro, G.; Castellucci, M.; Cinti, S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J. Lipid Res. 2008, 49, 1562–1568. [Google Scholar] [CrossRef]
- García-Segura, L.; Perezandrade, M.E.; Miranda-Ríos, J. The Emerging Role of MicroRNAs in the Regulation of Gene Expression by Nutrients. J. Nutr. Nutr. 2013, 6, 16–31. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hwang, S.J.; Bae, Y.C.; Jung, J.S. miR-21 Regulates Adipogenic Differentiation Through the Modulation of TGF-β Signaling in Mesenchymal Stem Cells Derived from Human Adipose Tissue. Stem Cells 2009, 27, 3093–3102. [Google Scholar] [CrossRef]
- Choudhury, S.N. miR-21 and let-7 in the Ras and NF-κB Pathways. MicroRNA 2012, 1, 65–69. [Google Scholar] [CrossRef]
- Esau, C.; Kang, X.; Peralta, E.; Hanson, E.; Marcusson, E.G.; Ravichandran, L.V.; Sun, Y.; Koo, S.; Perera, R.J.; Jain, R.; et al. MicroRNA-143 Regulates Adipocyte Differentiation. J. Biol. Chem. 2004, 279, 52361–52365. [Google Scholar] [CrossRef]
- Liang, L.; Liu, X.; He, J.; Shao, Y.; Liu, J.; Wang, Z.; Xia, L.; Han, T.; Wu, P. Cyanidin-3-glucoside induces mesenchymal to epithelial transition via activating Sirt1 expression in triple negative breast cancer cells. Biochimie 2019, 162, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.P.; Foletta, V.C.; Snow, R.J.; Wadley, G. Skeletal muscle mitochondria: A major player in exercise, health and disease. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 1276–1284. [Google Scholar] [CrossRef]
- Bonnard, C.; Durand, A.; Peyrol, S.; Chanseaume, E.; Chauvin, M.-A.; Morio, B.; Vidal, H.; Rieusset, J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Investig. 2008, 118, 789–800. [Google Scholar] [CrossRef]
- Koves, T.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.R.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2016, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Canto, C.; Auwerx, J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-Cardiovasc. Res. 2008, 79, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.; Brand, M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar] [CrossRef]
- Matsukawa, T.; Motojima, H.; Sato, Y.; Takahashi, S.; Villareal, M.O.; Isoda, H. Upregulation of skeletal muscle PGC-1α through the elevation of cyclic AMP levels by Cyanidin-3-glucoside enhances exercise performance. Sci. Rep. 2017, 7, 44799. [Google Scholar] [CrossRef] [PubMed]
- Mogalli, R.; Matsukawa, T.; Shimomura, O.; Isoda, H.; Ohkohchi, N. Cyanidin-3-glucoside enhances mitochondrial function and biogenesis in a human hepatocyte cell line. Cytotechnology 2018, 70, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Yuan, X.; Lee, H.J.; Huang, W.; Jin, W.; Zhan, J. Mulberry and mulberry wine extract increase the number of mitochondria during brown adipogenesis. Food Funct. 2014, 6, 401–408. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Xu, J.; Nelson, L.; Cacicedo, J.M.; Saha, A.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef] [PubMed]
- Ou, T.-T.; Hsu, M.-J.; Chan, K.-C.; Huang, C.-N.; Ho, H.-H.; Wang, C.-J. Mulberry extract inhibits oleic acid-induced lipid accumulation via reduction of lipogenesis and promotion of hepatic lipid clearance. J. Sci. Food Agric. 2011, 91, 2740–2748. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Lee, H.A.; Park, M.H.; Han, J.-S. Mulberry (Morus alba L.) Fruit Extract Containing Anthocyanins Improves Glycemic Control and Insulin Sensitivity via Activation of AMP-Activated Protein Kinase in Diabetic C57BL/Ksj-db/db Mice. J. Med. Food 2016, 19, 737–745. [Google Scholar] [CrossRef] [PubMed]

| Variable | LFD | HFD | ME-L | ME-H |
|---|---|---|---|---|
| Initial body weight (g) | 54.53 ± 0.77 | 55.47 ± 1.15 | 54.80 ± 2.10 | 54.56 ± 2.10 |
| Final body weight (g) | 517.15 ± 13.25 b | 593.80 ± 5.74 a | 552.71 ± 24.94 ab | 547.92 ± 11.98 ab |
| Body weight gain (g/14 weeks) | 462.62 ± 13.21 b | 538.33 ± 6.46 a | 497.91 ± 24.79 ab | 493.36 ± 12.23 ab |
| Food intake (g/day) | 25.07 ± 0.50 a | 22.66 ± 0.39 b | 20.53 ± 0.84 b | 20.57 ± 0.41 b |
| Energy intake (kcal/day) | 90.24 ± 1.81 b | 104.21 ± 1.79 a | 94.45 ± 3.87 b | 94.62 ± 1.87 ab |
| Energy efficiency ratio | 0.052 ± 0.001 | 0.053 ± 0.001 | 0.054 ± 0.001 | 0.053 ± 0.000 |
| Liver weight (g) | 13.23 ± 0.31 | 15.43 ± 0.65 | 14.17 ± 0.85 | 14.04 ± 0.44 |
| Serum AST (IU/L) | 49.76 ± 1.46 | 61.62 ± 7.37 | 56.24 ± 2.94 | 49.48 ± 1.88 |
| Serum ALT (IU/L) | 9.06 ± 0.17 b | 10.49 ± 0.54 a | 9.84 ± 0.17 ab | 8.76 ± 0.33 b |
| Variable | LFD | HFD | ME-L | ME-H |
|---|---|---|---|---|
| Serum | ||||
| TG (mmol/L) | 1.12 ± 0.06 a | 1.18 ± 0.05 a | 0.61 ± 0.13 b | 0.58 ± 0.06 b |
| TC (mmol/L) | 3.56 ± 0.07 b | 4.07 ± 0.10 a | 3.65 ± 0.07 b | 3.59 ± 0.11 b |
| HDL-C (mmol/L) | 2.19 ± 0.01 c | 2.25 ± 0.03 bc | 2.45 ± 0.07 ab | 2.61 ± 0.06 a |
| LDL-C (mmol/L) | 0.86 ± 0.07 ab | 1.28 ± 0.10 a | 0.92 ± 0.09 ab | 0.71 ± 0.16 b |
| Atherogenic index | 0.63 ± 0.03 b | 0.81 ± 0.05 a | 0.49 ± 0.03 bc | 0.38 ± 0.06 c |
| Liver | ||||
| Total lipids (mg/g) | 33.76 ± 1.03 c | 64.72 ± 5.62 a | 52.34 ± 3.71 ab | 42.55 ± 3.74 bc |
| TG (μmol/g) | 6.51 ± 0.45 c | 17.37 ± 2.19 a | 13.31 ± 1.50 ab | 10.68 ± 1.38 bc |
| TC (μmol/g) | 4.81 ± 0.36 b | 9.07 ± 1.04 a | 5.41 ± 0.42 b | 4.72 ± 0.53 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.; Lee, M.-S.; Chang, E.; Kim, C.-T.; Kim, Y. Mulberry (Morus alba L.) Fruit Extract Ameliorates Inflammation via Regulating MicroRNA-21/132/143 Expression and Increases the Skeletal Muscle Mitochondrial Content and AMPK/SIRT Activities. Antioxidants 2021, 10, 1453. https://doi.org/10.3390/antiox10091453
Jung S, Lee M-S, Chang E, Kim C-T, Kim Y. Mulberry (Morus alba L.) Fruit Extract Ameliorates Inflammation via Regulating MicroRNA-21/132/143 Expression and Increases the Skeletal Muscle Mitochondrial Content and AMPK/SIRT Activities. Antioxidants. 2021; 10(9):1453. https://doi.org/10.3390/antiox10091453
Chicago/Turabian StyleJung, Sunyoon, Mak-Soon Lee, Eugene Chang, Chong-Tai Kim, and Yangha Kim. 2021. "Mulberry (Morus alba L.) Fruit Extract Ameliorates Inflammation via Regulating MicroRNA-21/132/143 Expression and Increases the Skeletal Muscle Mitochondrial Content and AMPK/SIRT Activities" Antioxidants 10, no. 9: 1453. https://doi.org/10.3390/antiox10091453
APA StyleJung, S., Lee, M.-S., Chang, E., Kim, C.-T., & Kim, Y. (2021). Mulberry (Morus alba L.) Fruit Extract Ameliorates Inflammation via Regulating MicroRNA-21/132/143 Expression and Increases the Skeletal Muscle Mitochondrial Content and AMPK/SIRT Activities. Antioxidants, 10(9), 1453. https://doi.org/10.3390/antiox10091453






