Verbascoside Protects Gingival Cells against High Glucose-Induced Oxidative Stress via PKC/HMGB1/RAGE/NFκB Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Chemicals
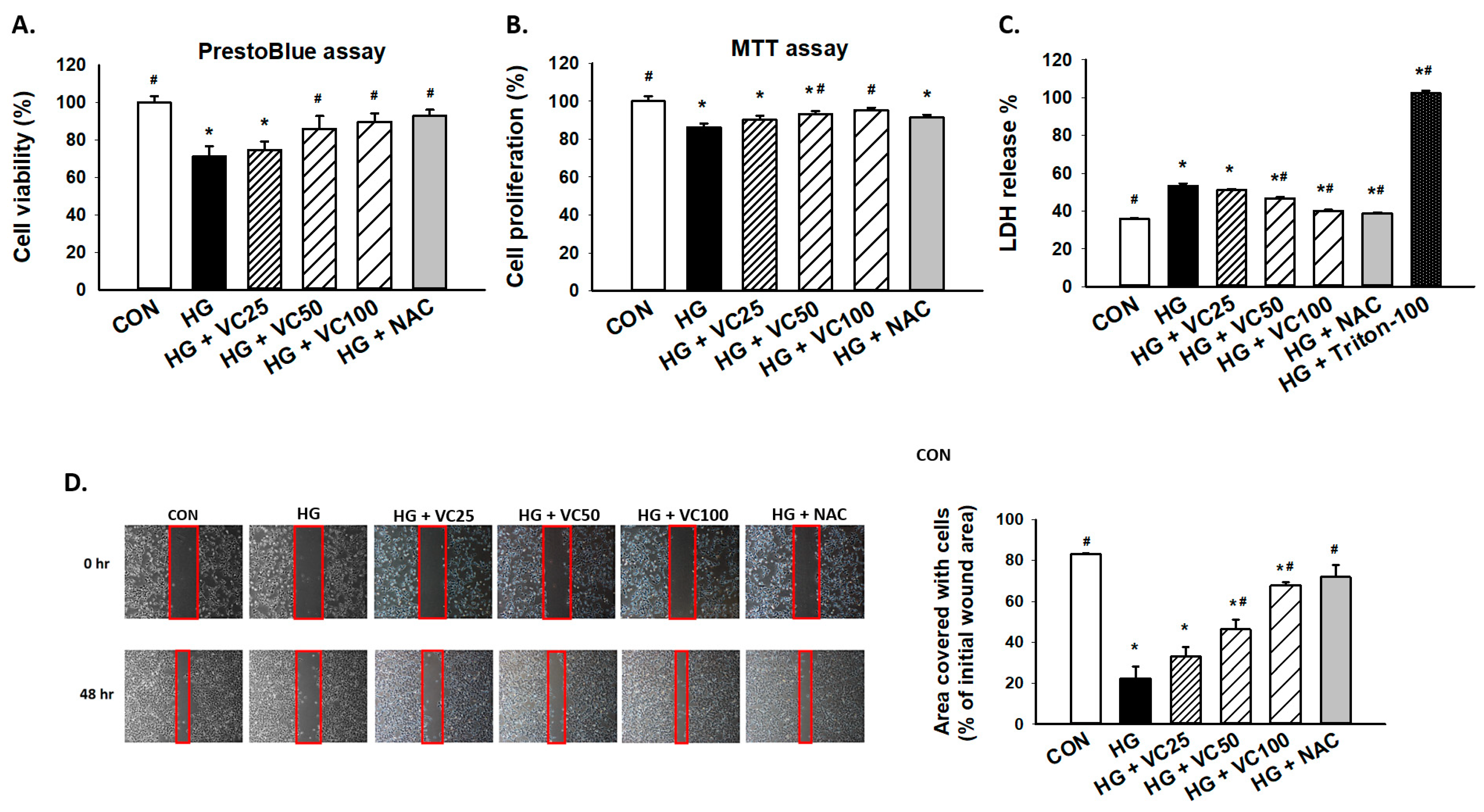
2.2. Prestoblue, MTT, and LDH Assays
2.3. Wound Healing Assay
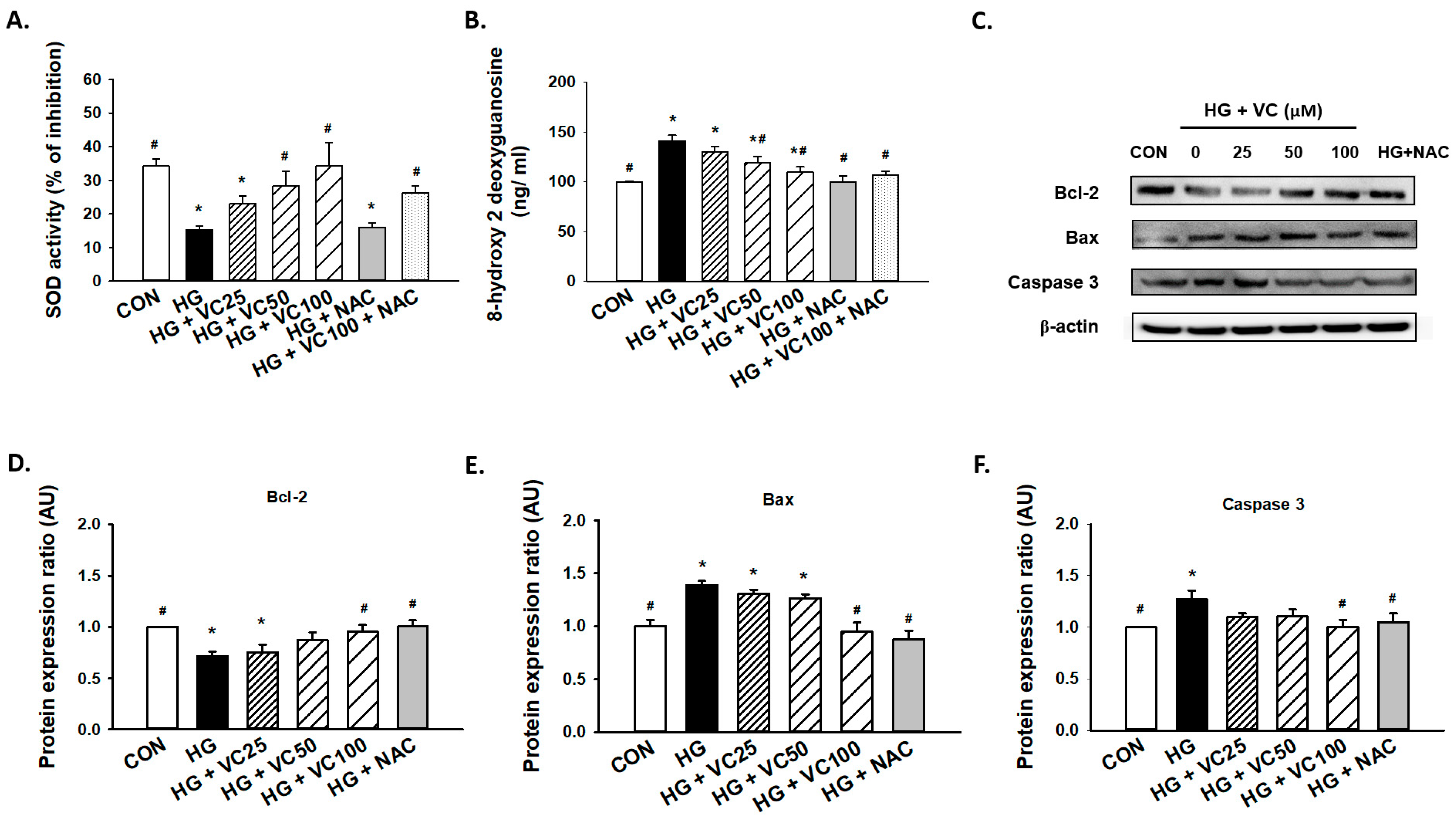
2.4. Examination of Antioxidant Capacity and Oxidative Stress
2.5. Western Blot
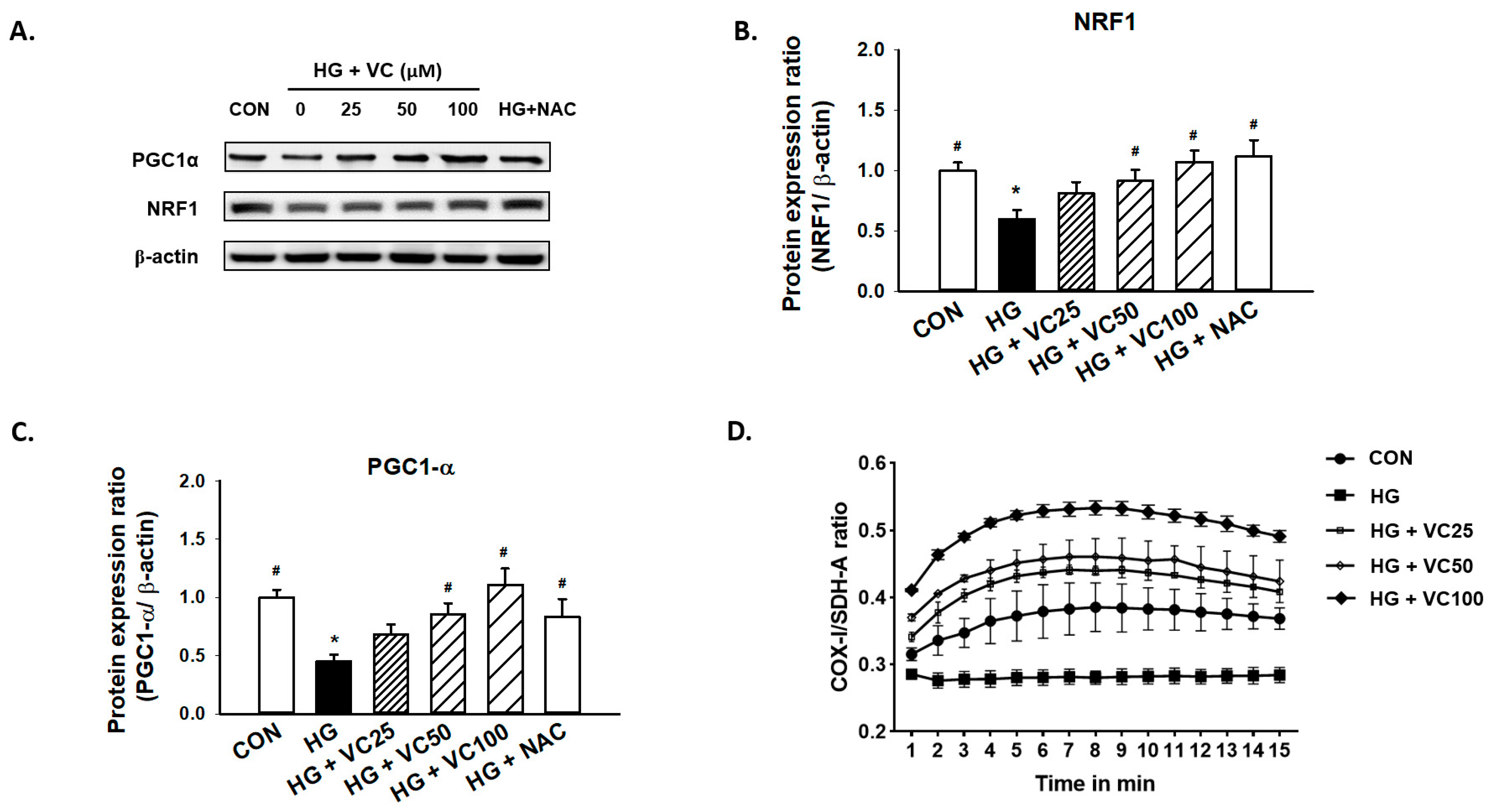
2.6. Assessment of Mitochondrial Biogenesis
2.7. Quantitative Real-Time PCR (qRT-PCR)
2.8. Measurement of Inflammatory Cytokine Production
2.9. Statistical Analysis
3. Results
3.1. Treatment of Verbascoside Mitigates the Suppressed Cell Proliferation and Wound Healing Capacity of Gingival Epithelial Cells under High Glucose Condition
3.2. Verbascoside Attenuates the Oxidative Stress and Apoptosis in High Glucose-Cultured Gingival Epithelial Cells
3.3. The High Glucose-Induced Mitochondrial Dysfunction Is Ameliorated by Verbascoside
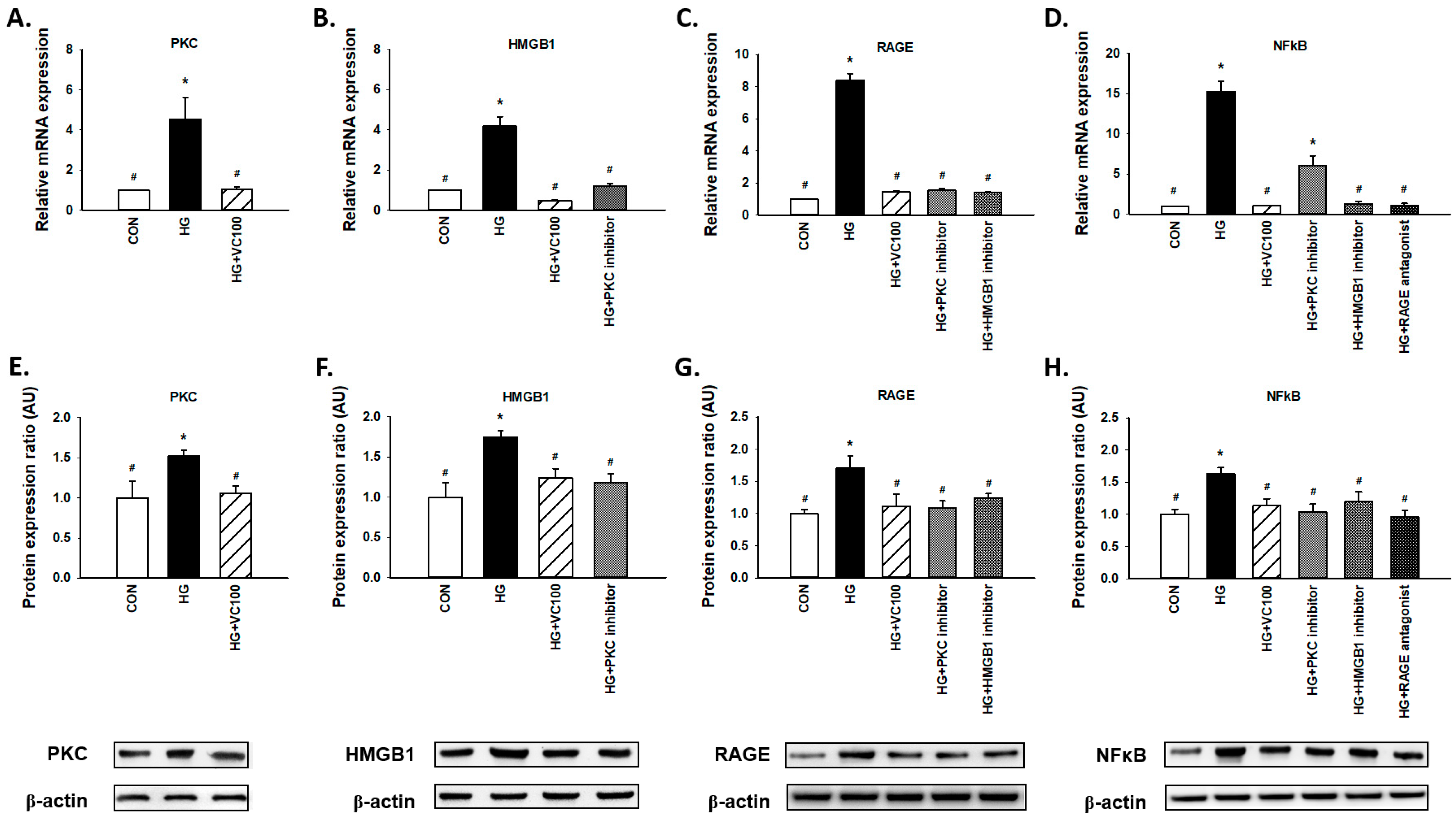
3.4. Verbascoside Inhibits the High Glucose-Elicited PKC/HMGB1/RAGE/NFκB Pathway
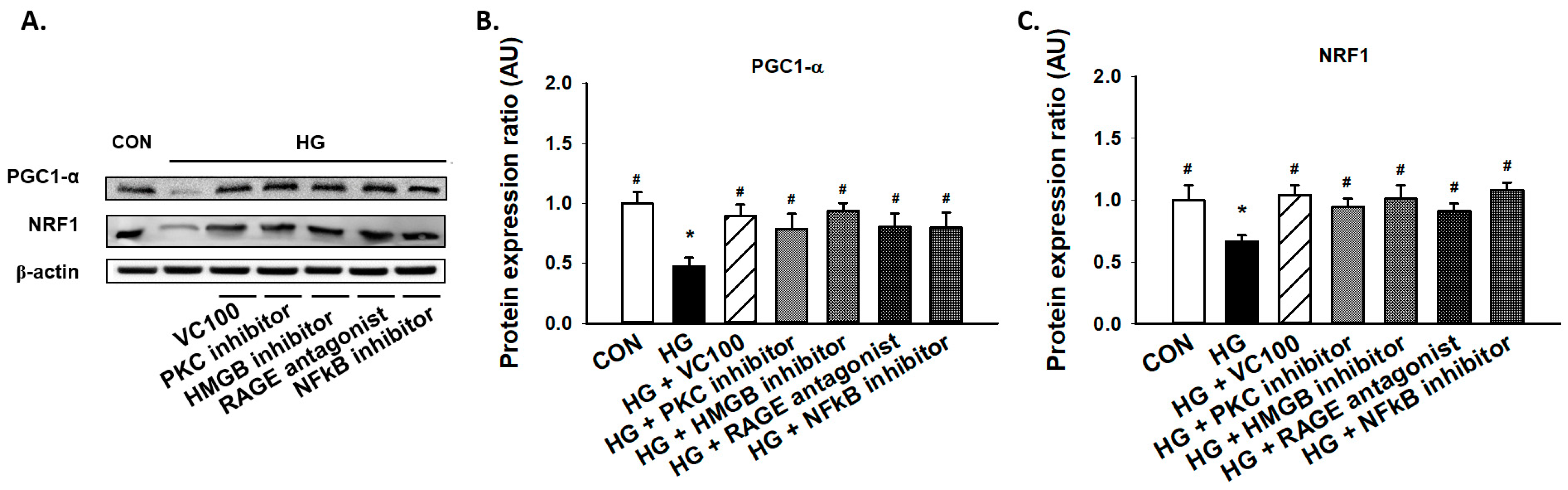
3.5. Suppression of PKC/HMGB1/RAGE/NFκB Signaling Reverses the Downregulation of PGC1-α and NRF1
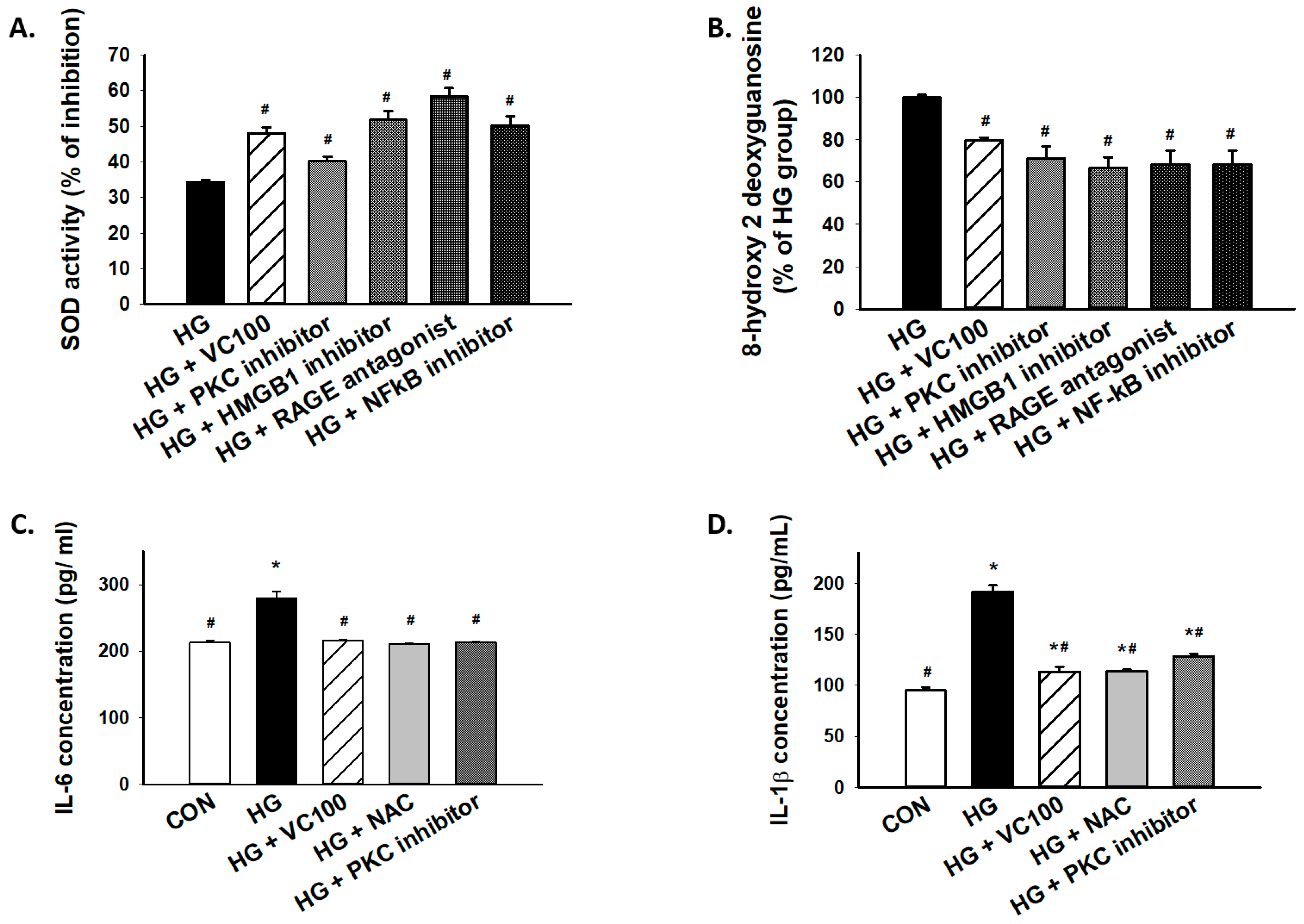
3.6. Inhibition of the PKC/HMGB1/RAGE/NFκB Pathway Diminishes High Glucose-Induced Oxidative Stress and the Subsequent Inflammation
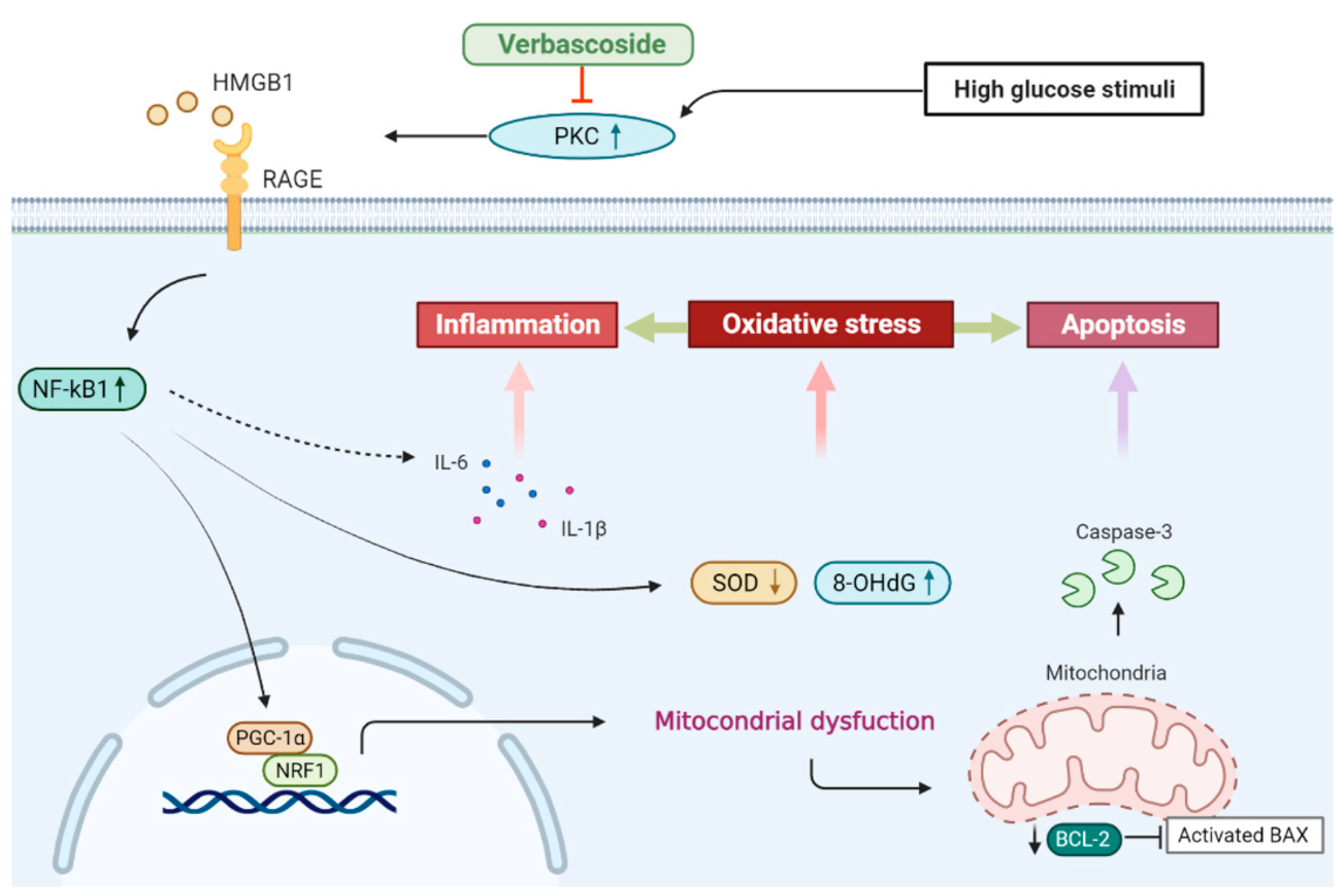
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogurtsova, K.; da Rocha Fernandes, J.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, S.; Vassy, J.; Ho, Y.; Song, R.J.; Gagnon, D.R.; Cho, K.; Wilson, P.W.F.; Phillips, L.S. Diabetes Mellitus–Related All-Cause and Cardiovascular Mortality in a National Cohort of Adults. J. Am. Hear. Assoc. 2019, 8, e011295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, K.I.; Sculean, A.; Graves, D.T. Diabetic wound healing in soft and hard oral tissues. Transl. Res. 2021, 236, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2011, 55, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.C.; Cáceres, M.; Martínez, C.; Oyarzún, A. Gingival Wound Healing: An essential response disturbed by aging? J. Dent. Res. 2014, 94, 395–402. [Google Scholar] [CrossRef]
- Leoni, G.; Neumann, P.-A.; Sumagin, R.; Denning, T.; Nusrat, A. Wound repair: Role of immune–epithelial interactions. Mucosal Immunol. 2015, 8, 959–968. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Xie, J.; Kang, L.-N.; Wang, L.; Xu, B. High Mobility Group Box-1: A Missing Link between Diabetes and Its Complications. Mediat. Inflamm. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, D.; Brownlee, M. Hyperglycemia-Induced Reactive Oxygen Species Increase Expression of the Receptor for Advanced Glycation End Products (RAGE) and RAGE Ligands. Diabetes 2009, 59, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Arcaroli, J.; Yum, H.-K.; Yang, H.; Wang, H.; Yang, K.-Y.; Choe, K.-H.; Strassheim, D.; Pitts, T.M.; Tracey, K.J.; et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am. J. Physiol. Physiol. 2003, 284, C870–C879. [Google Scholar] [CrossRef] [Green Version]
- Klimek, B. 6′-0-apiosyl-verbascoside in the flowers of mullein (Verbascum species). Acta Pol. Pharm.-Drug Res. 1996, 53, 137–140. [Google Scholar]
- Pardo, F.; Perich, F.; Villarroel, L.; Torres, R. Isolation of verbascoside, an antimicrobial constituent of Buddleja globosa leaves. J. Ethnopharmacol. 1993, 39, 221–222. [Google Scholar] [CrossRef]
- Tian, X.-Y.; Li, M.-X.; Lin, T.; Qiu, Y.; Zhu, Y.-T.; Li, X.-L.; Tao, W.-D.; Wang, P.; Ren, X.-X.; Chen, L.-P. A review on the structure and pharmacological activity of phenylethanoid glycosides. Eur. J. Med. Chem. 2021, 209, 112563. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.M.; Maffrand, J.P.; Taoubi, K.; Augereau, J.M.; Fouraste, I.; Gleye, J. Verbascoside Isolated from Lantana camara, an Inhibitor of Protein Kinase C. J. Nat. Prod. 1991, 54, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Khamaisi, M.; Katagiri, S.; Keenan, H.; Park, K.; Maeda, Y.; Li, Q.; Qi, W.; Thomou, T.; Eschuk, D.; Tellechea, A.; et al. PKCδ inhibition normalizes the wound-healing capacity of diabetic human fibroblasts. J. Clin. Investig. 2016, 126, 837–853. [Google Scholar] [CrossRef] [Green Version]
- Oh, Y.J.; Youn, J.H.; Ji, Y.; Lee, S.E.; Lim, K.J.; Choi, J.E.; Shin, J.-S. HMGB1 Is Phosphorylated by Classical Protein Kinase C and Is Secreted by a Calcium-Dependent Mechanism. J. Immunol. 2009, 182, 5800–5809. [Google Scholar] [CrossRef] [Green Version]
- Smulow, J.B.; Glickman, I. An Epithelial-Like Cell Line in Continuous Culture from Normal Adult Human Gingiva. Exp. Biol. Med. 1966, 121, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Kasten, F.H.; Soileau, K.; Meffert, R.M. Quantitative evaluation of human gingival epithelial cell attachment to implant surfaces in vitro. Int. J. Periodontics Restor. Dent. 1990, 10, 68–79. [Google Scholar]
- Toullec, D.; Pianetti, P.; Coste, H.; Bellevergue, P.; Grand-Perret, T.; Ajakane, M.; Baudet, V.; Boissin, P.; Boursier, E.; Loriolle, F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 1991, 266, 15771–15781. [Google Scholar] [CrossRef]
- Mollica, L.; De Marchis, F.; Spitaleri, A.; Dallacosta, C.; Pennacchini, D.; Zamai, M.; Agresti, A.; Trisciuoglio, L.; Musco, G.; Bianchi, M.E. Glycyrrhizin Binds to High-Mobility Group Box 1 Protein and Inhibits Its Cytokine Activities. Chem. Biol. 2007, 14, 431–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.; Shen, C.; Yin, Q.; Sun, M.; Ma, Y.; Liu, X. Effects of RAGE-Specific Inhibitor FPS-ZM1 on Amyloid-β Metabolism and AGEs-Induced Inflammation and Oxidative Stress in Rat Hippocampus. Neurochem. Res. 2016, 41, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Horie, R.; Watanabe, M.; Okamura, T.; Taira, M.; Shoda, M.; Motoji, T.; Utsunomiya, A.; Higashihara, M.; Umezawa, K.; Watanabe, T. DHMEQ, a new NF-κB inhibitor, induces apoptosis and enhances fludarabine effects on chronic lymphocytic leukemia cells. Leukemia 2006, 20, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Vivo, M.; Ranieri, M.; Sansone, F.; Santoriello, C.; Calogero, R.A.; Calabro, V.; Pollice, A.; La Mantia, G. Mimicking p14ARF Phosphorylation Influences Its Ability to Restrain Cell Proliferation. PLoS ONE 2013, 8, e53631. [Google Scholar] [CrossRef]
- Downs, C.A.; Kreiner, L.H.; Johnson, N.M.; Brown, L.A.; Helms, M.N. Receptor for Advanced Glycation End-Products Regulates Lung Fluid Balance via Protein Kinase C–gp91phox Signaling to Epithelial Sodium Channels. Am. J. Respir. Cell Mol. Biol. 2015, 52, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Ariga, A.; Namekawa, J.-I.; Matsumoto, N.; Inoue, J.-I.; Umezawa, K. Inhibition of Tumor Necrosis Factor-α-induced Nuclear Translocation and Activation of NF-κB by Dehydroxymethylepoxyquinomicin. J. Biol. Chem. 2002, 277, 24625–24630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrotte, E.J.; Chen, D.-D.; Hakim, J.; Chen, A.F. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J. Clin. Investig. 2010, 120, 4207–4219. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P. Mitochondrial Dysfunction Indirectly Elevates ROS Production by the Endoplasmic Reticulum. Cell Metab. 2013, 18, 145–146. [Google Scholar] [CrossRef] [Green Version]
- Patti, M.E.; Butte, A.J.; Landaker, E.J.; Goldfine, A.B.; Mun, E.; DeFronzo, R.; Finlayson, J.; Kahn, C.R.; Mandarino, L.J.; Crunkhorn, S.; et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role ofPGC1andNRF1. Proc. Natl. Acad. Sci. USA 2003, 100, 8466–8471. [Google Scholar] [CrossRef] [Green Version]
- Cottrell, D.A.; Blakely, E.L.; Borthwick, G.M.; Johnson, M.A.; Taylor, G.A.; Brierley, E.J.; Ince, P.; Turnbull, D.M. Role of Mitochondrial DNA Mutations in Disease and Aging. Ann. N. Y. Acad. Sci. 2006, 908, 199–207. [Google Scholar] [CrossRef]
- Edgar, D.; Shabalina, I.; Camara, Y.; Wredenberg, A.; Calvaruso, M.A.; Nijtmans, L.; Nedergaard, J.; Cannon, B.; Larsson, N.-G.; Trifunovic, A. Random Point Mutations with Major Effects on Protein-Coding Genes Are the Driving Force behind Premature Aging in mtDNA Mutator Mice. Cell Metab. 2009, 10, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, G.; Bakajsova, D.; Clifton, G.L. Protein kinase C-ϵ modulates mitochondrial function and active Na+transport after oxidant injury in renal cells. Am. J. Physiol. Physiol. 2004, 286, F307–F316. [Google Scholar] [CrossRef]
- Cherry, A.D.; Piantadosi, C.A. Regulation of Mitochondrial Biogenesis and Its Intersection with Inflammatory Responses. Antioxidants Redox Signal. 2015, 22, 965–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Yan, Y.; Liu, H.; Hu, J. Protective effects of acteoside against X-ray-induced damage in human skin fibroblasts. Mol. Med. Rep. 2015, 12, 2301–2306. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.; Kim, D.K.; Kim, T.-H.; Kang, K.-R.; Seo, J.-Y.; Cho, S.S.; Yun, Y.; Choi, Y.-Y.; Leem, J.; Kim, H.-W.; et al. Acteoside Counteracts Interleukin-1β-Induced Catabolic Processes through the Modulation of Mitogen-Activated Protein Kinases and the NFκB Cellular Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Zhu, K.; Meng, Z.; Tian, Y.; Gu, R.; Xu, Z.; Fang, H.; Liu, W.; Huang, W.; Ding, G.; Xiao, W. Hypoglycemic and hypolipidemic effects of total glycosides of Cistanche tubulosa in diet/streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2021, 276, 113991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, M.; Chen, Q.; Wang, T.; Yu, H.; Xu, J.; Wang, T. Leaves of Lippia triphylla improve hepatic lipid metabolism via activating AMPK to regulate lipid synthesis and degradation. J. Nat. Med. 2019, 73, 707–716. [Google Scholar] [CrossRef]
- Galli, A.; Marciani, P.; Marku, A.; Ghislanzoni, S.; Bertuzzi, F.; Rossi, R.; Di Giancamillo, A.; Castagna, M.; Perego, C. Verbascoside Protects Pancreatic β-Cells against ER-Stress. Biomedicines 2020, 8, 582. [Google Scholar] [CrossRef]
- Ayo, S.H.; Radnik, R.; Garoni, J.A.; Troyer, D.A.; Kreisberg, J.I. High glucose increases diacylglycerol mass and activates protein kinase C in mesangial cell cultures. Am. J. Physiol. Physiol. 1991, 261, F571–F577. [Google Scholar] [CrossRef]
- Shmueli, E.; Alberti, K.G.M.M.; Record, C.O. Diacylglycerol/protein kinase C signalling: A mechanism for insulin resistance? J. Intern. Med. 1993, 234, 397–400. [Google Scholar] [CrossRef]
- Ha, H.; Yu, M.-R.; Choi, Y.J.; Lee, H.B. Activation of protein kinase C-δ and C-ϵ by oxidative stress in early diabetic rat kidney. Am. J. Kidney Dis. 2001, 38, S204–S207. [Google Scholar] [CrossRef]
- Scivittaro, V.; Ganz, M.B.; Weiss, M.F. AGEs induce oxidative stress and activate protein kinase C-βII in neonatal mesangial cells. Am. J. Physiol. Physiol. 2000, 278, F676–F683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordo, R.; Nasrallah, G.K.; Posadino, A.M.; Galimi, F.; Capobianco, G.; Eid, A.H.; Pintus, G. Resveratrol-Elicited PKC Inhibition Counteracts NOX-Mediated Endothelial to Mesenchymal Transition in Human Retinal Endothelial Cells Exposed to High Glucose. Antioxidants 2021, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Peng, Z.; Yuan, Q.; Li, Q.; Peng, Y.; Wen, R.; Hu, Z.; Liu, J.; Xia, X.; Deng, H.; et al. AKF-PD alleviates diabetic nephropathy via blocking the RAGE/AGEs/NOX and PKC/NOX Pathways. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Dasu, M.R.; Devaraj, S.; Zhao, L.; Hwang, D.H.; Jialal, I. High Glucose Induces Toll-Like Receptor Expression in Human Monocytes: Mechanism of Activation. Diabetes 2008, 57, 3090–3098. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.-Y.; Wei, C.-C.; Shang, T.-T.; Lian, Q.; Wu, C.-X.; Deng, J.-Y. High glucose induces inflammatory cytokine through protein kinase C-induced toll-like receptor 2 pathway in gingival fibroblasts. Biochem. Biophys. Res. Commun. 2012, 427, 666–670. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Zeh, H.J., III; Lotze, M.T. High-Mobility Group Box 1, Oxidative Stress, and Disease. Antioxid. Redox Signal. 2011, 14, 1315–1335. [Google Scholar] [CrossRef] [Green Version]
- Youn, J.H.; Shin, J.-S. Nucleocytoplasmic Shuttling of HMGB1 Is Regulated by Phosphorylation That Redirects It toward Secretion. J. Immunol. 2006, 177, 7889–7897. [Google Scholar] [CrossRef] [Green Version]
- Sohn, E.; Kim, J.; Kim, C.-S.; Lee, Y.M.; Kim, J.S. Extract of Polygonum cuspidatum Attenuates Diabetic Retinopathy by Inhibiting the High-Mobility Group Box-1 (HMGB1) Signaling Pathway in Streptozotocin-Induced Diabetic Rats. Nutrients 2016, 8, 140. [Google Scholar] [CrossRef]
- Han, R.; Liu, Z.; Sun, N.; Liu, S.; Li, L.; Shen, Y.; Xiu, J.; Xu, Q. BDNF Alleviates Neuroinflammation in the Hippocampus of Type 1 Diabetic Mice via Blocking the Aberrant HMGB1/RAGE/NF-κB Pathway. Aging Dis. 2019, 10, 611–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akutagawa, K.; Fujita, T.; Ouhara, K.; Takemura, T.; Tari, M.; Kajiya, M.; Matsuda, S.; Kuramitsu, S.; Mizuno, N.; Shiba, H.; et al. Glycyrrhizic acid suppresses inflammation and reduces the increased glucose levels induced by the combination of Porphyromonas gulae and ligature placement in diabetic model mice. Int. Immunopharmacol. 2019, 68, 30–38. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, J.; Yang, W.; Zheng, H.; Xue, S. High Mobility Group Box 1 (HMGB1) Mediates High-Glucose-Induced Calcification in Vascular Smooth Muscle Cells of Saphenous Veins. Inflammation 2013, 36, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Xie, P.; Gong, P.; Tang, X.-H.; Ding, Y.; Deng, L.-X. Expression of HMGB1 and HMGN2 in gingival tissues, GCF and PICF of periodontitis patients and peri-implantitis. Arch. Oral Biol. 2011, 56, 1106–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atieh, M.A.; Faggion, C.M.; Seymour, G.J. Cytokines in patients with type 2 diabetes and chronic periodontitis: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2014, 104, e38–e45. [Google Scholar] [CrossRef] [PubMed]
- Andriankaja, O.; Barros, S.; Moss, K.; Panagakos, F.; DeVizio, W.; Beck, J.; Offenbacher, S. Levels of Serum Interleukin (IL)-6 and Gingival Crevicular Fluid of IL-1β and Prostaglandin E2Among Non-Smoking Subjects With Gingivitis and Type 2 Diabetes. J. Periodontol. 2009, 80, 307–316. [Google Scholar] [CrossRef]
- Katz, J.; Bhattacharyya, I.; Farkhondeh-Kish, F.; Perez, F.M.; Caudle, R.M.; Heft, M.W. Expression of the receptor of advanced glycation end products in gingival tissues of type 2 diabetes patients with chronic periodontal disease: A study utilizing immunohistochemistry and RT-PCR. J. Clin. Periodontol. 2005, 32, 40–44. [Google Scholar] [CrossRef]
- Morimoto, Y.; Kawahara, K.-I.; Tancharoen, S.; Kikuchi, K.; Matsuyama, T.; Hashiguchi, T.; Izumi, Y.; Maruyama, I. Tumor necrosis factor-α stimulates gingival epithelial cells to release high mobility-group box 1. J. Periodontal Res. 2007, 43, 76–83. [Google Scholar] [CrossRef]
- Ito, Y.; Bhawal, U.K.; Sasahira, T.; Toyama, T.; Sato, T.; Matsuda, D.; Nishikiori, H.; Kobayashi, M.; Sugiyama, M.; Hamada, N.; et al. Involvement of HMGB1 and RAGE in IL-1β-induced gingival inflammation. Arch. Oral Biol. 2012, 57, 73–80. [Google Scholar] [CrossRef]
- Tancharoen, S.; Gando, S.; Binita, S.; Nagasato, T.; Kikuchi, K.; Nawa, Y.; Dararat, P.; Yamamoto, M.; Narkpinit, S.; Maruyama, I. HMGB1 Promotes Intraoral Palatal Wound Healing through RAGE-Dependent Mechanisms. Int. J. Mol. Sci. 2016, 17, 1961. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Shen, J.; Chai, Y.; Chen, H. IL-1β Impaired Diabetic Wound Healing by Regulating MMP-2 and MMP-9 through the p38 Pathway. Mediat. Inflamm. 2021, 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Buranasin, P.; Mizutani, K.; Iwasaki, K.; Na Mahasarakham, C.P.; Kido, D.; Takeda, K.; Izumi, Y. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS ONE 2018, 13, e0201855. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Franco, S.; Padilla-Tejeda, D.E.; Carrillo-Ibarra, S.; Miranda-Díaz, A.G. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int. J. Endocrinol. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Franco, S.; Pacheco-Moisés, F.P.; Rodríguez-Carrizalez, A.D.; Miranda-Díaz, A.G. The Role of Oxidative Stress, Mitochondrial Function, and Autophagy in Diabetic Polyneuropathy. J. Diabetes Res. 2017, 2017, 1673081. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, P.-F.; Yu, C.-C.; Chu, P.-M.; Hsieh, P.-L. Verbascoside Protects Gingival Cells against High Glucose-Induced Oxidative Stress via PKC/HMGB1/RAGE/NFκB Pathway. Antioxidants 2021, 10, 1445. https://doi.org/10.3390/antiox10091445
Hsieh P-F, Yu C-C, Chu P-M, Hsieh P-L. Verbascoside Protects Gingival Cells against High Glucose-Induced Oxidative Stress via PKC/HMGB1/RAGE/NFκB Pathway. Antioxidants. 2021; 10(9):1445. https://doi.org/10.3390/antiox10091445
Chicago/Turabian StyleHsieh, Pei-Fang, Cheng-Chia Yu, Pei-Ming Chu, and Pei-Ling Hsieh. 2021. "Verbascoside Protects Gingival Cells against High Glucose-Induced Oxidative Stress via PKC/HMGB1/RAGE/NFκB Pathway" Antioxidants 10, no. 9: 1445. https://doi.org/10.3390/antiox10091445
APA StyleHsieh, P.-F., Yu, C.-C., Chu, P.-M., & Hsieh, P.-L. (2021). Verbascoside Protects Gingival Cells against High Glucose-Induced Oxidative Stress via PKC/HMGB1/RAGE/NFκB Pathway. Antioxidants, 10(9), 1445. https://doi.org/10.3390/antiox10091445






