Noble 3,4-Seco-triterpenoid Glycosides from the Fruits of Acanthopanax sessiliflorus and Their Anti-Neuroinflammatory Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. General Experimental Procedures
2.3. Extraction and Isolation
2.4. UPLC-QTOF/MS and UPLC-MS/MS Analyses
2.5. Cell Culture and Viability Assays
2.6. Determination of Nitrite
2.7. PGE2 Assay
2.8. Assays for IL-1β, IL-6, and TNF-α
2.9. Western Blotting Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical Structure Elucidation of Compounds 1–11
3.2. Analyses of Compounds 1–11 in ASFE by UPLC-QTOF/MS and Tandem Mass Spectrometry (MS/MS)
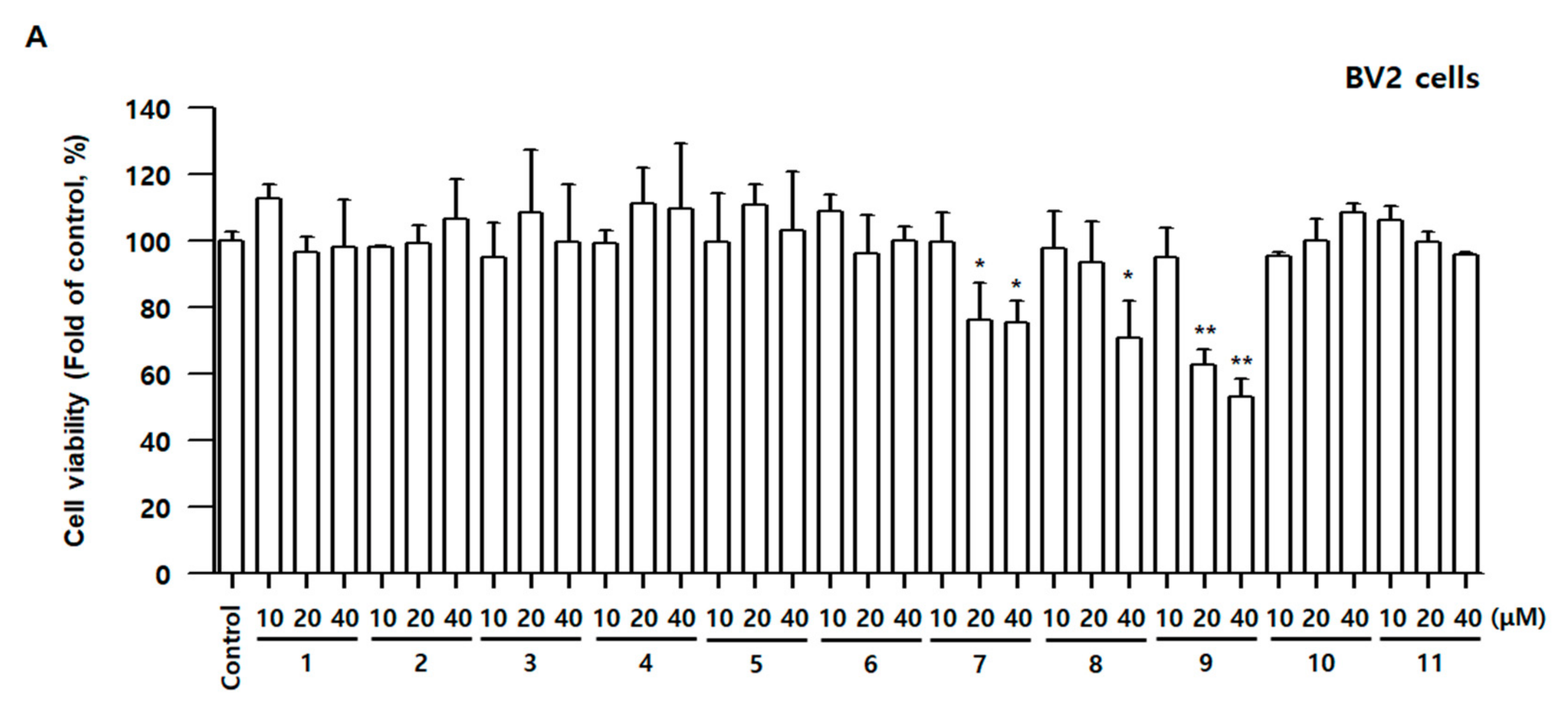
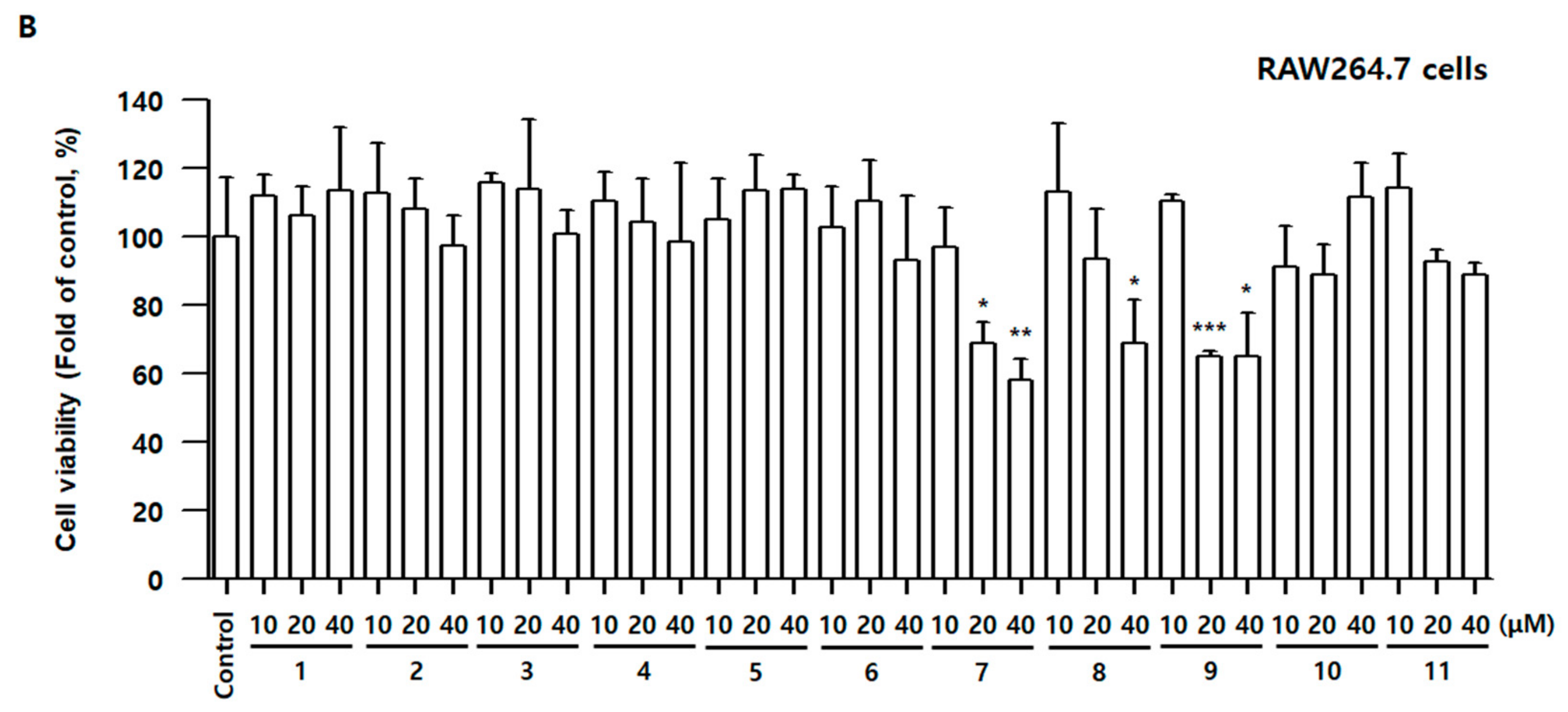
3.3. Effects of Compounds 1–11 on Cell Viability and Nitrite Contents in BV2 and RAW264.7 Cells
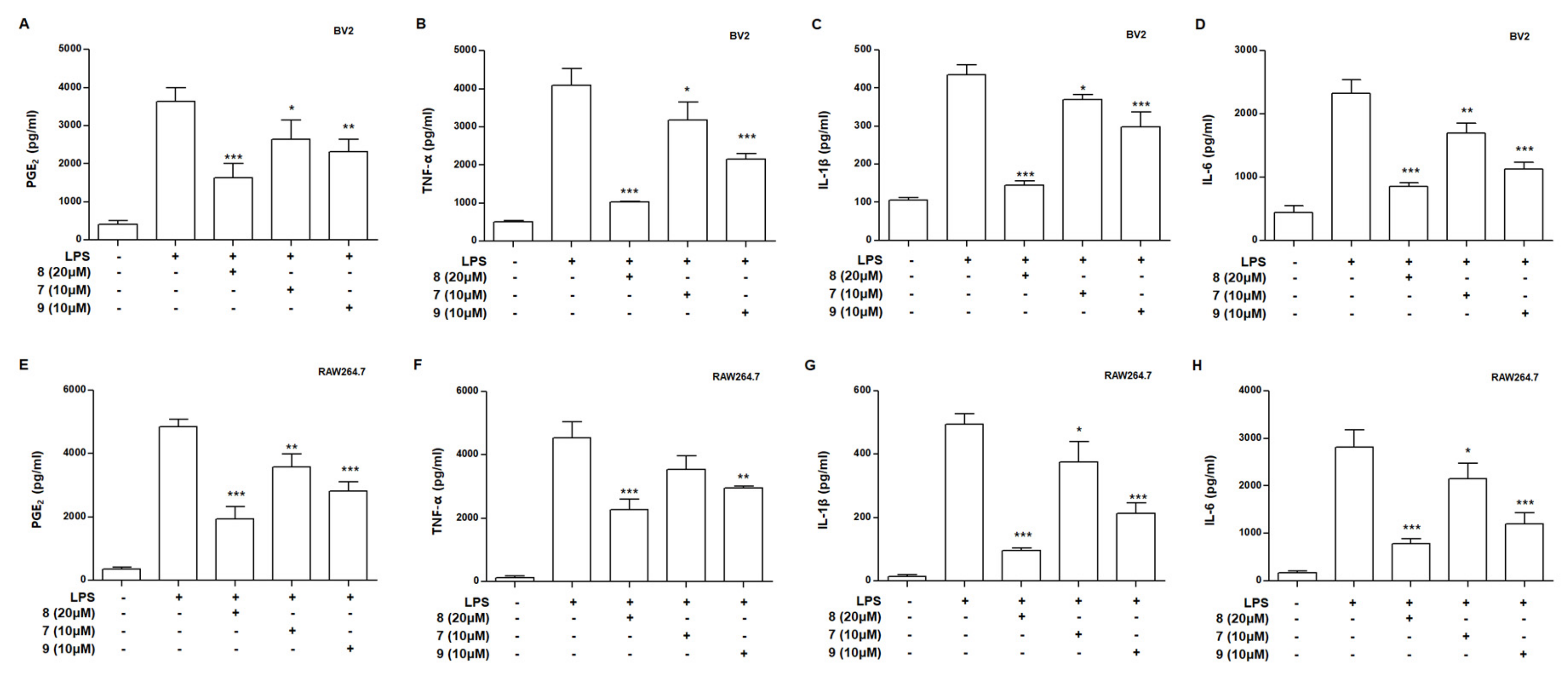
3.4. Effects of Compounds 7–9 on Levels of Prostaglandin E2 (PGE2), Tumor Necrosis Factor (TNF)-α, Interleukin (IL)-1β, and Interleukin (IL)-6 in LPS-Stimulated BV2 and RAW264.7 Cells
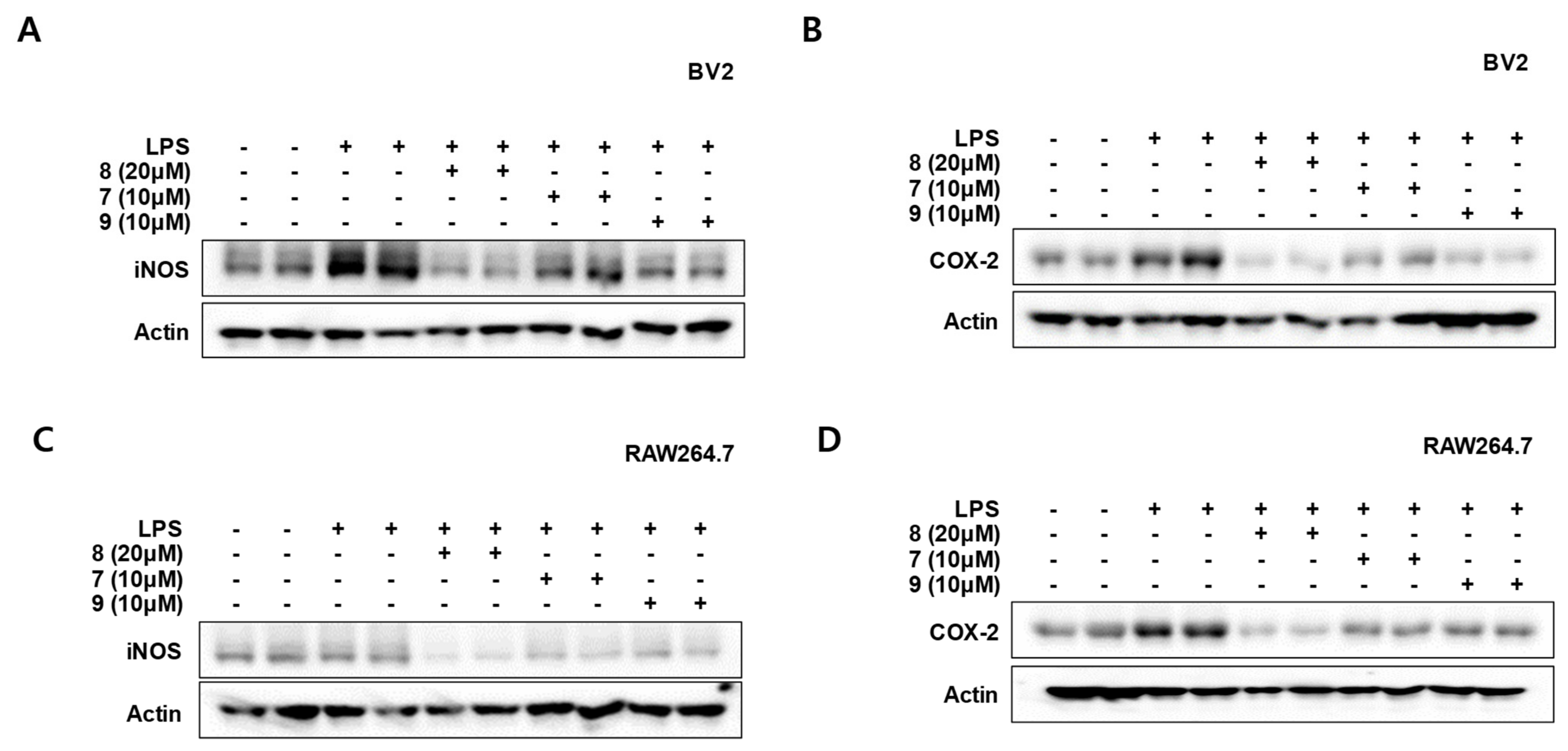
3.5. Effects of Compounds 7–9 on the Protein Expression Levels of Inducible Nitric Oxide Synthase (iNOS) and Cyclooxygenase-2 (COX-2) in LPS-Stimulated BV2 and RAW264.7 Cells
3.6. Effects of Compounds 7–9 on p38, c-Jun N-Terminal Kinase (JNK)-1/2, and Extracellular Signal-Regulated Kinase (ERK)-1/2 Phosphorylation in BV2 and RAW264.7 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFB | Acanthopanax sessiliflorus fruits n-butanol fraction |
| AFE | Acanthopanax sessiliflorus fruits ethyl acetate fraction |
| AFH | Acanthopanax sessiliflorus fruits aqueous fraction |
| ASFEx | Acanthopanax sessiliflorus fruits extraction |
| CC | column chromatography |
| DW | dry weight |
| EtOAc | ethyl acetate |
| FAB | subfractions from Acanthopanax sessiliflorus fruits n-butanol fraction |
| gHMBC | gradient heteronuclear multiple bond coherence |
| IFN- γ | interferon-gamma |
| LPS | lipopolysaccharide |
| MeOH | methanol |
| n-BuOH | n-butanol |
| NMR | nuclear magnetic resonance |
| NO | nitric oxide |
| ODS | octadecyl silica gel |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SiO2 | silica gel |
| TLC | thin layer chromatography |
| TNF-α | tumor necrosis factor-alpha |
| UV | ultraviolet |
| 13C-NMR | carbon-13 nuclear magnetic resonance |
| 1D-NMR | one-dimensional (1D) NMR |
| 2D-NMR | two-dimensional (2D) NMR |
| 1H-NMR | proton-1 nuclear magnetic resonance |
References
- Rha, C.S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.O. Antioxidative, anti-Inflammatory, and anticancer effects of purified flavonol glycosides and aglycones in green tea. Antioxidants 2019, 8, 278. [Google Scholar] [CrossRef] [Green Version]
- Sohn, E.S.; Sohn, E.H. A study on the R&D trend and patent analysis of treatments for degenerative brain diseases. J. Korea Acad. Industr. Coop. Soc. 2011, 12, 4411–4417. [Google Scholar]
- Nakamura, Y.; Si, Q.S.; Kataoka, K. Lipopolysaccharide-induced microglial activation in culture: Temporal profiles of morphological change and release of cytokines and nitric oxide. Neurosci. Res. 1999, 35, 95–100. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, J.; Liu, H.; Babu-Khan, S.; Vassar, R.; Biere, A.L.; Citron, M.; Landreth, G. Anti-inflammatory drug therapy alters â-amyloid processing and deposition in an animal model of Alzheimer’s disease. J. Neurosci. 2003, 23, 7504–7509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, M.È.; Stevens, B.; Sierra, A.; Wake, H.; Bessis, A.; Nimmerjahn, A. The role of microglia in the healthy brain. J. Neurosci. 2011, 31, 16064–16069. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Tumilowics, J.; Banaszczak, P. Woody species of Araliaceae at the Rogow Arboretum. Rocz. Dendrologiczny. 2006, 54, 35–50. [Google Scholar]
- Lee, D.Y.; Seo, K.H.; Lee, D.S.; Kim, Y.C.; Chung, I.S.; Kim, G.W.; Cheoi, D.S.; Baek, N.I. Bioactive 3,4-seco-Triterpenoids from the Fruits of Acanthopanax sessiliflorus. J. Nat. Prod. 2012, 75, 1138–1144. [Google Scholar] [CrossRef]
- Park, J.K.; Kim, C.K.; Gong, S.K.; Yu, A.R.; Lee, M.Y.; Park, S.K. Acanthopanax sessiliflorus stem confers increased resistance to environmental stresses and lifespan extension in Caenorhabditis elegans. Nutr. Res. Pract. 2014, 8, 526–532. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Li, W.; Fu, H.; Zhang, Q.; Koike, K. Pancreatic Lipase-Inhibiting Triterpenoid Saponins from Fruits of Acanthopanax senticosus. Chem. Pharm. Bull. 2007, 55, 1087–1089. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Son, D.W.; Ryu, J.Y.; Lee, Y.S.; Jung, S.H.; Kang, J.G.; Lee, S.Y.; Kim, H.S.; Shin, K.H. Anti-oxidant activities of Acanthopanax senticosus stems and their lignan components. Arch. Pharm. Res. 2004, 27, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, K.; Hirano, K.; Ando, H.; Hirai, Y.; Ida, Y.; Tsuji, T.; Tanaka, T.; Satouchi, K.; Terao, J. Lupane-Type Saponins from Leaves of Acanthopanax sessiliflorus and Their Inhibitory Activity on Pancreatic Lipase. J. Agric. Food Chem. 2006, 54, 335–341. [Google Scholar] [CrossRef]
- Hwang, E.; Kim, G.W.; Song, K.D.; Lee, H.K.; Kim, S.J. The enhancing effect of Acanthopanax sessiliflorus fruit extract on the antibacterial activity of porcine alveolar 3D4/31 macrophages via nuclear factor kappa Bl and lipid metabolism regulation. Asian-Australas. J. Anim. Sci. 2019, 32, 1776–1788. [Google Scholar] [CrossRef] [Green Version]
- Jung, I.H.; Kim, S.E.; Lee, Y.G.; Kim, D.H.; Kim, H.; Kim, G.S.; Baek, N.I.; Lee, D.Y. Antihypertensive Effect of Ethanolic Extract from Acanthopanax sessiliflorus Fruits and Quality Control of Active Compounds. Oxid. Med. Cell Longev. 2018, 2018, 5158243. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.J.; Nam, J.H.; Choi, J.W.; Lee, K.T.; Park, H.J. Anti-inflammatory effects of chiisanoside and chiisanogenin obtained from the leaves of Acanthopanax chiisanensis in the carrageenan- and Freund’s complete adjuvant-induced rats. J. Ethnopharmacol. 2005, 97, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Oh, H.J.; Ko, J.H.; Song, H.S.; Lee, Y.G.; Kang, S.C.; Lee, D.Y.; Baek, N.I. Lanceoleins A–G, hydroxychalcones, from the flowers of Coreopsis lanceolata and their chemopreventive effects against human colon cancer cells. Bioorg. Chem. 2019, 85, 274–281. [Google Scholar] [CrossRef]
- Quang, T.H.; Ngan, N.T.; Yoon, C.S.; Cho, K.H.; Kang, D.G.; Lee, H.S.; Kim, Y.C.; Oh, H. Protein tyrosine phosphatase 1B inhibitors from the Roots of Cudrania tricuspidata. Molecules 2015, 20, 11173–11183. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Chang, S.Y.; Yook, C.S.; Nohara, T. New 3,4-seco-lupane-type triterpene glycosides from Acanthopanax senticosus forma inermis. J. Nat. Prod. 2000, 63, 1630–1633. [Google Scholar] [CrossRef]
- Shirasuna, K.; Miyakoshi, M.; Mimoto, S.; Isoda, S.; Satoh, Y.; Hirai, Y.; Ida, Y.; Shoji, J. Lupane triterpenoid glycosyl esters from leaves of Acanthopanax divaricatus. Phytochemistry 1997, 45, 579–584. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, M.-J.; Yoon, D.; Lee, Y.-S.; Kim, G.-S.; Yoo, Y.C. Ginseng Berry Prevents Alcohol-Induced Liver Damage by Improving the Anti-Inflammatory System Damage in Mice and Quality Control of Active Compounds. Int. J. Mol. Sci. 2019, 20, 3522. [Google Scholar] [CrossRef] [Green Version]
- Ko, W.; Sohn, J.H.; Jang, J.H.; Ahn, J.S.; Kang, D.G.; Lee, H.S.; Kim, J.S.; Kim, Y.C.; Oh, H. Inhibitory effects of alternaramide on inflammatory mediator expression through TLR4-MyD88-mediated inhibition of NF-κB and MAPK pathway signaling in lipopolysaccharide-stimulated RAW264.7 and BV2 cells. Chem. Biol. Interact. 2016, 244, 16–26. [Google Scholar] [CrossRef]
- Lee, H.; Ko, W.; Chowdhury, A.; Li, B.; Kim, S.C.; Oh, H.; Kim, Y.C.; Woo, E.R.; Baek, N.I.; Lee, D.S. Brassicaphenanthrene A from Brassica rapa protects HT22 neuronal cells through the regulation of Nrf2-mediated heme oxygenase-1 expression. Mol. Med. Rep. 2020, 21, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Titheradge, M.A. The enzymatic measurement of nitrate and nitrite. Methods Mol. Biol. 1998, 100, 83–91. [Google Scholar] [PubMed]
- Pandur, E.; Varga, E.; Tamasi, K.; Pap, R.; Nagy, J.; Sipos, K. Effect of inflammatory mediators lipopolysaccharide and lipoteichoic acid on iron metabolism of differentiated SH-SY5Y cells alters in the presence of BV-2 microglia. Int. J. Mol. Sci. 2018, 20, 17. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Ko, Y.H.; Lee, Y.; Lee, S.Y.; Jang, C.G. Antineuroinflammatory Effects of 7,3′,4′-Trihydroxyisoflavone in Lipopolysaccharide-Stimulated BV2 Microglial Cells through MAPK and NF-κB Signaling Suppression. Biomol. Ther. 2021, 29, 127–134. [Google Scholar] [CrossRef]
- Boka, G.; Anglade, P.; Wallach, D.; Javoy-Agid, F.; Agid, Y.; Hirsch, E.C. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci. Lett. 1994, 172, 151–154. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Park, J.; Min, J.S.; Kim, B.; Chae, U.B.; Yun, J.W.; Choi, M.S.; Kong, I.K.; Chang, K.T.; Lee, D.S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-kappaB pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Ripmeester, E.G.J.; Timur, U.T.; Caron, M.M.J.; Welting, T.J.M. Recent insights into the contribution of the changing hypertrophic chondrocyte phenotype in the development and progression of osteoarthritis. Front. Bioeng. Biotechnol. 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
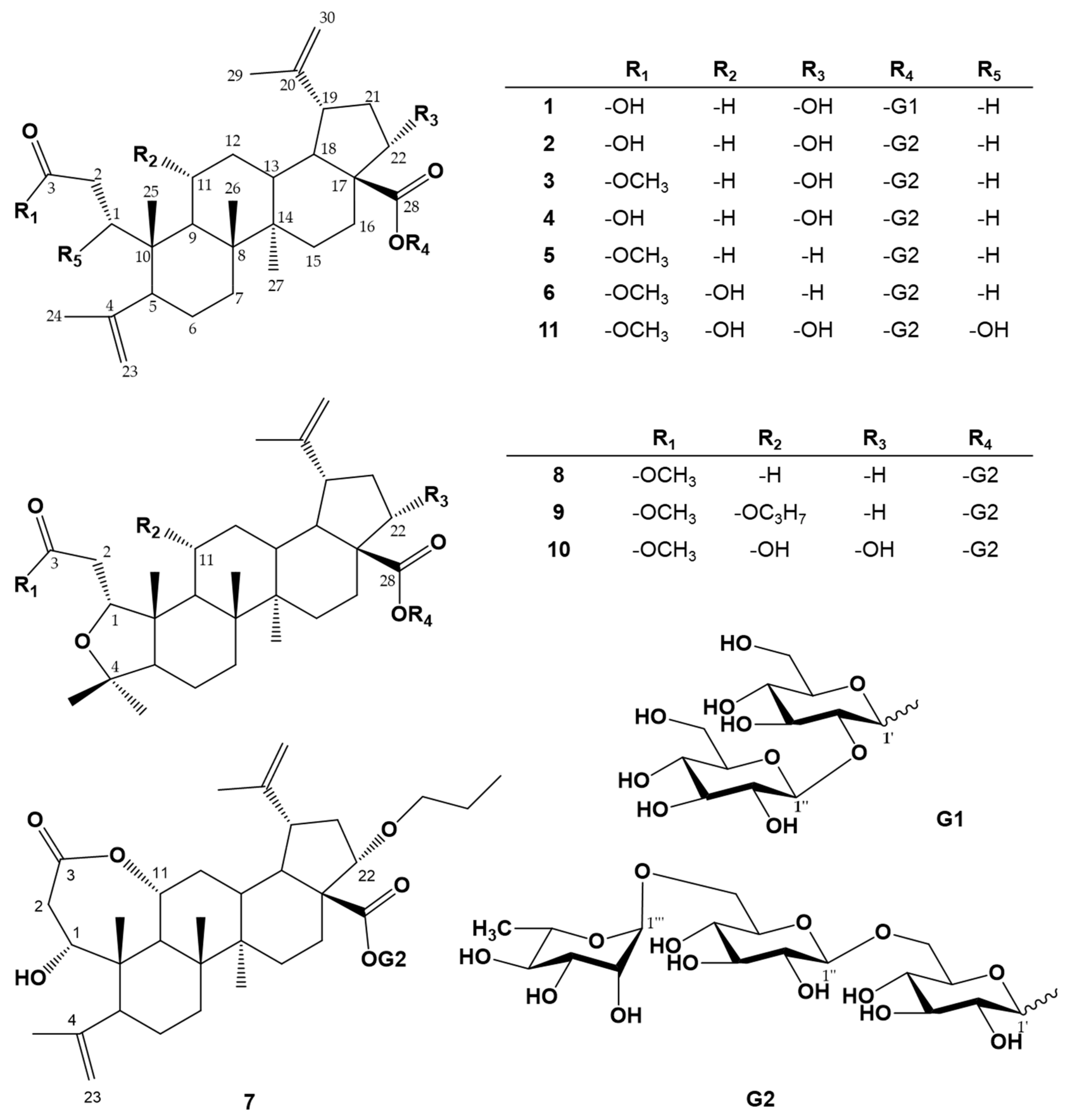




| No. | δH, Coupling Pattern, J in Hza) | ||||
|---|---|---|---|---|---|
| G (1) | H (2) | I (3) | J (5) | K (7) | |
| 1 | 1.82, overlap | 1.91, overlap | 1.91, overlap | 2.17, overlap 1.54, overlap | 3.70, overlap |
| 2 | 1.39, overlap 1.36, overlap | 1.37, overlap 1.33, overlap | 1.37, overlap 1.33, overlap | 1.80, overlap 1.24, overlap | 2.86, overlap 2.58, overlap |
| 9 | 1.73, overlap | 1.77, overlap | 1.77, overlap | 1.75, overlap | 2.63, d, 9.2 |
| 11 | 1.47, overlap | 1.51, overlap | 1.51, overlap | 1.25, overlap | 4.55, overlap |
| 18 | 2.69, overlap | 2.69, overlap | 2.69, overlap | 1.84, overlap | 2.51, overlap |
| 19 | 3.70, m | 3.57, m | 3.60, m | 3.48, m | 3.52, m |
| 22 | 4.83, overlap | 4.83, overlap | 4.84, overlap | 1.73, overlap 1.36, overlap | 4.80, overlap |
| 23 | 5.12, d, 2.0 4.90, d, 2.0 | 5.04, d, 2.0 4.81, d, 2.0 | 5.00, d, 2.0 4.81, d, 2.0 | 4.94, br. s 4.78, br. s | 5.00, d, 2.0 4.80, d, 2.0 |
| 24 | 1.71, s | 1.81, s | 1.71, s | 1.71, s | 1.74, s |
| 25 | 1.14, s | 1.22, s | 1.15, s | 1.03, s | 1.17, s |
| 26 | 1.32, s | 1.24, s | 1.17, s | 1.13, s | 1.19, s |
| 27 | 0.81, s | 0.88, s | 0.79, s | 0.77, s | 0.82, s |
| 29 | 2.13, s | 1.99, s | 1.95, s | 1.72, s | 1.95, s |
| 30a | 4.90, overlap 4.79, overlap | 4.95, overlap 4.89, overlap | 4.90, overlap 4.81, overlap | 4.79, overlap 4.75, overlap | 4.96, overlap 4.89, overlap |
| OCH3 | - | - | 3.63, s | 3.64, s | - |
| 1′ | 6.37, d, 8.0 | 6.38, d, 8.0 | 6.32, d, 8.0 | 6.31, d, 8.0 | 6.33, d, 8.0 |
| 2′ | 4.30, overlap | 4.09, overlap | 4.10, overlap | 4.09, overlap | 4.10, overlap |
| 3′ | 4.37, overlap | 4.38, overlap | 4.32, overlap | 4.38, overlap | 4.35, overlap |
| 4′ | 4.30, overlap | 4.30, overlap | 4.28, overlap | 4.30, overlap | 4.29, overlap |
| 5′ | 4.00, overlap | 4.13, overlap | 4.14, overlap | 4.13, overlap | 4.15, overlap |
| 6′ | 4.58, dd, 12.0, 2.4 4.46, dd, 12.0, 4.8 | 4.69, overlap 4.30, overlap | 4.64, overlap 4.30, overlap | 4.69, overlap 4.30, overlap | 4.66, overlap 4.29, overlap |
| 1′′ | 5.35, d, 8.0 | 4.94, d, 8.0 | 4.83, d, 8.0 | 4.96, d, 8.0 | 4.94, d, 8.0 |
| 2′′ | 4.07, dd, 8.0, 8.0 | 4.10, overlap | 4.10, overlap | 4.10, overlap | 4.10, overlap |
| 3′′ | 4.27, overlap | 4.25, overlap | 4.25, overlap | 4.25, overlap | 4.25, overlap |
| 4′′ | 4.30, overlap | 4.38, overlap | 4.31, overlap | 4.38, overlap | 4.35, overlap |
| 5′′ | 4.00, overlap | 4.12, overlap | 4.15, overlap | 4.12, overlap | 4.15, overlap |
| 6′′ | 4.42, dd, 12.0, 2.0 4.34, dd, 12.0, 5.2 | 4.19, overlap 4.09, overlap | 4.21, overlap 4.08, overlap | 4.19, overlap 4.09, overlap | 4.18, overlap 4.06, overlap |
| 1‴ | - | 5.83, br. s | 5.75, br. s | 5.79, br. s | 5.79, br. s |
| 2‴ | - | 4.66, overlap | 4.61, overlap | 4.66, overlap | 4.66, overlap |
| 3‴ | - | 4.53, dd, 8.4, 3.2 | 4.47, dd, 8.4, 3.2 | 4.53, dd, 8.4, 3.2 | 4.55, dd, 8.4, 3.2 |
| 4‴ | - | 3.93, dd, 8.4, 8.4 | 3.88, dd, 8.4, 8.4 | 3.93, dd, 8.4, 8.4 | 3.89, dd, 8.4, 8.4 |
| 5‴ | - | 4.93, overlap | 4.92, overlap | 4.93, overlap | 4.93, overlap |
| 6‴ | - | 1.71, d, 6.0 | 1.65, d, 6.0 | 1.67, d, 6.4 | 1.67, d, 6.0 |
| 1⁗ | 4.42, overlap | ||||
| 2⁗ | 2.05, overlap | ||||
| 3⁗ | 0.79, overlap | ||||
| No. | δH, Coupling Pattern, J in Hza) | ||||
| L (8) | M (9) | N (10) | O (11) | ||
| 1 | 4.86, dd, 12.0, 2.4 | 4.86, dd, 12.0, 2.8 | 4.87, overlap | 4.86, overlap | |
| 2 | 3.65, overlap 2.66, dd, 12.0, 8.0 | 3.65, dd, 12.0, 2.8 2.66, dd, 12.0, 8.0 | 3.65, overlap 2.66, overlap | 3.65, overlap 2.64, overlap | |
| 9 | 1.88, d, 11.2 | 1.90, d, 10.8 | 1.95, overlap | 1.95, overlap | |
| 11 | 1.25, overlap | 4.07, overlap | 4.07, overlap | 4.07, overlap | |
| 18 | 1.72, overlap | 1.80, dd, 12.0, 11.2 | 1.72, overlap | 1.72, overlap | |
| 19 | 3.50, m | 3.34, m | 3.58, m | 3.49, m | |
| 22 | 2.45, overlap 1.42, overlap | 2.35, overlap 1.44, overlap | 4.80, overlap | 4.79, overlap | |
| 23 | 1.24, s | 1.16, s | 1.24, s | 4.93, d, 2.0 4.80, d, 2.0 | |
| 24 | 1.37, s | 1.39, s | 1.37, s | 1.37, s | |
| 25 | 1.32, s | 1.31, s | 1.33, s | 1.32, s | |
| 26 | 1.21, s | 1.15, s | 1.21, s | 1.24, s | |
| 27 | 1.10, s | 1.13, s | 1.15, s | 1.15, s | |
| 29 | 1.93, s | 1.68, s | 1.96, s | 1.93, s | |
| 30 | 4.91, br. s 4.66, br. s | 4.80, br. s 4.64, overlap | 4.83, overlap 4.63, overlap | 4.87, overlap 4.66, overlap | |
| OCH3 | 3.60, s | 3.58, s | 3.60, s | 3.60, s | |
| 1′ | 6.37, d, 8.4 | 6.29, d, 8.4 | 6.31, d, 8.4 | 6.31, d, 8.0 | |
| 2′ | 4.15, overlap | 4.16, overlap | 4.16, overlap | 4.17, overlap | |
| 3′ | 4.27, overlap | 4.27, overlap | 4.27, overlap | 4.28, overlap | |
| 4′ | 4.32, overlap | 4.34, overlap | 4.33, overlap | 4.32, overlap | |
| 5′ | 4.10, overlap | 4.09, overlap | 4.08, overlap | 4.09, overlap | |
| 6′ | 4.41, br. d, 12.0 4.38, dd, 12.0, 6.0 | 4.66, overlap 4.29, overlap | 4.67, overlap 4.29, overlap | 4.66, overlap 4.30, overlap | |
| 1′′ | 4.92, d, 8.0 | 4.92, d, 7.6 | 4.90, d, 8.0 | 4.90, d, 8.0 | |
| 2′′ | 4.12, overlap | 4.10, overlap | 4.10, overlap | 4.10, overlap | |
| 3′′ | 4.25, overlap | 4.25, overlap | 4.25, overlap | 4.25, overlap | |
| 4′′ | 4.35, overlap | 4.34, overlap | 4.33, overlap | 4.34, overlap | |
| 5′′ | 4.15, overlap | 4.16, overlap | 4.16, overlap | 4.17, overlap | |
| 6′′ | 4.19, overlap 4.07, overlap | 4.19, overlap 4.05, overlap | 4.18, overlap 4.06, overlap | 4.18, overlap 4.03, overlap | |
| 1‴ | 5.85, br. s | 5.79, d, 1.2 | 5.78, br. s | 5.77, br. s | |
| 2‴ | 4.68, overlap | 4.64, overlap | 4.63, overlap | 4.62, overlap | |
| 3‴ | 4.54, dd, 8.4, 3.2 | 4.49, dd, 8.4, 3.2 | 4.48, dd, 8.4, 3.2 | 4.49, dd, 8.4, 3.2 | |
| 4‴ | 3.91, dd, 8.4, 8.4 | 3.89, dd, 8.4, 8.4 | 3.88, dd, 8.4, 8.4 | 3.88, dd, 8.4, 8.4 | |
| 5‴ | 4.93, overlap | 4.88, overlap | 4.92, overlap | 4.92, overlap | |
| 6‴ | 1.69, d, 6.0 | 1.67, d, 6.0 | 1.66, d, 6.0 | 1.64, d, 6.0 | |
| 1⁗ | - | 4.37, overlap | - | - | |
| 2⁗ | - | 2.01, overlap | - | - | |
| 3⁗ | - | 0.76, overlap | - | - | |
| No. | δCa) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| G (1) | H (2) | I (3) | J (5) | K (7) | L (8) | M (9) | N (10) | O (11) | |
| 1 | 35.1 | 35.8 | 34.7 | 30.9 | 34.8 | 87.1 | 87.3 | 87.2 | 69.7 |
| 2 | 29.7 | 29.9 | 28.6 | 30.1 | 29.9 | 41.8 | 38.7 | 41.9 | 37.6 |
| 3 | 173.6 | 175.2 | 174.3 | 178.5 | 174.8 | 173.4 | 173.0 | 173.4 | 175.2 |
| 4 | 148.1 | 148.5 | 147.9 | 148.6 | 148.0 | 79.3 | 79.2 | 79.2 | 148.4 |
| 5 | 50.6 | 50.8 | 50.3 | 50.3 | 50.4 | 55.9 | 56.1 | 56.1 | 51.4 |
| 6 | 27.4 | 27.1 | 26.6 | 25.9 | 28.9 | 17.9 | 19.2 | 18.8 | 31.0 |
| 7 | 33.5 | 33.4 | 32.9 | 34.7 | 33.0 | 35.3 | 35.4 | 35.4 | 33.8 |
| 8 | 40.1 | 40.0 | 39.5 | 39.5 | 39.6 | 42.8 | 42.9 | 42.9 | 42.4 |
| 9 | 41.6 | 41.6 | 41.1 | 41.0 | 41.2 | 48.8 | 48.9 | 49.0 | 49.5 |
| 10 | 43.7 | 43.7 | 43.2 | 43.2 | 43.2 | 43.9 | 47.0 | 47.0 | 45.9 |
| 11 | 22.3 | 22.3 | 21.7 | 21.6 | 64.2 | 22.8 | 67.6 | 67.7 | 64.2 |
| 12 | 25.4 | 25.5 | 24.9 | 24.9 | 25.9 | 37.2 | 37.0 | 37.3 | 36.7 |
| 13 | 38.9 | 38.7 | 38.2 | 38.4 | 38.3 | 38.5 | 37.5 | 38.5 | 37.4 |
| 14 | 41.2 | 41.3 | 40.8 | 40.8 | 40.8 | 42.7 | 42.8 | 42.8 | 43.0 |
| 15 | 30.2 | 30.1 | 29.6 | 28.6 | 31.0 | 29.8 | 30.4 | 29.8 | 32.6 |
| 16 | 26.3 | 26.4 | 25.9 | 32.2 | 26.6 | 32.5 | 31.1 | 32.6 | 32.2 |
| 17 | 63.3 | 63.6 | 63.1 | 57.0 | 63.1 | 49.6 | 57.0 | 58.1 | 57.1 |
| 18 | 44.6 | 44.7 | 44.2 | 49.8 | 44.3 | 47.4 | 49.5 | 49.6 | 49.7 |
| 19 | 48.3 | 48.0 | 47.5 | 47.3 | 47.6 | 46.9 | 47.3 | 47.4 | 47.8 |
| 20 | 152.1 | 151.7 | 151.2 | 150.8 | 151.2 | 150.8 | 150.5 | 150.9 | 150.4 |
| 21 | 42.6 | 42.4 | 41.9 | 32.9 | 41.9 | 30.6 | 30.9 | 30.2 | 41.7 |
| 22 | 76.0 | 75.7 | 76.4 | 36.8 | 76.5 | 36.8 | 36.7 | 76.4 | 76.5 |
| 23 | 114.4 | 114.0 | 113.6 | 113.7 | 113.7 | 24.8 | 24.9 | 24.9 | 113.9 |
| 24 | 23.9 | 24.1 | 23.4 | 23.4 | 23.4 | 32.4 | 32.6 | 32.5 | 23.7 |
| 25 | 20.7 | 21.0 | 20.3 | 20.3 | 20.4 | 18.5 | 18.8 | 19.2 | 20.9 |
| 26 | 16.8 | 16.8 | 16.3 | 16.3 | 16.3 | 17.9 | 17.9 | 17.9 | 17.4 |
| 27 | 15.3 | 15.3 | 14.8 | 14.7 | 14.9 | 15.1 | 15.2 | 15.2 | 14.8 |
| 28 | 179.0 | 177.0 | 174.8 | 174.9 | 174.0 | 174.7 | 174.9 | 174.7 | 175.0 |
| 29 | 19.7 | 19.7 | 19.2 | 19.5 | 19.3 | 19.2 | 19.6 | 19.3 | 19.6 |
| 30 | 110.9 | 111.0 | 110.5 | 110.0 | 110.6 | 110.9 | 110.2 | 110.7 | 110.0 |
| OCH3 | - | - | 51.3 | 51.3 | - | 51.1 | 49.6 | 51.0 | 51.1 |
| 1′ | 94.3 | 95.8 | 95.3 | 95.3 | 95.4 | 95.4 | 95.3 | 95.4 | 95.4 |
| 2′ | 83.6 | 74.5 | 73.9 | 74.0 | 74.0 | 73.9 | 74.0 | 73.9 | 74.0 |
| 3′ | 78.6 | 77.5 | 77.9 | 78.0 | 78.0 | 77.9 | 78.1 | 78.2 | 78.1 |
| 4′ | 71.1 | 71.4 | 70.3 | 70.3 | 71.0 | 70.8 | 71.0 | 71.0 | 71.1 |
| 5′ | 79.2 | 78.8 | 78.4 | 78.4 | 78.4 | 78.0 | 78.5 | 78.4 | 78.5 |
| 6′ | 62.5 | 70.0 | 69.5 | 69.5 | 69.6 | 69.5 | 69.5 | 69.6 | 69.6 |
| 1′′ | 107.1 | 105.6 | 105.0 | 105.1 | 105.1 | 105.1 | 105.1 | 105.1 | 105.1 |
| 2′′ | 76.8 | 75.4 | 74.0 | 74.1 | 74.1 | 74.0 | 74.1 | 74.1 | 74.1 |
| 3′′ | 78.5 | 78.4 | 77.0 | 77.0 | 77.1 | 77.1 | 77.1 | 77.1 | 77.2 |
| 4′′ | 71.8 | 76.9 | 76.4 | 76.5 | 76.5 | 76.3 | 76.5 | 76.4 | 76.5 |
| 5′′ | 79.7 | 79.1 | 78.6 | 78.7 | 78.7 | 78.6 | 78.7 | 78.7 | 78.7 |
| 6′′ | 62.4 | 61.8 | 61.3 | 61.4 | 61.4 | 61.1 | 61.4 | 61.4 | 61.4 |
| 1‴ | - | 103.2 | 102.6 | 102.7 | 102.7 | 102.6 | 102.7 | 102.7 | 102.8 |
| 2‴ | - | 72.9 | 72.5 | 72.5 | 72.5 | 72.5 | 72.5 | 72.5 | 72.5 |
| 3‴ | - | 73.1 | 72.7 | 72.7 | 72.7 | 72.7 | 72.7 | 72.7 | 72.8 |
| 4‴ | - | 74.4 | 75.2 | 75.3 | 75.2 | 75.2 | 75.3 | 75.2 | 75.3 |
| 5‴ | - | 70.7 | 70.9 | 71.0 | 70.3 | 70.2 | 70.3 | 70.3 | 70.3 |
| 6‴ | - | 18.9 | 18.4 | 18.5 | 18.4 | 18.5 | 18.4 | 18.4 | 18.5 |
| 1⁗ | - | - | - | - | 69.5 | - | 69.4 | - | - |
| 2⁗ | - | - | - | - | 19.4 | - | 19.3 | - | - |
| 3⁗ | - | - | - | - | 13.7 | - | 13.7 | - | - |
| Acanthosessiliosides (No.) | MRM a | Time b | DP c | EP d | CEP e | CE f | CXP g |
|---|---|---|---|---|---|---|---|
| G (1) | 809.364/467.4 | 150 | –55 | –10.5 | –36 | –46 | –6 |
| H (2) | 955.436/485.4 | 150 | –60 | –11 | –42 | –50 | –6 |
| I (3) | 969.464/499.4 | 150 | –70 | –10 | –26 | –48 | –6 |
| J (5) | 953.473/483.3 | 150 | –75 | –10 | –42 | –56 | –8 |
| K (7) | 1011.509/541.5 | 150 | –110 | –10.5 | –28 | –44 | –6 |
| L (8) | 969.431/499.4 | 150 | –75 | –9.5 | –38 | –52 | –6 |
| M (9) | 1027.45/557.4 | 150 | –75 | –10.5 | –42 | –54 | –8 |
| N (10) | 1001.44/531.4 | 150 | –70 | –8.5 | –26 | –46 | –6 |
| O (11) | 1001.45/531.5 | 150 | –70 | –12 | –46 | –50 | –6 |
| Acanthosessilioside | Molecular Formula | MW | Measured Value a [M–H] | MS/MS Fragmentation a |
|---|---|---|---|---|
| G | C42H66O15 | 810.44 | 809.2 | 647.2 [M–H–Glc]–; 587.2 [M–Glu–C2H3O2]–; 485.2 [M–H–Glc–Glc]–; 467.4 [M–H–Glc–Glc–H2O]–; 423.0 [M–H–Glc–Glc–HCOO–H2O]–; 405.4 [M–H–Glc–Glc–HCOO– 2H2O]–; 179.2 [M–H–Glu–3,4-seco-Triterpenoid–C2H3O2–2C3H5–H2O–HCOO]–; 161.2[M–H–3,4-seco-Triterpenoid–Glu–C2H3O2–2C3H5–2H2O-HCOO]– |
| H | C48H76O19 | 956.50 | 955.6 | 485.4 [M–H–Rha–Glc–Glc]–; 469.4 [M–H–Rha–Glc–Glc–H2O]–; 439.4 [M–H–Rha–Glc–Glc–HCOO]–; 423.4 [M–H–Rha–Glc–Glc–HCOO–H2O]–; 405.2 [M–H–Rha–Glc–Glc–HCOO–2H2O]–; 367.0 [M–H–Rha–Glc–Glc–HCOO–H2O–2C3H5]–; 325.0 [M–H–Rha-3,4-seco-Triterpenoid–C2H3O2–H2O–2C3H5–HCOO]–; 161.2 [M–H–Rha–Glu–3,4-seco-Triterpenoid – C2H3O2–H2O–2C3H5–HCOO]– |
| I | C49H78O19 | 970.51 | 969.6 | 808.0 [M–H–Rha]–; 499.4 [M–H–Rha–Glc–Glc]–; 469.2 [M–H–3,4-seco-Triterpenoid –C3H5O2–2C3H5–H2O–HCOO]–; 455.0 [M–H–Rha–Glc–Glc–HCOO]–; 365.4 [M–H–Rha–Glc–Glc–HCOO–C3H5O2–H2O]–; 325.0 [M–H–Rha–3,4-seco-Triterpenoid–C3H5O2–2C3H5–H2O–HCOO]–; 160.6 [M–H–Rha–Glu–3,4-seco-Triterpenoid–C3H5O2–2C3H5–2H2O–HCOO]– |
| J | C49H78O18 | 954.52 | 953.6 | 483.2 [M–H–Rha–Glc–Glc]–; 469.2 [M–H–Rha–Glc–Glc–H2O]–; 409.2 [M–H–Rha–Glc–Glc–HCOO–C3H6O2]–; 367.2 [M–H–Rha–Glc–Glc–HCOO–C3H6O2–C3H5]–; 323.4 [M–H–Rha–Glc–Glc–HCOO–C3H6O2–C3H5–HCOO]-; 160.8 [M–H–Rha–Glu–3,4-seco-Triterpenoid–C3H5O2–2C3H5–HCOO–H2O]– |
| K | C51H80O20 | 1012.52 | 1011.5 | 849.6 [M–H–Rha–H2O]–; 541.4 [M–H–Rha–Glc–Glc]–; 479.6 [M–H–Rha–Glu–Glu–HCOO–H2O]–; 469.2 [M–H–3,4-seco-Triterpenoid–HCOO]–; 454.8 [M–H–Rha–Glu–Glu–HCOO–C3H5]–; 405.4 [M–H–Rha–Glu–Glu–C2H2O2–C3H7O–H2O]–; 364.8 [M–H–Rha–Glu–Glu–C2H2O2–C3H7O–H2O–C3H5]–; 325.0 [M–H–Rha–Glu–Glu–C2H2O2–C3H7O–H2O–2C3H5]–; 160.8 [M–H–Rha–Glu–3,4-seco-Triterpenoid–C2H2O2–2H2O–2C3H5–C3H7O–HCOO]– |
| L | C49H78O19 | 970.51 | 969.6 | 499.4 [M–H–Rha–Glc–Glc]–; 469.2 [M–H–3,4-seco-Triterpenoid–HCOO]–; 323.0[M–H–Rha–3,4-seco-Triterpenoid–C3H5O2–C3H5–HCOO]–;160.8[M–H–Rha–Glu–3,4-seco-Triterpenoid–C3H5O2–C3H5–HCOO]– |
| M | C52H84O20 | 1028.56 | 1027.6 | 557.4 [M–H–Rha–Glc–Glc]–; 468.8 [M–H–3,4-seco-Triterpenoid]–; 367.2 [M–H–Rha–Glc–Glc–C3H6O2–C3H8O–C3H6]–; 323.2 [M–H–Rha–3,4-seco-Triterpenoid–C3H5O2–C3H7O–C3H5–H2O–HCOO]–; 161.2 [M–H–Rha–Glu–3,4-seco-Triterpenoid–C3H5O2–C3H7O–C3H5–H2O–HCOO]- |
| N | C49H78O21 | 1002.50 | 1001.6 | 531.2 [M–H–Rha–Glc–Glc]–; 486.8 [M–H–Rha–Glc–Glc–HCOO]–; 469.2[M–H–Rha–Glu–Glu–HCOO–2H2O]–; 437.2 [M–H–Rha–Glu–Glu–2H2O–C2H3O2]–; 393.4 [M–H–Rha–Glu–Glu–HCOO–H2O–C2H3O2]–; 379.4 [M–H–Rha–Glu–Glu–HCOO–2H2O–C3H5O2]–; 367.4 [M–H–Rha–3,4-seco-Triterpenoid–2H2O–C3H5O2]–; 325.2 [M–H–3,4-seco-Triterpenoid–2H2O–C3H5O2–HCOO–Rha]–; 161.0[M–H–Rha–Glu–3,4-seco-Triterpenoid–2H2O–C3H5O2–HCOO]– |
| O | C49H78O21 | 1002.50 | 1001.6 | 531.4 [M–H–Rha–Glc–Glc]–; 469.0 [M–H–3,4-seco-Triterpenoid–C3H5O2–2H2O–2C3H5]–; 437.4 [M–H–Rha–Glc–Glc–C2H3O2–2H2O]–; 379.2 [M–H–Rha–Glu–Glu–C3H5O2–2H2O–HCOO]–; 367.2[M–H–Rha–3,4-seco-Triterpenoid–C3H5O2–3H2O–2C3H5]–; 325.2[M–H–Rha–3,4-seco-Triterpenoid–C3H5O2–3H2O–2C3H5–HCOO]−; 161.2[M–H–Rha–Glu–3,4-seco-Triterpenoid–C3H5O2–4H2O–2C3H5–HCOO]– |
| Compounds | Rt b (min) | Calibration Curve c | R2 d | Linear Range (μg/mL) | LOD e (ppm) | LOQ f (ppm) | Amount (mg/g) |
|---|---|---|---|---|---|---|---|
| 1 | 6.20 | y = 27.8x − 952 | 0.9988 | 0.1–2.5 | 0.0117 | 0.0357 | 0.010 |
| 2 | 6.20 | y = 161x − 2.31 × 104 | 0.9974 | 0.1–2.5 | 0.0028 | 0.0086 | 2.806 |
| 3 | 9.68 | y = 146x − 5.45 × 103 | 0.9982 | 0.1–2.5 | 0.0025 | 0.0077 | 0.059 |
| 4 | 7.77 | y = 198x − 1.46 × 104 | 0.9989 | 0.1–2.5 | 0.0014 | 0.0044 | 0.43 |
| 5 | 11.49 | y = 203x − 1.99 × 104 | 0.9987 | 0.1–2.5 | 0.0016 | 0.0049 | 0.026 |
| 6 | 10.12 | y = 231x − 1.63e × 104 | 0.9954 | 0.1–2.5 | 0.0026 | 0.008 | 0.009 |
| 7 | 12.75 | y = 3.21x − 16.2 | 0.9998 | 0.05–2.5 | 0.0263 | 0.0799 | 0.023 |
| 8 | 9.19 | y = 41.7x − 1.64 × 103 | 0.9888 | 0.1–2.5 | 0.0237 | 0.0718 | 0.102 |
| 9 | 10.15 | y = 97.5x − 1.02 × 104 | 0.9965 | 0.1–2.5 | 0.0054 | 0.0165 | 0.017 |
| 10 | 4.67 | y = 65.1x − 6.4 × 103 | 0.9988 | 0.1–2.5 | 0.0048 | 0.0146 | 0.031 |
| 11 | 4.67 | y = 91.9x − 1.38 × 104 | 0.9958 | 0.1–2.5 | 0.0063 | 0.0193 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.-R.; Kim, H.-G.; Ko, W.; Dong, L.; Yoon, D.; Oh, S.M.; Lee, Y.-S.; Lee, D.-S.; Baek, N.-I.; Lee, D.Y. Noble 3,4-Seco-triterpenoid Glycosides from the Fruits of Acanthopanax sessiliflorus and Their Anti-Neuroinflammatory Effects. Antioxidants 2021, 10, 1334. https://doi.org/10.3390/antiox10091334
Choi B-R, Kim H-G, Ko W, Dong L, Yoon D, Oh SM, Lee Y-S, Lee D-S, Baek N-I, Lee DY. Noble 3,4-Seco-triterpenoid Glycosides from the Fruits of Acanthopanax sessiliflorus and Their Anti-Neuroinflammatory Effects. Antioxidants. 2021; 10(9):1334. https://doi.org/10.3390/antiox10091334
Chicago/Turabian StyleChoi, Bo-Ram, Hyoung-Geun Kim, Wonmin Ko, Linsha Dong, Dahye Yoon, Seon Min Oh, Young-Seob Lee, Dong-Sung Lee, Nam-In Baek, and Dae Young Lee. 2021. "Noble 3,4-Seco-triterpenoid Glycosides from the Fruits of Acanthopanax sessiliflorus and Their Anti-Neuroinflammatory Effects" Antioxidants 10, no. 9: 1334. https://doi.org/10.3390/antiox10091334
APA StyleChoi, B.-R., Kim, H.-G., Ko, W., Dong, L., Yoon, D., Oh, S. M., Lee, Y.-S., Lee, D.-S., Baek, N.-I., & Lee, D. Y. (2021). Noble 3,4-Seco-triterpenoid Glycosides from the Fruits of Acanthopanax sessiliflorus and Their Anti-Neuroinflammatory Effects. Antioxidants, 10(9), 1334. https://doi.org/10.3390/antiox10091334







