4. Discussion
This study is the first devoted to innovating a phytogenic feed additive using nanotechnology approaches in the livestock field. The development of encapsulation techniques facilitates the protection, as well as the controlled and targeted release, of bioactive molecules applied in the pharmaceutical, nutraceutical and food industries to improve their bioavailability (absorption and cellular intake) and to enhance the stability of bioactive compounds during processing and storage processes [
14,
15]. For this purpose, many natural polymers are used to encapsulate different bioactive components, including phytogenic crude extracts, used as natural-functioning nutritional supplements with health benefits [
17,
26]. This study used the nanoencapsulation ionic-gelation method for innovating a new feed additive that could be used in the rabbit industry during periods of heat stress, aiming at the phytochemicals of
Moringa leaf extracts, specifically polyphenols. Based on several previous studies, a
Moringa leaf extract has several phytogenic bioactive compounds with antioxidant, antimicrobial and immunomodulatory activities that can improve animals’ reproductive performance and health [
10,
11,
13].
In this study we used sodium alginate as a natural polymer for the nanoencapsulation process to ensure the safety of the final product to animal and human health. This natural polymer has been used as an efficient coating material with efficient protection ability due to its high encapsulation efficiency for active components of plant extracts, mainly phenolic compounds [
27]. Alginate is a natural anionic polyelectrolyte polymer that encompasses unbranched binary copolymers of (1–4) linked D-mannuronic acid (M) and L-guluronic acid (G) residues with widely varying composition. Alginate is extracted from different species of brown algae (
Phaeophyceae). Based on the unique physicochemical properties (biodegradability, biocompatibility and capability of forming three-dimensional gels in the presence of divalent cations such as CaCl2), low cost and simplicity of use, this polymer is considered one of the suitable choice materials for the encapsulation process [
18].
The encapsulation technique used in this study was efficient to entrap approximately 57% of the bioactive compounds of the
Moringa leaf extract, as indicated by the encapsulation efficiency of the
Moringa extract phenolic compounds. The ionic-gelation method is an efficient and low-cost encapsulation technique that does not require specialized equipment, high temperature or organic solvents, making it suitable for encapsulating hydrophobic or hydrophilic compounds[
15].
In this study, the conjugation of MLEE with alginate–CaCl
2 nanoparticles allowed an 80% reduction in dose with satisfactory positive effects on the reproductive performance of female rabbits. These results agree with those obtained in previous studies, aiming to encapsulate active phytogenic compounds, mainly polyphenols. This method was rapid and easily adapted to the industrial scale [
28]. For example, the aqueous leaf extract of
Stevia rebaudiana Bertoni, which is rich in phenolic compounds, was successfully encapsulated in CaCl
2 beads and showed high encapsulation efficiency (>60%) [
18,
28] values, as well as satisfactory antioxidant storage stability. Furthermore, Calvo et al. [
29] found that using alginate–CaCl
2 as a nanocarrier for antioxidant compounds (betacyanin and polyphenols) derived from beetroot industrial wastes was efficient in encapsulating (between 20% and 40%) these active components, with good conservation of the antioxidant activity (up to 70%).
These findings support the relevance of the technique used to fabricate MLEE-conjugated alginate–CaCl2 nanoparticles with physicochemical characteristics (<100 nm), allowing better bioavailability for target sites.
For oral delivery into the GIT (
oral pathway), particle uptake in the GIT depends on diffusion and accessibility through mucus and contact with the cells of the GIT. The smaller particle diameter is fast diffused through GIT mucus to reach intestinal lining cells, followed by uptake through the GIT barrier to reach the blood [
30]. Existing evidence indicates that particles smaller than 100 nm are absorbed in various tissues and organs. Smaller particles capable of being taken up by the villus epithelium may directly enter the bloodstream and then be predominantly scavenged by the liver and the spleen [
31].
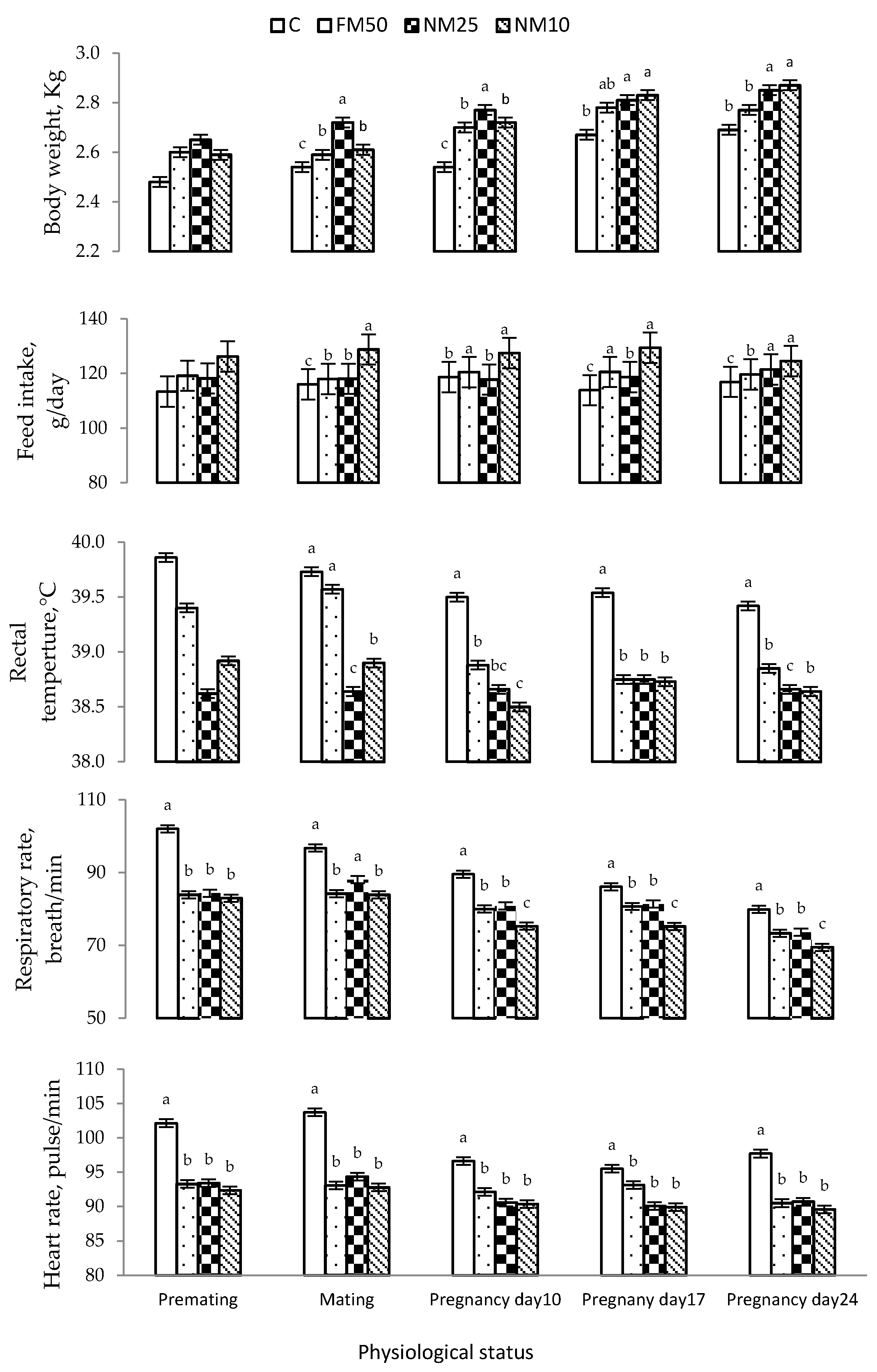
Heat stress induces various biological reactions and behavioral changes to cope with high ambient temperature and maintain thermal homeostasis [
3,
14]. Under heat stress, female rabbits express a high respiratory rate and water intake and low feed intake as adaptive mechanisms for high ambient temperature, which may negatively affect rabbit does’ reproductive performance if maintained for a long time [
32,
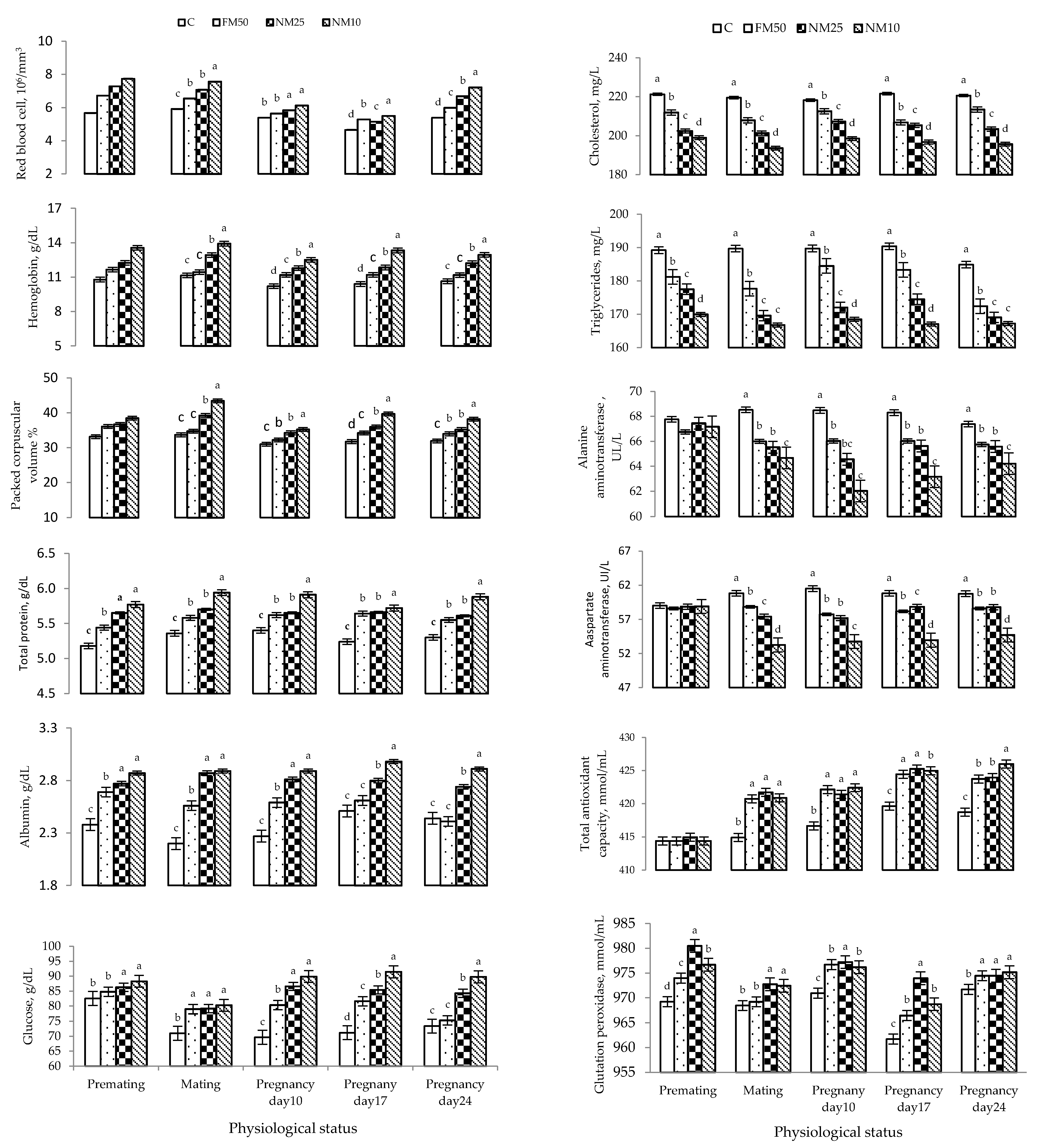
33]. For example, exposure of New Zealand rabbits to 41 °C led to an 18% decrease in RBC count; 20%, hemoglobin content; 22%, blood platelet count; 11.2%, total protein; 24%, albumin, and 21%, globulin [
4]. Under intensive production systems, as in most rabbit farms, these biological and behavioral responses could be more challenging when animals are housed in cages rather than in natural environments [
34]. Furthermore, heat stress may be a threat to females, specifically during sensitive reproductive windows, such as mating and pregnancy periods. Female rabbits are sensitive to heat stress, which is considered an important factor influencing their reproduction, fertility and physiological traits [
4,
35]. Overall, heat stress and accompanying elevated oxidative stress increase the risk of spontaneous abortion and reduce milk production; litter size and litter performance; and the longevity, welfare, and health status of females.
This study was conducted during the summer, when THI was 29.20, which is classified as severe heat stress for rabbits. Notably, MLEE significantly reduced the rectal temperature of female rabbits in the treated groups. Treatment with MLEE in either free or encapsulated form around mating time and pregnancy reduced heat-stress-related indicators, such as rectal temperatures, respiratory rates and heart rates. It also improved hematochemical attributes (RBC, Hb, PCV, total protein, albumin and glucose), redox status (TAC and GSH-Px) and hormones (progesterone and prolactin) and decreased cholesterol, triglycerides, ALT and AST, suggesting an improvement in the heat tolerance of the animals. These findings support the protective role of MLEE against the negative impacts of heat stress. Several mechanisms could mediate these effects, which could be due to several biologically active phytogenics in MLEE [
11,
13,
36]. Notably, the enhancements in physiological events in MLEE-treated groups were not associated with low feed intake as one of the adaptive behavioral mechanisms for heat stress. This effect is essential for animals during their reproductive cycles, especially mating and lactation, to maintain adequate performance. Under heat stress, animals decrease feed intake to reduce metabolic heat production, leading to changes in energy balance and nutrient availability and affecting reproductive cyclicity, pregnancy and fetal development [
37,
38]. Given that active components of MLEE, N-acetylneuraminic acid 2,3-dehydro-2-deoxy- (6.50%) were identified in our extract, this component is a sialylated glycoprotein that has prebiotic properties, promotes neurodevelopment and boosts immune function and gut maturation [
39,
40]. Additionally, our MLEE has many active components with antimicrobial/anticoccidial activity, including salinomycin [
41], 2- furoic acid [
42], 9S,11,15 S-trihydroxythrombox-13E-enoic acid [
43], rifabutin [
44] and lactacystin [
45]. Furthermore, active components can be used directly as valuable nutrients or to improve digestion (glycine, aminomethyl propanol, glycan le-a trisaccharide, N-acetyl leucyl-leucyl-methioninal and deoxycholic acid) or boost the energy status of animals (adenosine 5′-diphosphate and thiocyanic acid, L-arabinitol and D-gluconic acid) [
46,
47,
48,
49]. These components in MLEE explain the positive effects of the extract on digestion, the gut-intestinal microbiota ecosystem, nutrient availability and thus, the maintenance of the body weight of MLEE-treated does during pregnancy. These findings agree with that previously reported for
Moringa plants having several nutrients that stimulate growth and increase the bioavailability of the nutrients and feed use, such as high-quality protein, vitamins, minerals, antioxidants and cytokine-type hormone antioxidant components, particularly vitamin C [
12,
13,
50].
In this study, compared with the control, all MLEE-based treatments significantly increased hematological variables, including RBC and hemoglobin. The administration of MLEE improved redox status (higher TAC and GSH-Px), metabolism (higher energy-yielding nutrients: glucose; protein: total protein and albumin) and liver functions (ALT and AST) of does during mating and pregnancy.
Hemoglobin plays a vital role in carrying approximately 98% of oxygen throughout the animal body system, whereas PCV measures the proportion of blood made up of cells [
51]. These findings might explain the improved heat-stress tolerance and maintain the rectal temperature of MLLE-treated does, without changes in respiration rates and heart rates by improving the oxygen delivery to different organs [
4]. Furthermore, improved metabolism and the redox status of MLEE-treated does play vital roles in maintaining homeothermy during heat stress through different mechanisms. For example, albumin has osmoprotective properties in plasma, which regulates water balance and maintains protein and enzyme stability [
52]. Moreover, glucose availability provides an easy energy source to all body cells without the need for sophisticated catabolic and/or anabolic processes that can be combined with the rise of heat, thus increasing heat stress [
11]. The enhancements of the hematobiochemical attributes and redox status of MLEE-treated does could be attributed to the presence of some specific active components that stimulate the synthesis of some proteins/enzymes. For example, the increase in erythrocytes and hemoglobin concentration may be related to the effect of amino acids, vitamins [
36,
52] and minerals, particularly iron. Furthermore, a compound, such as ethidimuron, identified in our MLEE, acts as a precursor of intracellular glutathione. This is in line with some components’ free radical scavenging activity, such as diapocynin [
53]. These results explain the improved redox status of MLEE-treated does in our study.
Rabbit does are sensitive to heat stress, which is considered to be an important factor influencing their fertility and has negative effects on their reproductive and physiological traits [
3,
32,
54,
55]. In hot climates, the breeding of rabbits is stopped in most rabbit farms due to low reproductive performance and associated health problems. In this study, MLEE-treated does showed pronounced enhancements in sex hormones and reproductive performance compared with the control. These enhancements could be ascribed to the improved metabolism and health status of does around mating time and during pregnancy, as discussed earlier. In this context, some active components in MLEE positively affect specific reproductive events and functions. Sphingolipids, such as phytosphingosine (assembling 10.46% in our MLEE), are signaling molecules that regulate various physiological activities. In a study on pigs, exogenous phytosphingosine-1-phosphate (P1P) administration influenced animal reproduction by increasing porcine oocyte maturation and preimplantation embryo development through the regulation of oxidative stress and apoptosis signaling pathways [
56]. P1P treatment upregulated the gene expression involved in cumulus expansion (Has2 and epidermal growth factor), antioxidant enzymes (superoxide dismutase and catalase) and developmental competence (octamer-binding transcription factor 4 (Oct4)) while activating extracellular signal-regulated kinase1/2 and (serine/threonine) protein kinase B signaling. P1P treatment also influenced oocyte survival by shifting the ratio of B-cell lymphoma 2 to Bax while inactivating (C-Jun N-terminal kinase) signaling. Other components, such as phosphatidylethanolamine (phospholipid depravities) and palmitic acid, play a vital role in cell membrane integrity. They act as structural components in a bilayer cell membrane [
57,
58]. Furthermore, nailamaid with an antidepressant activity[
59]; folinic acid with antianemia, anticancer, anti-inflammatory, antitoxic activities[
60]; and leukotriene E4 methyl ester [
61] with anti-inflammatory and immunomodulatory effects were identified in MLEE. These components might attenuate the harmful effects of some inflammatory factors’ production, such as histamine and prostaglandins, which might negatively affect reproductive performance [
62,
63,
64]. These could be confirmed in our study, as kindling rates and litter sizes were significantly higher in MLEE-treated does. The enhancements in these two variables reflect the enhancement in oocyte quality, the fertilization rate and embryo survival through improved cell membrane integrity.
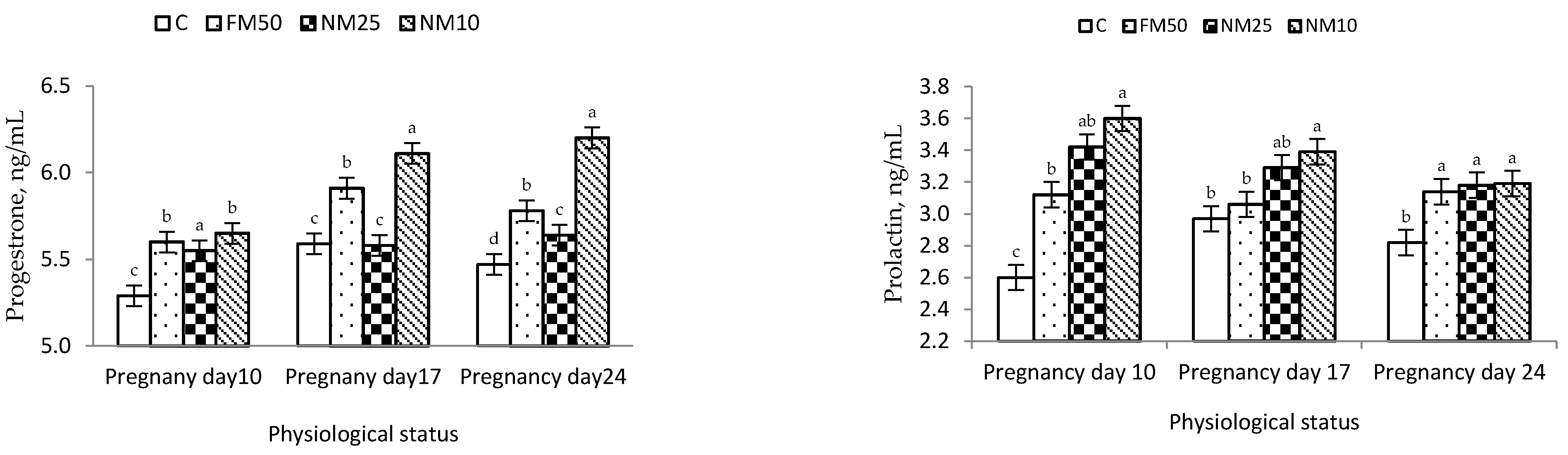
Finally, MLEE exerted positive effects on progesterone and prolactin levels during pregnancy. These results partially follow those obtained by Ajuogu et al. [
65]. The addition of a 15 mg/kg leaf powder of
Moringa significantly improved progesterone concentrations but did not affect prolactin concentrations during pregnancy. The authors suggested that
Moringa leaves may contain active components that can directly act on the uterus and ovary, interfering with the release of prostaglandins [
65]. The high level of progesterone during the first half of pregnancy plays a crucial role in preparing the uterus for embryo implantation by suppressing uterine motility/contractions during the first few days of pregnancy, thus increasing the opportunity for pregnancy maintenance [
24]. In rabbits, the levels of prolactin hormone increase from 3–4 d of gestation and remain elevated for 15–21 d of gestation. Such an increase is essential to maintain adequate concentrations of progesterone during pregnancy [
66].
Notably, the weights of litters born for encapsulated MLEE-treated does were higher than those recorded for control and nonencapsulated MLEE-treated does. This may refer to the higher bioavailability of MLEE components to fetuses through improved transfer via the placenta, as nanoencapsulation may facilitate the transfer of active components, specifically those with high molecular weight and low solubility, through the fetal–placental circulating system[
67].
It is worth note that the low dose of nanoencapsulated MLEE (NM
10) resulted in better responses, as indicated by most determined variables, than the high dose of nanoencapsulated MLEE (NM
25). This could be due to the improved bioavailability of some active components, which, though it is an advantage of the nanoencapsulation process may interfere with the competence of some biological processes. For example, MLEE is rich with phthalate, which has endocrine-disrupting effects [
11]. Moreover, increased antioxidant nutrients availability, mainly phenolic compounds and vitamins, can act as prooxidants by increasing oxidative stress [
6,
68].