Methyl Jasmonate Protects the PS II System by Maintaining the Stability of Chloroplast D1 Protein and Accelerating Enzymatic Antioxidants in Heat-Stressed Wheat Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement of Reactive Oxygen Species Content and Lipid Peroxidation
2.2. Assay of Antioxidant Enzymes Activities
2.3. Protein and Pigment Analysis
2.4. Chlorophyll a Fluorescence Measurement
2.5. Analysis of OJIP Chlorophyll a Fluroscense Transient
2.6. Photosynthetic and Growth Parameters
2.7. Western Blot Analysis
2.8. Quantitative RT-PCR Analysis
2.9. Statistical Analysis
3. Results
3.1. Screening of MeJA Concentration for Protection of Plants against Heat-Induced Oxidative Stress
3.2. MeJA Enhanced Antioxidant Enzymes Activity and Reduced Oxidative Damage under Heat Stress
3.3. Pigments and Protein Content
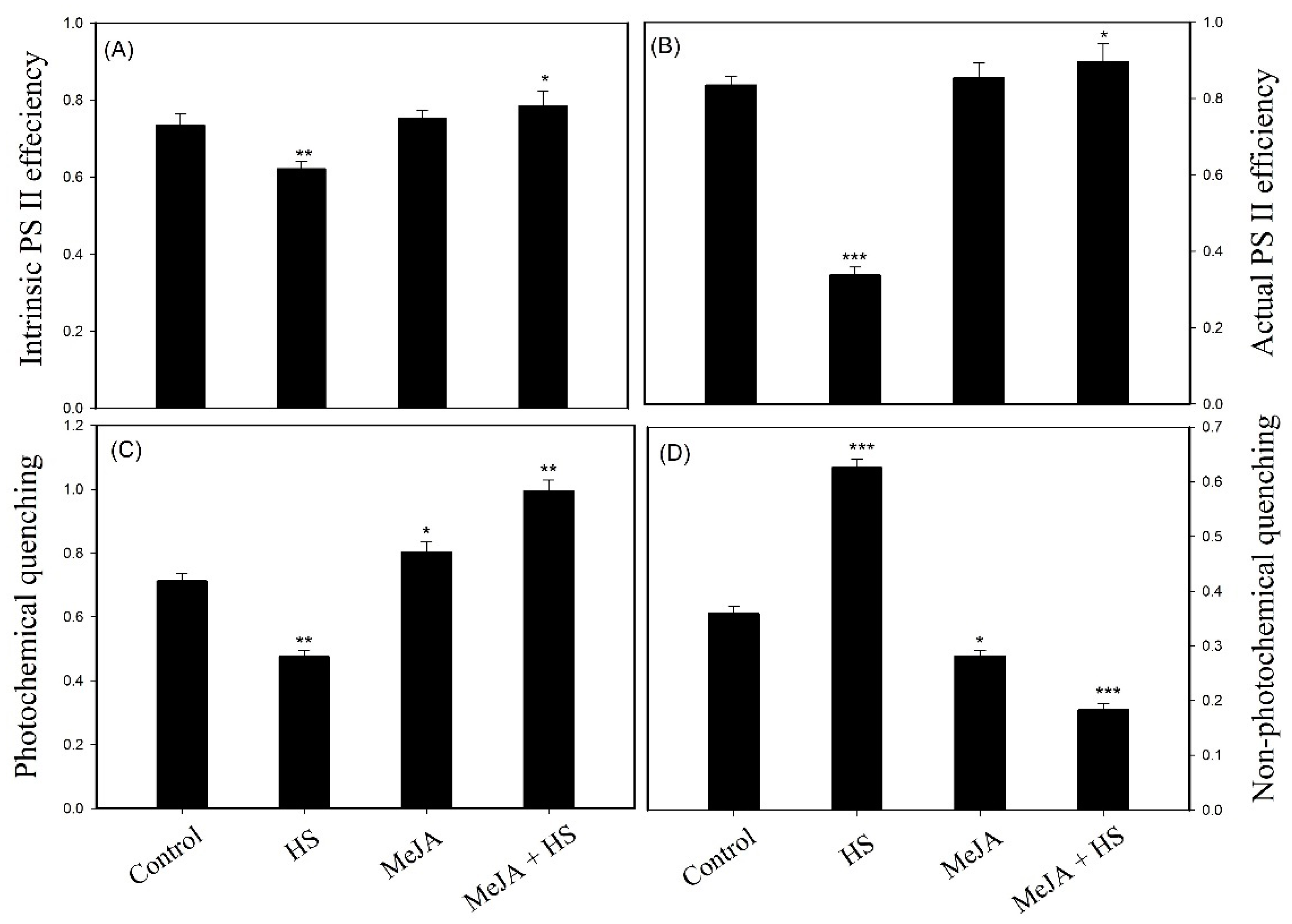
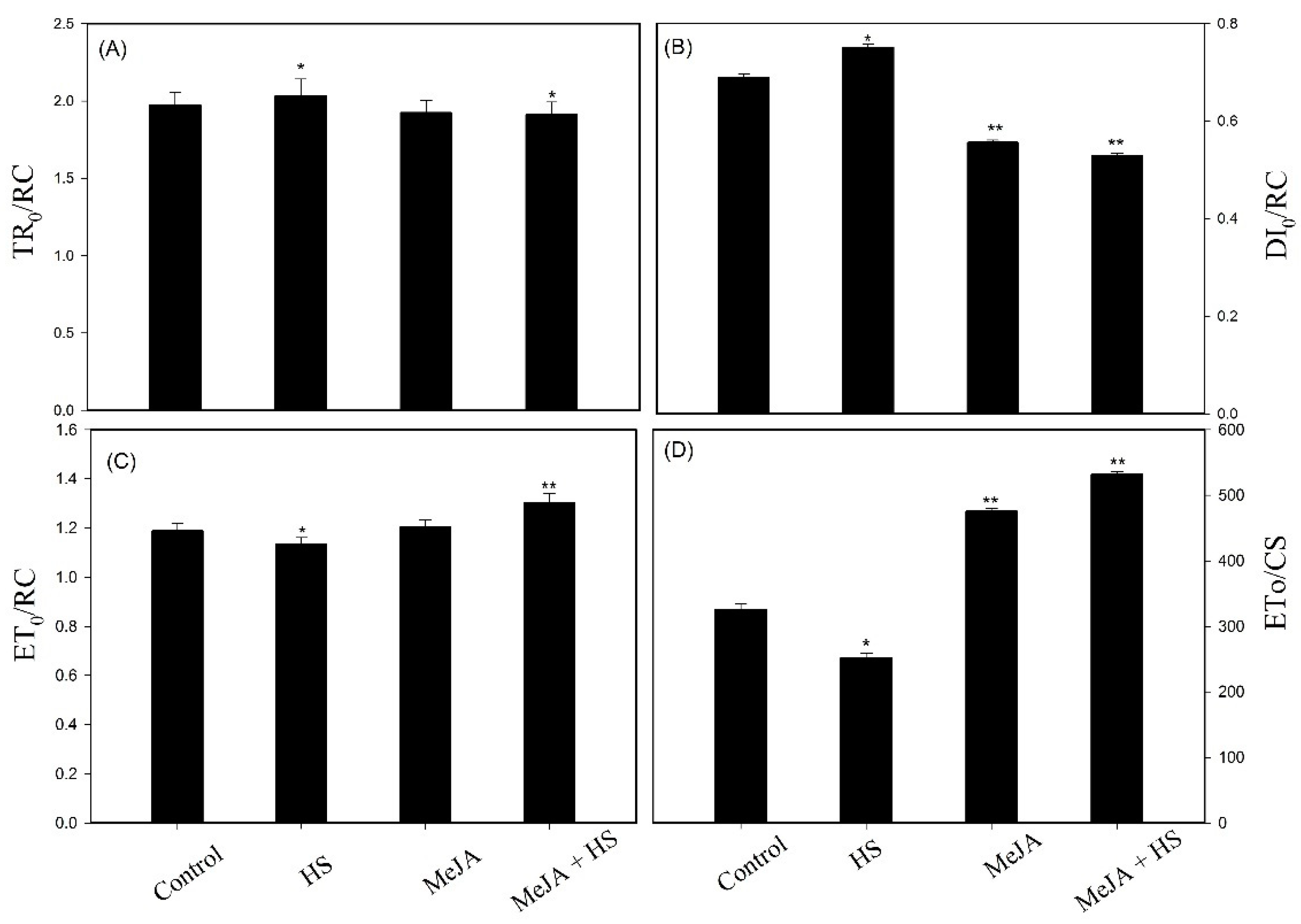
3.4. Influence of MeJA on Chlorophyll a Fluorescence
3.5. Impact of MeJA on Photosynthesis and Growth under Heat Stress or without Stress
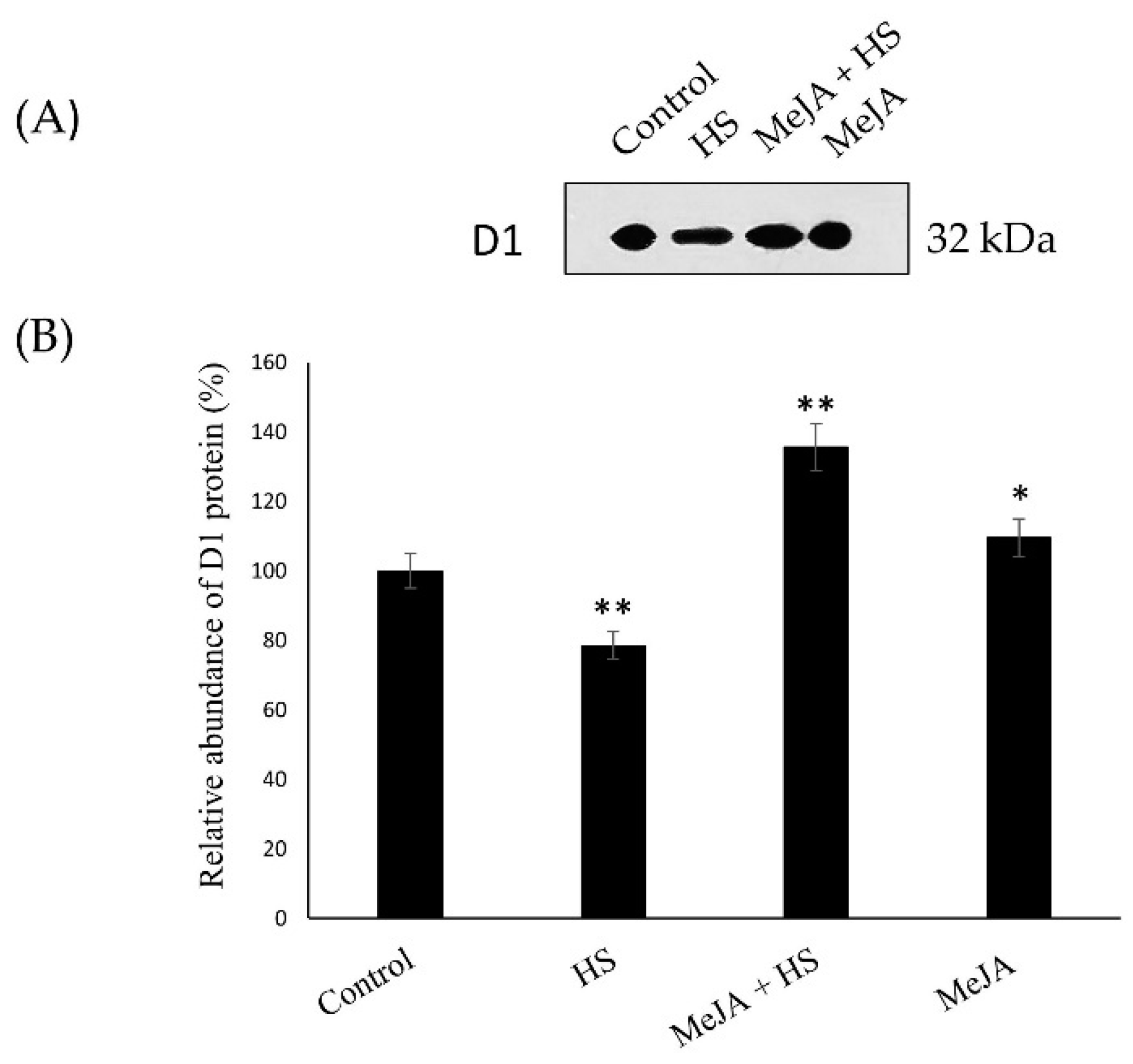
3.6. Effect of MeJA on D1 Protein Content Abundance and Gene Expression Relevant to the Photosynthetic System
4. Discussion
4.1. MeJA Increases Antioxidant System Activity to Mitigate the Oxidative Damage Induced by Heat Stress
4.2. MeJA Improved the Photosynthetic Efficiency under Heat Stress
4.3. MeJA Increases D1 Protein Content and Gene Expression Relevant to the Photosynthetic System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poudel, P.B.; Poudel, M.R. Heat stress effects and tolerance in wheat: A Review. J. Biol. Today World 2020, 9, 1–6. [Google Scholar]
- Zhao, H.J.; Zhao, X.J.; Ma, P.F.; Wang, Y.X.; Hu, W.W.; Li, L.H.; Zhao, Y.D. Effects of salicylic acid on protein kinase activity and chloroplast D1 protein degradation in wheat leaves subjected to heat and high light stress. Acta Ecol. Sin. 2011, 31, 259–263. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Nitric oxide and hydrogen sulfide coordinately reduce glucose sensitivity and decrease oxidative stress via ascorbate-glutathione cycle in heat-stressed wheat (Triticum aestivum L.) plants. Antioxidants 2021, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.H.; Chen, S.T.; He, N.Y.; Wang, Q.L.; Zhao, Y.; Gao, W.; Guo, F.Q. Nuclear-encoded synthesis of the D1 subunit of photosystem II increases photosynthetic efficiency and crop yield. Nat. Plants 2020, 6, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, T.; Amombo, E.; Wang, G.; Xie, Y.; Fu, J. The alleviation of heat damage to photosystem II and enzymatic antioxidants by exogenous spermidine in tall fescue. Front. Plant Sci. 2017, 8, 1747. [Google Scholar] [CrossRef] [Green Version]
- Bethmann, S.; Melzer, M.; Schwarz, N.; Jahns, P. The zeaxanthin epoxidase is degraded along with the D1 protein during photoinhibition of photosystem II. Plant Direct 2019, 3, e00185. [Google Scholar] [CrossRef] [Green Version]
- Per, T.S.; Khan, N.A.; Masood, A.; Fatma, M. Methyl jasmonate alleviates cadmium-induced photosynthetic damages through increased S-assimilation and glutathione production in mustard. Front. Plant Sci. 2016, 7, 1933. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.E.; Mao, H.T.; Wu, N.; Mohi Ud Din, A.; Khan, A.; Zhang, H.Y.; Yuan, S. Salicylic acid protects photosystem II by alleviating photoinhibition in Arabidopsis thaliana under high light. Int. J. Mol. Sci. 2020, 21, 1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahan, B.; Iqbal, N.; Fatma, M.; Sehar, Z.; Masood, A.; Sofo, A.; D’Ippolito, I.; Khan, N.A. Ethylene supplementation combined with split application of nitrogen and sulfur protects salt-inhibited photosynthesis through optimization of proline metabolism and antioxidant system in mustard (Brassica juncea L.). Plants 2021, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Huang, Y.; Dong, X.; Wang, R.; Tang, M.; Cai, J.; Chen, J.; Zhang, X.; Nie, G. Exogenous methyl jasmonate improves heat tolerance of perennial ryegrass through alteration of osmotic adjustment, antioxidant defense, and expression of jasmonic acid-responsive genes. Front. Plant Sci. 2021, 12, 664519. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Yi, X.Y.; Li, Y.T. Effect of pretreatment with hydrogen sulfide donor sodium hydrosulfide on heat tolerance in relation to antioxidant system in maize (Zea mays) seedlings. Biologia 2014, 69, 1001–1009. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Zhou, J.; Zhou, Y.H.; Yu, J.Q. Role of hormones in plant adaptation to heat stress. In Plant Hormones Under Challenging Environmental Factors, 1st ed.; Ahammed, G.J., Yu, J.Q., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–21. [Google Scholar]
- Lang, D.; Yu, X.; Jia, X.; Li, Z.; Zhang, X. Methyl jasmonate improves metabolism and growth of NaCl-stressed Glycyrrhiza uralensis seedlings. Sci. Horticul. 2020, 266, 109287. [Google Scholar] [CrossRef]
- Sirhindi, G.; Mushtaq, R.; Gill, S.S.; Sharma, P.; Allah, E.F.A.; Ahmad, P. Jasmonic acid and methyl jasmonate modulate growth, photosynthetic activity and expression of photosystem II subunit genes in Brassica oleracea L. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Faghih, S.; Zarei, A.; Ghobadi, C. Positive effects of plant growth regulators on physiology responses of Fragaria × ananassa cv. “Camarosa” under salt stress. Int. J. Fruit Sci. 2019, 19, 104–114. [Google Scholar] [CrossRef]
- Maksymiec, W.; Wojcik, M.; Krupa, Z. Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and ascorbate. Chemosphere 2007, 66, 421–427. [Google Scholar] [CrossRef]
- Qiu, X.; Xu, Y.; Xiong, B.; Dai, L.; Huang, S.; Dong, T.; Sun, G.; Liao, L.; Deng, Q.; Wang, X.; et al. Effects of exogenous methyl jasmonate on the synthesis of endogenous jasmonates and the regulation of photosynthesis in citrus. Physiol. Plant. 2020, 170, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Attaran, E.; Major, I.T.; Cruz, J.A.; Rosa, B.A.; Koo, A.J.; Chen, J.; Howe, G.A. Temporal dynamics of growth and photosynthesis suppression in response to jasmonate signaling. Plant Physiol. 2014, 165, 1302–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demming-Adams, B.; Adams, W.; Mattoo, A. Photoprotection, Photoinhibition, Gene Regulation and Environment; Springer: Dordrecht, The Netherlands, 2006; pp. 1–382. [Google Scholar]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition, 2nd ed.; Commonwealth Agricultural Bureaux, Farnham Royal: Bucks, UK; Cambridge University Press: East Malling, UK, 1966; pp. 187–190. [Google Scholar]
- Bu, R.; Xie, J.; Yu, J.; Liao, W.; Xiao, X.; Lv, J. Autotoxicity in cucumber (Cucumis sativus L.) seedlings is alleviated by silicon through an increase in the activity of antioxidant enzymes and by mitigating lipid peroxidation. J. Plant Biol. 2016, 59, 247–250. [Google Scholar] [CrossRef]
- Okuda, T.; Matsuda, Y.; Yamanaka, A.; Sagisaka, S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Fatma, M.; Asgher, M.; Masood, A.; Khan, N.A. Excess sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environ. Exp. Bot. 2014, 107, 55–63. [Google Scholar] [CrossRef]
- Fatma, M.; Masood, A.; Per, T.S.; Khan, N.A. Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front. Plant Sci. 2016, 7, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatma, M.; Iqbal, N.; Gautam, H.; Sehar, Z.; Sofo, A.; D’Ippolito, I.; Khan, N.A. Ethylene and sulfur coordinately modulate the antioxidant system and ABA accumulation in mustard plants under salt stress. Plants 2021, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents. In Advances Photosynthesis Research, 1st ed.; Sysbesma, C., Ed.; Springer: Dordrecht, The Netherlands, 1984; Volume 2, pp. 9–12. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Demmig, B.; Björkman, O. Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta 1987, 171, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence, 1st ed.; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. [Google Scholar]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B 2011, 104, 236–257. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Riznichenko, G.Y.; Rubin, A.B. Modeling chlorophyll a fluorescence transient: Relation to photosynthesis. Biochemist 2014, 79, 291–323. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Sun, X.; Amombo, E.; Zhu, Q.; Zhao, Z.; Chen, L.; Fu, J. High correlation between thermotolerance and photosystem II activity in tall fescue. Photosynth. Res. 2014, 122, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Tsimilli-Michael, M.; Eggenberg, P.; Biro, B.; Köves-Pechy, K.; Vörös, I.; Strasser, R.J. Synergistic and antagonistic effects of arbuscular mycorrhizal fungi and Azospirillum and Rhizobium nitrogen-fixers on the photosynthetic activity of alfalfa, probed by the polyphasic chlorophyll a fluorescence transient OJIP. Appl. Soil Ecol. 2000, 15, 169–182. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, F.E.; Ghirardi, M.L.; Sopory, S.K.; Mehta, A.M.; Edelman, M.; Mattoo, A.K. A novel metabolic form of the 32kDa-D1 protein in the grana localized reaction center of photosystem II. J. Biol. Chem. 1990, 265, 15357–15360. [Google Scholar] [CrossRef]
- Chen, Y.; Gelfond, J.A.; McManus, L.M.; Shireman, P.K. Reproducibility of quantitative RT-PCR array in miRNA expression profiling and comparison with microarray analysis. BMC Genom. 2009, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.A.; Gill, R.A.; Islam, F.; Ali, B.; Liu, H.; Xu, J.; Zhou, W. Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front. Plant Sci. 2016, 7, 468. [Google Scholar] [CrossRef] [Green Version]
- Verma, G.; Srivastava, D.; Narayan, S.; Shirke, P.A.; Chakrabarty, D. Exogenous application of methyl jasmonate alleviates arsenic toxicity by modulating its uptake and translocation in rice. Ecotoxicol. Environ. Saf. 2020, 201, 110735. [Google Scholar] [CrossRef] [PubMed]
- Serna-Escolano, V.; Martínez-Romero, D.; Giménez, M.J.; Serrano, M.; García-Martínez, S.; Valero, D.; Valverde, J.M.; Zapata, P.J. Enhancing antioxidant systems by preharvest treatments with methyl jasmonate and salicylic acid leads to maintain lemon quality during cold storage. Food Chem. 2021, 338, 128044. [Google Scholar] [CrossRef] [PubMed]
- Zuñiga, P.E.; Castañeda, Y.; Arrey-Salas, O.; Fuentes, L.; Aburto, F.; Figueroa, C.R. Methyl jasmonate applications from flowering to ripe fruit stages of strawberry (Fragaria × ananassa “Camarosa”) reinforce the fruit antioxidant response at post-harvest. Front. Plant Sci. 2020, 11, 538. [Google Scholar] [CrossRef]
- Singh, A.K.; Rana, H.K.; Pandey, A.K. Analysis of chlorophylls. In Recent Advances in Natural Products Analysis, 1st ed.; Silva, A.S., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Tehran, Iran, 2020; pp. 635–650. [Google Scholar]
- Yamasato, A.; Nagata, N.; Tanaka, R.; Tanaka, A. The N-terminal domain of chlorophyllide a oxygenase confers protein instability in response to chlorophyll b accumulation in Arabidopsis. Plant Cell 2005, 17, 1585–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmar, P.; Kumari, N.; Sharma, V. Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot. Stud. 2013, 54, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Cotado, A.; Müller, M.; Morales, M.; Munné-Bosch, S. Linking jasmonates with pigment accumulation and photoprotection in a high-mountain endemic plant, Saxifraga longifolia. Environ. Exp. Bot. 2018, 154, 56–65. [Google Scholar] [CrossRef]
- Bi, A.; Fan, J.; Hu, Z.; Wang, G.; Amombo, E.; Fu, J.; Hu, T. Differential acclimation of enzymatic antioxidant metabolism and photosystem II photochemistry in tall fescue under drought and heat and the combined stresses. Front. Plant Sci. 2016, 7, 453. [Google Scholar] [CrossRef] [Green Version]
- Giorio, P.; Sellami, M.H. Polyphasic OKJIP Chlorophyll a fluorescence transient in a landrace and a commercial cultivar of sweet pepper (Capsicum annuum, L.) under long-term salt stress. Plants 2021, 10, 887. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.; Pathre, U.; Mohanty, P. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Strasser, A., Srivastava, A., Tsimilli-Michael, M., Eds.; Taylor and Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Fan, Y.; Liu, Z.; Zhang, F.; Zhao, Q.; Wei, Z.; Fu, Q.; Li, H. Tunable mid-infrared coherent perfect absorption in a graphene meta-surface. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Chen, L.; Fan, J.; Fu, J. Alleviation of heat damage to photosystem II by nitric oxide in tall fescue. Photosynth. Res. 2013, 116, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Aminaka, R.; Yoshioka, M.; Khatoon, M.; Komayama, K.; Takenaka, D.; Yamamoto, Y. Quality control of photosystem II: Impact of light and heat stresses. Photosynth. Res. 2008, 98, 589–608. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hori, H.; Kai, S.; Ishikawa, T.; Ohnishi, A.; Tsumura, N.; Morita, N. Quality control of Photosystem II: Reversible and irreversible protein aggregation decides the fate of Photosystem II under excessive illumination. Front. Plant Sci. 2013, 4, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kale, R.; Hebert, A.E.; Frankel, L.K.; Sallans, L.; Bricker, T.M.; Pospíšil, P. Amino acid oxidation of the D1 and D2 proteins by oxygen radicals during photoinhibition of Photosystem II. Proc. Natl. Acad. Sci. USA 2017, 114, 2988–2993. [Google Scholar] [CrossRef] [Green Version]
- Vani, B.; Saradhi, P.P.; Mohanty, P. Alteration in chloroplast structure and thylakoid membrane composition due to in vivo heat treatment of rice seedlings: Correlation with the functional changes. J. Plant Physiol. 2000, 158, 583–592. [Google Scholar] [CrossRef]
- Sehar, Z.; Iqbal, N.; Khan, M.I.R.; Masood, A.; Rehman, M.T.; Hussain, A.; Alajmi, M.F.; Ahmad, A.; Khan, N.A. Ethylene reduces glucose sensitivity and reverses photosynthetic repression through optimization of glutathione production in salt-stressed wheat (Triticum aestivum L.). Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bredenkamp, G.J.; Baker, N.R. Temperature-sensitivity of D1 protein metabolism in isolated Zea mays chloroplasts. Plant Cell Environ. 1994, 17, 205–210. [Google Scholar] [CrossRef]
- Adamiec, M.; Misztal, L.; Kosicka, E.; Paluch-Lubawa, E.; Luciński, R. Arabidopsis thaliana egy2 mutants display altered expression level of genes encoding crucial photosystem II proteins. J. Plant Physiol. 2018, 231, 155–167. [Google Scholar] [CrossRef] [PubMed]


| Gene | Encoded Polypeptide | Gene ID | Forward(F) /Reverse (R) | Primer Sequences (5′–3′) | Size (bp) |
|---|---|---|---|---|---|
| psbA | D1 protein | 7095419 | F R | GTATTTATTATCGCCTTCATCG AGGACGCATACCCAAACG | 284 |
| psbB | CP47 | 7095420 | F R | TAGGCGTAACGGTGGA AACATCTCGGAACAAGG | 254 |
| psbC | CP43 | 7095484 | F R | TAATACGGCTTATCCGAGTGAGTTT TCTTGCCAAGGTTGTATGTCTTT | 288 |
| Treatments | H2O2 Content (nmol g−1 Leaf FW) | Total Protein (mg g−1 Leaf FW) | Net Photosynthesis (µmol CO2 m−2 s−1) | Plant Fresh Weight (g Plant−1) |
|---|---|---|---|---|
| Control | 15.2 ± 0.89 | 8.70 ± 0.86 | 16.8 ± 0.82 | 3.85 ± 0.12 |
| HS | 33.4 ± 0.98 ** | 3.60 ± 0.42 ** | 10.9 ± 0.56 ** | 1.55 ± 0.06 ** |
| 5 µM MeJA | 10.6 ± 0.76 * | 9.60 ± 0.88 | 18.1 ± 0.93 | 4.07 ± 0.11 |
| 10 µM MeJA | 07.8 ± 0.56 ** | 10.8 ± 1.21 * | 20.8 ± 0.96 ** | 4.83 ± 0.19 * |
| 20 µM MeJA | 19.3 ± 0.84 * | 5.84 ± 0.65 ** | 11.2 ± 0.64 ** | 2.83 ± 0.08 * |
| 5 µM MeJA + HS | 17.2 ± 0.73 | 6.65 ± 0.73 * | 13.1 ± 0.71 * | 3.79 ± 0.09 |
| 10 µM MeJA + HS | 06.2 ± 0.66 ** | 11.8 ± 1.42 ** | 23.1 ± 0.97 *** | 5.11 ± 0.22 ** |
| 20 µM MeJA+ HS | 40.1 ± 1.02 ** | 2.73 ± 0.13 ** | 09.2 ± 0.54 *** | 1.29 ± 0.05 ** |
| Treatments | ||||
|---|---|---|---|---|
| Parameters | Control | HS | MeJA | MeJA + HS |
| Production rate of O2− (µmol g FW−1 min−1) | 0.801 ± 0.05 | 1.118 ± 0.080 ** | 0.611 ± 0.060 * | 0.451 ± 0.02 ** |
| H2O2 content (nmol g−1 leaf FW) | 35.60 ± 1.60 | 86.80 ± 02.40 *** | 31.10 ± 01.1 * | 20.50 ± 0.09 ** |
| TBARS content (nmol g−1 leaf FW) | 08.2 ± 0.12 | 25.3 ± 0.19 ** | 07.2 ± 0.09 * | 04.1 ± 0.07 ** |
| CAT activity (U mg−1 protein min−1) | 119 ± 3.70 | 144 ± 4.00 * | 210 ± 4.30 ** | 222 ± 5.10 ** |
| SOD activity (U mg−1 protein min−1) | 05.34 ± 0.08 | 07.66 ± 0.11 * | 10.62 ± 0.18 ** | 11.5 ± 0.21 ** |
| APX activity (U mg−1 protein min−1) | 1.12 ± 0.04 | 1.57 ± 0.09 * | 2.58 ± 0.11 ** | 2.72 ± 0.11 *** |
| GR activity (U mg−1 protein min−1) | 0.197 ± 0.005 | 0.231 ± 0.008 * | 0.288 ± 0.009 ** | 0.318 ± 0.01 *** |
| Treatments | ||||
|---|---|---|---|---|
| Parameters | Control | HS | MeJA | MeJA + HS |
| Chl a (mg g−1 Leaf FW) | 1.71 ± 0.06 | 1.26 ± 0.04 ** | 1.75 ± 0.06 * | 1.92 ± 0.08 ** |
| Chl b (mg g−1 Leaf FW) | 0.45 ± 0.01 | 0.37 ± 0.01 ** | 0.46 ± 0.02 | 0.47 ± 0.04 * |
| Chl (a/b) | 3.79 ± 1.70 | 3.38 ± 1.56 ** | 3.80 ± 1.73 | 4.05 ± 1.77 ** |
| Total chl (mg g−1 Leaf FW) | 2.15 ± 0.07 | 1.62 ± 0.04 ** | 2.21 ± 0.07 * | 2.39 ± 0.08 ** |
| Carotenoids (mg g−1 Leaf FW) | 0.44 ± 0.01 | 0.38 ± 0.01 ** | 0.49 ± 0.02 * | 0.63 ± 0.05 *** |
| Total protein (mg g−1 Leaf FW) | 08.60 ± 1.18 | 03.80 ± 1.09 ** | 10.90 ± 1.39 ** | 11.90 ± 1.42 ** |
| Treatments | ||||
|---|---|---|---|---|
| Parameters | Control | HS | MeJA | MeJA + HS |
| Fo | 206 ± 03.80 | 187 ± 03.50 ** | 241 ± 04.10 ** | 246 ± 04.40 ** |
| Fm | 935 ± 07.30 | 816 ± 06.20 * | 1258 ± 08.10 ** | 1350 ± 08.50 *** |
| Fv/Fo | 2.863 ± 0.018 | 2.709 ± 0.011 * | 3.445 ± 0.021 ** | 3.639 ± 0.026 ** |
| Fv/Fm | 0.780 ± 0.04 | 0.760 ± 0.04 * | 0.808 ± 0.05 ** | 0.817 ± 0.05 ** |
| RC/ABS | 0.831 ± 0.07 | 0.754 ± 0.04 * | 0.956 ± 0.08 ** | 0.992 ± 0.08 ** |
| PI | 1.675 ± 0.014 | 1.383 ± 0.009 * | 2.423 ± 0.021 ** | 2.734 ± 0.023 *** |
| Area | 21,744 ± 505 | 15,989 ± 421 * | 23,100 ± 539 | 24,907 ± 611 * |
| Treatments | ||||
|---|---|---|---|---|
| Parameters | Control | HS | MeJA | MeJA + HS |
| Net photosynthesis (µmol CO2 m−2 s−1) | 16.2 ± 0.91 | 10.6 ± 0.52 *** | 20.5 ± 095 ** | 22.5 ± 0.99 *** |
| Intercellular CO2 concentration (µmol CO2 mol−1) | 230 ± 9.1 | 160 ± 7.3 ** | 258 ± 10.3 ** | 281 ± 11.1 ** |
| Stomatal conductance (mmol H2O m−2 s−1) | 310 ± 12.3 | 228 ± 8.7 ** | 345 ± 13.5 ** | 377 ± 14.1 ** |
| Leaf area (cm2 Plant−1) | 106 ± 4.1 | 60.1 ± 2.9 *** | 123 ± 4.3 ** | 140.6 ± 4.9 *** |
| Plant fresh weight (g Plant−1) | 5.37 ± 0.09 | 2.82 ± 0.05 ** | 6.01 ± 0.10 * | 7.23 ± 0.11 ** |
| Plant dry weight (g Plant−1) | 0.808 ± 0.04 | 0.461 ± 0.01 ** | 0.897 ± 0.06 * | 1.090 ± 0.09 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl Jasmonate Protects the PS II System by Maintaining the Stability of Chloroplast D1 Protein and Accelerating Enzymatic Antioxidants in Heat-Stressed Wheat Plants. Antioxidants 2021, 10, 1216. https://doi.org/10.3390/antiox10081216
Fatma M, Iqbal N, Sehar Z, Alyemeni MN, Kaushik P, Khan NA, Ahmad P. Methyl Jasmonate Protects the PS II System by Maintaining the Stability of Chloroplast D1 Protein and Accelerating Enzymatic Antioxidants in Heat-Stressed Wheat Plants. Antioxidants. 2021; 10(8):1216. https://doi.org/10.3390/antiox10081216
Chicago/Turabian StyleFatma, Mehar, Noushina Iqbal, Zebus Sehar, Mohammed Nasser Alyemeni, Prashant Kaushik, Nafees A. Khan, and Parvaiz Ahmad. 2021. "Methyl Jasmonate Protects the PS II System by Maintaining the Stability of Chloroplast D1 Protein and Accelerating Enzymatic Antioxidants in Heat-Stressed Wheat Plants" Antioxidants 10, no. 8: 1216. https://doi.org/10.3390/antiox10081216
APA StyleFatma, M., Iqbal, N., Sehar, Z., Alyemeni, M. N., Kaushik, P., Khan, N. A., & Ahmad, P. (2021). Methyl Jasmonate Protects the PS II System by Maintaining the Stability of Chloroplast D1 Protein and Accelerating Enzymatic Antioxidants in Heat-Stressed Wheat Plants. Antioxidants, 10(8), 1216. https://doi.org/10.3390/antiox10081216












