The Potential of Dietary Antioxidants from a Series of Plant Extracts as Anticancer Agents against Melanoma, Glioblastoma, and Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Cell Culture
2.3. Chemicals and Materials
2.4. Extraction
2.5. UPLC–ESI–MS/MS Conditions
2.6. Determination of Antioxidant Activity
2.7. Cell Viability Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Extract Composition
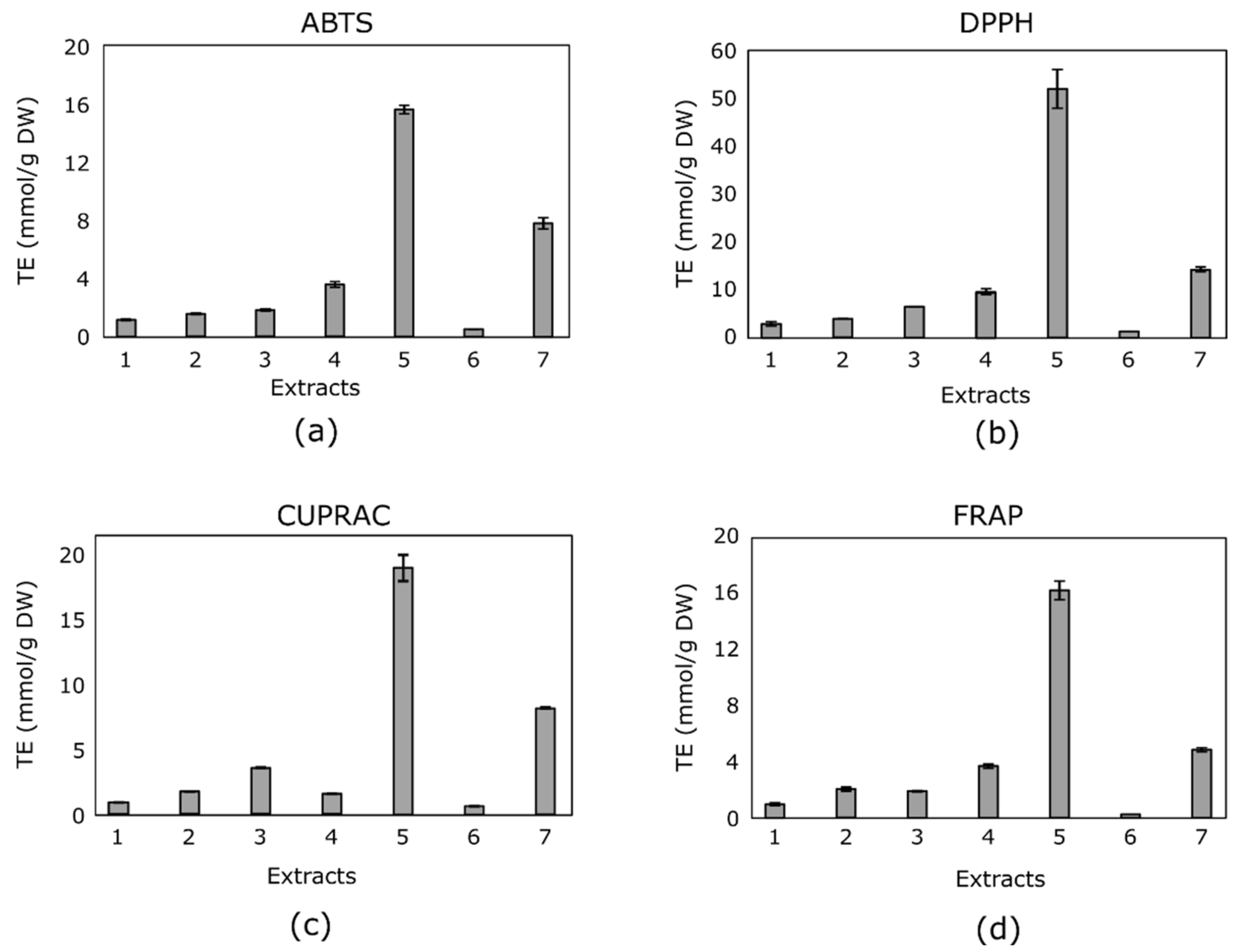
3.2. Antioxidant Activity
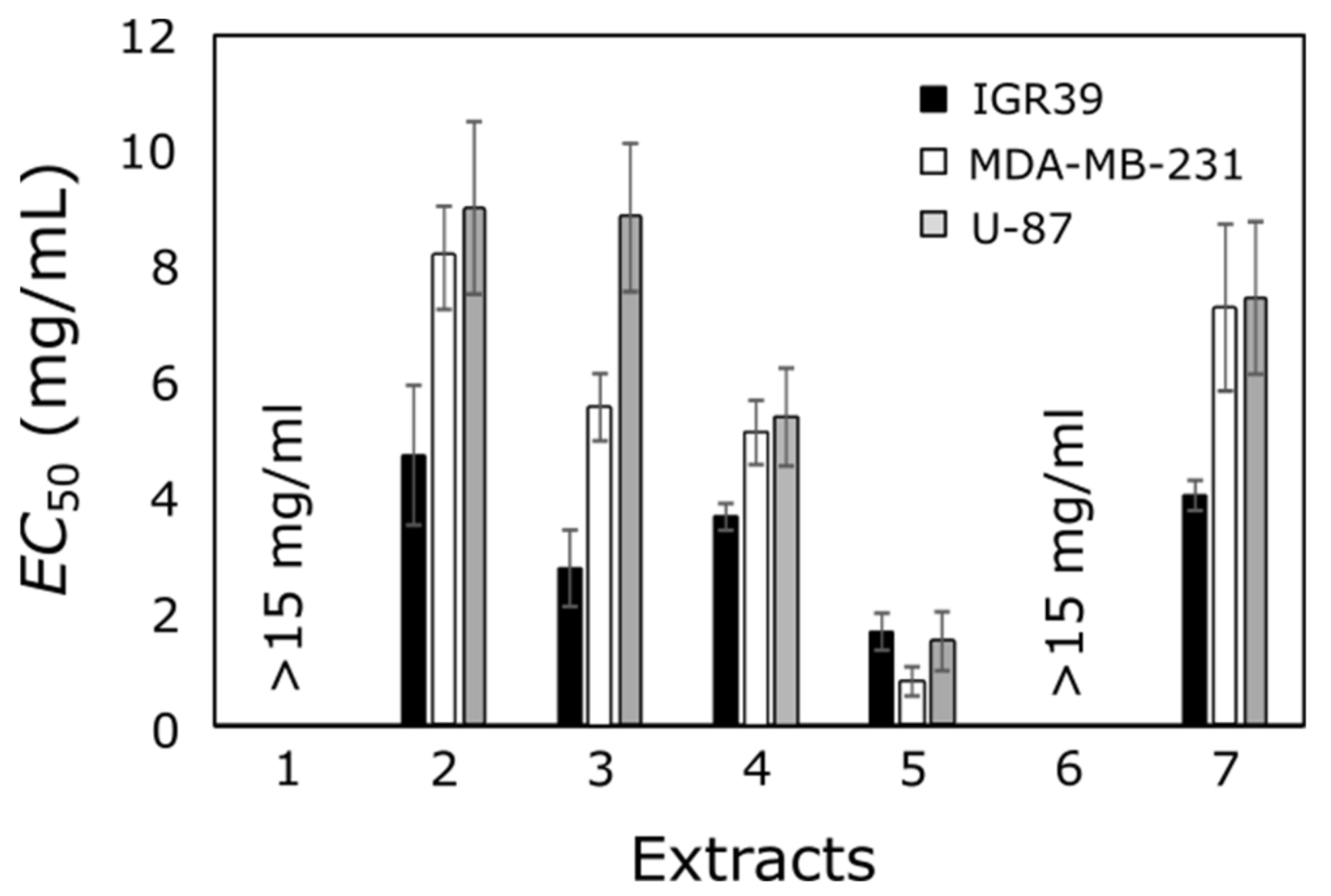
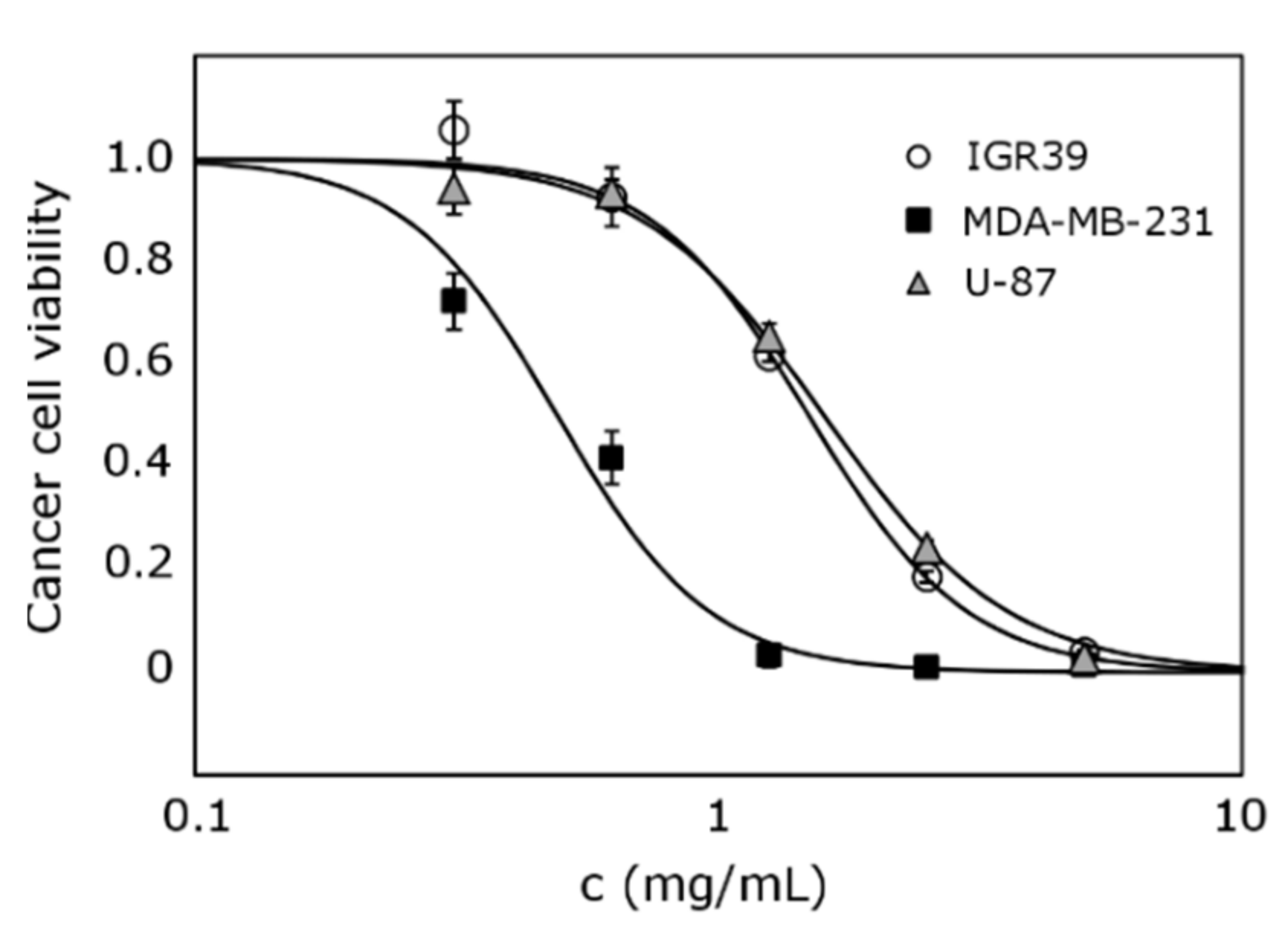
3.3. Anticancer Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, D.W.; Nash, P.; Buttar, H.S.; Griffiths, K.; Singh, R.; De Meester, F.; Horiuchi, R.; Takahashi, T. The Role of Food Antioxidants, Benefits of Functional Foods, and Influence of Feeding Habits on the Health of the Older Person: An Overview. Antioxidants 2017, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Aghajanpour, M.; Nazer, M.R.; Obeidavi, Z.; Akbari, M.; Ezati, P.; Kor, N.M. Functional foods and their role in cancer prevention and health promotion: A comprehensive review. Am. J. Cancer Res. 2017, 7, 740–769. [Google Scholar]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Plant-Based Diets Are Associated with a Lower Risk of Incident Cardiovascular Disease, Cardiovascular Disease Mortality, and All-Cause Mortality in a General Population of Middle-Aged Adults. J. Am. Heart Assoc. 2019, 8, e012865. [Google Scholar] [CrossRef]
- Kerley, C.P. A Review of Plant-based Diets to Prevent and Treat Heart Failure. Card. Fail. Rev. 2018, 4, 54–61. [Google Scholar] [CrossRef]
- Hu, N.; Yu, J.-T.; Tan, L.; Wang, Y.-L.; Sun, L.; Tan, L. Nutrition and the risk of Alzheimer’s disease. Biomed. Res. Int. 2013, 2013, 524820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prior, R.L. Fruits and vegetables in the prevention of cellular oxidative damage. Am. J. Clin. Nutr. 2003, 78, 570S–578S. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Shalaby, E.A.; Shanab, S.M.M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Mar. Sci. 2013, 42, 9. [Google Scholar]
- Çekiç, S.D.; Başkan, K.S.; Tütem, E.; Apak, R. Modified cupric reducing antioxidant capacity (CUPRAC) assay for measuring the antioxidant capacities of thiol-containing proteins in admixture with polyphenols. Talanta 2009, 79, 344–351. [Google Scholar] [CrossRef]
- Amorati, R.; Pedulli, G.F.; Cabrini, L.; Zambonin, L.; Landi, L. Solvent and pH Effects on the Antioxidant Activity of Caffeic and Other Phenolic Acids. J. Agric. Food Chem. 2006, 54, 2932–2937. [Google Scholar] [CrossRef]
- Staško, A.; Brezová, V.; Biskupič, S.; Mišík, V. The potential pitfalls of using 1,1-diphenyl-2-picrylhydrazyl to characterize antioxidants in mixed water solvents. Free. Radic. Res. 2007, 41, 379–390. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Liaudanskas, M.; Viškelis, J.; Žvikas, V.; Janulis, V.; Gómez-Serranillos, M.P. Antioxidant activity, neuro-protective properties and bioactive constituents analysis of varying polarity extracts from Eucalyptus globulus leaves. J. Food Drug Anal. 2018, 26, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, L.; Rupasinghe, H.V. The anticancer properties of phytochemical extracts from Salvia plants. Bot. Targets Ther. 2016, 6, 25–44. [Google Scholar]
- Mouhid, L.; De Cedrón, M.G.; García-Carrascosa, E.; Reglero, G.; Fornari, T.; De Molina, A.R. Yarrow supercritical extract exerts antitumoral properties by targeting lipid metabolism in pancreatic cancer. PLoS ONE 2019, 14, e0214294. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Medina, E.; Garcia-Lora, A.; Paco, L.; Algarra, I.; Collado, A.; Garrido, F. A new extract of the plant Calendula officinalis produces a dual in vitro effect: Cytotoxic anti-tumor activity and lymphocyte activation. BMC Cancer 2006, 6, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, E.; Vuoso, D.C.; D’Angelo, S.; Mele, L.; D’Onofrio, N.; Porcelli, M.; Cacciapuoti, G. Annurca apple polyphenol extract selectively kills MDA-MB-231 cells through ROS generation, sustained JNK activation and cell growth and survival inhibition. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Inhibition of proliferation of human carcinoma cell lines by phenolic compounds from a bear-berry-leaf crude extract and its fractions. J. Funct. Foods 2013, 5, 660–667. [Google Scholar] [CrossRef]
- Trumbeckaite, S.; Benetis, R.; Bumblauskiene, L.; Burdulis, D.; Janulis, V.; Toleikis, A.; Viškelis, P.; Jakštas, V. Achillea millefolium L. s.l. herb extract: Antioxidant activity and effect on the rat heart mitochondrial functions. Food Chem. 2011, 127, 1540–1548. [Google Scholar] [CrossRef]
- Preethi, K.C.; Kuttan, G.; Kuttan, R. Antioxidant Potential of an Extract of Calendula officinalis. Flowers in Vitro and in Vivo. Pharm. Biol. 2006, 44, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Raudone, L.; Raudonis, R.; Liaudanskas, M.; Janulis, V.; Viskelis, P. Phenolic antioxidant profiles in the whole fruit, flesh and peel of apple cultivars grown in Lithuania. Sci. Hortic. 2017, 216, 186–192. [Google Scholar] [CrossRef]
- Mohd Azman, N.A.; Gallego, M.G.; Segovia, F.; Abdullah, S.; Shaarani, S.M.; Almajano Pablos, M.P. Study of the Properties of Bearberry Leaf Extract as a Natural Antioxidant in Model Foods. Antioxidants 2016, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Pizzimenti, S.; Toaldo, C.; Pettazzoni, P.; Dianzani, M.U.; Barrera, G. The “Two-Faced” Effects of Reactive Oxygen Species and the Lipid Peroxidation Product 4-Hydroxynonenal in the Hallmarks of Cancer. Cancers 2010, 2, 338–363. [Google Scholar] [CrossRef] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Kooti, W.; Servatyari, K.; Behzadifar, M.; Asadi-Samani, M.; Sadeghi, F.; Nouri, B.; Marzouni, H.Z. Effective Medicinal Plant in Cancer Treatment, Part 2: Review Study. J. Evid. Based Integr. Med. 2017, 22, 982–995. [Google Scholar] [CrossRef]
- Deshmukh, V.N.; Sakarkar, D.M. Ethnopharmacological review of traditional medicinal plants for anticancer activity. Int. J. PharmTech Res. 2011, 3, 298–308. [Google Scholar]
- Roleira, F.M.F.; Tavares-da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-L. Dietary polyphenols as antioxidants and anticancer agents: More questions than answers. Chang. Gung Med. J. 2011, 34, 1–12. [Google Scholar]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Can phytochemical antioxidant rich foods act as anti-cancer agents? Food Res. Int. 2011, 44, 2545–2554. [Google Scholar] [CrossRef]
- Nepote, V.; Grosso, N.R.; Guzmán, C.A. Optimization of extraction of phenolic antioxidants from peanut skins. J. Sci. Food Agric. 2004, 85, 33–38. [Google Scholar] [CrossRef]
- Vatai, T.; Škerget, M.; Knez, Ž. Extraction of phenolic compounds from elder berry and different grape marc varieties using organic solvents and/or supercritical carbon dioxide. J. Food Eng. 2009, 90, 246–254. [Google Scholar] [CrossRef]
- Boulekbache-Makhlouf, L.; Medouni, L.; Medouni-Adrar, S.; Arkoub, L.; Madani, K. Effect of solvents extraction on phenolic content and antioxidant activity of the byproduct of eggplant. Ind. Crop. Prod. 2013, 49, 668–674. [Google Scholar] [CrossRef]
- Nasr, A.; Zhou, X.; Liu, T.; Yang, J.; Zhu, G.-P. Acetone-water mixture is a competent solvent to extract phenolics and antioxidants from four organs of Eucalyptus camaldulensis. Turk. J. Biochem. 2019, 44, 231–239. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Viškelis, P.; Jakštas, V.; Raudonis, R.; Kviklys, D.; Milašius, A.; Janulis, V. Application of an Optimized HPLC Method for the Detection of Various Phenolic Compounds in Apples from Lithuanian Cultivars. J. Chem. 2014, 2014. Available online: https://www.hindawi.com/journals/jchem/2014/542121/ (accessed on 10 January 2020). [CrossRef]
- Peterson, J.J.; Dwyer, J.T.; Jacques, P.F.; McCullough, M.L. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr. Rev. 2012, 70, 491–508. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Haskins, A.H.; Omata, C.; Aizawa, Y.; Kato, T.A. PARP Inhibition by Flavonoids Induced Selective Cell Killing to BRCA2-Deficient Cells. Pharmaceuticals 2017, 10, 80. [Google Scholar] [CrossRef]
- Konieczynski, P.; Viapiana, A.; Lysiuk, R.; Wesolowski, M. Chemical Composition of Selected Commercial Herbal Remedies in Relation to Geographical Origin and Inter-Species Diversity. Biol. Trace Elem. Res. 2017, 182, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef]
- Sadeghi Ekbatan, S.; Li, X.-Q.; Ghorbani, M.; Azadi, B.; Kubow, S. Chlorogenic Acid and Its Microbial Metabolites Exert Anti-Proliferative Effects, S-Phase Cell-Cycle Arrest and Apoptosis in Human Colon Cancer Caco-2 Cells. Int. J. Mol. Sci. 2018, 19, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.S.; Satsu, H.; Bae, M.-J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-N.; Mu, T.-H.; Xi, L.-S. Effect of pH, heat, and light treatments on the antioxidant activity of sweet potato leaf polyphenols. Int. J. Food Prop. 2017, 20, 318–332. [Google Scholar] [CrossRef]
- Cano, A.; Alcaraz, O.; Acosta, M.; Arnao, M.B. On-line antioxidant activity determination: Comparison of hydrophilic and lipophilic antioxidant activity using the ABTS•+ assay. Redox Rep. 2002, 7, 103–109. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Vastag, D. Flavonoids and Phenolic Acids as Potential Natural Antioxidants. In Antioxidants; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/books/antioxidants/flavonoids-and-phenolic-acids-as-potential-natural-antioxidants (accessed on 3 January 2020).
- Sak, K.; Nguyen, T.H.; Ho, V.D.; Do, T.T.; Raal, A. Cytotoxic effect of chamomile (Matricaria recutita) and marigold (Calendula officinalis) extracts on human melanoma SK-MEL-2 and epidermoid carcinoma KB cells. Cogent Med. 2017, 4. [Google Scholar] [CrossRef]
- Sun, J.; Liu, R.H. Apple phytochemical extracts inhibit proliferation of estrogen-dependent and estrogen-independent human breast cancer cells through cell cycle modulation. J. Agric. Food Chem. 2008, 56, 11661–11667. [Google Scholar] [CrossRef] [PubMed]
- Balça-Silva, J.; Matias, D.; Carmo, A.; Dubois, L.G.F.; Gonçalves, A.C.; Girao, H.; Canedo, N.H.S.; Correia, A.H.; De Souza, J.M.; Ribeiro, A.B.S.; et al. Glioblastoma entities express subtle differences in molecular composition and response to treatment. Oncol. Rep. 2017, 38, 1341–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.-L.; Kuo, Y.-C.; Ho, Y.-S.; Huang, Y.-H. Triple-Negative Breast Cancer: Current Understanding and Future Therapeutic Breakthrough Targeting Cancer Stemness. Cancers 2019, 11, 1334. [Google Scholar] [CrossRef] [Green Version]
- Grigalius, I.; Petrikaite, V. Relationship between Antioxidant and Anticancer Activity of Trihydroxyflavones. In Molecules; 2017; 22, Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6149854/ (accessed on 5 January 2020). [CrossRef] [Green Version]
- Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. In Int. J. Mol. Sci.; 2018; 19, p. 1573. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6032343/ (accessed on 5 January 2020). [CrossRef] [Green Version]
- Rauf, A.; Imran, M.; Ali Khan, I.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. In Molecules; 2019; 24, p. 2277. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6631472/ (accessed on 10 January 2020). [CrossRef] [Green Version]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. In Cell Biosci.; 2017; 7, pp. 1–16. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5629766/ (accessed on 6 January 2020). [CrossRef] [Green Version]
- Shukla, S.; Gupta, S. Apigenin: A Promising Molecule for Cancer Prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
| No. | Raw Material | Extraction Solvent | Concentration of Extraction Solvent, % (v/v) | The Ratio of Extraction Solvent to Raw Material |
|---|---|---|---|---|
| 1 | Dried calendula flowers | Ethanol | 40 | 1:5 |
| 2 | Dried sage leaves | Ethanol | 40 | 1:5 |
| 3 | Dried yarrow herb | Ethanol | 40 | 1:5 |
| 4 | Dried bearberry leaves | Ethanol | 70 | 1:20 |
| 5 | Dried eucalyptus leaves | Acetone | 70 | 1:2 |
| 6 | Lyophilized apples | Ethanol | 70 | 1:20 |
| 7 | Dried eucalyptus leaves | Ethanol | 40 | 1:2 |
| Active Substances | Extracts | ||||||
|---|---|---|---|---|---|---|---|
| mg/g DW | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Apigenin | 12.99 ± 0.47 | 20.32 ± 0.77 | 92.88 ± 4.32 | ||||
| Aviculiarin | 276.9 ± 10.23 | 0.75 ± 0.03 | 118.61 ± 5.42 | 2.58 ± 0.09 | |||
| Caffeic acid | 50.65 ± 2.03 | 30.74 ± 1.22 | 112.38 ± 4.93 | 0.06 ± 0.01 | |||
| (+)-Catechin | 0.03 ± 0.01 | 220.2 ± 10.41 | 0.32 ± 0.01 | 70.09 ± 2.98 | 0.03 ± 0.01 | ||
| Chlorogenic acid | 206.16 ± 8.35 | 11.84 ± 0.48 | 538.5 ± 24.11 | 184.05 ± 7.36 | 94.97 ± 4.01 | 677.6 ± 30.23 | 80.24 ± 2.47 |
| p-Coumaric acid | 16.24 ± 0.05 | 1.56 ± 0.05 | |||||
| Galangin | 0.90 ± 0.04 | ||||||
| Hyperoside | 93.96 ± 4.50 | 7.35 ± 0.31 | 273.6 ± 13.26 | 2.65 ± 0.07 | 114.24 ± 5.63 | 1.59 ± 0.06 | |
| Isorhamnetin | 241.65 ± 11.0 | 0.40 ± 0.02 | 10.60 ± 0.44 | 1.81 ± 0.07 | 0.66 ± 0.02 | ||
| Isorhamnetin 3-O-glucoside | 171.15 ± 8.03 | 0.78 ± 0.03 | 17.92 ± 0.82 | ||||
| Isorhamnetin 3-O-rutinoside | 320.41 ± 15.3 | 2.26 ± 0.09 | 82.54 ± 4.03 | 1.66 ± 0.06 | |||
| Kaempherol | 1.35 ± 0.06 | 0.15 ± 0.01 | 0.47 ± 0.02 | 0.49 ± 0.02 | 0.22 ± 0.01 | 1.49 ± 0.05 | |
| Kaempherol 3-O-glucoside | 8.79 ± 0.37 | 1.17 ± 0.05 | 0.12 ± 0.01 | 11.17 ± 0.47 | 0.70 ± 0.02 | ND | 0.32 ± 0.01 |
| Luteolin 7-O-glucoside | 7.54 ± 0.27 | 9.74 ± 0.41 | |||||
| Orientin | 0.48 ± 0.02 | 0.82 ± 0.03 | 14.60 ± 0.67 | 2.99 ± 0.14 | 3.75 ± 0.17 | ||
| Phloridzin | 3.83 ± 0.14 | 3.30 ± 0.12 | 35.51 ± 1.42 | 64.90 ± 3.01 | 20.93 ± 0.88 | ||
| Quercetin | 32.14 ± 1.47 | 5.76 ± 0.20 | 38.20 ± 1.70 | 2.59 ± 0.10 | 1.61 ± 0.06 | 28.40 ± 1.33 | |
| Quinic acid | 381.56 ± 16.3 | 4.09 ± 0.18 | 2342.1 ± 101.4 | 7824.8 ± 350.6 | 703.6 ± 32.03 | 5398.6 ± 213.36 | 627.94 ± 28.9 |
| Rosmarinic acid | 0.39 ± 0.01 | 1799.2 ± 80.5 | 7.17 ± 0.29 | 9.80 ± 0.42 | 0.34 ± 0.02 | 106.35 ± 4.69 | 0.07 ± 0.01 |
| Rutin | 153.32 ± 7.12 | 6.87 ± 0.29 | 29.21 ± 1.20 | 71.97 ± 2.98 | 12.53 ± 0.46 | 22.92 ± 1.03 | 7.30 ± 0.28 |
| Syringic acid | 48.59 ± 1.97 | 40.45 ± 1.76 | |||||
| Tiliroside | 1.21 ± 0.04 | 3.01 ± 0.11 | ND | 0.65 ± 0.02 | 0.15 ± 0.01 | ||
| Vitexin | 0.32 ± 0.02 | 1.46 ± 0.05 | 1.90 ± 0.08 | ND | 1.76 ± 0.06 | ||
| ABTS | DPPH | CUPRAC | FRAP | ||
|---|---|---|---|---|---|
| ABTS | Correlation coefficient | 1 | 0.972 ** | 0.981 ** | 0.976 ** |
| p value (two-tailed) | 0.000 | 0.000 | 0.000 | ||
| DPPH | Correlation coefficient | 0.972 ** | 1 | 0.976 ** | 0.997 ** |
| p value (two-tailed) | 0.000 | 0.000 | 0.000 | ||
| CUPRAC | Correlation coefficient | 0.981 ** | 0.976 ** | 1 | 0.971 ** |
| p value (two-tailed) | 0.000 | 0.000 | 0.005 | ||
| FRAP | Correlation coefficient | 0.976 ** | 0.997 ** | 0.971 ** | 1 |
| p value (two-tailed) | 0.000 | 0.000 | 0.005 | ||
| Antioxidant Activity, mmol TE/g DW | |||||
|---|---|---|---|---|---|
| Anticancer Activity | ABTS | DPPH | CUPRAC | FRAP | |
| IGR39 | Correlation coefficient | −0.583 | −0.558 | −0.517 | −0.600 |
| p value (two-tailed) | 0.129 | 0.151 | 0.190 | 0.116 | |
| MDA-MB-231 | Correlation coefficient | −0.791 * | −0.793 * | −0.744 * | −0.803 ** |
| p value (two-tailed) | 0.019 | 0.019 | 0.034 | 0.016 | |
| U-87 | Correlation coefficient | −0.827 * | −0.817 * | −0.738 * | −0.850 ** |
| p value (two-tailed) | 0.011 | 0.013 | 0.036 | 0.008 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liaudanskas, M.; Žvikas, V.; Petrikaitė, V. The Potential of Dietary Antioxidants from a Series of Plant Extracts as Anticancer Agents against Melanoma, Glioblastoma, and Breast Cancer. Antioxidants 2021, 10, 1115. https://doi.org/10.3390/antiox10071115
Liaudanskas M, Žvikas V, Petrikaitė V. The Potential of Dietary Antioxidants from a Series of Plant Extracts as Anticancer Agents against Melanoma, Glioblastoma, and Breast Cancer. Antioxidants. 2021; 10(7):1115. https://doi.org/10.3390/antiox10071115
Chicago/Turabian StyleLiaudanskas, Mindaugas, Vaidotas Žvikas, and Vilma Petrikaitė. 2021. "The Potential of Dietary Antioxidants from a Series of Plant Extracts as Anticancer Agents against Melanoma, Glioblastoma, and Breast Cancer" Antioxidants 10, no. 7: 1115. https://doi.org/10.3390/antiox10071115
APA StyleLiaudanskas, M., Žvikas, V., & Petrikaitė, V. (2021). The Potential of Dietary Antioxidants from a Series of Plant Extracts as Anticancer Agents against Melanoma, Glioblastoma, and Breast Cancer. Antioxidants, 10(7), 1115. https://doi.org/10.3390/antiox10071115








