Green One-Pot Synthesis of Coumarin-Hydroxybenzohydrazide Hybrids and Their Antioxidant Potency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Instruments
2.2. Synthesis of Coumarin-Hydroxybenzohydrazide Derivatives
2.3. DPPH Radical Scavenging Assay
2.4. Computational Methods
3. Results and Discussion
3.1. Synthesis
3.2. In Vitro Antioxidative Activity of Tested Compounds
3.3. Thermodynamic Parameters of Antioxidative Activity
3.3.1. Bond Dissociation Energy
3.3.2. Proton Affinity
3.3.3. Frontier Molecular Orbitals and HOMO–LUMO Gap
3.3.4. Ionization Potential
3.4. Theoretical Assessment of Mechanisms of Antioxidative Action
3.5. Radicals and Anions of Investigated Compounds
3.6. Radical Scavenging Activity against DPPH Radical
3.7. Potential Toxicology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Fiskum, G.; Schubert, D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002, 80, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cayuela, M. Oxygen free radicals and human disease. Biochimie 1995, 77, 147–161. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Sadeer, N.B.; Llorent-Martínez, E.J.; Bene, K.; Mahomoodally, M.F.; Mollica, A.; Sinan, K.I.; Zengin, G. Chemical profiling, antioxidant, enzyme inhibitory and molecular modelling studies on the leaves and stem bark extracts of three African medicinal plants. J. Pharm. Biomed. Anal. 2019, 174, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Costante, R.; Fiorito, S.; Genovese, S.; Stefanucci, A.; Mathieu, V.; Epifano, F. Synthesis and anti-cancer activity of naturally occurring 2, 5-diketopiperazines. Fitoterapia 2014, 98, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Uysal, A.; Ozer, O.Y.; Zengin, G.; Stefanucci, A.; Mollica, A.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Multifunctional approaches to provide potential pharmacophores for the pharmacy shelf: Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt. Comput. Biol. Chem. 2019, 78, 64–73. [Google Scholar] [CrossRef]
- Kirsch, G.; Abdelwahab, A.B.; Chaimbault, P. Natural and synthetic coumarins with effects on inflammation. Molecules 2016, 21, 1322. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Sethi, P.; Bansal, G. Coumarin: A potential nucleus for anti-inflammatory molecules. Med. Chem. Res. 2013, 22, 3049–3060. [Google Scholar] [CrossRef]
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 2016, 123, 236–255. [Google Scholar] [CrossRef]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef]
- de Souza, L.G.; Rennó, M.N.; Figueroa-Villar, J.D. Coumarins as cholinesterase inhibitors: A review. Chem.-Biol. Interact. 2016, 254, 11–23. [Google Scholar] [CrossRef] [PubMed]
- A Garro, H.; R Pungitore, C. Coumarins as potential inhibitors of DNA polymerases and reverse transcriptases. Searching new antiretroviral and antitumoral drugs. Curr. Drug Discov. Technol. 2015, 12, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Dadashpour, S. Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem. 2015, 102, 611–630. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. Coumarin-containing hybrids and their anticancer activities. Eur. J. Med. Chem. 2019, 181, 111587. [Google Scholar] [CrossRef]
- Menezes, J.C.; Diederich, M. Translational role of natural coumarins and their derivatives as anticancer agents. Future Med. Chem. 2019, 11, 1057–1082. [Google Scholar] [CrossRef]
- Kotali, A.; Nasiopoulou, D.A.; Harris, P.A.; Helliwell, M.; Joule, J.A. Transformation of a hydroxyl into an acyl group on α-pyrone ring: A novel route to 3, 4-diacylcoumarins. Tetrahedron 2012, 68, 761–766. [Google Scholar] [CrossRef]
- Kotali, A.; Nasiopoulou, D.A.; Tsoleridis, C.A.; Harris, P.A.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.J. Antioxidant Activity of 3-[N-(Acylhydrazono) ethyl]-4-hydroxy-coumarins. Molecules 2016, 21, 138. [Google Scholar] [CrossRef] [Green Version]
- Govindaiah, P.; Dumala, N.; Mattan, I.; Grover, P.; Prakash, M.J. Design, synthesis, biological and in silico evaluation of coumarin-hydrazone derivatives as tubulin targeted antiproliferative agents. Bioorg. Chem. 2019, 91, 103143. [Google Scholar] [CrossRef] [PubMed]
- Avdović, E.H.; Petrović, I.P.; Stevanović, M.J.; Saso, L.; Dimitrić Marković, J.M.; Filipović, N.D.; Živić, M.Ž.; Cvetić Antić, T.N.; Žižić, M.V.; Todorović, N.V.; et al. Synthesis and Biological Screening of New 4-Hydroxycoumarin Derivatives and Their Palladium (II) Complexes. Oxidative Med. Cell. Longev. 2021, 2021, 8849568. [Google Scholar] [CrossRef]
- Milovanović, V.M.; Petrović, Z.D.; Novaković, S.; Bogdanović, G.A.; Petrović, V.P.; Simijonović, D. Green synthesis of benzamide-dioxoisoindoline derivatives and assessment of their radical scavenging activity–Experimental and theoretical approach. Tetrahedron 2020, 76, 131456. [Google Scholar] [CrossRef]
- Kontogiorgis, C.; Hadjipavlou-Litina, D. Biological evaluation of several coumarin derivatives designed as possible anti-inflammatory/antioxidant agents. J. Enzym. Inhib. Med. Chem. 2003, 18, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Dimić, D.; Milenković, D.; Marković, J.D.; Marković, Z. Antiradical activity of catecholamines and metabolites of dopamine: Theoretical and experimental study. Phys. Chem. Chem. Phys. 2017, 19, 12970–12980. [Google Scholar] [CrossRef] [PubMed]
- Prihantini, A.I.; Tachibana, S.; Itoh, K. Antioxidant active compounds from elaeocarpussylvestris and their relationship between structure and activity. Procedia Environ. Sci. 2015, 28, 758–768. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B. Gaussian 09 (Revision E. 01); Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Walker, M.; Harvey, A.J.; Sen, A.; Dessent, C.E. Performance of M06, M06-2X, and M06-HF density functionals for conformationally flexible anionic clusters: M06 functionals perform better than B3LYP for a model system with dispersion and ionic hydrogen-bonding interactions. J. Phys. Chem. A 2013, 117, 12590–12600. [Google Scholar] [CrossRef]
- Takano, Y.; Houk, K.N. Benchmarking the conductor-like polarizable continuum model (CPCM) for aqueous solvation free energies of neutral and ionic organic molecules. J. Chem. Theory Comput. 2005, 1, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z. Study of the mechanisms of antioxidative action of different antioxidants. J. Serb. Soc. Comput. Mech. 2016, 10, 135–150. [Google Scholar] [CrossRef]
- Milenković, D.A.; Dimić, D.S.; Avdović, E.H.; Amić, A.D.; Marković, J.M.D.; Marković, Z.S. Advanced oxidation process of coumarins by hydroxyl radical: Towards the new mechanism leading to less toxic products. Chem. Eng. J. 2020, 395, 124971. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food antioxidants: Chemical insights at the molecular level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef]
- Galano, A. Free radicals induced oxidative stress at a molecular level: The current status, challenges and perspectives of computational chemistry based protocols. J. Mex. Chem. Soc. 2015, 59, 231–262. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Xue, Y.; An, L.; Zheng, Y.; Dou, Y.; Zhang, L.; Liu, Y. Theoretical study on the structural and antioxidant properties of some recently synthesised 2, 4, 5-trimethoxy chalcones. Food Chem. 2015, 171, 89–97. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Zhao, X.; Li, Y. Effects of nitro-and amino-group on the antioxidant activity of genistein: A theoretical study. Food Chem. 2019, 275, 339–345. [Google Scholar] [CrossRef]
- Marković, Z.; Tošović, J.; Milenković, D.; Marković, S. Revisiting the solvation enthalpies and free energies of the proton and electron in various solvents. Comput. Theor. Chem. 2016, 1077, 11–17. [Google Scholar] [CrossRef]
- Avdović, E.H.; Stojković, D.L.; Jevtić, V.V.; Kosić, M.; Ristić, B.; Harhaji-Trajković, L.; Vukić, M.; Vuković, N.; Marković, Z.S.; Potočňák, I.; et al. Synthesis, characterization and cytotoxicity of a new palladium (II) complex with a coumarin-derived ligand 3-(1-(3-hydroxypropylamino) ethylidene) chroman-2, 4-dione. Crystal structure of the 3-(1-(3-hydroxypropylamino) ethylidene)-chroman-2, 4-dione. Inorg. Chim. Acta 2017, 466, 188–196. [Google Scholar] [CrossRef]
- Dimić, D.S.; Marković, Z.S.; Saso, L.; Avdović, E.H.; Đorović, J.R.; Petrović, I.P.; Stanisavljević, D.D.; Stevanović, M.J.; Potočňák, I.; Samoľová, E.; et al. Synthesis and Characterization of 3-(1-((3, 4-Dihydroxyphenethyl) amino) ethylidene)-chroman-2, 4-dione as a Potential Antitumor Agent. Oxidative Med. Cell. Longev. 2019, 2019, 2069250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avdović, E.H.; Milenković, D.; Marković, J.M.D.; Đorović, J.; Vuković, N.; Vukić, M.D.; Jevtić, V.V.; Trifunović, S.R.; Potočňák, I.; Marković, Z. Synthesis, spectroscopic characterization (FT-IR, FT-Raman, and NMR), quantum chemical studies and molecular docking of 3-(1-(phenylamino) ethylidene)-chroman-2, 4-dione. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 195, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C.; Johnson, E.R.; Vinqvist, M.R.; Wright, J.S.; Barclay, L.R.C.; Ingold, K.U. Naphthalene diols: A new class of antioxidants intramolecular hydrogen bonding in catechols, naphthalene diols, and their aryloxyl radicals. J. Org. Chem. 2002, 67, 5190–5196. [Google Scholar] [CrossRef] [Green Version]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Simijonović, D.; Petrović, Z.D.; Milovanović, V.M.; Petrović, V.P.; Bogdanović, G.A. A new efficient domino approach for the synthesis of pyrazolyl-phthalazine-diones. Antiradical activity of novel phenolic products. RSC Adv. 2018, 8, 16663–16673. [Google Scholar] [CrossRef] [Green Version]
- Cornard, J.P.; Boudet, A.C.; Merlin, J.C. Theoretical investigation of the molecular structure of the isoquercitrin molecule. J. Mol. Struct. 1999, 508, 37–49. [Google Scholar] [CrossRef]
- Sadasivam, K.; Kumaresan, R. Antioxidant behavior of mearnsetin and myricetin flavonoid compounds—A DFT study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Vagánek, A.; Rimarčík, J.; Dropková, K.; Lengyel, J.; Klein, E. Reaction enthalpies of OH bonds splitting-off in flavonoids: The role of non-polar and polar solvent. Comput. Theor. Chem. 2014, 1050, 31–38. [Google Scholar] [CrossRef]
- Petrović, Z.D.; Đorović, J.; Simijonović, D.; Petrović, V.P.; Marković, Z. Experimental and theoretical study of antioxidative properties of some salicylaldehyde and vanillic Schiff bases. RSC Adv. 2015, 5, 24094–24100. [Google Scholar] [CrossRef]
- Zheng, Y.; An, L.; Dou, Y.; Liu, Y. Density functional theory study of the structure–antioxidant activity of polyphenolic deoxybenzoins. Food Chem. 2014, 151, 198–206. [Google Scholar]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [Green Version]
- Gansukh, E.; Nile, A.; Kim, D.H.; Oh, J.W.; Nile, S.H. New insights into antiviral and cytotoxic potential of quercetin and its derivatives–a biochemical perspective. Food Chem. 2021, 334, 127508. [Google Scholar] [CrossRef]
- Keizo, S.; Hiromichi, O.; Shigeru, A. Selective inhibition of platelet lipoxygenase by esculetin. Biochim. Et Biophys. Acta (BBA)-Lipids Lipid Metab. 1982, 713, 68–72. [Google Scholar] [CrossRef]
- Milanović, Ž.B.; Antonijević, M.R.; Amić, A.D.; Avdović, E.H.; Dimić, D.S.; Milenković, D.A.; Marković, Z.S. Inhibitory activity of quercetin, its metabolite, and standard antiviral drugs towards enzymes essential for SARS-CoV-2: The role of acid–base equilibria. RSC Adv. 2021, 11, 2838–2847. [Google Scholar] [CrossRef]
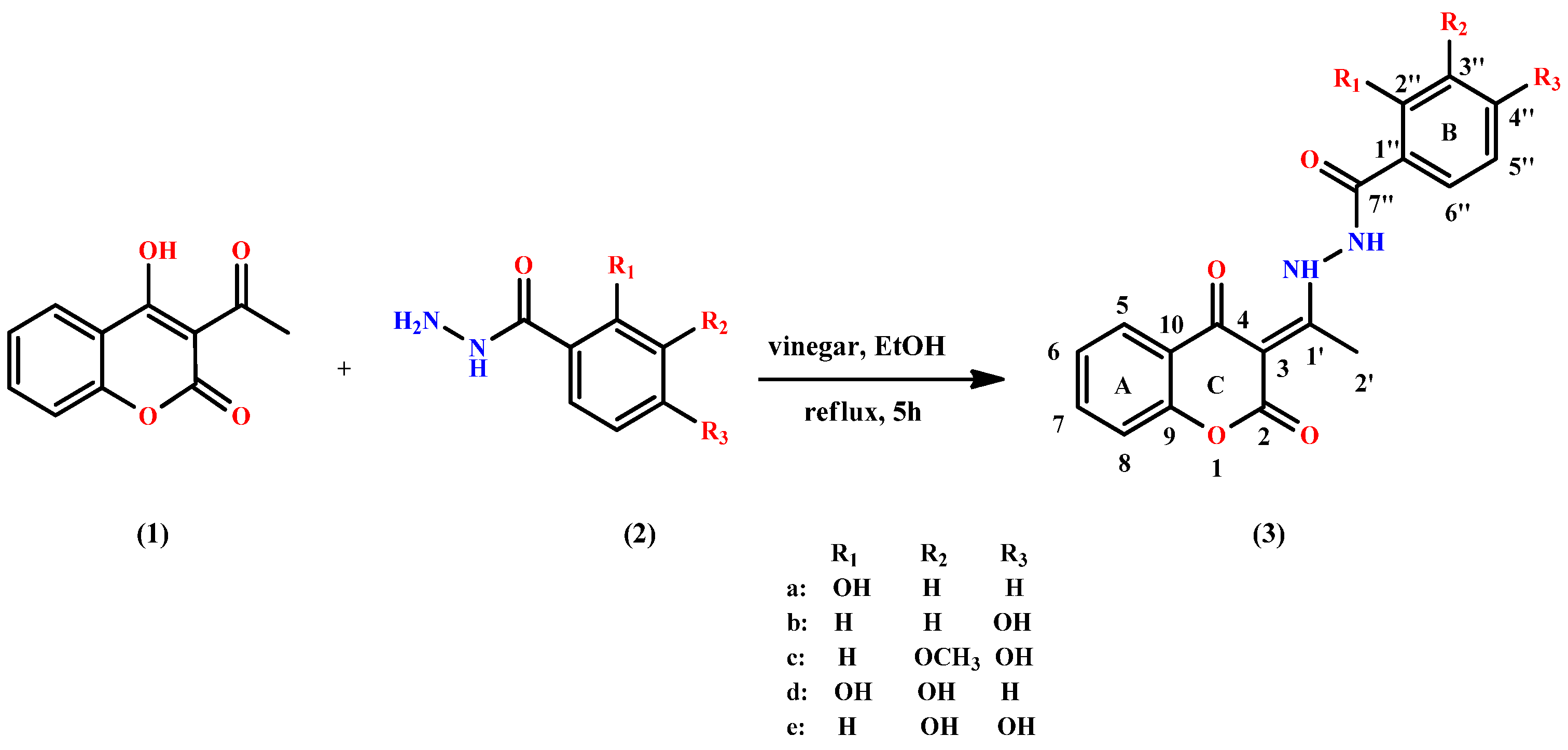
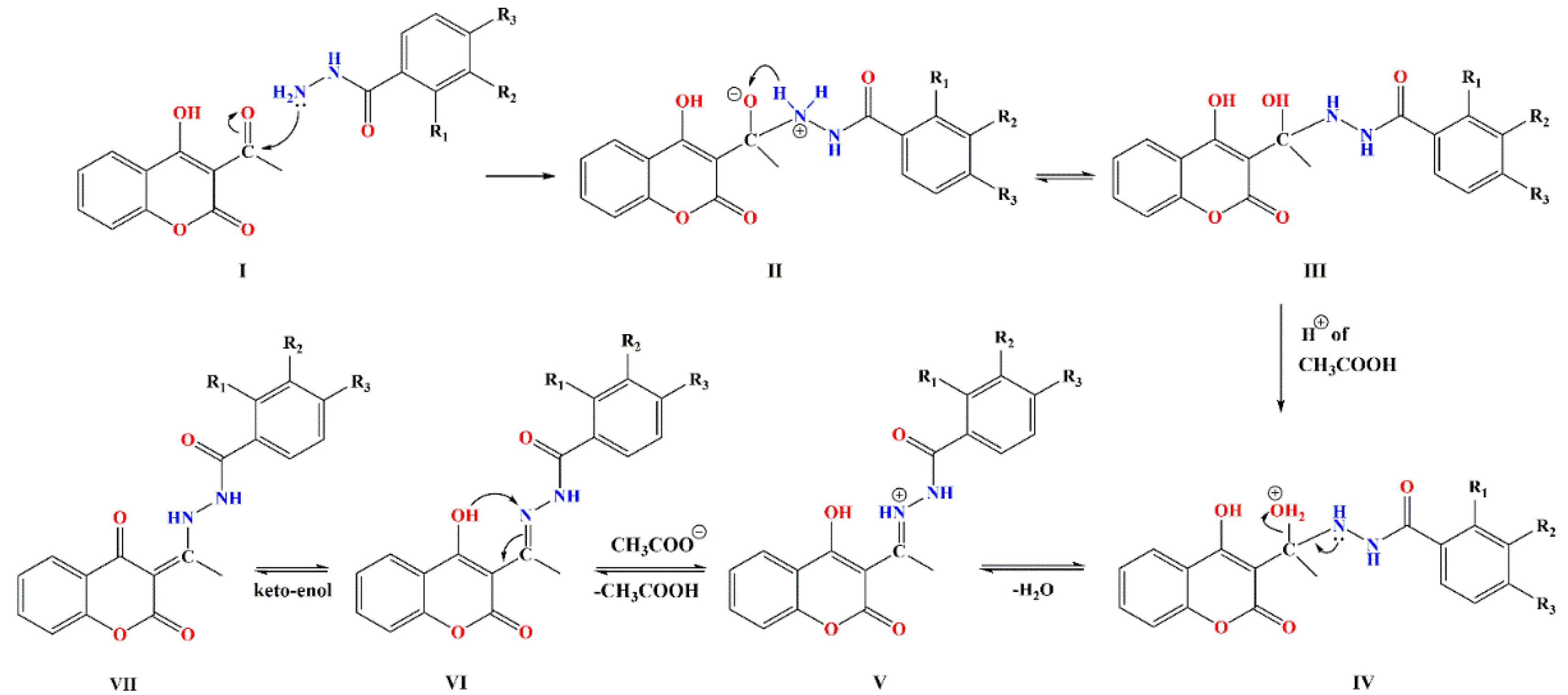
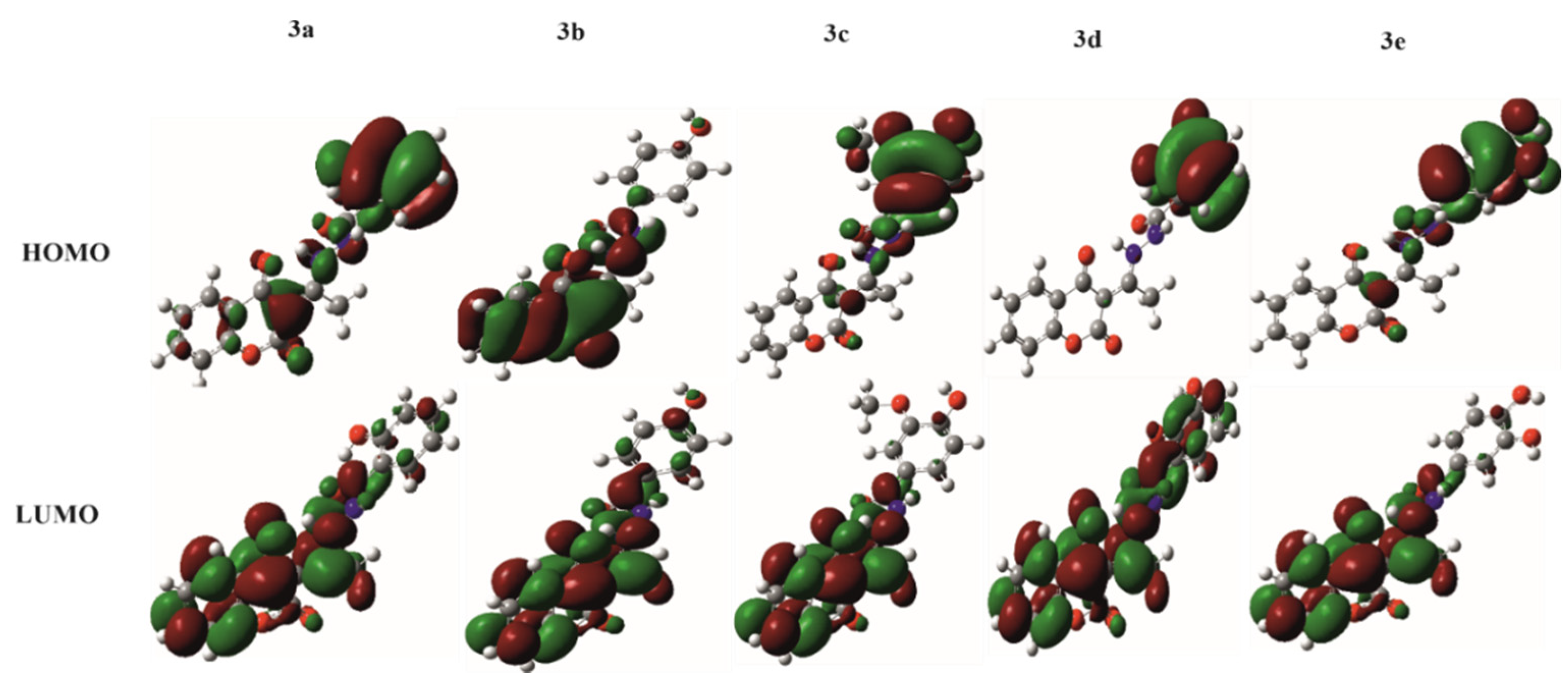


| Entry | Compound | Isolated Yield (%) | HPLC Purity (%) |
|---|---|---|---|
| 1 | 3a | 65.31 | 91.44 |
| 2 | 3b | 63.51 | 97.79 |
| 3 | 3c | 66.83 | 98.50 |
| 4 | 3d | 62.85 | 99.10 |
| 5 | 3e | 65.71 | 97.46 |
| Compound | DPPH Scavenging Ability (%) | IC50 (µM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 µM | 50 µM | 25 µM | * 150 µM | * 10 µM | * 2.5 µM | SF | |||||
| 20 Min | 60 Min | 20 Min | 60 Min | 20 Min | 60 Min | 20 Min | 20 Min | 20 Min | |||
| 3a | 2.9 ± 0.7 | 8.7 ± 0.7 | 0.6 ± 0.8 | 1.2 ± 0.7 | 0.6 ± 0.8 | 1.2 ± 0.7 | nd | nd | nd | nd | nd |
| 3b | 4.5 ± 1.8 | 15.8 ± 0.2 | 0.1 ± 1.0 | 4.7 ± 1.7 | 0.1 ± 1.0 | 4.7 ± 1.7 | nd | nd | nd | nd | nd |
| 3c | 31.5 ± 0.4 | 41.5 ± 0.6 | 20.4 ± 1.2 | 26.7 ± 0.9 | 20.4 ± 1.2 | 26.7 ± 0.9 | 56.4 ± 1.0 | nd | nd | 142.2 ± 2.7 | nd |
| 3d | 94.9 ± 0.6 | 94.3 ± 0.9 | 92.9 ± 0.1 | 92.5 ± 0.4 | 92.9 ± 0.1 | 92.5 ± 0.4 | nd | nd | 42.9 ± 1.3 | 2.9 ± 0.1 | 4.4 |
| 3e | 66.2 ± 1.8 | 75.3 ± 0.4 | 63.1 ± 1.1 | 73.6 ± 0.9 | 63.1 ± 1.1 | 73.6 ± 0.9 | nd | 47.7 ± 0.3 | 32.5 ± 0.7 | 12.9 ± 0.4 | 1.0 |
| NDGA | 94.5 ± 0.2 | 94.1 ± 0.7 | 94.6 ± 0.7 | 94.6 ± 0.6 | 94.6 ± 0.7 | 94.6 ± 0.6 | nd | nd | 59.9 ± 0.1 | 1.7 ± 0.1 | 7.4 |
| Quercetin | 95.1 ± 0.9 | 95.4 ± 0.8 | 95.3 ± 0.8 | 95.1 ± 0.9 | 95.3 ± 0.8 | 95.1 ± 0.9 | nd | nd | 60.9 ± 7.2 | 1.9 ± 0.1 | 6.6 |
| BENZENE | METHANOL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Position | HAT | SET-PT | SPLET | HAT | SET-PT | SPLET | ||||
| BDE | IP | PDE | PA | ETE | BDE | IP | PDE | PA | ETE | |
| 3a | ||||||||||
| C2″-OH | 404 | 714 | 123 | 446 | 391 | 373 | 552 | 19 | 191 | 380 |
| C7″-NH | 383 | 102 | 413 | 402 | 362 | 7 | 161 | 398 | ||
| 3b | ||||||||||
| C4″-OH | 382 | 688 | 127 | 407 | 408 | 381 | 551 | 28 | 185 | 394 |
| C7″-NH | 348 | 94 | 372 | 409 | 355 | 3 | 156 | 398 | ||
| 3c | ||||||||||
| C4″-OH | 377 | 693 | 118 | 422 | 388 | 377 | 528 | 47 | 200 | 375 |
| C7″-NH | 348 | 88 | 376 | 405 | 355 | 25 | 158 | 395 | ||
| 3d | ||||||||||
| C2″-OH | 372 | 706 | 99 | 420 | 385 | 362 | 519 | 41 | 191 | 369 |
| C3″-OH | 374 | 101 | 430 | 376 | 365 | 44 | 200 | 363 | ||
| C7″-NH | 348 | 75 | 343 | 438 | 355 | 34 | 133 | 420 | ||
| 3e | ||||||||||
| C3″-OH | 342 | 703 | 72 | 393 | 382 | 346 | 535 | 9 | 178 | 367 |
| C4″-OH | 344 | 74 | 380 | 397 | 348 | 11 | 168 | 378 | ||
| C7″-NH | 346 | 77 | 370 | 410 | 355 | 19 | 154 | 399 | ||
| Ligand | METHANOL | BENZENE | ||||
|---|---|---|---|---|---|---|
| HOMO | LUMO | HL Gap (eV) | HOMO | LUMO | HL Gap (eV) | |
| 3a | −8.09 | −1.41 | 6.68 | −8.10 | −1.37 | 6.72 |
| 3b | −8.09 | −1.36 | 6.74 | −8.01 | −1.26 | 6.75 |
| 3c | −7.80 | −1.35 | 6.45 | −7.85 | −1.26 | 6.59 |
| 3d | −7.81 | −1.42 | 6.39 | −7.87 | −1.41 | 6.46 |
| 3e | −7.86 | −1.36 | 6.50 | −7.94 | −1.28 | 6.66 |
| METHANOL | HAT | SET-PT | SPLET | ||
|---|---|---|---|---|---|
| ∆HBDE | ∆HIP | ∆HPDE | ∆HPA | ∆HETE | |
| 3a | |||||
| C2″-OH | 47 | 201 | −142 | 50 | 7 |
| C7″-NH | 27 | −163 | 2 | 34 | |
| 3b | |||||
| C4″-OH | 59 | 184 | −134 | 23 | 28 |
| C7″-NH | 38 | −159 | −6 | 29 | |
| 3c | |||||
| C4″-OH | 50 | 161 | −115 | 38 | 3 |
| C7″-NH | 24 | −137 | −4 | 25 | |
| 3d | |||||
| C3″-OH | 34 | 152 | −118 | 38 | −4 |
| C2″-OH | 31 | −121 | 29 | 0.1 | |
| C7″-NH | 24 | −128 | −29 | 53 | |
| 3e | |||||
| C4″-OH | 17 | 168 | −151 | 6 | 9 |
| C3″-OH | 15 | −152 | 16 | −1.8 | |
| C7″-NH | 25 | −143 | −8 | 32 | |
| Compound: | Predicted Toxicity Class | Predicted LD50 (mg/kg) |
|---|---|---|
| 3a | Class V | 3000 |
| 3b | Class IV | 1460 |
| 3c | Class IV | 721 |
| 3d | Class V | 3000 |
| 3e | Class IV | 2000 |
| Warfarin | Class I | 2 |
| Esculetin | Class IV | 945 |
| 4-hydroxy coumarin | Class IV | 2000 |
| Quercetin | Class III | 159 |
| Ethanol | Class V | 3450 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonijević, M.R.; Simijonović, D.M.; Avdović, E.H.; Ćirić, A.; Petrović, Z.D.; Marković, J.D.; Stepanić, V.; Marković, Z.S. Green One-Pot Synthesis of Coumarin-Hydroxybenzohydrazide Hybrids and Their Antioxidant Potency. Antioxidants 2021, 10, 1106. https://doi.org/10.3390/antiox10071106
Antonijević MR, Simijonović DM, Avdović EH, Ćirić A, Petrović ZD, Marković JD, Stepanić V, Marković ZS. Green One-Pot Synthesis of Coumarin-Hydroxybenzohydrazide Hybrids and Their Antioxidant Potency. Antioxidants. 2021; 10(7):1106. https://doi.org/10.3390/antiox10071106
Chicago/Turabian StyleAntonijević, Marko R., Dušica M. Simijonović, Edina H. Avdović, Andrija Ćirić, Zorica D. Petrović, Jasmina Dimitrić Marković, Višnja Stepanić, and Zoran S. Marković. 2021. "Green One-Pot Synthesis of Coumarin-Hydroxybenzohydrazide Hybrids and Their Antioxidant Potency" Antioxidants 10, no. 7: 1106. https://doi.org/10.3390/antiox10071106
APA StyleAntonijević, M. R., Simijonović, D. M., Avdović, E. H., Ćirić, A., Petrović, Z. D., Marković, J. D., Stepanić, V., & Marković, Z. S. (2021). Green One-Pot Synthesis of Coumarin-Hydroxybenzohydrazide Hybrids and Their Antioxidant Potency. Antioxidants, 10(7), 1106. https://doi.org/10.3390/antiox10071106










