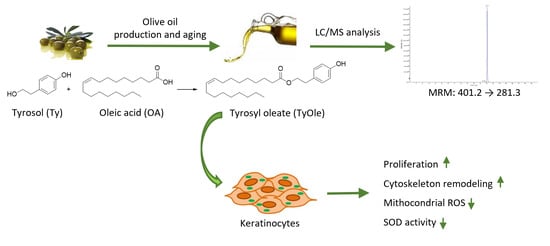
Identification of Tyrosyl Oleate as a Novel Olive Oil Lipophenol with Proliferative and Antioxidant Properties in Human Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentations
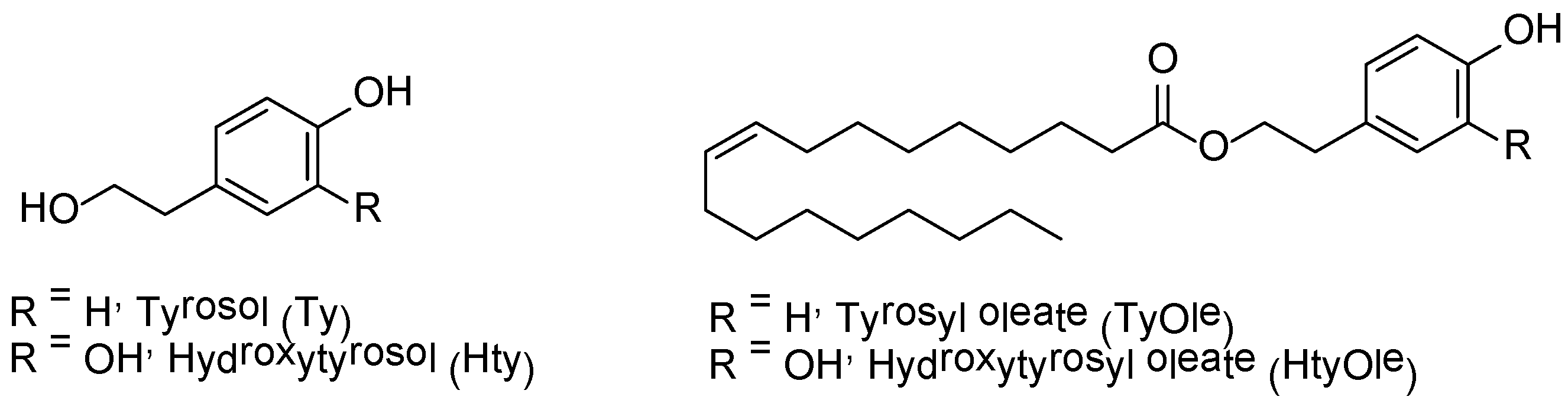
2.3. Chemistry
2.4. Olive and Olive Oil Samples
2.5. Extraction of Phenolics from Olives and Olive Oils
2.5.1. Method 1
2.5.2. Method 2
2.6. Preparation of Standard Solutions
2.7. High Performance Liquid Chromatography/Tandem Mass Spectrometry
2.8. Method Validation
2.9. Cell Culture
2.10. Cell Viability Assay
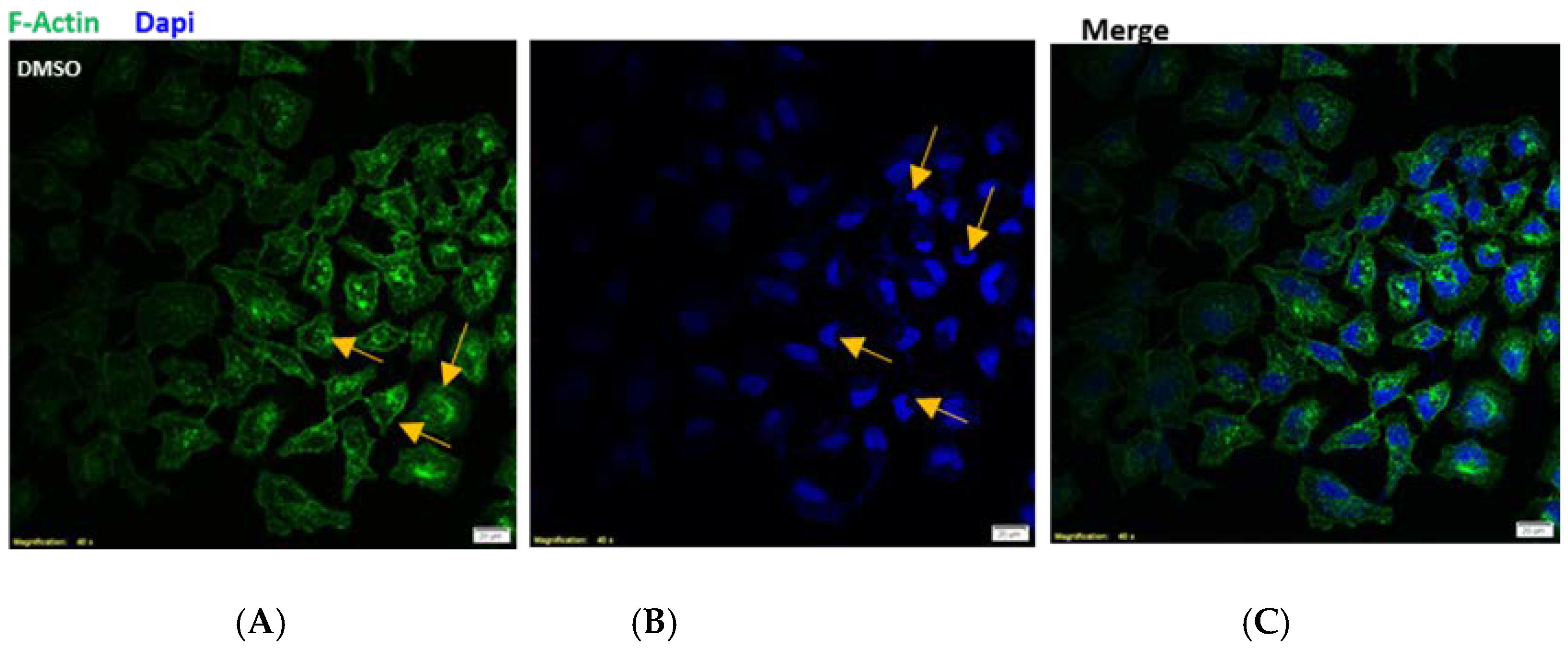
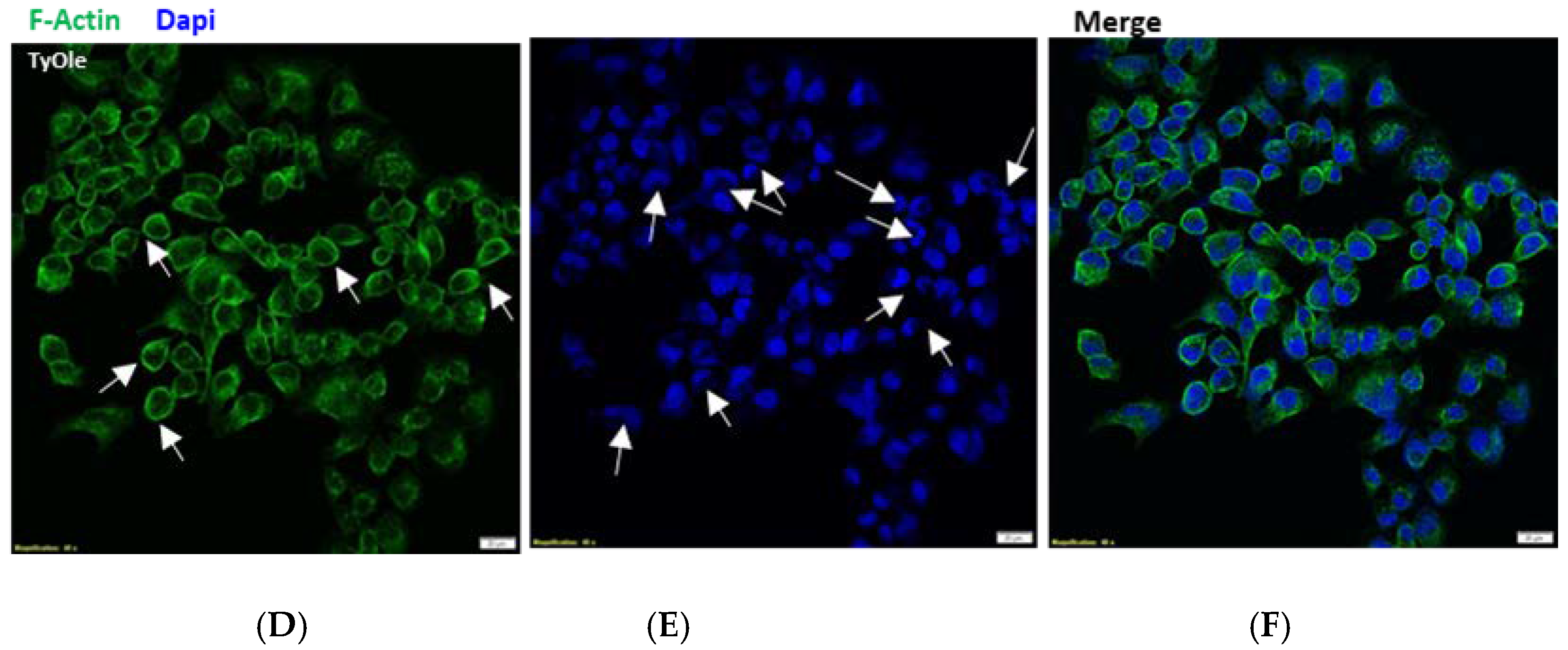
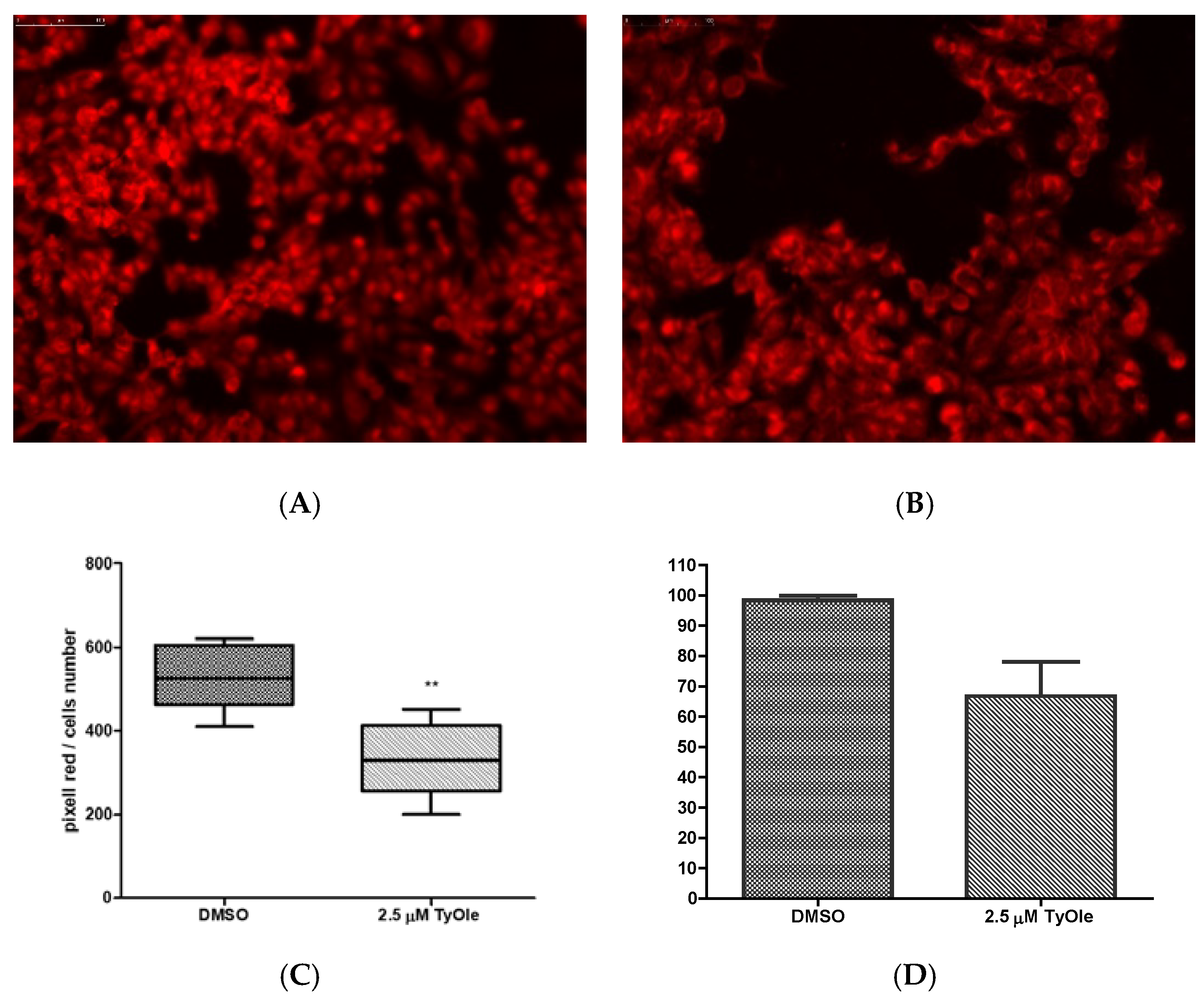
2.11. Fluorescent Staining
2.12. Superoxide Dismutase Enzymatic Activity
2.13. Statistical Analysis
3. Results
3.1. HPLC/MS Analyses
3.1.1. Method Validation
3.1.2. Identification and Quantification of TyOle in Olive Oils
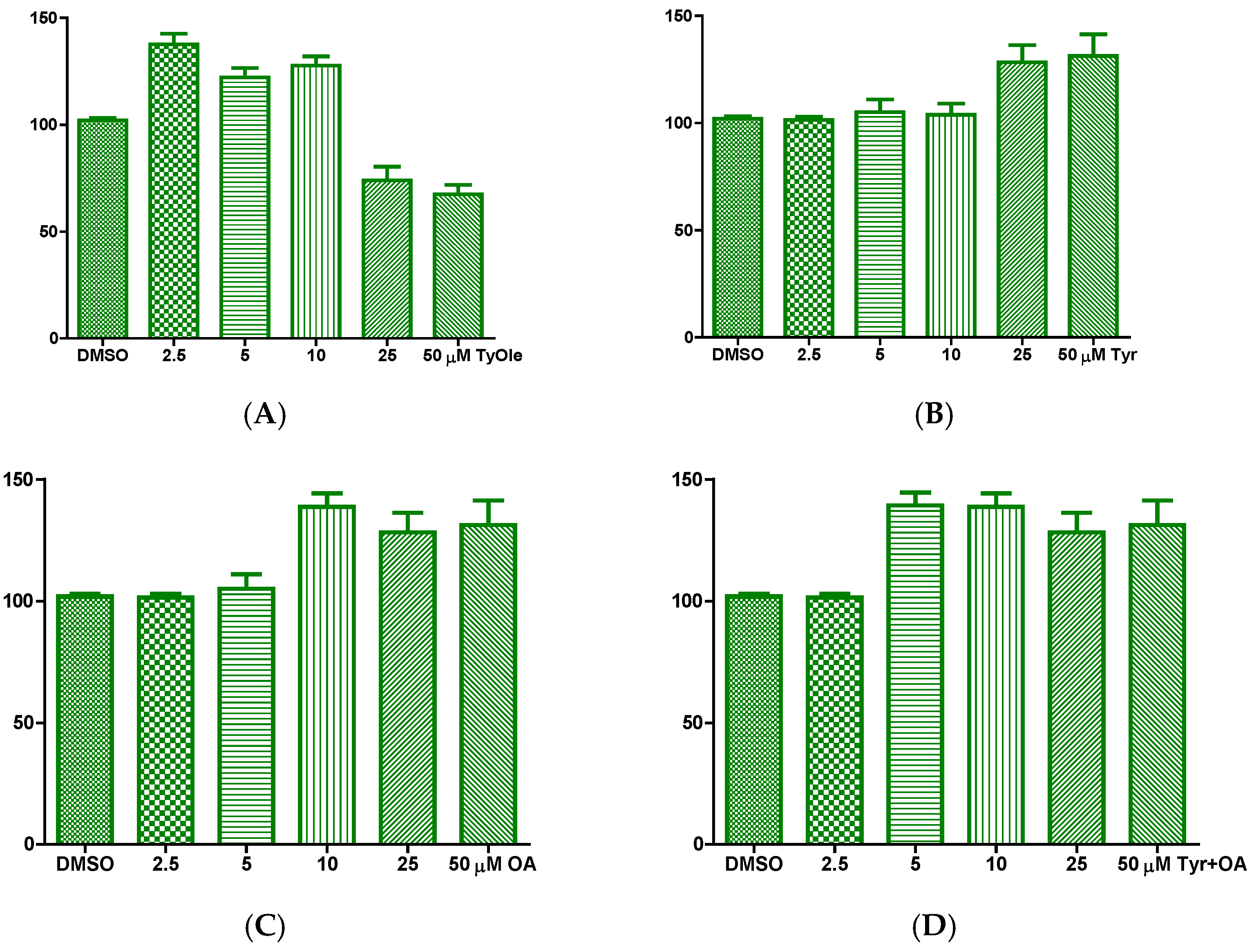
3.2. Tyrosyl Oleate Affects Cell Viability of Human Keratinocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Available online: https://ec.europa.eu/info/food-farming-fisheries/plants-and-plant-products/plant-products/olive-oil (accessed on 25 May 2021).
- Martinez-Gonzalez, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health A Critical Review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Visioli, F.; Davalos, A.; López de Las Hazas, M.C.; Crespo, M.C.; Tomé-Carneiro, J. An overview of the pharmacology of olive oil and its active ingredients. Br. J. Pharmacol. 2020, 177, 1316–1330. [Google Scholar] [CrossRef] [Green Version]
- Gavahian, M.; Khaneghah, A.M.; Lorenzo, J.M.; Munekata, P.E.S.; Garcia-Mantrana, I.; Collado, M.C.; Melendez-Martinez, A.J.; Barba, F.J. Health benefits of olive oil and its components: Impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci. Technol. 2019, 88, 220–227. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano-Castellon, J.; Lopez-Yerena, A.; Rinaldi de Alvarenga, J.F.; del Castillo-Alba, J.R.; Vallverdu-Queralt, A.; Escribano-Ferrer, E.; Lamuela-Raventos, R.M. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. Commission Regulation No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other Than those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Off. J. Eur. Union 2012, 43, 281–320. [Google Scholar]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL-cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (ID 3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar]
- Cicerale, S.; Conlan, X.A.; Sinclair, A.J.; Keast, R.S. Chemistry and health of olive oil phenolics. Crit. Rev. Food Sci. Nutr. 2009, 49, 218–236. [Google Scholar] [CrossRef] [Green Version]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M.C. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; García, A.; García, P.; Rios, J.J.; Garrido, A. Phenolic compounds in Spanish olive oils. J. Agric. Food Chem. 1999, 47, 3535–3540. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Crauste, C.; Wang, H.; Leung, H.H.; Vercauteren, J.; Galano, J.M.; Oger, C.; Durand, T.; Wan, J.M.; Lee, J.C. Extra Virgin Olive Oil Reduced Polyunsaturated Fatty Acid and Cholesterol Oxidation in Rodent Liver: Is This Accounted for Hydroxytyrosol-Fatty Acid Conjugation? Chem. Res. Toxicol. 2016, 29, 1689–1698. [Google Scholar] [CrossRef] [Green Version]
- Benincasa, C.; La Torre, C.; Plastina, P.; Fazio, A.; Perri, E.; Caroleo, M.C.; Gallelli, L.; Cannataro, R.; Cione, E. Hydroxytyrosyl Oleate: Improved Extraction Procedure from Olive Oil and By-Products, and In Vitro Antioxidant and Skin Regenerative Properties. Antioxidants 2019, 8, 233. [Google Scholar] [CrossRef] [Green Version]
- Plastina, P.; Benincasa, C.; Perri, E.; Fazio, A.; Augimeri, G.; Poland, M.; Witkamp, R.; Meijerink, J. Identification of hydroxytyrosyl oleate, a derivative of hydroxytyrosol with anti-inflammatory properties, in olive oil by-products. Food Chem. 2019, 279, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Munnier, E.; Al Assaad, A.; David, S.; Mahut, F.; Vayer, M.; Van Gheluwe, L.; Yvergnaux, F.; Sinturel, C.; Soucé, M.; Chourpa, I.; et al. Homogeneous distribution of fatty ester-based active cosmetic ingredients in hydrophilic thin films by means of nanodispersion. Int. J. Cosmet. Sci. 2020, 42, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Bayrasy, C.; Chabi, B.; Laguerre, M.; Lecomte, J.; Jublanc, E.; Villeneuve, P.; Wrutniak-Cabello, C.; Cabello, G. Boosting antioxidants by lipophilization: A strategy to increase cell uptake and target mitochondria. Pharm. Res. 2013, 30, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Procopio, A.; Celia, C.; Nardi, M.; Oliverio, M.; Paolino, D.; Sindona, G. Lipophilic Hydroxytyrosol Esters: Fatty Acid Conjugates for Potential Topical Administration. J. Nat. Prod. 2011, 74, 2377–2381. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Trujillo, M.; Pereira-Caro, G.; Madrona, A.; Cert, A.; Espartero, J.L. New Lipophilic Tyrosyl Esters. Comparative Antioxidant Evaluation with Hydroxytyrosyl Esters. J. Agric. Food Chem. 2008, 56, 10960–10966. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Melchioni, C.; Ramunno, A.; Romeo, G.; Uccella, N. Phenolic components of Olea Europaea isolation of tyrosol derivatives. Nat. Prod. Res. 2004, 18, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Perri, M.; Yap, J.L.; Fletcher, S.; Cione, E.; Kane, M.A. Therapeutic potential of Bcl-xL/Mcl-1 synthetic inhibitor JY-1-106 and retinoids for human triple-negative breast cancer treatment. Oncol. Lett. 2018, 15, 7231–7236. [Google Scholar] [CrossRef] [PubMed]
- Ferri, F.; Olivieri, F.; Cannataro, R.; Caroleo, M.C.; Cione, E. Phytomelatonin Regulates Keratinocytes Homeostasis Counteracting Aging Process. Cosmetics 2019, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Perri, M.; Pingitore, A.; Cione, E.; Vilardi, E.; Perrone, V.; Genchi, G. Proliferative and anti-proliferative effects of retinoic acid at doses similar to endogenous levels in Leydig MLTC-1/R2C/TM-3 cells. Biochim. Biophys. Acta 2010, 1800, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Cione, E.; Pingitore, A.; Perri, M.; Genchi, G. Influence of all-trans-retinoic acid on oxoglutarate carrier via retinoylation reaction. Biochim. Biophys. Acta 2009, 1791, 3–7. [Google Scholar] [CrossRef]
- Bernini, R.; Gilardini Montani, M.S.; Merendino, N.; Romani, A.; Velotti, F. Hydroxytyrosol-derived compounds: A basis for the creation of new pharmacological agents for cancer prevention and therapy. J. Med. Chem. 2015, 58, 9089–9107. [Google Scholar] [CrossRef]
- Olajide, T.M.; Liu, T.; Liu, H.; Weng, X. Antioxidant properties of two novel lipophilic derivatives of hydroxytyrosol. Food Chem. 2020, 315, 126197. [Google Scholar] [CrossRef] [PubMed]
- Plastina, P. Hydroxytyrosol and hydroxytyrosyl fatty esters: Occurrence and antiinflammatory properties. In Olives and Olive Oil in Health and Disease Prevention, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 547–555. [Google Scholar]
- Bernini, R.; Carastro, I.; Palmini, G.; Tanini, A.; Zonefrati, R.; Pinelli, P.; Brandi, M.L.; Romani, A. Lipophilization of Hydroxytyrosol-Enriched Fractions from Olea europaea L. Byproducts and Evaluation of the in Vitro Effects on a Model of Colorectal Cancer Cells. J. Agric. Food Chem. 2017, 65, 6506–6512. [Google Scholar] [CrossRef]
- Bernini, R.; Crisante, F.; Barontini, M.; Tofani, D.; Balducci, V.; Gambacorta, A. Synthesis and Structure/Antioxidant Activity Relationship of Novel Catecholic Antioxidant Structural Analogues to Hydroxytyrosol and Its Lipophilic Esters. J. Agric. Food Chem. 2012, 60, 7408–7416. [Google Scholar] [CrossRef] [PubMed]
- Bouallagui, Z.; Bouaziz, M.; Lassoued, S.; Engasser, J.M.; Ghoul, M.; Sayadi, S. Hydroxytyrosol acyl esters: Biosynthesis and activities. Appl. Biochem. Biotechnol. 2011, 163, 592–599. [Google Scholar] [CrossRef]
- Burattini, S.; Salucci, S.; Baldassarri, V.; Accorsi, A.; Piatti, E.; Madrona, A.; Espartero, J.L.; Candiracci, M.; Zappia, G.; Falcieri, E. Anti-apoptotic activity of hydroxytyrosol and hydroxytyrosyl laurate. Food Chem. Toxicol. 2013, 55, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Candiracci, M.; Madrona, A.; Espartero, J.L.; Zappia, G.; Piatti, E. Lipophilic hydroxytyrosol esters significantly improve the oxidative state of human red blood cells. J. Funct. Food 2016, 23, 339–347. [Google Scholar] [CrossRef]
- Funakohi-Tago, M.; Sakata, T.; Fujiwara, S.; Sakakura, A.; Sugai, T.; Tago, K.; Tamura, H. Hydroxytyrosol butyrate inhibits 6-OHDA-induced apoptosis through activation of the Nrf2/HO-1 axis in SH-SY5Y cells. Eur. J. Pharmacol. 2018, 834, 246–256. [Google Scholar] [CrossRef]
- Grasso, S.; Siracusa, L.; Spatafora, C.; Renis, M.; Tringali, C. Hydroxytyrosol lipophilic analogues: Enzymatic synthesis, radical scavenging activity and DNA oxidative damage protection. Bioorg. Chem. 2007, 35, 137–152. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, D.; Shahidi, F. Antioxidant properties of tyrosol and hydroxytyrosol saturated fatty acid esters. Food Chem. 2018, 245, 1262–1268. [Google Scholar] [CrossRef]
- Tofani, D.; Balducci, V.; Gasperi, T.; Incerpi, S.; Gambacorta, A. Fatty Acid Hydroxytyrosyl Esters: Structure/Antioxidant Activity Relationship by ABTS and in Cell-Culture DCF Assays. J. Agric. Food Chem. 2010, 58, 5292–5299. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Sun, Y.X.; Shahidi, F. Preparation and antioxidant activity of tyrosol and hydroxytyrosol esters. J. Funct. Food 2017, 37, 66–73. [Google Scholar] [CrossRef]
- Mateos, R.; Pereira-Caro, G.; Saha, S.; Cert, R.; Redondo-Horcajo, M.; Bravo, L.; Kroon, P.A. Acetylation of hydroxytyrosol enhances its transport across differentiated Caco-2 cell monolayers. Food Chem. 2011, 125, 865–872. [Google Scholar] [CrossRef]
- Tabernero, M.; Sarriá, B.; Largo, C.; Martínez-López, S.; Madrona, A.; Espartero, J.L.; Bravo, L.; Mateos, R. Comparative evaluation of the metabolic effects of hydroxytyrosol and its lipophilic derivatives (hydroxytyrosyl acetate and ethyl hydroxytyrosyl ether) in hypercholesterolemic rats. Food Funct. 2014, 5, 1556–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, F.; Hu, X.; Zhou, D.; Ma, X.; Tian, X.; Huo, X.; Rakariyatham, K.; Shahidi, F.; Zhu, B. Hydrolysis and Transport Characteristics of Tyrosol Acyl Esters in Rat Intestine. J. Agric. Food Chem. 2018, 66, 12521–12526. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Wang, X.; Hu, Y.; Xie, H.; Liu, X.; Qin, L.; Zhang, J.; Zhou, D.; Shahidi, F. Evaluation of Absorption and Plasma Pharmacokinetics of Tyrosol Acyl Esters in Rats. J. Agric. Food Chem. 2020, 68, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Akanbi, T.O.; Barrow, C.J. Lipase-Produced Hydroxytyrosyl Eicosapentaenoate is an Excellent Antioxidant for the Stabilization of Omega-3 Bulk Oils, Emulsions and Microcapsules. Molecules 2018, 23, 275. [Google Scholar] [CrossRef] [Green Version]
- Balducci, V.; Incerpi, S.; Stano, P.; Tofani, D. Antioxidant activity of hydroxytyrosyl esters studied in liposome models. Biochim. Biophys. Acta Biomembr. 2018, 1860, 600–610. [Google Scholar] [CrossRef]
- Almeida, J.; Losada-Barreiro, S.; Costa, M.; Paiva-Martins, F.; Bravo-Díaz, C.; Romsted, L.S. Interfacial Concentrations of Hydroxytyrosol and Its Lipophilic Esters in Intact Olive Oil-in-Water Emulsions: Effects of Antioxidant Hydrophobicity, Surfactant Concentration, and the Oil-to-Water Ratio on the Oxidative Stability of the Emulsions. J. Agric. Food Chem. 2016, 64, 5274–5283. [Google Scholar] [CrossRef]
- Evans, K.O.; Laszlo, J.A.; Compton, D.L. Hydroxytyrosol and tyrosol esters partitioning into, location within, and effect on DOPC liposome bilayer behavior. Biochim. Biophys. Acta 2015, 1848, 1175–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, R.; Comelles, F.; Alcántara, D.; Maldonado, O.S.; Curcuroze, M.; Parra, J.L.; Moreales, J.C. Surface-active properties of lipophilic antioxidants tyrosol and hydroxytyrosol fatty acid esters: A potential explanation for the nonlinear hypothesis of the antioxidant activity in oil-in-water emulsions. J. Agric. Food Chem. 2010, 58, 8021–8026. [Google Scholar] [CrossRef]
- Marzocchi, S.; Caboni, M.F. Study of the Effect of Tyrosyl Oleate on Lipid Oxidation in a Typical Italian Bakery Product. J. Agric. Food Chem. 2018, 66, 12555–12560. [Google Scholar] [CrossRef] [PubMed]
- Medina, I.; Lois, S.; Alcántara, D.; Lucas, R.; Morales, J.C. Effect of lipophilization of hydroxytyrosol on its antioxidant activity in fish oils and fish oil-in-water emulsions. J. Agric. Food Chem. 2009, 57, 9773–9779. [Google Scholar] [CrossRef]
- Torres de Pinedo, A.; Peñalver, P.; Pérez-Victoria, I.; Rondón, D.; Morales, J.C. Synthesis of new phenolic fatty acid esters and their evaluation as lipophilic antioxidants in an oil matrix. Food Chem. 2007, 105, 657–665. [Google Scholar] [CrossRef]
- Trujillo, M.; Mateos, R.; de Teran, L.C.; Espartero, J.L.; Cert, R.; Jover, M.; Alcudia, F.; Bautista, J.; Cert, A.; Parrado, J. Lipophilic hydroxytyrosyl esters. Antioxidant activity in lipid matrices and biological systems. J. Agric. Food Chem. 2006, 54, 3779–3785. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Aissa, I.; Kharrat, N.; Aloui, F.; Sellami, M.; Bouaziz, M.; Gargouri, Y. Valorization of antioxidants extracted from olive mill wastewater. Biotechnol. Appl. Biochem. 2017, 64, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545. [Google Scholar] [CrossRef] [Green Version]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Kwon, M.; Joung, E.-J.; Shin, T.; Oh, C.-W.; Choi, J.S.; Kim, H.-R. Meroterpenoid-Rich Fraction of the Ethanolic Extract from Sargassum serratifolium Suppressed Oxidative Stress Induced by Tert-Butyl Hydroperoxide in HepG2 Cells. Mar. Drugs 2018, 16, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Gomes, E.C.; Silva, A.N.; de Oliveira, M.R. Oxidants, Antioxidants, and the Beneficial Roles of Exercise-Induced Production of Reactive Species. Oxid. Med. Cell. Longev. 2012, 2012, 756132. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnar, J.A.; Underdown, M.J.; Clark, W.A. Nutrition and Chronic Wounds. Adv. Wound Care 2014, 3, 663–681. [Google Scholar] [CrossRef] [PubMed]

| Sample | Cultivar | Campaign | ExtractionMethod | TyOle(mg·kg−1) |
|---|---|---|---|---|
| Fruit#1 | Carolea | 2017/2018 | 1 and 2 | <LOD |
| Fruit#2 | Cassanese | 2017/2018 | 1 and 2 | <LOD |
| Fruit#3 | Coratina | 2017/2018 | 1 and 2 | <LOD |
| Fruit#4 | Grossa di Spagna | 2017/2018 | 1 and 2 | <LOD |
| Fruit#5 | Nocellara del Belice | 2017/2018 | 1 and 2 | <LOD |
| EVOO#1 | Carolea | 2017/2018 | 2 | 1.18 ± 0.08 |
| EVOO#2 | Dolce di Rossano | 2017/2018 | 2 | 0.7 ± 0.2 |
| EVOO#3 | Nocellara del Belice | 2017/2018 | 2 | 0.17 ± 0.08 |
| DOO#1 | Blend | 2016/2017 | 2 | 1.0 ± 0.3 |
| DOO#2 | Blend | 2014/2015 | 2 | 5.0 ± 0.8 |
| DOO#3 | Blend | 2014/2015 | 2 | 7.0 ± 0.4 |
| DOO#3 | Blend | 2014/2015 | 1 | 2.4 ± 0.1 |
| DDVOP | 2017/2018 | 2 | 8.2 ± 0.2 | |
| DDVOPO | 2017/2018 | 2 | 33.2 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benincasa, C.; La Torre, C.; Fazio, A.; Perri, E.; Caroleo, M.C.; Plastina, P.; Cione, E. Identification of Tyrosyl Oleate as a Novel Olive Oil Lipophenol with Proliferative and Antioxidant Properties in Human Keratinocytes. Antioxidants 2021, 10, 1051. https://doi.org/10.3390/antiox10071051
Benincasa C, La Torre C, Fazio A, Perri E, Caroleo MC, Plastina P, Cione E. Identification of Tyrosyl Oleate as a Novel Olive Oil Lipophenol with Proliferative and Antioxidant Properties in Human Keratinocytes. Antioxidants. 2021; 10(7):1051. https://doi.org/10.3390/antiox10071051
Chicago/Turabian StyleBenincasa, Cinzia, Chiara La Torre, Alessia Fazio, Enzo Perri, Maria Cristina Caroleo, Pierluigi Plastina, and Erika Cione. 2021. "Identification of Tyrosyl Oleate as a Novel Olive Oil Lipophenol with Proliferative and Antioxidant Properties in Human Keratinocytes" Antioxidants 10, no. 7: 1051. https://doi.org/10.3390/antiox10071051
APA StyleBenincasa, C., La Torre, C., Fazio, A., Perri, E., Caroleo, M. C., Plastina, P., & Cione, E. (2021). Identification of Tyrosyl Oleate as a Novel Olive Oil Lipophenol with Proliferative and Antioxidant Properties in Human Keratinocytes. Antioxidants, 10(7), 1051. https://doi.org/10.3390/antiox10071051