Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review
Abstract
:1. Introduction
2. Chokeberries Nutritional Profile
2.1. Dietary Fiber
2.2. Fat
2.3. Organic Acids
2.4. Proteins and Amino Acids
2.5. Sugar
2.6. Vitamins, Minerals and Trace Compounds
2.7. Aroma Components
2.8. Polyphenols
2.9. Procyanidins
2.10. Anthocyanins
2.11. Phenolic Acids
2.12. Flavonols
| Phenolic Constituents | Substituents | Chemical Structure | ||
|---|---|---|---|---|
| Cyanidin-3-O-galactoside | R1 = galactose | R2 = OH | R3 = OH | 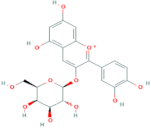 |
| Cyanidin-3-O-glucoside | R1 = glucose | R2 = OH | R3 = OH | 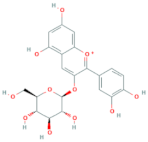 |
| Cyanidin-3-O-xyloside | R1 = xylose | R2 = OH | R3 = OH | 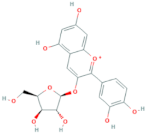 |
| Cyanidin-3-O-arabinoside | R1 = arabinose | R2 = OH | R3 = OH | 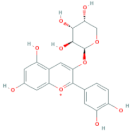 |
| Pelargonidin-3-O-arabinoside | R1 = arabinose | R2 = H | R3 = OH | 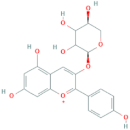 |
| Pelargonidin-3-O-galactoside | R1 = galactose | R2 = H | R3 = OH | 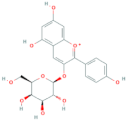 |
| Phenolic Constituents | Substituents | Chemical Structures |
|---|---|---|
| Quercetin-3-O-vicianoside | R = vicianose | 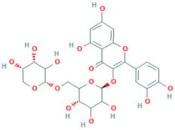 |
| Quercetin-3-O-robinobioside | R = robinose |  |
| Quercetin-3-O-rutinoside | R = rutinose | 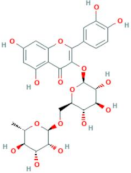 |
| Quercetin-3-O-galactoside | R = galactose | 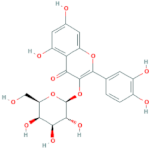 |
| Quercetin-3-O-glucoside | R = glucose | 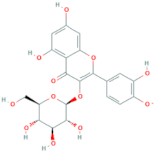 |
| Quercetin-3-arabinoside | R = arabinose | 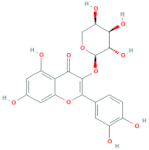 |
| Quercetin-3-O-glucuronide | R = glucuronide |  |
| Quercetin-3-O-xyloside | R = Xylose | 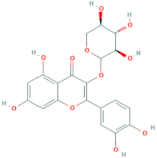 |
| Quercetin-3-O-arabinoglucoside | R = arabinoglucoside |  |
| Quercetin-3-O-(6′-malonyl)-glucoside | R = malonyl-glucoside | 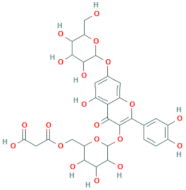 |
3. Beneficial Health Properties of Chokeberry Fruits
3.1. Antioxidant Effects
3.2. Anti-Inflammatory Activity
3.3. Antidiabetic Activity
3.4. Antibacterial Activity
3.5. Antiviral Activity
3.6. Antimutagenic and Anticancer Activity
3.7. Cardiovascular Disorder
3.8. Hepatoprotective Activity
3.9. Overweight and Obesity
3.10. Other Health Problems
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABTS | 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonate) |
| CCl4 | Carbon tetrachloride |
| COXs | Cyclooxygenases |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DM | Dry matter |
| DP | Degree of polymerization |
| DW | Dry weight |
| FW | Fresh weight |
| HFD | High fat diet |
| IL-1 | Interleukin-1 |
| LDL | Low-density lipoprotein |
| iNOS | Inducible nitric oxide synthetase |
| ORAC | Oxygen radical absorbance capacity |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| TNF-α | Tumor necrosis factor α |
| TRAP | Total radical-trapping antioxidant parameters |
References
- Buda, V.; Andor, M.; Diana, A.; Ardelean, F.; Pavel, I.Z.; Dehelean, C.; Soica, C.; Folescu, R.; Andrei, F.; Danciu, C. Cardioprotective Effects of Cultivated Black Chokeberries (Aronia spp.): Traditional Uses, Phytochemistry and Therapeutic Effects. In Natural Products-From Bioactive Molecules to Human Health; IntechOpen: London, UK, 2020. [Google Scholar]
- Borowska, S.; Brzóska, M.M. Chokeberries (Aronia Melanocarpa) and Their Products as a Possible Means for the Prevention and Treatment of Noncommunicable Diseases and Unfavorable Health Effects Due to Exposure to Xenobiotics. Compr. Rev. Food Sci. Food Saf. 2016, 15, 982–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. AroniaPlants: A Review of Traditional Use, Biological Activities, and Perspectives for Modern Medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef] [Green Version]
- Kulling, S.E.; Rawel, H. Chokeberry (Aronia Melanocarpa)—A Review on the Characteristic Components and Potential Health Effects. Planta Medica 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ćujić, N.; Kardum, N.; Šavikin, K.; Zdunić, G.; Janković, T.; Menković, N. Potential of Chokeberry (Aronia Melanocarpa L.) as a Therapeutic Food. In Handbook of Food Bioengieneering; Holban, A.M., Grumezescu, A.M., Eds.; Andre Gerhard Wolff: London, UK, 2018; Volume 8, pp. 209–237. [Google Scholar]
- Ochmian, I.D.; Grajkowski, J.; Smolik, M. Comparison of Some Morphological Features, Quality and Chemical Content of Four Cultivars of Chokeberry Fruits (Aronia Melanocarpa). Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 253. [Google Scholar] [CrossRef] [Green Version]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavová, J. Fruits of Black Chokeberry Aronia Melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Kokotkiewicz, A.; Kubica, P.; Banaszczak, P.; Wojtanowska-Krośniak, A.; Krosniak, M.; Marzec-Wróblewska, U.; Badura, A.; Zagrodzki, P.; Bucinski, A.; et al. Comparative analysis of different groups of phenolic compounds in fruit and leaf extracts of Aronia sp.: A. melanocarpa, A. arbutifolia, and A. ×prunifolia and their antioxidant activities. Eur. Food Res. Technol. 2017, 243, 1645–1657. [Google Scholar] [CrossRef] [Green Version]
- Oszmiański, J.; Wojdylo, A. Aronia Melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Tolić, M.-T.; Krbavčić, I.P.; Vujević, P.; Milinović, B.; Jurčević, I.L.; Vahčić, N. Effects of Weather Conditions on Phenolic Content and Antioxidant Capacity in Juice of Chokeberries (Aronia Melanocarpa L.). Pol. J. Food Nutr. Sci. 2017, 67, 67–74. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Skupien, K.; Oszmianski, J. The effect of mineral fertilization on nutritive value and biological activity of chokeberry fruit. Agric. Food Sci. 2007, 16, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Mayer-Miebach, E.; Adamiuk, M.; Behsnilian, D. Stability of Chokeberry Bioactive Polyphenols during Juice Processing and Stabilization of a Polyphenol-Rich Material from the By-Product. Agriculture 2012, 2, 244–258. [Google Scholar] [CrossRef] [Green Version]
- Sójka, M.; Kołodziejczyk, K.; Milala, J. Polyphenolic and basic chemical composition of black chokeberry industrial by-products. Ind. Crop. Prod. 2013, 51, 77–86. [Google Scholar] [CrossRef]
- Yamane, T.; Kozuka, M.; Wada-Yoneta, M.; Sakamoto, T.; Nakagaki, T.; Nakano, Y.; Ohkubo, I. Aronia juice suppresses the elevation of postprandial blood glucose levels in adult healthy Japanese. Clin. Nutr. Exp. 2017, 12, 20–26. [Google Scholar] [CrossRef] [Green Version]
- German Nutrition Society. D-A-CH-Referenzwerte Für Die Nährstoffzufuhr. 2018. Available online: https://www.dge.de/wissenschaft/referenzwerte/ (accessed on 29 September 2020).
- Wawer, I.; Wolniak, M.; Paradowska, K. Solid state NMR study of dietary fiber powders from aronia, bilberry, black currant and apple. Solid State Nucl. Magn. Reson. 2006, 30, 106–113. [Google Scholar] [CrossRef]
- Nawirska, A.; Kwaśniewska, M. Dietary fibre fractions from fruit and vegetable processing waste. Food Chem. 2005, 91, 221–225. [Google Scholar] [CrossRef]
- Zlatanov, M.D. Lipid composition of Bulgarian chokeberry, black currant and rose hip seed oils. J. Sci. Food Agric. 1999, 79, 1620–1624. [Google Scholar] [CrossRef]
- Pieszka, M.; Gogol, P.; Pietras, M.; Pieszka, M. Valuable Components of Dried Pomaces of Chokeberry, Black Currant, Strawberry, Apple and Carrot as a Source of Natural Antioxidants and Nutraceuticals in the Animal Diet. Ann. Anim. Sci. 2015, 15, 475–491. [Google Scholar] [CrossRef] [Green Version]
- Boncheva, M.; Georgiev, G.; Shishov, V. Effects of Aronia Melanocarpa fruit juice in improving medical test results and creating a feeling of health in patients with non-alcoholic fatty liver disease-NAFLD (steatosis). J Gen Med. Bulgaria 2013, 2, 21–30. [Google Scholar]
- Denev, P.; Kratchanova, M.; Petrova, I.; Klisurova, D.; Georgiev, Y.; Ognyanov, M.; Yanakieva, I. Black Chokeberry (Aronia Melanocarpa (Michx.) Elliot) Fruits and Functional Drinks Differ Significantly in Their Chemical Composition and Antioxidant Activity. J. Chem. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sidor, A.; Gramza-Michałowska, A. Black Chokeberry Aronia Melanocarpa L.—A Qualitative Composition, Phenolic Profile and Antioxidant Potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [Green Version]
- Pavlovic, A.N.; Brcanović, J.M.; Veljković, J.N.; Mitic, S.S.; Tošić, S.B.; Kaličanin, B.M.; Kostic, D.A.; Ðorđević, M.S.; Velimirović, D.S. Characterization of commercially available products of aronia according to their metal content. Fruits 2015, 70, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Romani, A.; Vignolini, P.; Ieri, F.; Heimler, D. Polyphenols and Volatile Compounds in Commercial Chokeberry (Aronia Melanocarpa) Products. Nat. Prod. Commun. 2016, 11, 99–102. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Oke, M.O.; Olaniyan, S.A.; Ajala, A.S. A Review of Cyanogenic Glycosides in Edible Plants. In Toxicology—New Aspects to This Scientific Conundrum; Larramendy, M.L., Soleneski, S., Eds.; InTech: London, UK, 2016; pp. 179–191. [Google Scholar]
- Jakobek, L.; Šeruga, M.; Medvidović-Kosanović, M.; Novak, I. Antioxidant activity and polyphenols of Aronia in comparison to other berry species. Agric. Conspec. Sci. 2007, 72, 301–306. [Google Scholar]
- Gralec, I.W.M. Aronia Melanocarpa berries: Phenolics composition and antioxidant properties changes during fruit development and ripening. Emir. J. Food Agric. 2019, 31, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Kobus, Z.; Nadulski, R.; Wilczyński, K.; Kozak, M.; Guz, T.; Rydzak, L. Effect of the black chokeberry (Aronia Melanocarpa (Michx.) Elliott) juice acquisition method on the content of polyphenols and antioxidant activity. PLoS ONE 2019, 14, e0219585. [Google Scholar] [CrossRef] [Green Version]
- Tolić, M.-T.; Jurčević, I.L.; Krbavčić, I.P.; Marković, K.; Vahčić, N. Phenolic Content, Antioxidant Capacity and Quality of Chokeberry (Aronia Melanocarpa) Products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-Rich Extract From Aronia meloncarpa E. Induces a Cell Cycle Block in Colon Cancer but Not Normal Colonic Cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, D. Black chokeberry (Aronia Melanocarpa) polyphenols reveal differ-ent antioxidant, antimicrobial and neutrophil-modulating activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, Anthocyanins and Procyanidins from Aronia Melanocarpa as Radical Scavengers and Enzyme Inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef] [Green Version]
- National Library of Medicine, National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 23 June 2021).
- Pilaczynska-Szczesniak, L.; Skarpanska-Steinborn, A.; Deskur, E.; Basta, P.; Horoszkiewicz-Hassan, M. The Influence of Chokeberry Juice Supplementation on the Reduction of Oxidative Stress Resulting from an Incremental Rowing Ergometer Exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, K.; Ilieva, I.; Shiratori, K.; Koyama, Y.; Yoshida, K.; Kase, S.; Suzuki, Y.; Ohno, S.; Jin, X.-H.; Kitaichi, N.; et al. Anti-inflammatory Effects of Aronia Extract on Rat Endotoxin-Induced Uveitis. Investig. Opthalmology Vis. Sci. 2005, 46, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zapolska-Downar, D.; Bryk, D.; Małecki, M.; Hajdukiewicz, K.; Sitkiewicz, D. Aronia Melanocarpa fruit extract exhibits anti-inflammatory activity in human aortic endothelial cells. Eur. J. Nutr. 2012, 51, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Jang, B.-K.; Lee, J.-W.; Choi, H.; Yim, S.-V. Aronia melanocarpa Fruit Bioactive Fraction Attenuates LPS-Induced Inflammatory Response in Human Bronchial Epithelial Cells. Antioxidants 2020, 9, 816. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Tancheva, S.; Belcheva, A. Hypoglycemic and hypolipidemic effects of Aronia Melanocarpa fruit juice in streptozotocin-induced diabetic rats. Methods Find Exp. Clin. Pharmacol. 2007, 29, 101–106. [Google Scholar] [CrossRef]
- Kim, S.-S.; Shin, Y. Antibacterial and in vitro antidementia effects of aronia (Aronia Melanocarpa) leaf extracts. Food Sci. Biotechnol. 2020, 29, 1295–1300. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.I.; Lee, I.; Lee, S.; Hwang, M.-W.; Bae, J.-Y.; Heo, J.; Kim, D.; Han, S.-Z.; Park, M.-S. Aronia Melanocarpa and its components demonstrate antiviral activity against influenza viruses. Biochem. Biophys. Res. Commun. 2013, 440, 14–19. [Google Scholar] [CrossRef]
- Chojnacka, K.; Witek-Krowiak, A.; Skrzypczak, D.; Mikula, K.; Młynarz, P. Phytochemicals containing biologically active polyphenols as an effective agent against Covid-19-inducing coronavirus. J. Funct. Foods 2020, 73, 104146. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Wu, K.-L.; Zhang, X.; Deng, S.-Q.; Peng, B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J. Integr. Med. 2020, 18, 152–158. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Thi, N.D. Anti-cancer and anti-inflammatory activities of aronia (Aronia Melanocarpa) leaves. Asian Pac. J. Trop. Biomed. 2018, 8, 586. [Google Scholar] [CrossRef]
- Sharif, T.; Alhosin, M.; Auger, C.; Minker, C.; Kim, J.-H.; Etienne-Selloum, N.; Bories, P.; Gronemeyer, H.; Lobstein, A.; Bronner, C.; et al. Aronia Melanocarpa Juice Induces a Redox-Sensitive p73-Related Caspase 3-Dependent Apoptosis in Human Leukemia Cells. PLoS ONE 2012, 7, e32526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciocoiu, M.; Badescu, L.; Miron, A.; Badescu, M. The Involvement of a Polyphenol-Rich Extract of Black Chokeberry in Oxidative Stress on Experimental Arterial Hypertension. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Skoczyñska, A.; Jêdrychowska, I.; Porêba, R.; Affelska-Jercha, A.; Turczyn, B.; Wojakowska, A.; Andrzejak, R. Influ-ence of chokeberry juice on arterial blood pressure and lipid parameters in men with mild hypercholesterolemia. Pharmacol. Rep. 2007, 59, 177–182. [Google Scholar]
- Poreba, R.; Skoczynska, A.; Gac, P.; Poreba, M.; Jedrychowska, I.; Affelska-Jercha, A.; Turczyn, B.; Wojakowska, A.; Oszmianski, J.; Andrzejak, R. Drinking of chokeberry juice from the ecological farm Dzieciolowo and distensibility of brachial artery in men with mild hypercholesterolemia. Ann. Agric. Environ. Med. 2009, 16, 305–308. [Google Scholar]
- Bijak, M.; Bobrowski, M.; Borowiecka, M.; Podsędek, A.; Golański, J.; Nowak, P. Anticoagulant effect of polyphenols-rich extracts from black chokeberry and grape seeds. Fitoterapia 2011, 82, 811–817. [Google Scholar] [CrossRef]
- Olas, B.; Wachowicz, B.; Tomczak, A.; Erler, J.; Stochmal, A.; Oleszek, W. Comparative anti-platelet and antioxidant properties of polyphenol-rich extracts from: Berries of Aronia Melanocarpa, seeds of grape and bark of Yucca schidigerain vitro. Platelets 2008, 19, 70–77. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Borisova, P.; Galunska, B.; Krasnaliev, I.; Belcheva, A. Hepatoprotective effect of the natural fruit juice from Aronia Melanocarpa on carbon tetrachloride-induced acute liver damage in rats. Exp. Toxicol. Pathol. 2004, 56, 195–201. [Google Scholar] [CrossRef]
- Yang, J.; Gao, J.; Yu, W.; Hao, R.; Fan, J.; Wei, J. The effects and mechanism of Aronia Melanocarpa Elliot anthocyanins on hepatic fibrosis. J. Funct. Foods 2020, 68, 103897. [Google Scholar] [CrossRef]
- Kowalczyk, E.; Kopff, A.; Fijałkowski, P.; Kopff, M.; Niedworok, J.; Błaszczyk, J.; Kedziora, J.; Tyślerowicz, P. Effect of anthocyanins on selected biochemical parameters in rats exposed to cadmium. Acta Biochim. Pol. 2003, 50, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Kozłowska, M.; Brzóska, M.M.; Rogalska, J.; Galicka, A. The Impact of a Polyphenol-Rich Extract from the Berries of Aronia melanocarpa L. on Collagen Metabolism in the Liver: A Study in an In Vivo Model of Human Environmental Exposure to Cadmium. Nutrients 2020, 12, 2766. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhao, X.; Liu, S.; Wang, D. Aronia Melanocarpa Prevents Alcohol-Induced Chronic Liver Injury via Regulation of Nrf2 Signaling in C57BL/6 Mice. Oxidative Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [Green Version]
- World Health Organisation. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 November 2019).
- Kim, N.-H.; Jegal, J.; Na Kim, Y.; Heo, J.-D.; Rho, J.-R.; Yang, M.H.; Jeong, E.J. Chokeberry Extract and Its Active Polyphenols Suppress Adipogenesis in 3T3-L1 Adipocytes and Modulates Fat Accumulation and Insulin Resistance in Diet-Induced Obese Mice. Nutrients 2018, 10, 1734. [Google Scholar] [CrossRef] [Green Version]
- Sosnowska, D.; Podsędek, A.; Kucharska, A.Z.; Redzynia, M.; Opęchowska, M.; Koziołkiewicz, M. Comparison of in vitro anti-lipase and antioxidant activities, and composition of commercial chokeberry juices. Eur. Food Res. Technol. 2016, 242, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Sonoda, K.; Aoi, W.; Iwata, T.; Li, Y. Anthocyanin-rich Aronia Melanocarpa extract improves body temperature maintenance in healthy women with a cold constitution. SpringerPlus 2013, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
| Chemical Composition | Berries g/kg | References | Juice g/L | References | Pomace g/kg | References |
|---|---|---|---|---|---|---|
| Dry matter % | 15–31 | [6,13] | 11–17 | [14] | 45–50 | [14] |
| pH | 3.3–3.7 | [4] | ||||
| Titratable acidity (g citric acid per 100 g) | 0.5–1 | [6,13] | 0.9–1 | [11] | 0.5–0.6 | [15] |
| Total sugar | 68–158 | [14] | 110–143 | [14] | 84 | [14] |
| Glucose | 11–40 | 32–40 | 22 | |||
| Fructose | 14–42 | 30–39 | 24 | |||
| Sorbitol | 44–76 | 48–64 | 38 | |||
| Total polyphenols (Chromatographic method) | 79 | [9] | 4.7–9.0 | [14] | 31–63 | [14] |
| Fiber | 56 | [4] | 3 | [16] | 630–780 | [15] |
| Minerals | 4–6 | 5 | 14–39 | |||
| Fat | 1.4 | <1 | 29–139 | |||
| Proteins | 7 | 2 | 49–241 | |||
| Amygdalin mg/100 g | 20 | [15] | 5.8 | [15] | 7–185 | [15] |
| Ingredient | Amount in 100 g [4] | Recommended Daily Intake [17] | Percent Daily Value | |||
|---|---|---|---|---|---|---|
| 1–4 Years Old | 4–15 Years Old | After 15 Years Old | Average | % | ||
| Energy (kcal) | ||||||
| Carbohydrates (Kcal) | 60 | 1100–1300 | 1300–2900 | 2000–3400 | 2000 | 3 |
| Proteins (g) | 0.7 | 14 | 18–50 | 48–67 | 40 | 2 |
| Fats (g) | 0.14 | - | - | - | - | |
| Minerals (mg) | ||||||
| Sodium (Na) | 2.6 | 400 | 500–1400 | 1500 | 950 | 0.3 |
| Calcium (Ca) | 32 | 600 | 750–1200 | 1200 | 900 | 4 |
| Potassium (K) | 218 | 1100 | 1300–3600 | 4000 | 2550 | 9 |
| Magnesium (Mg) | 16 | 80 | 120–310 | 350–400 | 240 | 7 |
| Zinc (Zn) | 0.2 | 3 | 4–12 | 11–16 | 10 | 2 |
| Iron (Fe) | 0.9 | 8 | 8–15 | 12–15 | 12 | 8 |
| Vitamins (mg) | ||||||
| Thiamin (B1) | 0.02 | 0.6 | 0.7–1.2 | 1.1–1.4 | 1 | 2 |
| Riboflavin (B2) | 0.02 | 0.7 | 0.8–1.4 | 1.2–1.6 | 1 | 2 |
| Pyridoxin (B6) | 0.03 | 0.6 | 0.7–1.5 | 1.4–1.6 | 1 | 3 |
| Niacin | 0.3 | 8 | 9–15 | 13–17 | 12 | 3 |
| Phantothenic acid | 0.3 | 4 | 4–6 | 6 | 5 | 6 |
| Ascorbic acid (C) | 14 | 20 | 30–85 | 90–110 | 65 | 22 |
| Tocopherols (E) | 1.7 | 5–6 | 8–14 | 12–15 | 10 | 17 |
| Folate/µg | 20 | 120 | 140–300 | 300 | 210 | 10 |
| Phyllochinon (K)/µg | 24 | 15 | 20–50 | 60–80 | 48 | 50 |
| Elements | Berries [25] | Juice [25] | Pomace [15] | Leaves [25] |
|---|---|---|---|---|
| K | 271–498 | 85–320 | 181–308 | 262 |
| Ca | 60–117 | 14–123 | 219–408 | 373 |
| P | 24–96 | 17–104 | 239 | 151 |
| Mg | 16–58 | 21–59 | 37–250 | 83 |
| Na | 1–2 | 2–6 | 5–9 | 2 |
| Zn | 0.4–0.8 | 0.1–0.3 | 0.6–3.7 | 1.2 |
| Fe | 0.9–1.4 | 0.7–2.5 | 7.5–8.6 | 2.2 |
| Se | 0.02 | 0.07–0.1 | 0.05 | |
| Cu | 0.08–0.2 | 0.1–0.5 | 0.5–1.2 | 0.4 |
| Mo | 0.002 | 0.005–0.006 | 0.008 | |
| Cr | 0.05 | 0.06–0.07 | 0.05 | |
| Mn | 0.5–1.8 | 0.3–1.2 | 3.2 | 0.6 |
| Si | 0.2–0.6 | 0.3–0.7 | 0.6 | |
| Ni | 0.01–0.07 | 0.01–0.09 | 0.01 | |
| B | 0.3–1.4 | 0.1–0.9 | 0.5 | |
| V | 0.04–0.2 | 0.1 | 0.2 | |
| Pb | 0.005–0.009 | 0.006–0.01 | 0.005 | |
| Cd | 0.02–0.004 | 0.005–0.006 | 0.002 | |
| As | 0.03–0.04 | 0.06–0.08 | 0.03 |
| Phenolic Constituents | Fruit mg/100 g DW [8,9] | Fruit mg/100 g FW [6,13,29] | Pomace mg/100 g DW [9] | Juice mg/100 g DW [9] | Leaves mg/100 g DW [8] |
|---|---|---|---|---|---|
| Flavan-3-ol | |||||
| (−)-Epicatechin | 15 | 32 | 11 | 13 | |
| Procyanidins | 5182 | 1646 | 8192 | 1579 | |
| Degree polymerization (DP) | 23 | 59 | 34 | 23 | |
| Anthocyanins | |||||
| Cyanidin-3-O-galactoside | 19–1282 | 417–636 | 1120 | 787 | 0.2–2 |
| Cyanidin-3-O-glucoside | 0.3–42 | 8–27 | 79 | 28 | |
| Cyanidin-3-O-arabinoside | 6.2–582 | 129–299 | 533 | 324 | 0.2 |
| Cyanidin-3-O-xyloside | 53 | 29–38 | 105 | 34 | |
| Phenolic acids | |||||
| Chlorogenic acid | 16–302 | 72–111 | 204 | 416 | 184–706 |
| Neochlorogenic acid | 92–291 | 59–100 | 169 | 393 | 143–483 |
| 3,4-Dihydroxyphenylacetic acid | 4–26 | 5,8–66 | |||
| Protocatechuic acid | 0.4–31 | 2–9 | |||
| Rosmarinic acid | 9–18 | 23–155 | |||
| Flavonols | |||||
| Quercetin | 12–44 | 7.1 | 83–316 | ||
| Quercetin-3-O-galactoside | 37 | 7–13 | 47 | 50 | |
| Quercetin-3-O-glucoside | 22 | 4 | 27 | 31 | |
| Quercetin-3-O-rutinoside | 15 | 4 | 14 | 28 | 62–103 |
| Quercetin-3-O-rhamnoside | 97–367 | ||||
| Quercetin-3-O-vicianoside | 3–5 | ||||
| Quercetin-3-O-robinobioside | 1–5 | ||||
| Quercetin derivates unidentified | 27 | 82 | 47 | ||
| Kaempferol | 0,5 | ||||
| Flavanon | |||||
| Eriodictyol-7-O-glucuronide | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurendić, T.; Ščetar, M. Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants 2021, 10, 1052. https://doi.org/10.3390/antiox10071052
Jurendić T, Ščetar M. Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants. 2021; 10(7):1052. https://doi.org/10.3390/antiox10071052
Chicago/Turabian StyleJurendić, Tomislav, and Mario Ščetar. 2021. "Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review" Antioxidants 10, no. 7: 1052. https://doi.org/10.3390/antiox10071052
APA StyleJurendić, T., & Ščetar, M. (2021). Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants, 10(7), 1052. https://doi.org/10.3390/antiox10071052







