Mint Oils: In Vitro Ability to Perform Anti-Inflammatory, Antioxidant, and Antimicrobial Activities and to Enhance Intestinal Barrier Integrity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vitro Anti-Inflammatory Assays
2.2.1. Collection of Porcine Alveolar Macrophages
2.2.2. Cell Culture and Experimental Design
2.2.3. Detection of Cell Viability
2.2.4. Measurements of Pro-Inflammatory Cytokines
2.3. Transepithelial Electrical Resistance Assay
2.4. Antioxidant Assays
2.4.1. DPPH Radical Scavenging Capacity Assay
2.4.2. Reducing Power Assay
2.5. Escherichia coli Growth Inhibitory Activity
2.6. Statistical Analysis
3. Results
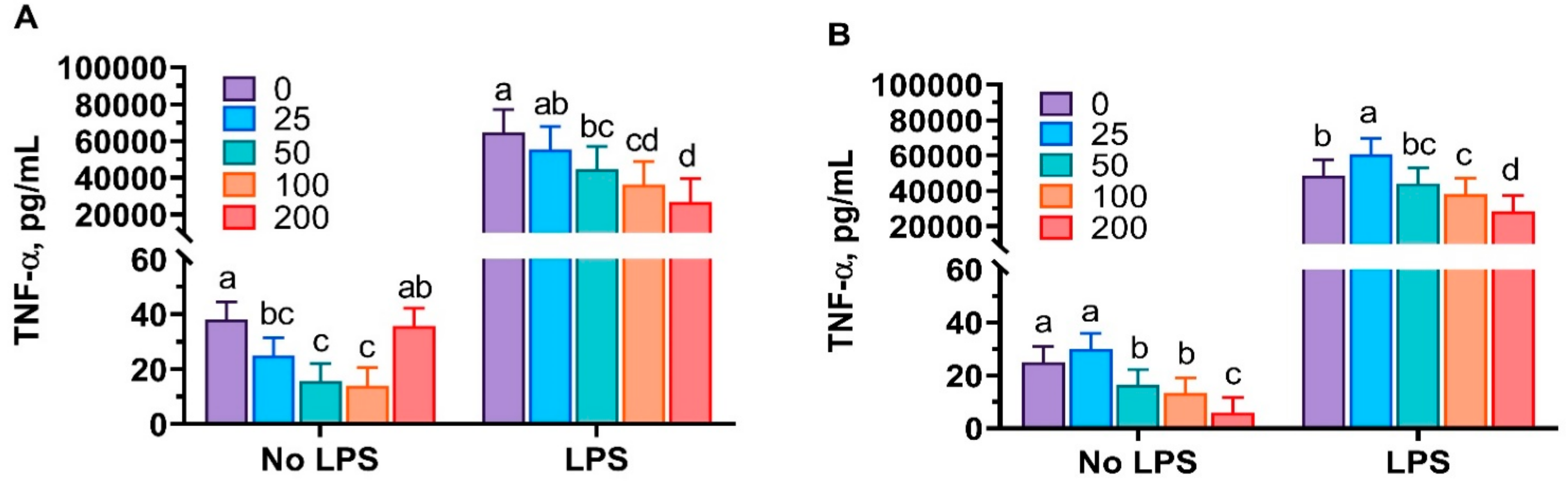
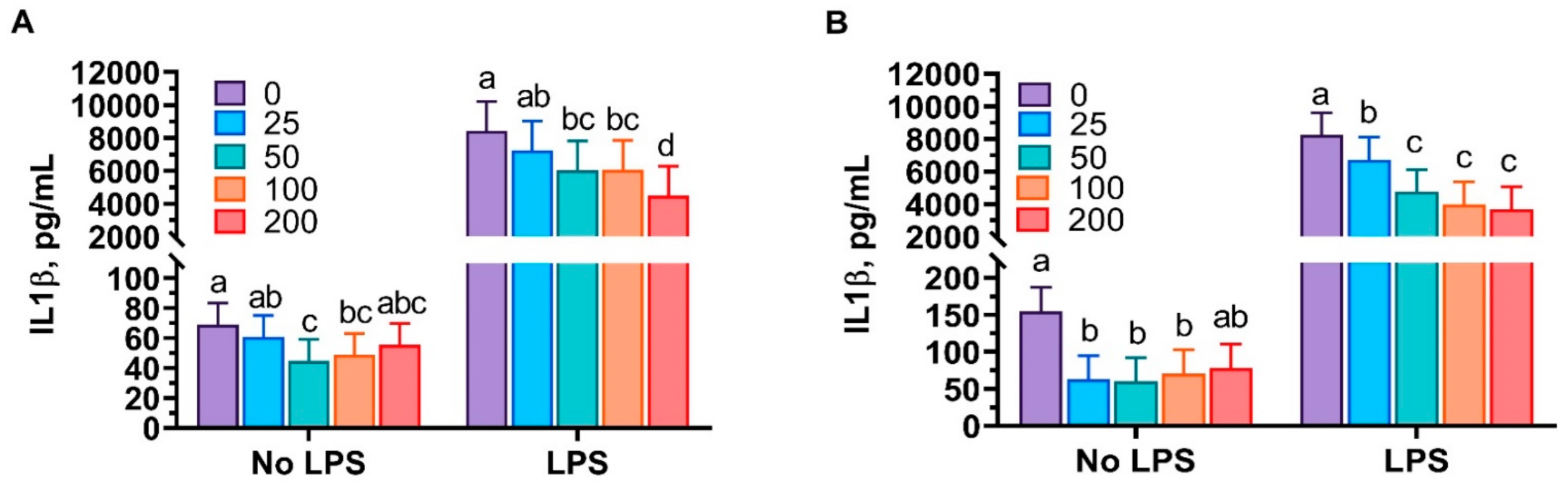
3.1. Anti-Inflammatory Properties of Mint Oils
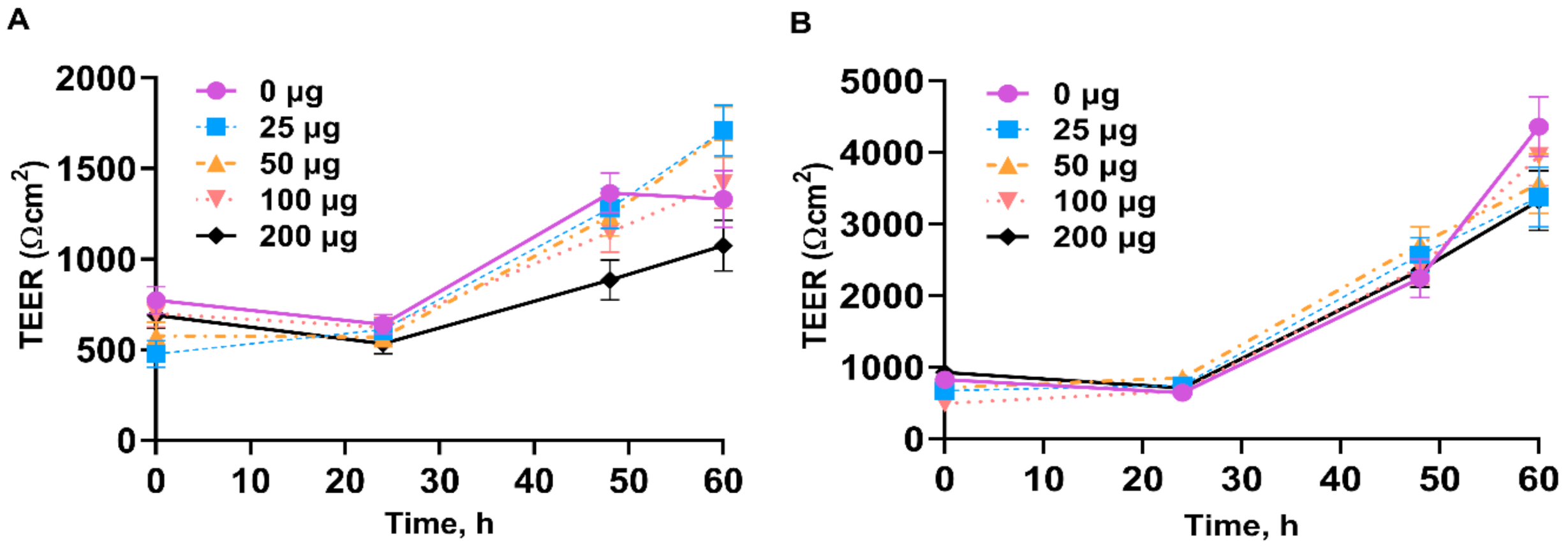
3.2. Transepithelial Electrical Resistance of IPEC-J2
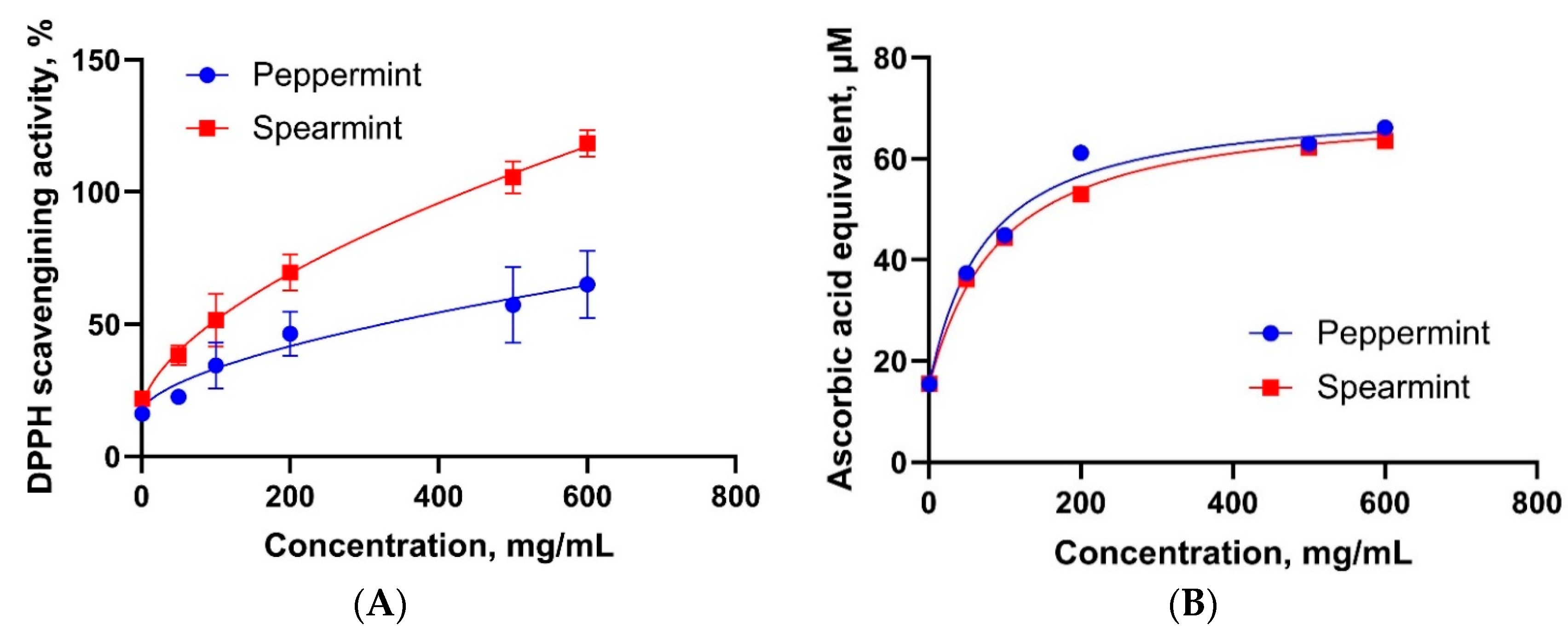
3.3. Antioxidant Properties of Mint Oils
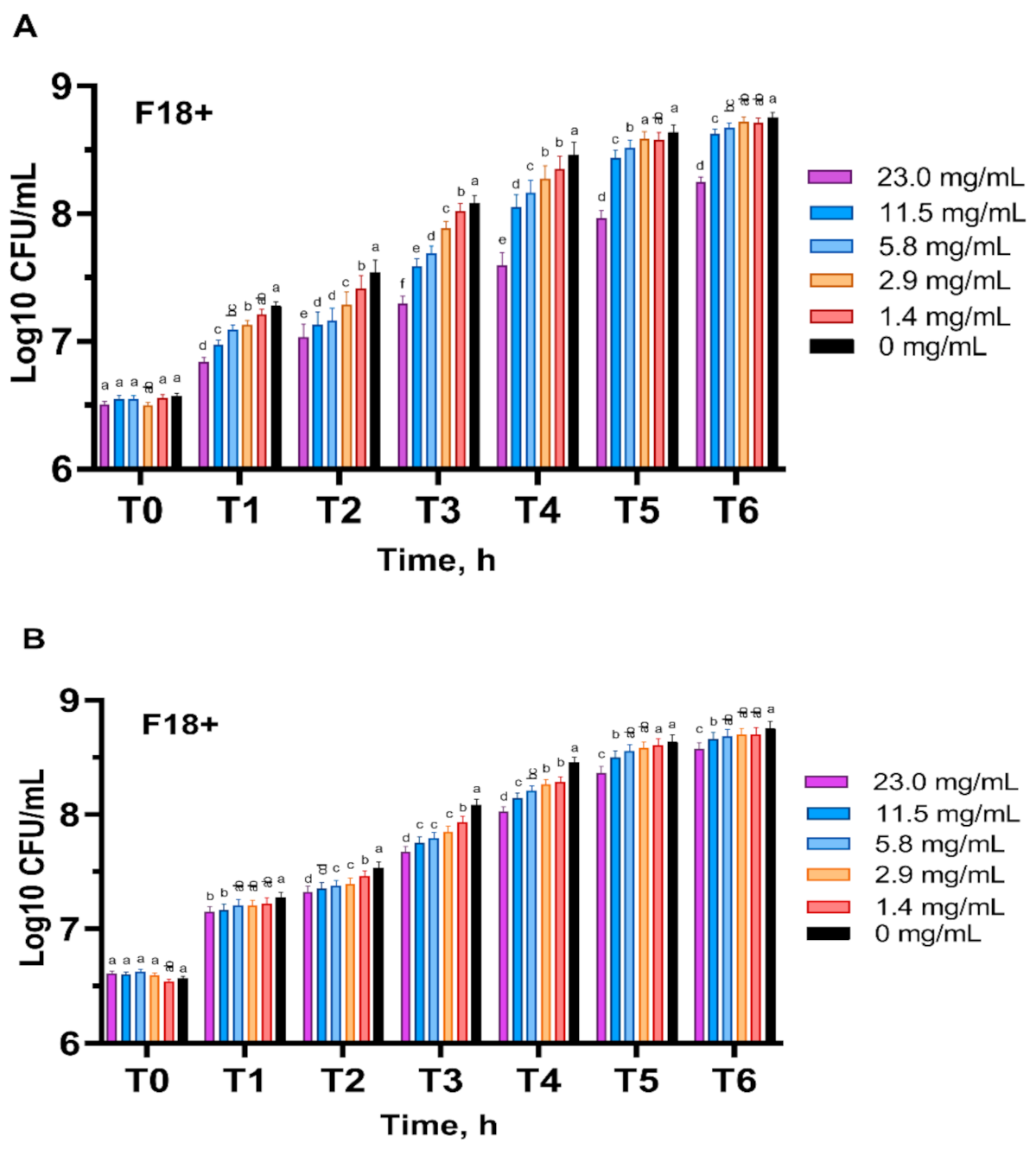
3.4. Escherichia coli Growth Inhibitory Activity
4. Discussion
4.1. Anti-Inflammatory Properties of Mint Oils
4.2. Transepithelial Electrical Resistance of IPEC-J2
4.3. Antioxidant Properties of Mint Oils
4.4. Escherichia coli Growth Inhibitory Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.; He, Y.; Xiong, X.; Ehrlich, A.; Li, X.; Raybould, H.; Atwill, E.R.; Maga, E.A.; Jørgensen, J.; Liu, Y. Dietary supplementation of Bacillus subtilis influenced intestinal health of weaned pigs experimentally infected with a pathogenic E. coli. J. Anim. Sci. Biotechnol. 2019, 10, 52. [Google Scholar] [CrossRef]
- Moeser, A.J.; Klok, C.V.; Ryan, K.A.; Wooten, J.G.; Little, D.; Cook, V.L.; Blikslager, A.T. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, 173–181. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Principles for the Containment of Antimicrobial Resistance in Animals Intended for Food: Report of a WHO Consultation with the Participation of the Food and Agriculture Organization of the United Nations and the Office International des Epizooties; WHO: Geneva, Switzerland, 2000; pp. 5–9. [Google Scholar]
- European Parliament and the Council. Regulation (EC) 1831/2003 of 22 September 2003 on Additives for Use in Animal Nutrition; Off J. L: 268/29; European Parliament and the Council: Strasbourg, France, 2003. [Google Scholar]
- Hejna, M.; Gottardo, D.; Baldi, A.; Dell’Orto, V.; Cheli, F.; Zaninelli, M.; Rossi, L. Review: Nutritional ecology of heavy metals. Animal 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hejna, M.; Moscatelli, A.; Onelli, E.; Baldi, A.; Pilu, S.; Rossi, L. Evaluation of concentration of heavy metals in animal rearing system. Ital. J. Anim. Sci. 2019, 18, 1372–1384. [Google Scholar] [CrossRef]
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef] [PubMed]
- Yazdankhah, S.; Rudi, K.; Bernhoft, A. Zinc and copper in animal feed-development of resistance and co-resistance to anti-microbial agents in bacteria of animal origin. Microb. Ecol. Health Dis. 2014, 25. [Google Scholar] [CrossRef]
- European Medicine Agency (EMA) N° 394961/2017. Questions and Answers on Veterinary Medicinal Products Containing Zinc Oxide to Be Administered Orally to Food-Producing Species; European Medicine Agency: Amsterdam, The Netherlands, 2017.
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef]
- Baydar, N.G.; Özkan, G.; Sagdiç, O. Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control 2004, 15, 335–339. [Google Scholar] [CrossRef]
- Dundar, E.; Olgun, E.G.; Isiksoy, S.; Kurkcuoglu, M.; Baser, K.H.C.; Bal, C. The effects of intra-rectal and intra-peritoneal application of Origanum onites L. essential oil on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in the rat. Exp. Toxicol. Pathol. 2008, 59, 399–408. [Google Scholar] [CrossRef]
- Liu, Y.; Song, M.; Che, T.; Bravo, D.; Pettigrew, J. Anti-inflammatory effects of several plant extracts on porcine alveolar macrophages in vitro. J. Anim. Sci. 2012, 90, 2774–2783. [Google Scholar] [CrossRef]
- Wu, Z.; Tan, B.; Liu, Y.; Dunn, J.; Martorell Guerola, P.; Tortajada, M.; Cao, Z.; Ji, P. Chemical composition and antioxidant properties of essential oils from peppermint, native spearmint and scotch spearmint. Molecules 2019, 24, 2825. [Google Scholar] [CrossRef]
- Caprarulo, V.; Hejna, M.; Giromini, C.; Liu, Y.; Dell’Anno, M.; Sotira, S.; Reggi, S.; Sgoifo-Rossi, C.A.; Callegari, M.L.; Rossi, L. Evaluation of dietary administration of chestnut and quebracho tannins on growth, serum metabolites and fecal parameters of weaned piglets. Animals 2020, 22, 1945. [Google Scholar] [CrossRef]
- Dell’Anno, M.; Hejna, M.; Sotira, S.; Caprarulo, V.; Reggi, S.; Pilu, R. Evaluation of leonardite as a feed additive on lipid metabolism and growth of weaned piglets. Anim. Feed Sci. Technol. 2020, 266, 114519. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Kalemba, D.; Synowiec, A. Agrobiological interactions of essential oils of two menthol mints: Mentha piperita and Mentha arvensis. Molecules 2020, 25, 59. [Google Scholar] [CrossRef]
- Park, Y.J.; Baek, S.A.; Choi, Y.; Kim, J.K.; Park, S.U. Metabolic profiling of nine Mentha species and prediction of their antioxidant properties using chemometrics. Molecules 2019, 24, 258. [Google Scholar] [CrossRef]
- Lv, J.; Huang, H.; Yu, L.; Whent, M.; Niu, Y.; Shi, H.; Wang, T.T.; Luthria, D.; Charles, D.; Yu, L.L. Phenolic composition and nutraceutical properties of organic and conventional cinnamon and peppermint. Food Chem. 2012, 132, 1442–1450. [Google Scholar] [CrossRef]
- Kapp, K.; Hakala, E.; Orav, A.; Pohjala, L.; Vuorela, P.; Püssa, T.; Vuorela, H.; Raal, A. Commercial peppermint (Mentha × piperita L.) teas: Antichlamydial effect and polyphenolic composition. Food Res. J. 2013, 53, 758–766. [Google Scholar] [CrossRef]
- Pereira, E.; Pimenta, A.I.; Calhelha, R.C.; Antonio, A.L.; Verde, S.C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C. Effects of gamma irradiation on cytotoxicity and phenolic compounds of Thymus vulgaris L. and Mentha × piperita. LLWT Food Sci. Technol. 2016, 71, 370–377. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Zengin, G.; Bahadori, S.; Dinparast, L.; Movahhedin, N. Phenolic composition and functional properties of wild mint (Mentha longifolia var. calliantha). Int. J. Food Prop. 2018, 21, 183–193. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic compounds and biological activity of selected Mentha species. Plants 2021, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Z.; Liu, K.; Qi, P.; Xu, J.; Wei, J.; Li, B.; Shao, D.; Shi, Y.; Qiu, Y.; et al. Proteomic analysis of the secretome of porcine alveolar macrophages infected with porcine reproductive and respiratory syndrome virus. Proteomics 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, S.; Liu, Q.; Li, Y.; Xu, L.; Zhang, Z.; Cai, X.; He, X. Porcine alveolar macrophage polarization is involved in inhibition of porcine reproductive and respiratory syndrome virus (PRRSV) replication. J. Vet. Med. Sci. 2017, 79, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Pröll, M.; Hölker, M.; Tholen, E.; Tesfaye, D. Alveolar macrophage phagocytic activity is enhanced with LPS priming, and combined stimulation of LPS and lipoteichoic acid synergistically induce pro-inflammatory cytokines in pigs. Innate Immun. 2013, 19, 631–643. [Google Scholar] [CrossRef]
- Murphy, K.; Weaver, C. Janeway’s Immunobiology, 9th ed.; Garland Science: New York, NY, USA; Taylor & Francis Group: London, UK, 2017. [Google Scholar]
- Berschneider, H.M. Development of normal cultured small intestinal epithelial cell lines which transport Na and Cl. Gastroenterology 1989, 96 Pt 2, A41. [Google Scholar]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–22. [Google Scholar] [CrossRef]
- Chen, S.; Einspanier, R.; Schoen, J. Transepithelial electrical resistance (TEER): A functional parameter to monitor the quality of oviduct epithelial cells cultured on filter supports. Histochem. Cell Biol. 2015, 144, 509–515. [Google Scholar] [CrossRef]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef]
- Sharafi, S.M.; Rasooli, I.; Owlia, P.; Taghizadeh, M.; Astaneh, S.D. Protective effects of bioactive phytochemicals from Mentha piperita with multiple health potentials. Pharmacogn. Mag. 2010, 6, 147–153. [Google Scholar] [CrossRef]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evid. Based Complement Alternat. Med. 2015, 795435. [Google Scholar] [CrossRef]
- Marjanović-Balaban, Ž.; Stanojević, L.; Kalaba, V.; Stanojević, J.; Cvetković, D.; Cakić, M.; Gojković, V. Chemical composition and antibacterial activity of the essential oil of Menthae piperitae L. Qual. Life 2018, 9, 5–12. [Google Scholar] [CrossRef]
- Muntean, D.; Licker, M.; Alexa, E.; Popescu, I.; Jianu, C.; Buda, V.; Dehelean, C.A.; Ghiulai, R.; Horhat, F.; Horhat, D.; et al. Evaluation of essential oil obtained from Mentha × piperita L. against multidrug-resistant strains. Infect. Drug Resist. 2019, 13, 2905–2914. [Google Scholar] [CrossRef]
- Rossi, L.; Di Giancamillo, A.; Reggi, S.; Domeneghini, C.; Baldi, A.; Sala, V. Expression of verocytotoxic Escherichia coli antigens in tobacco seeds and evaluation of gut immunity after oral administration in mouse model. J. Vet. Sci. 2013, 14, 263–270. [Google Scholar] [CrossRef]
- Giromini, C.; Cheli, F.; Rebucci, R.; Baldi, A. Invited review: Dairy proteins and bioactive peptides: Modeling digestion and the intestinal barrier. J. Dairy Sci. 2019, 102, 929–942. [Google Scholar] [CrossRef]
- Baarsch, M.J.; Wannemuehler, M.J.; Molitor, T.W.; Murtaugh, M.P. Detection of tumor necrosis factor α from porcine alveolar macrophages using an L929 fibroblast bioassay. J. Immunol. Methods 1991, 140, 15–22. [Google Scholar] [CrossRef]
- Dickie, R.; Tasat, D.R.; Fernandez Alanis, E.; Delfosse, V.; Tsuda, A. Age-dependent changes in porcine alveolar macrophage function during the postnatal period of alveolarization. Dev. Comp. Immunol. 2009, 33, 145–151. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Geens, M.M.; Niewold, T.A. Optimizing culture conditions of a porcine epithelial cell line IPEC-J2 through a histological and physiological characterization. Cytotechnology 2011, 63, 415–423. [Google Scholar] [CrossRef]
- Zhou, H.C.; Lin, Y.M.; Wei, S.D.; Tam, N.F. Structural diversity and antioxidant activity of condensed tannins fractionated from mangosteen pericarp. Food Chem. 2011, 129, 1710–1720. [Google Scholar] [CrossRef]
- Chung, S.I.; Kang, M.Y.; Lee, S.C. In vitro and in vivo antioxidant activity of aged ginseng (Panax ginseng). Prev. Nutr. Food Sci. 2016, 21, 24–30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhalodia, N.R.; Nariya, P.B.; Acharya, R.N.; Shukla, V.J. In vitro antioxidant activity of hydro alcoholic extract from the fruit pulp of Cassia fistula Linn. Ayu 2013, 34, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Dell’Orto, V.; Vagni, S.; Sala, V.; Reggi, S.; Baldi, A. Protective effect of oral administration of transgenic tobacco seeds against verocytotoxic Escherichia coli strain in piglets. Vet. Res. Commun. 2014, 38, 39–49. [Google Scholar] [CrossRef]
- Dell’Anno, M.; Sotira, S.; Rebucci, R.; Reggi, S.; Castiglioni, B.; Rossi, L. In vitro evaluation of antimicrobial and antioxidant activities of algal extracts. Ital. J. Anim. Sci. 2020, 19, 103–113. [Google Scholar] [CrossRef]
- Min, B.R.; Attwood, G.T.; McNabb, W.C.; Molan, A.L.; Barry, T.N. The effect of condensed tannins from Lotus corniculatus on the proteolytic activities and growth of rumen bacteria. Anim. Feed Sci. Technol. 2005, 121, 45–58. [Google Scholar] [CrossRef]
- Reggi, S.; Giromini, C.; Dell’Anno, M.; Baldi, A.; Rebucci, R.; Rossi, L. In vitro digestion of chestnut and quebracho tannin extracts: Antimicrobial effect, antioxidant capacity and cytomodulatory activity in swine intestinal IPEC-J2 cells. Animals 2020, 23, 195. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Guo, J.; Yu, Y.; Li, J.; Guo, Y.; Liu, C. Comparing two functions for optical density and cell numbers in bacterial exponential growth phase. J. Pure Appl. Microbiol. 2015, 9, 299–305. [Google Scholar]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Salud Pérez, G.; Miguel Zavala, S.; Lucina Arias, G.; Miguel Ramos, L. Anti-inflammatory activity of some essential oils. J. Essent. Oil. Res. 2011, 23, 5–38. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhu, L.; Zeng, D.; Long, W.; Zhu, S.M. Chemical composition and anti-inflammatory activities of essential oil from Trachydium roylei. J. Food Drug Anal. 2016, 24, 602–609. [Google Scholar] [CrossRef]
- Mogosan, C.; Vostinaru, O.; Oprean, R.; Heghes, C.; Filip, L.; Balica, G.; Moldovan, R.I. A comparative analysis of the chemical composition, anti-inflammatory, and antinociceptive effects of the essential oils from three species of Mentha cultivated in Romania. Molecules 2017, 22, 263. [Google Scholar] [CrossRef]
- Choi, C.Y.; Park, K.R.; Lee, J.H.; Jeon, Y.J.; Liu, K.H.; Oh, S. Isoeugenol suppression of inducible nitric oxide synthase expression is mediated by down-regulation of NF-kappaB, ERK1/2, and p38 kinase. Eur. J. Pharmacol. 2007, 8, 151–159. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect Biol. 2009, 1, 001651. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal. Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D.C. NF-κB signaling in macrophages: Dynamics, crosstalk, and signal integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Wan, M.L.; Ling, K.H.; Wang, M.F.; El-Nezami, H. Green tea polyphenol epigallocatechin-3-gallate improves epithelial barrier function by inducing the production of antimicrobial peptide pBD-1 and pBD-2 in monolayers of porcine intestinal epithelial IPEC-J2 cells. Mol. Nutr. Food Res. 2016, 60, 1048–1058. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Liu, S.; Hui, Q.; Zhang, H.; Lahaye, L.; Bodin, J.C.; Gong, J.; Nyachoti, M.; Yang, C. Thymol improves barrier function and attenuates inflammatory responses in porcine intestinal epithelial cells during lipopolysaccharide (LPS)-induced inflammation. J. Agric. Food Chem. 2019, 16, 615–624. [Google Scholar] [CrossRef]
- Hui, Q.; Ammeter, E.; Liu, S.; Yang, R.; Lu, P.; Lahaye, L.; Yang, C. Eugenol attenuates inflammatory response and enhances barrier function during lipopolysaccharide-induced inflammation in the porcine intestinal epithelial cells. J. Anim. Sci. 2020, 1, 245. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 956792. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell Longev. 2016, 1245049. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Brown, N.; John, J.A.; Shahidi, F. Polyphenol composition and antioxidant potential of mint leaves. Food Prod. Process Nutr. 2019, 1, 1. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Kosar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant properties and composition of aqueous extracts from mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Bail, S.; Buchbauer, G.; Stoilova, I.; Atanasova, T.; Stoyanova, A.; Krastanov, A.; Jirovetz, L. Chemical composition, olfactory evaluation and antioxidant effects of essential oil from Mentha × piperita. Nat. Prod. Commun. 2009, 4, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Naidu, J.R.; Ismail, R.; Yeng, C.; Sasidharan, S.; Kumar, P. Chemical composition and antioxidant activity of the crude methanolic extracts of Mentha spicata. J. Phytol. 2012, 4, 13–18. [Google Scholar]
- Teissedre, P.L.; Waterhouse, A.L. Inhibition of oxidation of human low-density lipoproteins by phenolic substances in different essential oils varieties. J. Agric. Food Chem. 2000, 48, 3801–3805. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Janda, B.; Pecio, L.; Stochmal, A.; Oleszek, W.; Czubacka, A.; Przybys, M.; Doroszewska, T. Determination of polyphenols in Mentha Longifolia and M. Piperita field-grown and in vitro plant samples using UPLC-TQ-MS. J. AOAC Int. 2011, 94, 43–50. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crop Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Nickavar, B.; Alinaghi, A.; Kamalinejad, M. Evaluation of the antioxidant properties of five mentha species. Iran. J. Pharm. Sci. 2010, 10, 203–209. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Wong, S.Y.; Grant, I.R.; Friedman, M.; Elliott, C.T.; Situ, C. Antibacterial activities of naturally occurring compounds against Mycobacterium avium subsp. paratuberculosis. Appl. Environ. Microbiol. 2008, 74, 5986–5990. [Google Scholar] [CrossRef]
- Degenhardt, J.; Kollner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef]
- Cosentino, S.C.I.G.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.L.; Mascia, V.; Arzedi, E.; Palmas, F. In vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 2002, 29, 130–135. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foodsea review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharopov, F.; Antolak, H.; Kręgiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R.; et al. Plants of genus mentha: From farm to food factory. Plants 2018, 7, 70. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Sokovic, M.D.; Vukojevic, J.; Marin, P.D.; Brkic, D.D.; Vajs, V.; van Griensven, L.J. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef]
- Aridogan, B.C.; Baydar, H.; Kaya, S.; Demirci, M.; Ozbasar, D.; Mumcu, E. Antimicrobial activity and chemical composition of some essential oils. Arch. Pharm. Res. 2002, 25, 860–864. [Google Scholar] [CrossRef]
- Işcan, G.; Kirimer, N.; Kürkcüoğlu, M.; Başer, K.H.; Demirci, F. Antimicrobial screening of Mentha piperita essential oils. J. Agric. Food Chem. 2002, 3, 3943–3946. [Google Scholar] [CrossRef]
| Assay 2 | EC50 1 (Goodness of Fit), mg/mL | |
|---|---|---|
| Peppermint Oil | Spearmint Oil | |
| DPPH scavenging activity | Not detected | Not detected |
| Reducing power assay | 71.30 (0.7765) | 71.30 (0.9722) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hejna, M.; Kovanda, L.; Rossi, L.; Liu, Y. Mint Oils: In Vitro Ability to Perform Anti-Inflammatory, Antioxidant, and Antimicrobial Activities and to Enhance Intestinal Barrier Integrity. Antioxidants 2021, 10, 1004. https://doi.org/10.3390/antiox10071004
Hejna M, Kovanda L, Rossi L, Liu Y. Mint Oils: In Vitro Ability to Perform Anti-Inflammatory, Antioxidant, and Antimicrobial Activities and to Enhance Intestinal Barrier Integrity. Antioxidants. 2021; 10(7):1004. https://doi.org/10.3390/antiox10071004
Chicago/Turabian StyleHejna, Monika, Lauren Kovanda, Luciana Rossi, and Yanhong Liu. 2021. "Mint Oils: In Vitro Ability to Perform Anti-Inflammatory, Antioxidant, and Antimicrobial Activities and to Enhance Intestinal Barrier Integrity" Antioxidants 10, no. 7: 1004. https://doi.org/10.3390/antiox10071004
APA StyleHejna, M., Kovanda, L., Rossi, L., & Liu, Y. (2021). Mint Oils: In Vitro Ability to Perform Anti-Inflammatory, Antioxidant, and Antimicrobial Activities and to Enhance Intestinal Barrier Integrity. Antioxidants, 10(7), 1004. https://doi.org/10.3390/antiox10071004








