Urinary Spermidine Predicts and Associates with In-Hospital Acute Kidney Injury after Cardiac Surgery
Abstract
1. Introduction
2. Materials and Methods
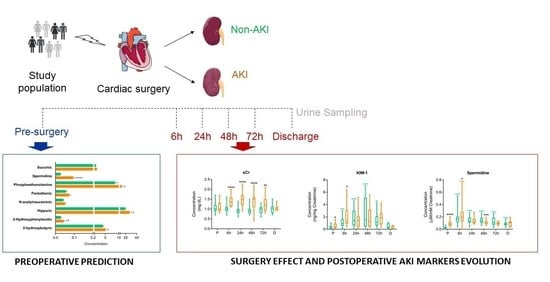
2.1. Study Population, Clinical Data, and Sample Collection
2.2. Clinical Outcome Definitions
2.3. Metabolic Screening of In-Hospital Kidney Injury
2.4. Quantification of Urinary Metabolites Pre- and Post-Surgery. Identification of Metabolic Markers of Renal injury
2.5. Analysis of Protein Markers of Renal Tubular Damage
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Altered Urine Metabolites in AKI vs. Non-AKI Patients
3.3. Surgery Is Reflected in the Metabolic Urinary Profile Independently of AKI
3.4. sCr, sCysC, uNGAL and uKIM-1 Variation after Cardiovascular Surgery in AKI and Non-AKI Patients
3.5. Molecular Marker Correlation with sCr and eGFR
3.6. uKIM-1 and Spermidine Predict AKI Development after Cardiovascular Surgery
3.7. Pre-Surgery Concentrations of Urinary Predictors Associate with AKI Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosner, M.H.; Okusa, M.D. Acute kidney injury associated with cardiac surgery. Clin. J. Am. Soc. Nephrol. 2006, 1, 19–32. [Google Scholar] [CrossRef]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef]
- Hobson, C.; Ozrazgat-Baslanti, T.; Kuxhausen, A.; Thottakkara, P.; Efron, P.A.; Moore, F.A.; Moldawer, L.L.; Segal, M.S.; Bihorac, A. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann. Surg. 2015, 261, 1207–1214. [Google Scholar] [CrossRef]
- Lafrance, J.P.; Miller, D.R. Acute kidney injury associates with increased long-term mortality. J. Am. Soc. Nephrol. 2010, 21, 345–352. [Google Scholar] [CrossRef]
- Dasta, J.F.; Kane-Gill, S.L.; Durtschi, A.J.; Pathak, D.S.; Kellum, J.A. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol. Dial. Transplant. 2008, 23, 1970–1974. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, K.; Watanabe, Y.; Inoue, T.; Ohno, Y.; Nakajima, H.; Okada, H. Predictors of long-term prognosis in acute kidney injury survivors who require continuous renal replacement therapy after cardiovascular surgery. PLoS ONE 2019, 14, e0211429. [Google Scholar] [CrossRef]
- Wu, B.; Chen, J.; Yang, Y. Biomarkers of Acute Kidney Injury after Cardiac Surgery: A Narrative Review. BioMed Res. Int. 2019, 2019, 7298635. [Google Scholar] [CrossRef]
- Neyra, J.A.; Hu, M.C.; Minhajuddin, A.; Nelson, G.E.; Ahsan, S.A.; Toto, R.D.; Jessen, M.E.; Moe, O.W.; Fox, A.A. Kidney Tubular Damage and Functional Biomarkers in Acute Kidney Injury Following Cardiac Surgery. Kidney Int. Rep. 2019, 4, 1131–1142. [Google Scholar] [CrossRef]
- Schaub, J.A.; Garg, A.X.; Coca, S.G.; Testani, J.M.; Shlipak, M.G.; Eikelboom, J.; Kavsak, P.; McArthur, E.; Shortt, C.; Whitlock, R.; et al. Perioperative heart-type fatty acid binding protein is associated with acute kidney injury after cardiac surgery. Kidney Int. 2015, 88, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.S.; Mahmoud, K.; Hanoura, S.; Osman, H.; Sivadasan, P.; Sudarsanan, S.; Shouman, Y.; Singh, R.; AlKhulaifi, A. Acute kidney injury induces high-sensitivity troponin measurement changes after cardiac surgery. BMC Anesthesiol. 2017, 17, 15. [Google Scholar] [CrossRef]
- Coca, S.G.; Nadkarni, G.N.; Garg, A.X.; Koyner, J.; Thiessen-Philbrook, H.; McArthur, E.; Shlipak, M.G.; Parikh, C.R. First Post-Operative Urinary Kidney Injury Biomarkers and Association with the Duration of AKI in the TRIBE-AKI Cohort. PLoS ONE 2016, 11, e0161098. [Google Scholar] [CrossRef]
- Zacharias, H.U.; Hochrein, J.; Vogl, F.C.; Schley, G.; Mayer, F.; Jeleazcov, C.; Eckardt, K.U.; Willam, C.; Oefner, P.J.; Gronwald, W. Identification of Plasma Metabolites Prognostic of Acute Kidney Injury after Cardiac Surgery with Cardiopulmonary Bypass. J. Proteome Res. 2015, 14, 2897–2905. [Google Scholar] [CrossRef]
- Martin-Lorenzo, M.; Gonzalez-Calero, L.; Ramos-Barron, A.; Sanchez-Niño, M.D.; Gomez-Alamillo, C.; García-Segura, J.M.; Ortiz, A.; Arias, M.; Vivanco, F.; Alvarez-Llamas, G. Urine metabolomics insight into acute kidney injury point to oxidative stress disruptions in energy generation and H(2)S availability. J. Mol. Med. 2017, 95, 1399–1409. [Google Scholar] [CrossRef]
- Section 2: AKI Definition. Kidney Int. Suppl. (2011) 2012, 2, 19–36. [CrossRef]
- Gonzalez-Calero, L.; Martin-Lorenzo, M.; Martínez, P.J.; Baldan-Martin, M.; Ruiz-Hurtado, G.; Segura, J.; de la Cuesta, F.; Barderas, M.G.; Ruilope, L.M.; Vivanco, F.; et al. Hypertensive patients exhibit an altered metabolism. A specific metabolite signature in urine is able to predict albuminuria progression. Transl. Res. 2016, 178, 25–37.e27. [Google Scholar] [CrossRef]
- Martin-Lorenzo, M.; Martinez, P.J.; Baldan-Martin, M.; Ruiz-Hurtado, G.; Prado, J.C.; Segura, J.; de la Cuesta, F.; Barderas, M.G.; Vivanco, F.; Ruilope, L.M.; et al. Citric Acid Metabolism in Resistant Hypertension: Underlying Mechanisms and Metabolic Prediction of Treatment Response. Hypertension 2017, 70, 1049–1056. [Google Scholar] [CrossRef]
- Martin-Lorenzo, M.; Zubiri, I.; Maroto, A.S.; Gonzalez-Calero, L.; Posada-Ayala, M.; de la Cuesta, F.; Mourino-Alvarez, L.; Lopez-Almodovar, L.F.; Calvo-Bonacho, E.; Ruilope, L.M.; et al. KLK1 and ZG16B proteins and arginine-proline metabolism identified as novel targets to monitor atherosclerosis, acute coronary syndrome and recovery. Metabolomics 2015, 11, 1056–1067. [Google Scholar] [CrossRef]
- Martinez, P.J.; Agudiez, M.; Molero, D.; Martin-Lorenzo, M.; Baldan-Martin, M.; Santiago-Hernandez, A.; García-Segura, J.M.; Madruga, F.; Cabrera, M.; Calvo, E.; et al. Urinary metabolic signatures reflect cardiovascular risk in the young, middle-aged, and elderly populations. J. Mol. Med. 2020, 98, 1603–1613. [Google Scholar] [CrossRef]
- Posada-Ayala, M.; Zubiri, I.; Martin-Lorenzo, M.; Sanz-Maroto, A.; Molero, D.; Gonzalez-Calero, L.; Fernandez-Fernandez, B.; de la Cuesta, F.; Laborde, C.M.; Barderas, M.G.; et al. Identification of a urine metabolomic signature in patients with advanced-stage chronic kidney disease. Kidney Int. 2014, 85, 103–111. [Google Scholar] [CrossRef]
- Tulpan, D.; Léger, S.; Belliveau, L.; Culf, A.; Cuperlović-Culf, M. MetaboHunter: An automatic approach for identification of metabolites from 1H-NMR spectra of complex mixtures. BMC Bioinform. 2011, 12, 400. [Google Scholar] [CrossRef]
- Rodrigo, E.; Ruiz, J.C.; Fernández-Fresnedo, G.; Fernández, M.D.; Piñera, C.; Palomar, R.; Monfá, E.; Gómez-Alamillo, C.; Arias, M. Cystatin C and albuminuria as predictors of long-term allograft outcomes in kidney transplant recipients. Clin. Transplant. 2013, 27, E177–E183. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- O’Neal, J.B.; Shaw, A.D.; Billings, F.T.T. Acute kidney injury following cardiac surgery: Current understanding and future directions. Crit Care 2016, 20, 187. [Google Scholar] [CrossRef]
- Vives, M.; Hernandez, A.; Parramon, F.; Estanyol, N.; Pardina, B.; Muñoz, A.; Alvarez, P.; Hernandez, C. Acute kidney injury after cardiac surgery: Prevalence, impact and management challenges. Int. J. Nephrol. Renovasc. Dis. 2019, 12, 153–166. [Google Scholar] [CrossRef]
- Gameiro, J.; Fonseca, J.A.; Neves, M.; Jorge, S.; Lopes, J.A. Acute kidney injury in major abdominal surgery: Incidence, risk factors, pathogenesis and outcomes. Ann. Intensive Care 2018, 8, 22. [Google Scholar] [CrossRef]
- Han, W.K.; Wagener, G.; Zhu, Y.; Wang, S.; Lee, H.T. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin. J. Am. Soc. Nephrol. 2009, 4, 873–882. [Google Scholar] [CrossRef]
- Arthur, J.M.; Hill, E.G.; Alge, J.L.; Lewis, E.C.; Neely, B.A.; Janech, M.G.; Tumlin, J.A.; Chawla, L.S.; Shaw, A.D. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int. 2014, 85, 431–438. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359. [Google Scholar] [CrossRef]
- Goek, O.N.; Prehn, C.; Sekula, P.; Römisch-Margl, W.; Döring, A.; Gieger, C.; Heier, M.; Koenig, W.; Wang-Sattler, R.; Illig, T.; et al. Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrol. Dial. Transplant. 2013, 28, 2131–2138. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Soda, K.; Dobashi, Y.; Kano, Y.; Tsujinaka, S.; Konishi, F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp. Gerontol. 2009, 44, 727–732. [Google Scholar] [CrossRef]
- Kim, J. Spermidine rescues proximal tubular cells from oxidative stress and necrosis after ischemic acute kidney injury. Arch. Pharm. Res. 2017, 40, 1197–1208. [Google Scholar] [CrossRef]
- Aragno, M.; Cutrin, J.C.; Mastrocola, R.; Perrelli, M.G.; Restivo, F.; Poli, G.; Danni, O.; Boccuzzi, G. Oxidative stress and kidney dysfunction due to ischemia/reperfusion in rat: Attenuation by dehydroepiandrosterone. Kidney Int. 2003, 64, 836–843. [Google Scholar] [CrossRef]
- Murray Stewart, T.; Dunston, T.T.; Woster, P.M.; Casero, R.A., Jr. Polyamine catabolism and oxidative damage. J. Biol. Chem. 2018, 293, 18736–18745. [Google Scholar] [CrossRef]
- Zahedi, K.; Barone, S.; Soleimani, M. Polyamine Catabolism in Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 4790. [Google Scholar] [CrossRef]
- Basile, D.P.; Bonventre, J.V.; Mehta, R.; Nangaku, M.; Unwin, R.; Rosner, M.H.; Kellum, J.A.; Ronco, C. Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J. Am. Soc. Nephrol. 2016, 27, 687–697. [Google Scholar] [CrossRef]
- Smith, L.E.; Smith, D.K.; Yancey, P.G.; Kon, V.; Remaley, A.T.; Billings, F.T.T.; Linton, M.F. Perioperative high density lipoproteins, oxidative stress, and kidney injury after cardiac surgery. J. Lipid Res. 2021, 62, 100024. [Google Scholar] [CrossRef]
- Meersch, M.; Schmidt, C.; Hoffmeier, A.; Van Aken, H.; Wempe, C.; Gerss, J.; Zarbock, A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial. Intensiv. Care Med. 2017, 43, 1551–1561. [Google Scholar] [CrossRef] [PubMed]


| Baseline Characteristics (Pre-Surgery) | CVS-C | CVS-AKI | p-Value |
|---|---|---|---|
| Number of patients | 40 | 20 | |
| Age, years (†) | 68 ± 12 | 73 ± 8 | 0.097 |
| Gender (males) (*) | 30 (75) | 11 (55) | 0.116 |
| Comorbidities (*) | |||
| Hypertension | 32 (80) | 16 (80) | 1 |
| Diabetes mellitus | 7 (17.5) | 8 (40) | 0.058 |
| Cerebrovascular accident | 2 (5) | 0 (0) | 0.309 |
| AMI | 9 (22.5) | 3 (15) | 0.494 |
| Peripheral vascular disease | 13 (32.5) | 15 (75) | 0.002 |
| Chronic kidney disease | 6 (15) | 7 (35) | 0.076 |
| Congestive heart failure | 8 (20) | 7 (35) | 0.206 |
| Endocarditis | 1 (2.6) | 0 | 0.470 |
| PAH | 2 (5.1) | 4 (20) | 0.074 |
| Charlson comorbidity score (†) | 4.08 ± 2.08 | 5.85 ± 1.98 | 0.002 |
| Weight (kg) (†) | 80 ± 11 | 76 ± 11 | 0.217 |
| BMI (kg/m2) (†) | 29 ± 3 | 29 ± 3 | 0.687 |
| Total cholesterol (mg/dL) (†) | 178 ± 36 | 180 ± 31 | 0.872 |
| LDL cholesterol (mg/dL) (†) | 108 ± 29 | 102 ± 26 | 0.510 |
| HDL cholesterol (mg/dL) (†) | 46 ± 13 | 55 ± 24 | 0.129 |
| Triglycerides (mg/dL) (†) | 120 ± 43 | 113 ± 57 | 0.661 |
| Previous hyperlipidemia (*) | 25 (71) | 16 (80) | 0.483 |
| Previous hyperlipidemia treatment (*) | 27 (73) | 16 (80) | 0.556 |
| Nephrotoxic drugs (≥1) (*) | 36 (90) | 16 (80) | 0.283 |
| Previous angiography (*) | 29 (72.5) | 17 (85) | 0.281 |
| Type of surgery | |||
| Only valvular (*) | 25 (62.5) | 18 (90) | 0.026 |
| Only coronary(*) | 16 (40) | 8 (40) | 1 |
| ByPass (*) | 38 (95) | 19 (95) | 1 |
| Ischemia time (min) (†) | 79 ± 28 | 90 ± 39 | 0.252 |
| CPB time (min) (†) | 105 ± 34 | 120 ± 50 | 0.166 |
| Left ventricular ejection fraction (%) (**) | 60 (10) | 60 (10) | 1 |
| ICU stay, days (**) | 2 (2) | 2 (3) | 0.927 |
| Markers of renal function | |||
| sCreatinine (mg/dL) (†) | 1.05 ± 0.23 | 1.12 ± 0.28 | 0.325 |
| eGFR (CKD-EPI) (†) | 71 ± 18 | 61 ± 19 | 0.070 |
| eGFR (MDRD) (†) | 73 ± 17 | 64 ± 20 | 0.096 |
| Urine output* (≤400 mL/24 h) | 0 (0) | 0 (0) | 1 |
| Markers of Renal Function at 24 h Post-Surgery | CVS-C | CVS-AKI | p-Value |
| KDIGO * | 0.000 | ||
| 0 | 40 (100) | 5 (25) | |
| 1 | 0 | 15 (75) | |
| sCreatinine (†) | 0.98 ± 0.24 | 1.49 ± 0.36 | 0.000 |
| eGFR (†) | 77 ± 20 | 44 ± 15 | 0.000 |
| Urine output * (≤400 mL/24 h) | 3 (7.5) | 0 (0) | 0.209 |
| sCr (mg/dL) | eGFR (mL/min) | |||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| AKI Post-CVS Metabolic Markers | ||||
| 2-hydroxybutyric acid | −0.272 | <0.001 | 0.2151 | <0.001 |
| 2-hydroxyphenylacetic acid | −0.01721 | 0.80 | −0.02630 | 0.70 |
| Hippuric acid | 0.03536 | 0.57 | −0.1050 | 0.10 |
| N-acetylneuraminic acid | 0.2193 | <0.001 | −0.3373 | <0.001 |
| Pantothenic acid | −0.1391 | 0.03 | 0.09505 | 0.14 |
| Phosphoethanolamine | −0.001941 | 0.98 | −0.01219 | 0.85 |
| Spermidine | −0.04087 | 0.52 | 0.004941 | 0.94 |
| Succinic acid | −0.3313 | <0.001 | 0.2447 | <0.001 |
| Renal Functional Markers | ||||
| Cystatin C | 0.5847 | <0.001 | −0.6126 | <0.001 |
| uNGAL | 0.2964 | <0.001 | −0.2407 | <0.001 |
| uKIM-1 | 0.04008 | 0.54 | 0.06392 | 0.33 |
| OR [95% CI] | p-Value | ||
|---|---|---|---|
| Renal Functional Markers | |||
| eGFR | 0.4582 [0.1529–1.372] | 0.18 | |
| sCysC | 4.714 [1.317–16.87] | 0.02 | |
| uKIM-1 | 5.333 [1.226–23.20] | 0.03 | |
| uNGAL | 3.330 [0.9174–12.11] | 0.11 | |
| AKI Post-CVS Surgery Metabolic Markers | |||
| 2-hydroxybutyric acid | 3.302 [1.349–8.084] | 0.01 | |
| 2-hydroxyphenylacetic acid | 2.623 [1.008–6.370] | 0.047 | |
| Hippuric acid | 3.302 [1.349–8.084] | 0.01 | |
| N-acetylneuraminic acid | 2.178 [0.8944–5.302] | 0.12 | |
| Pantothenic acid | 3.500 [1.401–8.744] | 0.008 | |
| Phosphoethanolamine | 2.521 [0.9877–6.434] | 0.07 | |
| Spermidine | 69.75 [17.17–283.4] | <0.001 | |
| Succinic acid | 2.000 [0.8167–4.898] | 0.19 | |
| Adjusted Best Predictor Features by eGFR | |||
| Spermidine | 65.83 [9.312–465.4] | <0.001 | |
| Pantothenic acid | 5.769 [1.267–26.25] | 0.02 | |
| uKIM-1 | 4.552 [0.9980–20.76] | 0.05 | |
| Adjusted Best Predictor Features by uKIM-1 | |||
| Spermidine | 158.1 [11.97–2088] | 0.0001 | |
| Pantothenic acid | 5.454 [1.251–23.77] | 0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Lorenzo, M.; Ramos-Barron, A.; Gutierrez-Garcia, P.; Martin-Blazquez, A.; Santiago-Hernandez, A.; Rodrigo Calabia, E.; Gomez-Alamillo, C.; Alvarez-Llamas, G. Urinary Spermidine Predicts and Associates with In-Hospital Acute Kidney Injury after Cardiac Surgery. Antioxidants 2021, 10, 896. https://doi.org/10.3390/antiox10060896
Martin-Lorenzo M, Ramos-Barron A, Gutierrez-Garcia P, Martin-Blazquez A, Santiago-Hernandez A, Rodrigo Calabia E, Gomez-Alamillo C, Alvarez-Llamas G. Urinary Spermidine Predicts and Associates with In-Hospital Acute Kidney Injury after Cardiac Surgery. Antioxidants. 2021; 10(6):896. https://doi.org/10.3390/antiox10060896
Chicago/Turabian StyleMartin-Lorenzo, Marta, Angeles Ramos-Barron, Paula Gutierrez-Garcia, Ariadna Martin-Blazquez, Aranzazu Santiago-Hernandez, Emilio Rodrigo Calabia, Carlos Gomez-Alamillo, and Gloria Alvarez-Llamas. 2021. "Urinary Spermidine Predicts and Associates with In-Hospital Acute Kidney Injury after Cardiac Surgery" Antioxidants 10, no. 6: 896. https://doi.org/10.3390/antiox10060896
APA StyleMartin-Lorenzo, M., Ramos-Barron, A., Gutierrez-Garcia, P., Martin-Blazquez, A., Santiago-Hernandez, A., Rodrigo Calabia, E., Gomez-Alamillo, C., & Alvarez-Llamas, G. (2021). Urinary Spermidine Predicts and Associates with In-Hospital Acute Kidney Injury after Cardiac Surgery. Antioxidants, 10(6), 896. https://doi.org/10.3390/antiox10060896