The Effects of Beverage Intake after Exhaustive Exercise on Organ Damage, Inflammation and Oxidative Stress in Healthy Males
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Exercise Protocol
2.3. Blood and Urine Sampling
2.4. Assays for Biochemistry, Organ Damage Markers, Inflammatory Mediators and Oxidative Stress
2.5. Statistics
3. Results
3.1. Exercise Duration and Hydration Status
3.2. Exercise-Induced Organ Damage
3.3. Exercise-Induced Inflammation
3.4. Exercise-Induced Oxidative Stress and Antioxidant Substances
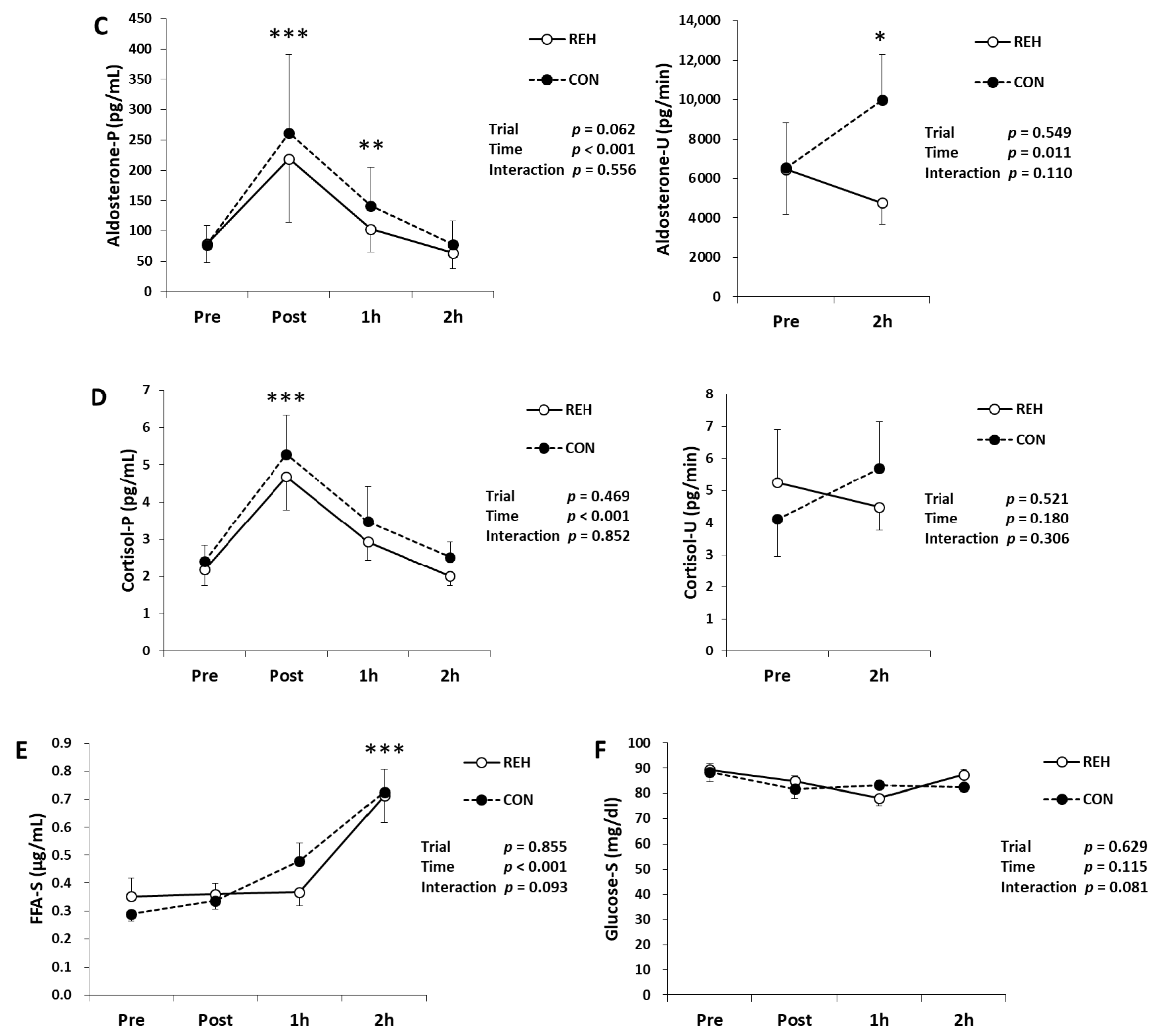
3.5. Hormones, Glucose and FFA
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef]
- Tominaga, T.; Ma, S.; Sugama, K.; Kanda, K.; Omae, C.; Choi, W.; Hashimoto, S.; Aoyama, K.; Yoshikai, Y.; Suzuki, K. Changes in Urinary Biomarkers of Organ Damage, Inflammation, Oxidative Stress, and Bone Turnover Following a 3000-m Time Trial. Antioxidants 2021, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a Competitive Marathon Race on Systemic Cytokine and Neutrophil Responses. Med. Sci. Sports Exerc. 2003, 35, 348–355. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle Damage and Inflammation during Recovery from Exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic Review: Exercise-Induced Gastrointestinal Syndrome-Implications for Health and Intestinal Disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef]
- Moses, F.M. Exercise-Associated Intestinal Ischemia. Curr. Sports Med. Rep. 2005, 4, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Kometani, T. Urinary Excretion of Cytokines versus Their Plasma Levels after Endurance Exercise. Exerc. Immunol. Rev. 2013, 19, 29–48. [Google Scholar]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Totsuka, M.; Sato, K.; Sugawara, K. Systemic Inflammatory Response to Exhaustive Exercise. Cytokine Kinetics. Exerc. Immunol. Rev. 2002, 8, 6–48. [Google Scholar]
- Suzuki, K.; Yamada, M.; Kurakake, S.; Okamura, N.; Yamaya, K.; Liu, Q.; Kudoh, S.; Kowatari, K.; Nakaji, S.; Sugawara, K. Circulating Cytokines and Hormones with Immunosuppressive but Neutrophil-Priming Potentials Rise after Endurance Exercise in Humans. Eur. J. Appl. Physiol. 2000, 81, 281–287. [Google Scholar] [CrossRef]
- Lim, C.L.; Suzuki, K. Systemic inflammation mediates the effects of endotoxemia in the mechanisms of heat stroke. Biol. Med. 2017, 9, 376. [Google Scholar] [CrossRef]
- Leon, L.R.; Helwig, B.G. Heat Stroke: Role of the systemic inflammatory response. J. Appl. Physiol. 2010, 109, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J. Effect of exercise on oxidative stress biomarkers. Adv. Clin. Chem. 2008, 46, 1–50. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Protective effects of sulforaphane on exercise-induced organ damage via inducing antioxidant defense responses. Antioxidants 2020, 9, 136. [Google Scholar] [CrossRef]
- Yada, K.; Roberts, L.A.; Oginome, N.; Suzuki, K. Effect of acacia polyphenol supplementation on exercise-induced oxidative stress in mice liver and skeletal muscle. Antioxidants 2019, 9, 29. [Google Scholar] [CrossRef]
- Yada, K.; Suzuki, K.; Oginome, N.; Ma, S.; Fukuda, Y.; Iida, A.; Radak, Z. Single dose administration of taheebo polyphenol enhances endurance capacity in mice. Sci. Rep. 2018, 8, 14625. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T.; Ma, S.; Saitou, K.; Suzuki, K. Glucose ingestion inhibits endurance exercise-induced il-6 producing macrophage infiltration in mice muscle. Nutrients 2019, 11, 1496. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Davis, J.M.; Henson, D.A.; Gross, S.J.; Dumke, C.L.; Utter, A.C.; Vinci, D.M.; Carson, J.A.; Brown, A.; McAnulty, S.R.; et al. Muscle cytokine mRNA Changes after 2.5 h of cycling: Influence of carbohydrate. Med. Sci. Sports Exerc. 2005, 37, 1283–1290. [Google Scholar] [CrossRef]
- Peake, J.; Wilson, G.; Mackinnon, L.; Coombes, J.S. Carbohydrate supplementation and alterations in neutrophils, and plasma cortisol and myoglobin concentration after intense exercise. Eur. J. Appl. Physiol. 2005, 93, 672–678. [Google Scholar] [CrossRef]
- Valentine, R.J.; Saunders, M.J.; Todd, M.K.; St Laurent, T.G. Influence of carbohydrate-protein beverage on cycling endurance and indices of muscle disruption. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 363–378. [Google Scholar] [CrossRef]
- Snipe, R.M.J.; Khoo, A.; Kitic, C.M.; Gibson, P.R.; Costa, R.J.S. Carbohydrate and protein intake during exertional heat stress ameliorates intestinal epithelial injury and small intestine permeability. Appl. Physiol. Nutr. Metab. 2017, 42, 1283–1292. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Camões-Costa, V.; Snipe, R.M.J.; Dixon, D.; Russo, I.; Huschtscha, Z. Impact of exercise-induced hypohydration on gastrointestinal integrity, function, symptoms, and systemic endotoxin and inflammatory profile. J. Appl. Physiol. 2019, 126, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Hashimoto, H.; Oh, T.; Ishijima, T.; Mitsuda, H.; Peake, J.M.; Sakamoto, S.; Muraoka, I.; Higuchi, M. The effects of sports drink osmolality on fluid intake and immunoendocrine responses to cycling in hot conditions. J. Nutr. Sci. Vitaminol. 2013, 59, 206–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ikemura, T.; Suzuki, K.; Nakamura, N.; Yada, K.; Hayashi, N. Fluid intake restores retinal blood flow early after exhaustive exercise in healthy subjects. Eur. J. Appl. Physiol. 2018, 118, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Dill, D.B.; Costill, D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974, 37, 247–248. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Ogden, H.B.; Child, R.B.; Fallowfield, J.L.; Delves, S.K.; Westwood, C.S.; Layden, J.D. The gastrointestinal exertional heat stroke paradigm: Pathophysiology, assessment, severity, aetiology and nutritional countermeasures. Nutrients 2020, 12, 537. [Google Scholar] [CrossRef] [PubMed]
- Nemes, R.; Koltai, E.; Taylor, A.W.; Suzuki, K.; Gyori, F.; Radak, Z. Reactive oxygen and nitrogen species regulate key metabolic, anabolic, and catabolic pathways in skeletal muscle. Antioxidants 2018, 7, 85. [Google Scholar] [CrossRef]
- Tanisawa, K.; Suzuki, K.; Ma, S.; Kondo, S.; Okugawa, S.; Higuchi, M. Effects of ingestion of different amounts of carbohydrate after endurance exercise on circulating cytokines and markers of neutrophil activation. Antioxidants 2018, 7, 51. [Google Scholar] [CrossRef]
- Afroundeh, R.; Siahkouhian, M.; Khalili, A. The effect of post-exercise carbohydrate ingestion on inflammatory responses to short time, high-force eccentric exercise. J. Sports Med. Phys. Fit. 2010, 50, 182–188. [Google Scholar]
- Depner, C.M.; Kirwan, R.D.; Frederickson, S.J.; Miles, M.P. Enhanced inflammation with high carbohydrate intake during recovery from eccentric exercise. Eur. J. Appl. Physiol. 2010, 109, 1067–1076. [Google Scholar] [CrossRef]
- Ross, M.L.R.; Halson, S.L.; Suzuki, K.; Garnham, A.; Hawley, J.A.; Cameron-Smith, D.; Peake, J.M. Cytokine responses to carbohydrate ingestion during recovery from exercise-induced muscle injury. J. Interferon Cytokine Res. 2010, 30, 329–337. [Google Scholar] [CrossRef]
- van Wijck, K.; Lenaerts, K.; van Loon, L.J.C.; Peters, W.H.M.; Buurman, W.A.; Dejong, C.H.C. Exercise-Induced Splanchnic Hypoperfusion Results in Gut Dysfunction in Healthy Men. PLoS ONE 2011, 6, e22366. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.N.; Impey, S.G.; Doran, D.A.; Fleming, S.C.; Morton, J.P.; Close, G.L. Acute High-Intensity Interval Running Increases Markers of Gastrointestinal Damage and Permeability but Not Gastrointestinal Symptoms. Appl. Physiol. Nutr. Metab. 2017, 42, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Kinoshita, S.; Kira, S. Effects of Acute Moderate Exercise on the Phagocytosis of Kupffer Cells in Rats. Acta Physiol. 2004, 182, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Suzuki, K.; Coombes, J.S. The Influence of Antioxidant Supplementation on Markers of Inflammation and the Relationship to Oxidative Stress after Exercise. J. Nutr. Biochem. 2007, 18, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Hayashida, H. Effect of Exercise Intensity on Cell-Mediated Immunity. Sports 2021, 9, 8. [Google Scholar] [CrossRef]
- Hashimoto, H.; Ishijima, T.; Hayashida, H.; Suzuki, K.; Higuchi, M. Menstrual cycle phase and carbohydrate ingestion alter immune response following endurance exercise and high intensity time trial performance test under hot conditions. J. Int. Soc. Sports Nutr. 2014, 11, 39. [Google Scholar] [CrossRef]
- Suzuki, K. Characterization of exercise-induced cytokine release, the impacts on the body, the mechanisms and modulations. Int. J. Sports Exerc. Med. 2019, 5, 122. [Google Scholar] [CrossRef]
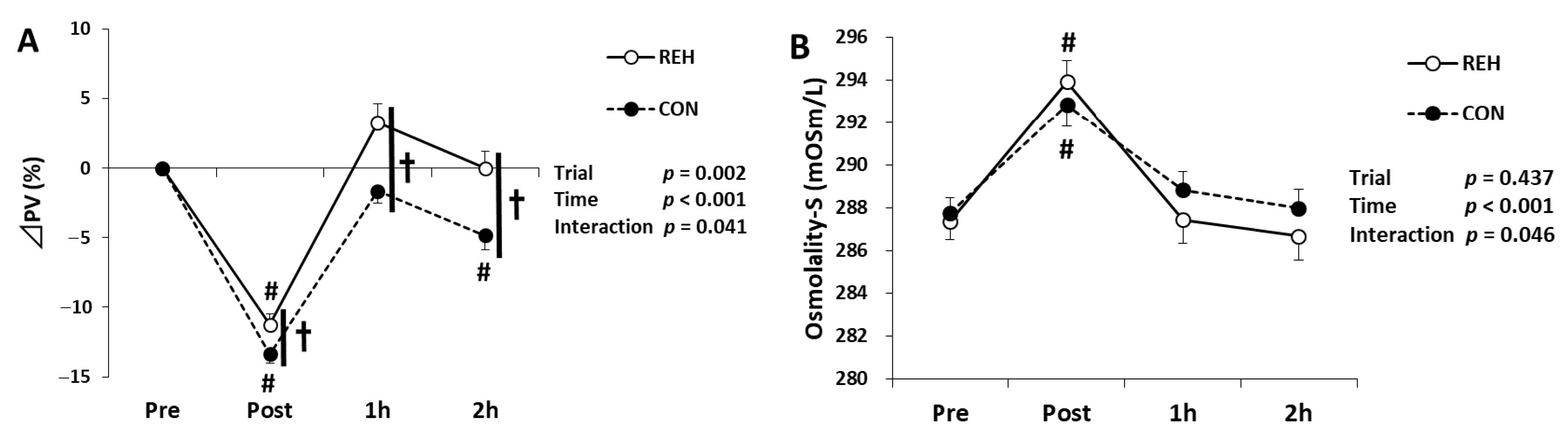
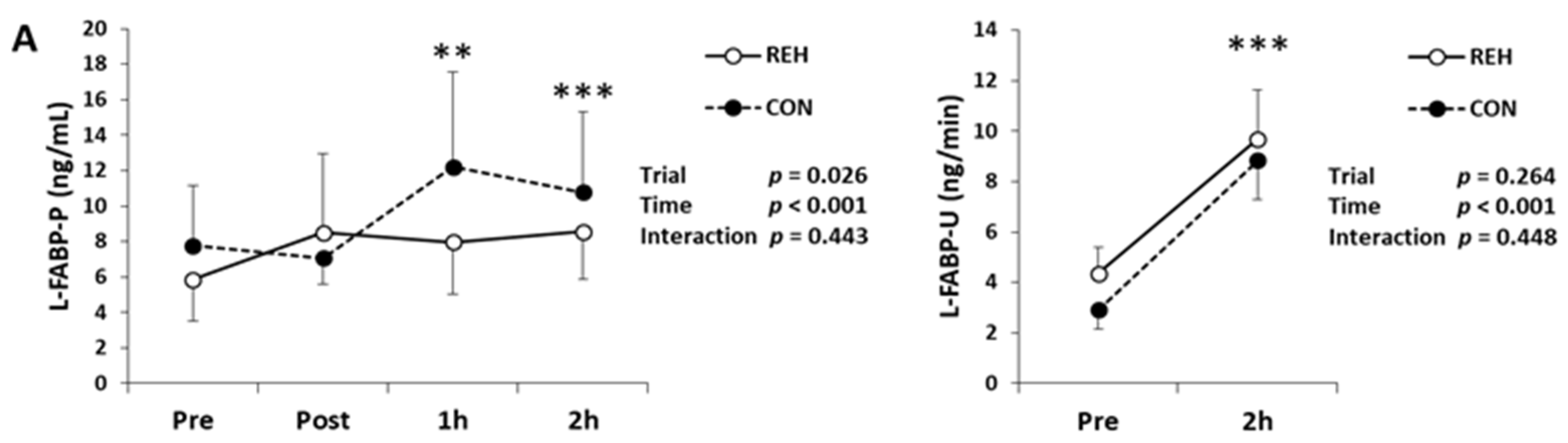
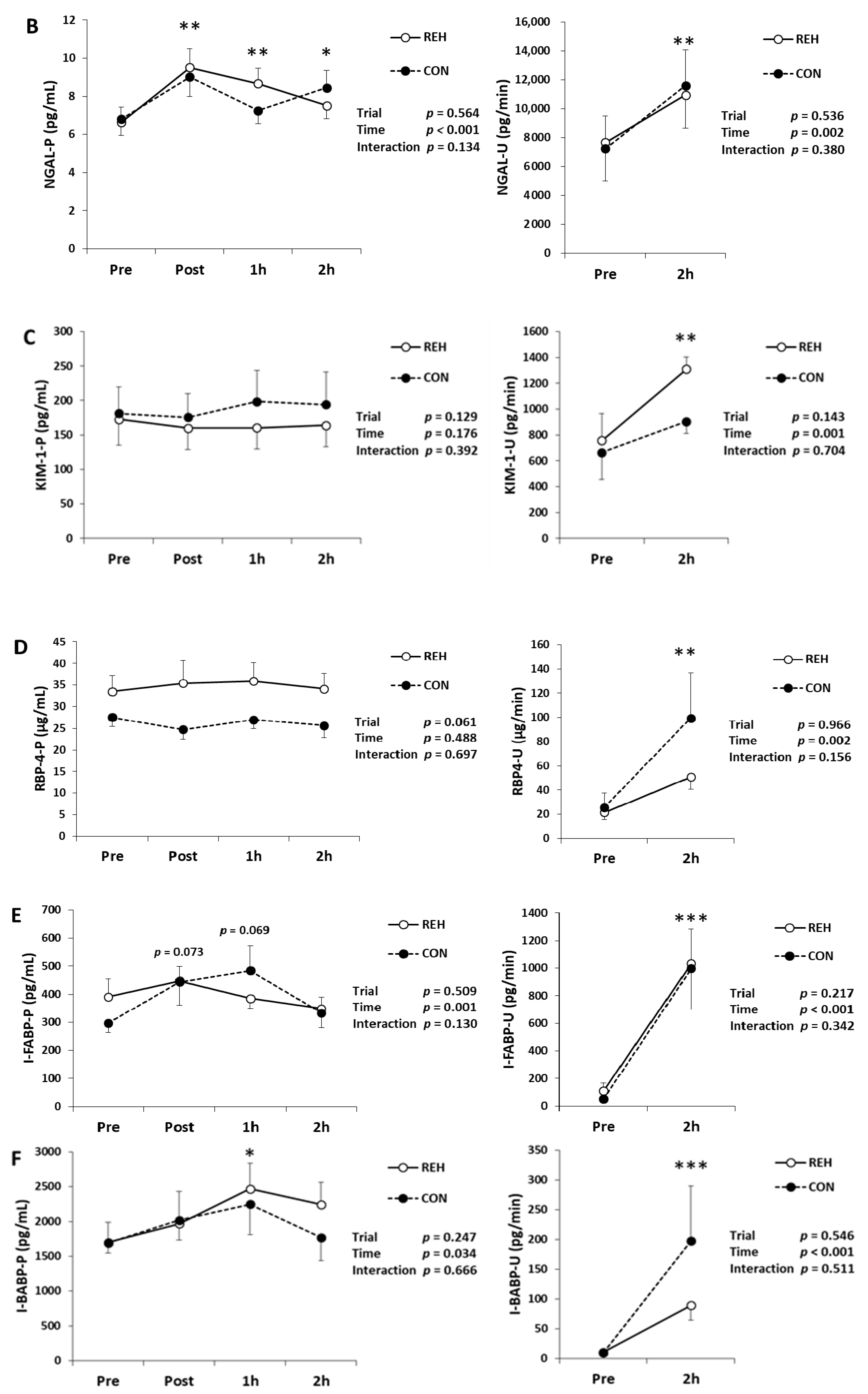

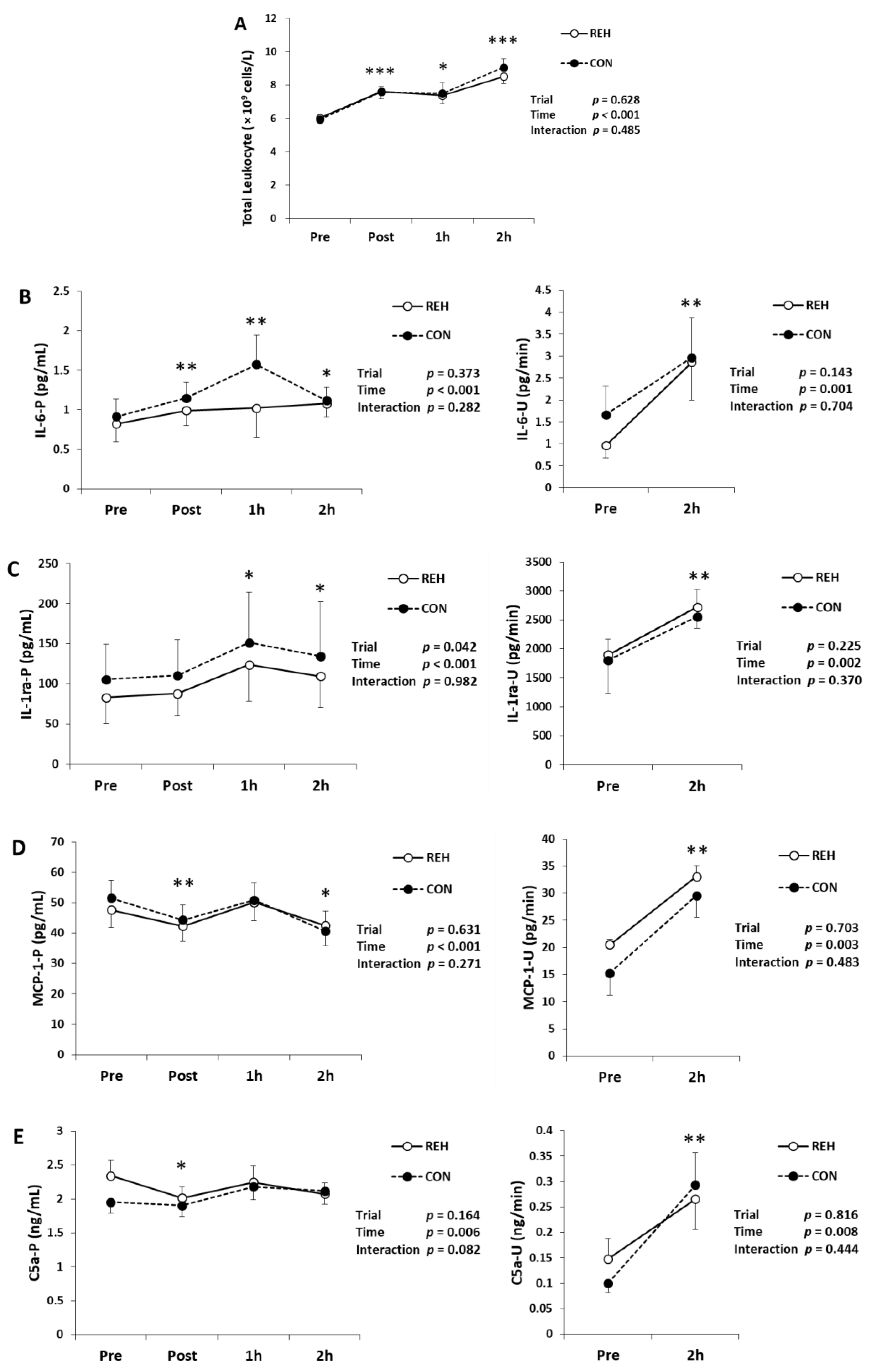
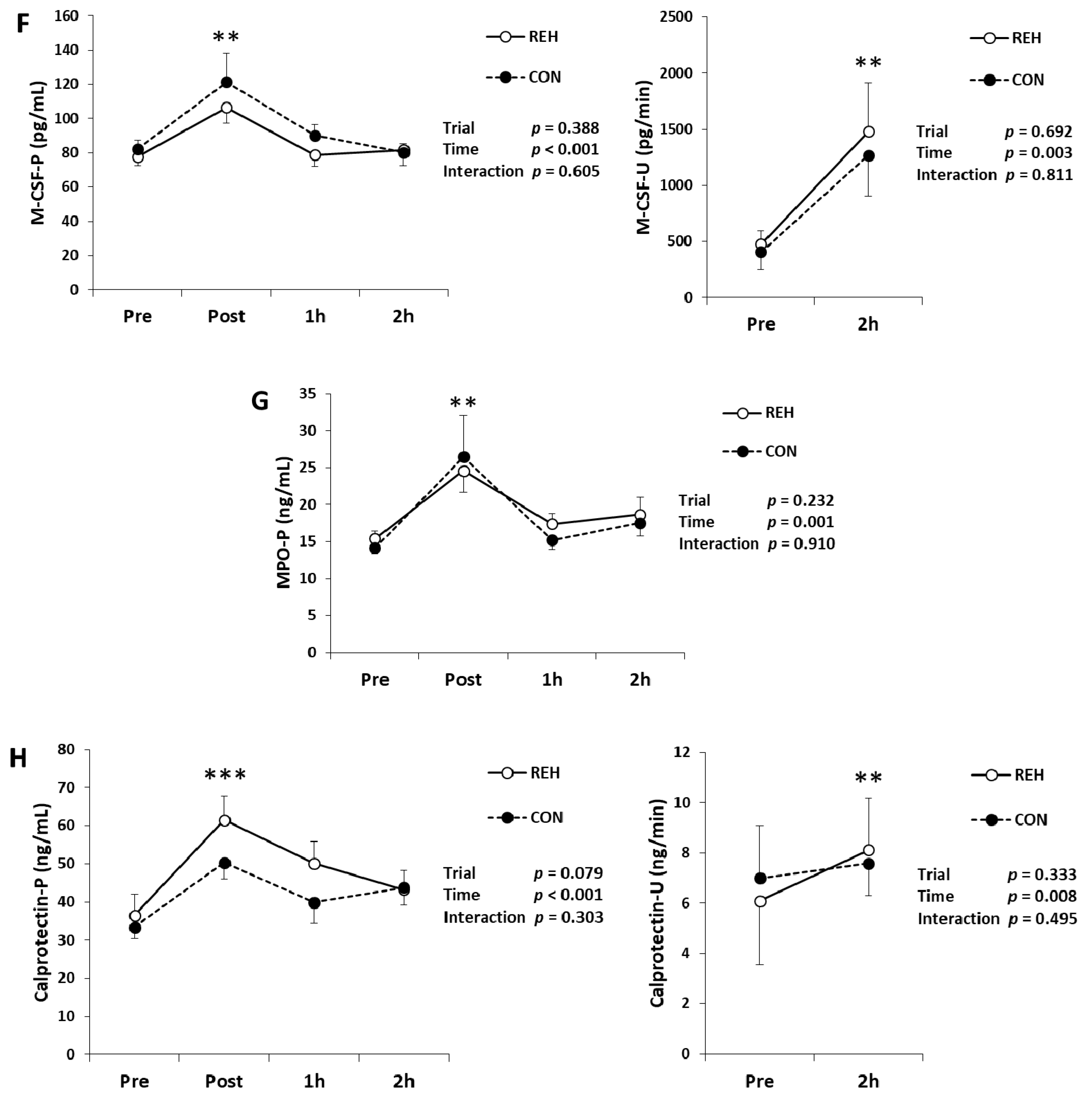
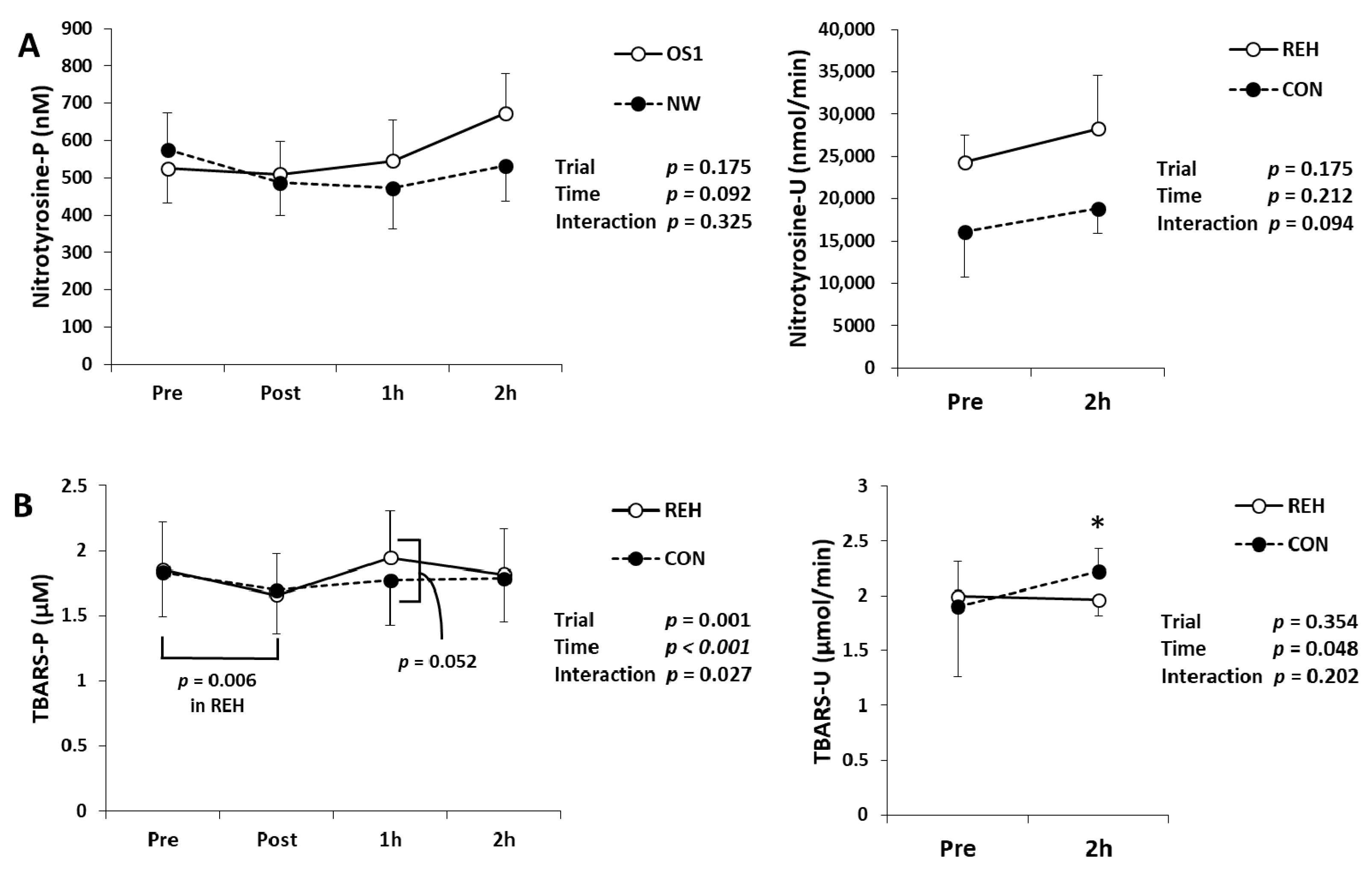
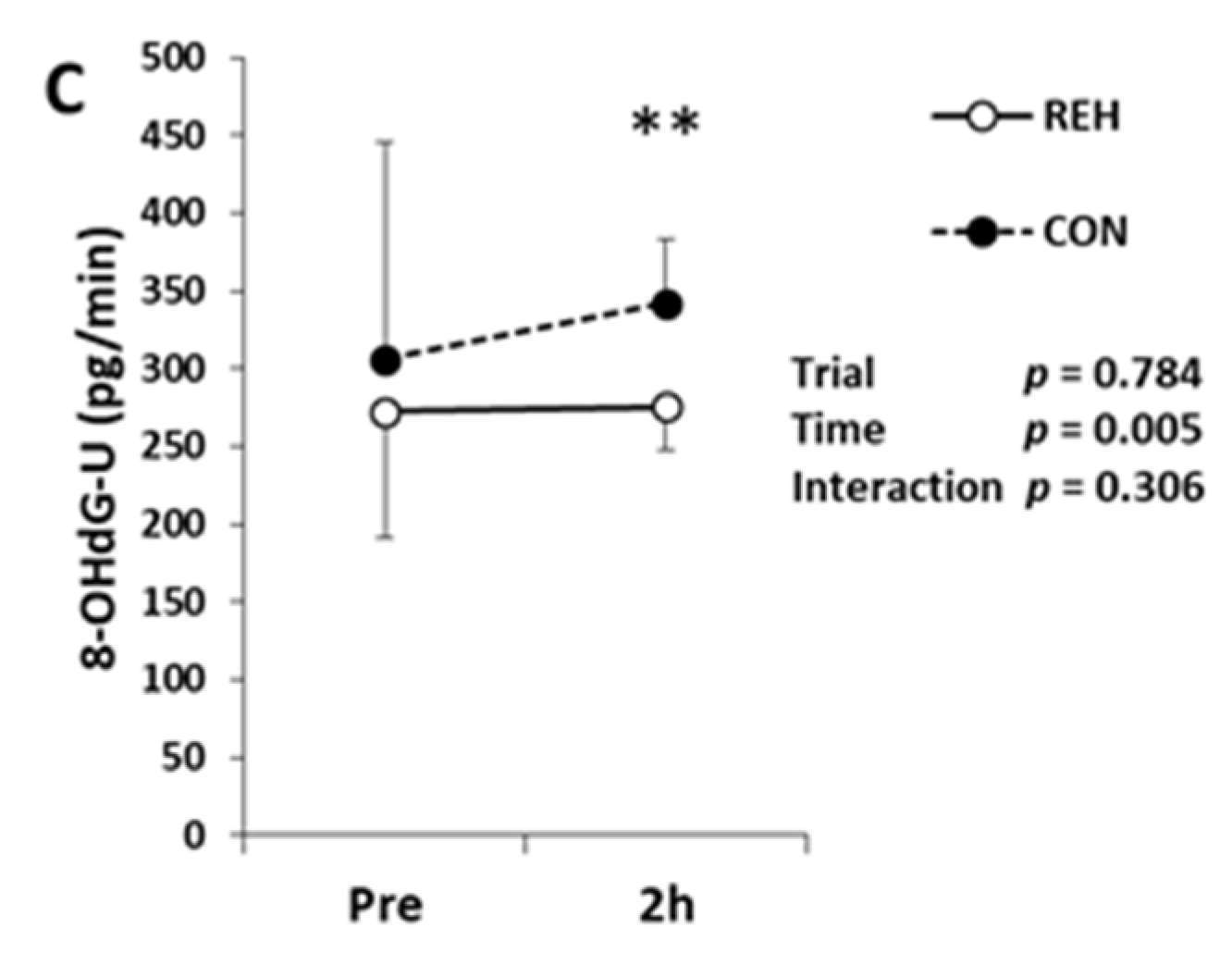
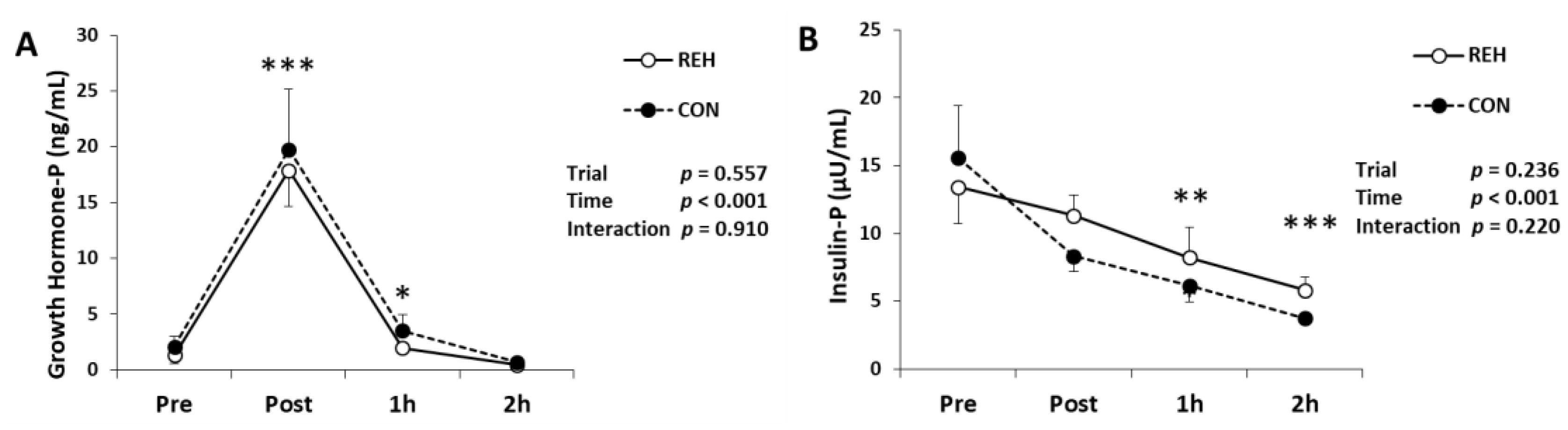
| Unit | Trial | Pre | Post | 1h | 2h | Trial | Time | Interaction | |
|---|---|---|---|---|---|---|---|---|---|
| BUN-S | mg/dL | ** | 0.907 | <0.001 | 0.902 | ||||
| REH | 18.88 ± 1.05 | 17.76 ± 1.12 | 19.80 ± 1.12 | 18.90 ± 1.16 | |||||
| CON | 19.02 ± 1.23 | 17.67 ± 1.12 | 20.05 ± 1.47 | 19.13 ± 1.36 | |||||
| Creatinine-S | mg/dL | ** | ** | 0.429 | <0.001 | 0.105 | |||
| REH | 0.92 ± 0.03 | 0.84 ± 0.03 | 0.98 ± 0.03 | 0.92 ± 0.03 | |||||
| CON | 0.95 ± 0.04 | 0.88 ± 0.03 | 0.98 ± 0.03 | 0.93 ±0.04 | |||||
| Cystatin C-P | mg/mL | *** | 0.232 | <0.001 | 0.326 | ||||
| REH | 0.74 ± 0.01 | 0.72 ± 0.01 | 0.80 ± 0.01 | 0.76 ± 0.02 | |||||
| CON | 0.74 ± 0.01 | 0.71 ± 0.02 | 0.77 ± 0.02 | 0.73 ± 0.02 | |||||
| Urine Protein-U | µg/min | ** | 0.528 | 0.001 | 0.183 | ||||
| REH | 41.08 ±10.48 | 73.23 ± 6.93 | |||||||
| CON | 26.31 ± 5.84 | 75.10 ± 8.93 | |||||||
| NAG-U | mU/min | ** | 0.930 | 0.001 | 0.624 | ||||
| REH | 29.88 ± 7.05 | 54.77 ± 6.13 | |||||||
| CON | 26.52 ± 7.26 | 56.82 ± 6.68 | |||||||
| Alubmin-U | mU/min | *** | 0.609 | <0.001 | 0.469 | ||||
| REH | 43.82 ± 11.29 | 338.26 ± 67.74 | |||||||
| CON | 47.80 ± 12.31 | 386.24 ± 74.32 | |||||||
| Endotoxin-P | IU/mL | REH | ND | ND | ND | ND | |||
| CON | ND | ND | ND | ND |
| Unit | Trial | Pre | Post | 1h | 2h | Trial | Time | Interaction | |
|---|---|---|---|---|---|---|---|---|---|
| TNF-α-P | pg/mL | REH | ND | ND | ND | ND | |||
| CON | ND | ND | ND | ND | |||||
| TNF-α-U | pg/mL | REH | ND | ND | |||||
| CON | ND | ND | |||||||
| IL-2-P | pg/mL | REH | ND | ND | ND | ND | |||
| CON | ND | ND | ND | ND | |||||
| IL-2-U | pg/mL | REH | ND | ND | |||||
| CON | ND | ND | |||||||
| IL-4-P | pg/mL | REH | ND | ND | ND | ND | |||
| CON | ND | ND | ND | ND | |||||
| IL-4-U | pg/mL | REH | ND | ND | |||||
| CON | ND | ND | |||||||
| IL-10-P | pg/mL | REH | 5.96 ± 1.00 | 5.61 ± 0.89 | 5.78 ± 0.64 | 4.64 ± 0.66 | 0.540 | 0.091 | 0.655 |
| CON | 5.34 ± 0.73 | 5.74 ± 1.07 | 6.61 ± 0.91 | 5.61 ± 0.98 | |||||
| IL-10-U | pg/min | REH | 9.85 ± 1.33 | 13.83 ± 1.19 | 0.006 | 0.214 | 0.707 | ||
| CON | 7.98 ± 2.18 | 7.84 ± 0.83 | |||||||
| IL-18-P | pg/mL | REH | ND | ND | ND | ND | |||
| CON | ND | ND | ND | ND | |||||
| IL-18-U | pg/mL | REH | ND | ND | |||||
| CON | ND | ND | |||||||
| IL-18BPa-P | ng/mL | REH | 79.53 ± 11.01 | 79.56 ± 11.55 | 66.39 ± 12.52 | 75.52 ± 11.94 | 0.904 | 0.146 | 0.683 |
| CON | 74.10 ± 10.17 | 81.61 ± 11.93 | 70.10 ± 9.19 | 72.63 ± 5.25 | |||||
| IL-18BPa-U | ng/min | REH | 94.55 ± 21.41 | 133.49 ± 30.00 | 0.025 | 0.287 | 0.838 | ||
| CON | 62.17 ± 24.42 | 52.75 ± 11.45 | |||||||
| G-CSF-P | pg/mL | REH | 14.17 ± 2.62 | 12.69 ± 2.30 | 14.41 ± 2.32 | 13.16 ± 2.23 | 0.789 | 0.292 | 0.246 |
| CON | 13.49 ± 2.53 | 12.32 ± 2.01 | 15.37 ± 2.15 | 13.36 ± 2.08 | |||||
| G-CSF-U | pg/mL | REH | ND | ND | |||||
| CON | ND | ND | |||||||
| MPO-U | ng/mL | REH | ND | ND | |||||
| CON | ND | ND |
| Unit | Trial | Pre | Post | 1h | 2h | Trial | Time | Interaction | |
|---|---|---|---|---|---|---|---|---|---|
| d-ROMs-S | U-CARR | REH | 237.77 ± 10.67 | 234.38 ± 10.36 | 237.37 ± 10.64 | 233.00 ± 11.37 | 0.138 | 0.327 | 0.567 |
| CON | 230.00 ± 9.50 | 218.87 ± 9.87 | 224.70 ± 10.90 | 227.84 ± 10.73 | |||||
| BAP-S | mmol/L | REH | 2.28 ± 0.05 | 2.32 ± 0.05 | 2.31 ± 0.07 | 2.36 ± 0.06 | 0.218 | 0.343 | 0.474 |
| CON | 2.20 ± 0.05 | 2.26 ± 0.04 | 2.32 ± 0.06 | 2.23 ± 0.03 | |||||
| Uric Acid-S | mg/dL | *** | *** | ** | 0.427 | <0.001 | 0.602 | ||
| REH | 5.43 ± 0.35 | 5.11 ± 0.34 | 7.01 ± 0.58 | 6.52 ± 0.52 | |||||
| CON | 5.76 ± 0.29 | 5.26 ± 0.26 | 7.04 ± 0.35 | 6.65 ± 0.37 | |||||
| Uric Acid-U | µg/min | * | 0.229 | 0.025 | 0.994 | ||||
| REH | 564.32 ± 86.24 | 775.35 ± 80.89 | |||||||
| CON | 445.87 ± 19.81 | 658.69 ± 68.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tominaga, T.; Ikemura, T.; Yada, K.; Kanda, K.; Sugama, K.; Ma, S.; Choi, W.; Araya, M.; Huang, J.; Nakamura, N.; et al. The Effects of Beverage Intake after Exhaustive Exercise on Organ Damage, Inflammation and Oxidative Stress in Healthy Males. Antioxidants 2021, 10, 866. https://doi.org/10.3390/antiox10060866
Tominaga T, Ikemura T, Yada K, Kanda K, Sugama K, Ma S, Choi W, Araya M, Huang J, Nakamura N, et al. The Effects of Beverage Intake after Exhaustive Exercise on Organ Damage, Inflammation and Oxidative Stress in Healthy Males. Antioxidants. 2021; 10(6):866. https://doi.org/10.3390/antiox10060866
Chicago/Turabian StyleTominaga, Takaki, Tsukasa Ikemura, Koichi Yada, Kazue Kanda, Kaoru Sugama, Sihui Ma, Wonjun Choi, Mayu Araya, Jiapeng Huang, Nobuhiro Nakamura, and et al. 2021. "The Effects of Beverage Intake after Exhaustive Exercise on Organ Damage, Inflammation and Oxidative Stress in Healthy Males" Antioxidants 10, no. 6: 866. https://doi.org/10.3390/antiox10060866
APA StyleTominaga, T., Ikemura, T., Yada, K., Kanda, K., Sugama, K., Ma, S., Choi, W., Araya, M., Huang, J., Nakamura, N., & Suzuki, K. (2021). The Effects of Beverage Intake after Exhaustive Exercise on Organ Damage, Inflammation and Oxidative Stress in Healthy Males. Antioxidants, 10(6), 866. https://doi.org/10.3390/antiox10060866









