Natural Antioxidant Application on Fat Accumulation: Preclinical Evidence
Abstract
1. Introduction
2. Natural Antioxidant Compounds
2.1. Flavonoids
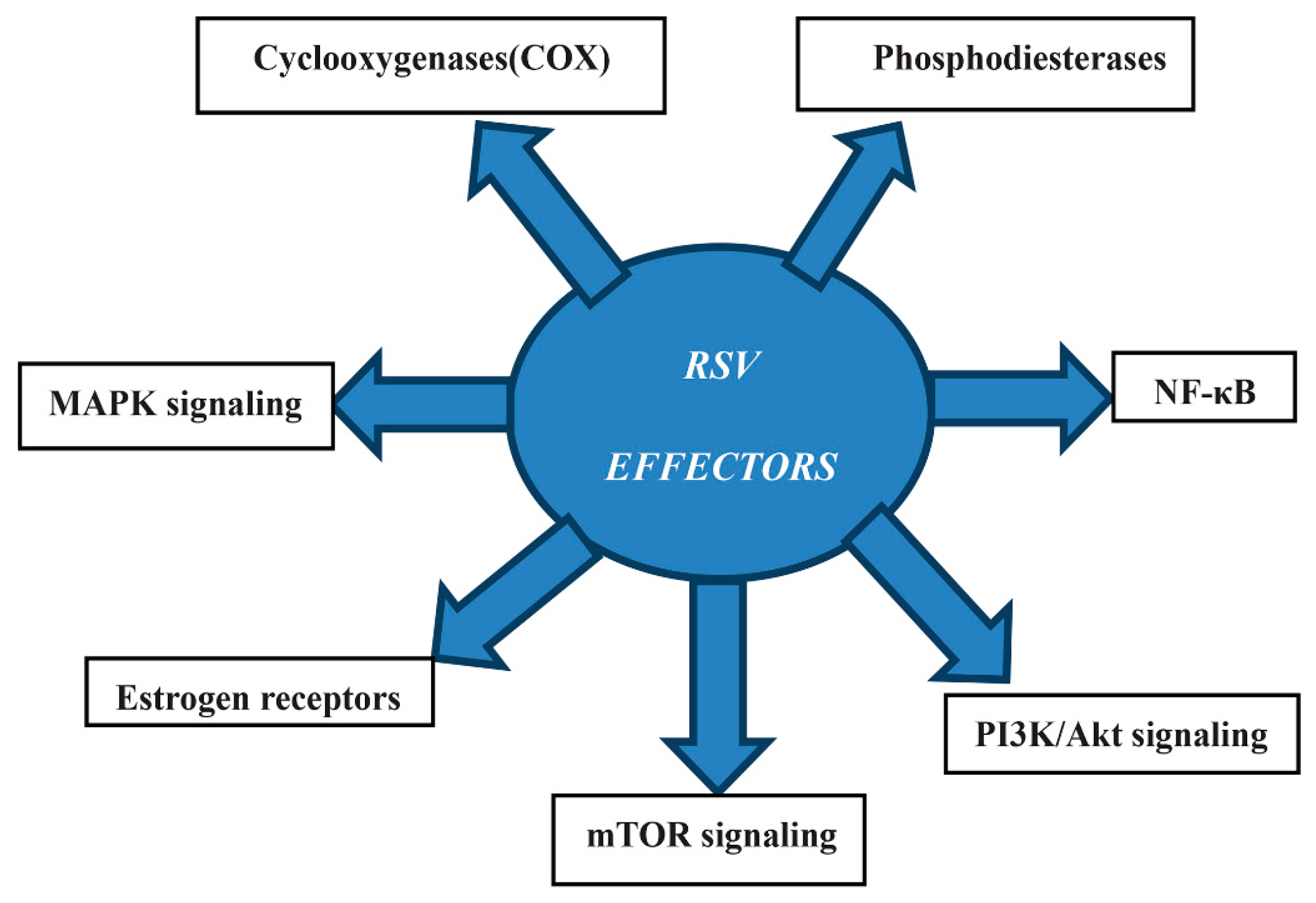
2.2. Resveratrol
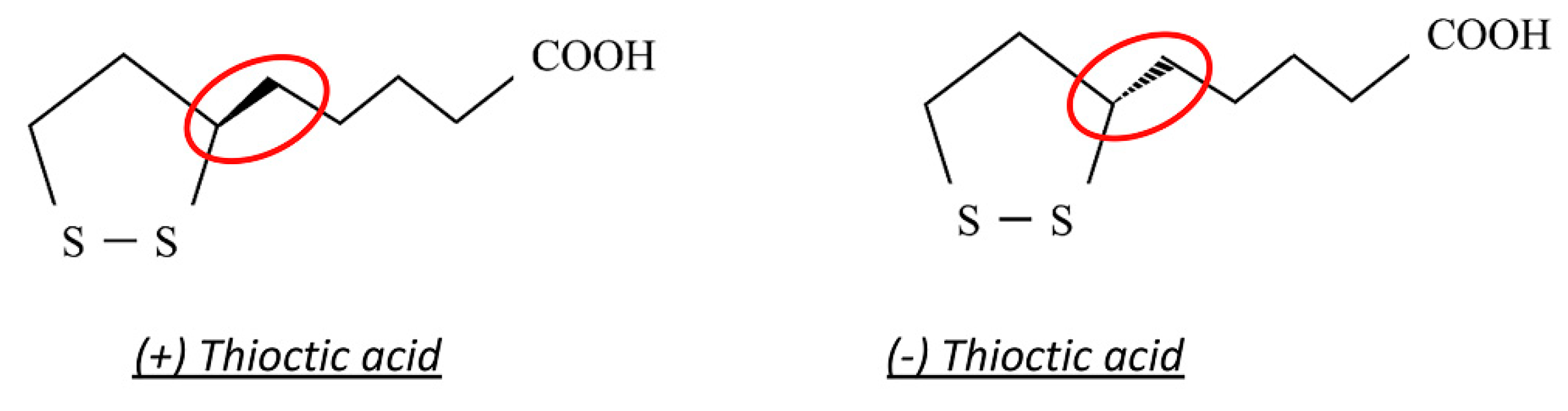
2.3. Thioctic Acid
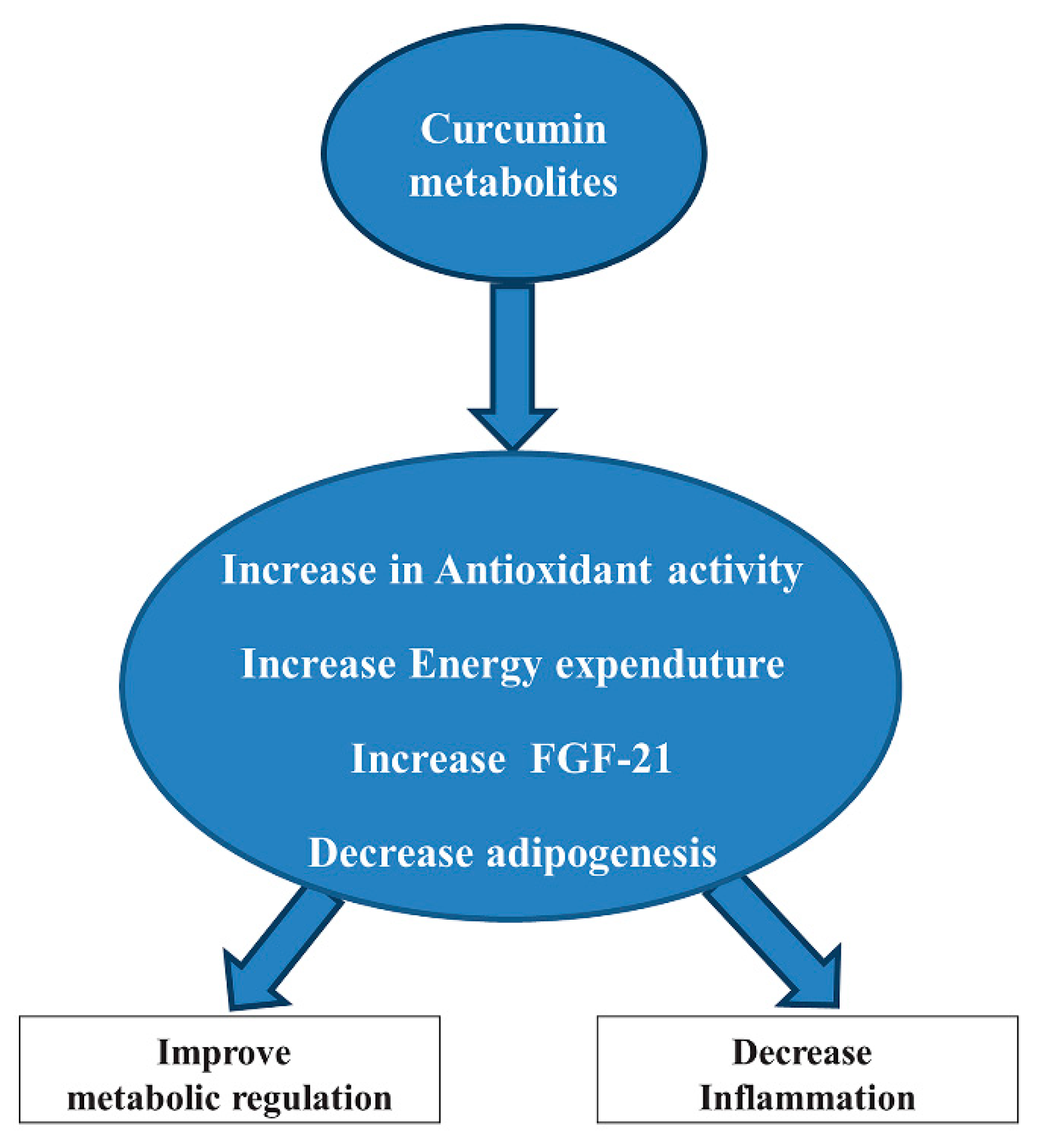
2.4. Curcumin
2.5. Caffeine and Catechin
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef] [PubMed]
- Wormser, D.; Kaptoge, S.; Di Angelantonio, E.; Wood, A.M.; Pennells, L.; Thompson, A.; Sarwar, N.; Kizer, J.R.; Lawlor, D.A.; Nordestgaard, B.G.; et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet 2011, 377, 1085–1095. [Google Scholar]
- Nimptsch, K.; Konigorski, S.; Pischon, T. Diagnosis of obesity and use of obesity biomarkers in science and clinical medicine. Metabolism 2019, 92, 61–70. [Google Scholar] [CrossRef]
- Hoyas, I.; Leon-Sanz, M. Nutritional challenges in metabolic syndrome. J. Clin. Med. 2019, 8, 1301. [Google Scholar] [CrossRef]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Boccuto, L.; Federico, A.; Dallio, M.; Loguercio, C.; Di Renzo, L.; De Lorenzo, A. Diet and non-alcoholic fatty liver disease: The mediterranean way. Int. J. Environ. Res. Public Health 2019, 16, 3011. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: An updated review. Oxid. Med. Cell Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef] [PubMed]
- Sensoy, I. A review on the relationship between food structure, processing, and bioavailability. Crit. Rev. Food Sci. Nutr. 2014, 54, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Karakaya, S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef]
- Lith, R.V.; Ameer, G.A. Antioxidant polymers as biomaterial. Oxidative Stress Biomater. 2016, 2, 251–296. [Google Scholar]
- Hollman, P.C.H.; Arts, I.C.W. Flavonols, flavones, and flavanols—Nature, occurrence, and dietary burden. J. Sci. Food Agric. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Zhang, L.; Zuo, Z.; Lin, G. Intestinal and hepatic glucuronidation of flavonoids. Mol. Pharm. 2007, 4, 833–845. [Google Scholar] [CrossRef]
- Vamanu, E.; Gatea, F. Correlations between Microbiota Bioactivity and Bioavailability of Functional Compounds: A Mini-Review. Biomedicines 2020, 20, 39. [Google Scholar] [CrossRef]
- Angelino, D.; Berhow, M.; Ninfali, P.; Jeffery, E.H. Caecal absorption of vitexin-2-O-xyloside and its aglycone apigenin, in the rat. Food Funct. 2013, 4, 1339–1345. [Google Scholar] [CrossRef]
- Martín, J.; Kuskoski, E.M.; Navas, M.J.; Asuero, A.G. Antioxidant capacity of anthocyanin pigments. In Flavonids: From Biosynthesis to Human Health; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Kirakosyan, A.; Seymour, E.M.; UrcuyoLlanes, D.E.; Kaufman, P.B.; Bolling, S.F. Chemical profile and antioxidant capacities of tart cherry products. Chemical profile and antioxidant capacities of tart cherry products. Food Chem. 2009, 30, 20–25. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Laskowski, P.; Oszmiański, J. Evaluation of sour cherry (Prunus cerasus L.) fruits for their polyphenol content, antioxidant properties, and nutritional components. J. Agric. Food Chem. 2014, 62, 12332–12345. [Google Scholar] [CrossRef]
- Tall, J.M.; Seeram, N.P.; Zhao, C.; Nair, M.G.; Meyer, R.A.; Raja, S.N. Tart cherry anthocyanins suppress inflammation-induced pain behavior in rat. Behav. Brain Res. 2004, 153, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, K.S.; Perrier, E.T.; Elliot, D.L.; Chesnutt, J.C. Efficacy of tart cherry juice in reducing muscle pain during running: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2010, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Recent progress in anti-obesity and anti-diabetes effect of berries. Antioxidants 2016, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Seeram, N.P.; Nair, M.G.; Bourquin, L.D. Tart cherry anthocyanins inhibit tumor development in Apc (Min) mice and reduce proliferation of human colon cancer cells. Cancer Lett. 2003, 194, 13–19. [Google Scholar] [CrossRef]
- Traustadóttir, T.; Davies, S.S.; Stock, A.A.; Su, Y.; Heward, C.B.; Roberts, L.J., 2nd; Harman, S.M. Tart cherry juice decreases oxidative stress in healthy older men and women. J. Nutr. 2009, 139, 1896–1900. [Google Scholar] [CrossRef]
- Howatson, G.B.; Tallent, P.G.J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 2012, 51, 909–916. [Google Scholar] [CrossRef]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Miniati, E. Anthocyanins in Fruits, Vegetables and Grains, 1st ed.; CRC Press: Boca Raton, FL, USA, 1993; p. 384. [Google Scholar]
- Pascual-Teresa De, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Kähkonen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999, 62, 294–296. [Google Scholar] [CrossRef]
- Saric, A.; Sobocanec, S.; Balog, T.; Kusic, B.; Sverko, V.; Dragovic-Uzelec, V.; Levaj, B.; Cosic, Z.; Safranko, Z.M.; Marotti, T. Improved antioxidant and anti-inflammatory potential in mice consuming sour cherry juice (Prunus Cerasus cv. Maraska). Plant Foods Hum. Nutr. 2009, 64, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Tsuda, T.; Shiga, K.; Ohshima, K.; Kawakishi, S.; Osawa, T. Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L. Biochem. Pharmacol. 1996, 52, 1033–1039. [Google Scholar] [CrossRef]
- Tedesco, I.; Luigi Russo, G.; Nazzaro, F.; Russo, M.; Palumbo, R. Antioxidant effect of red wine anthocyanins in normal and catalase-inactive human erythrocytes. J. Nutr. Biochem. 2001, 12, 505–511. [Google Scholar] [CrossRef]
- Heo, H.J.; Lee, C.Y. Strawberry and its anthocyanins reduce oxidative stress-induced apoptosis in pc12 cells. J. Agric. Food Chem. 2005, 53, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Isaak, C.K.; Petkau, J.C.; Blewett, H.; Karmin, O.; Siow, Y.L. Lingonberry anthocyanins protect cardiac cells from oxidative-stress-induced apoptosis. Can. J. Physiol. Pharmacol. 2017, 95, 904–910. [Google Scholar] [CrossRef]
- Thoppil, R.J.; Bhatia, D.; Barnes, K.F.; Haznagy-Radnai, E.; Hohmann, J.; Darvesh, A.S.; Bishayee, A. Black currant anthocyanins abrogate oxidative stress through nrf2-mediated antioxidant mechanisms in a rat model of hepatocellular carcinoma. Curr. Cancer Drug Targets 2012, 12, 44–1257. [Google Scholar]
- Afaq, F.; Saleem, M.; Krueger, C.G.; Reed, J.D.; Mukhtar, H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates mapk and nf-kappab pathways and inhibits skin tumorigenesis in cd-1 mice. Int. J. Cancer 2005, 113, 423–433. [Google Scholar] [CrossRef]
- Jeong, J.W.; Lee, W.S.; Shin, S.C.; Kim, G.Y.; Choi, B.T.; Choi, Y.H. Anthocyanins down regulate lipopolysaccharide-induced inflammatory responses in bv2 microglial cells by suppressing the nf-kappab and akt/mapks signaling pathways. Int. J. Mol. Sci. 2013, 14, 1502–1515. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Klimis-Zacas, D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-kappab and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Booren, A.M.; Gray, J.I. Novel antioxidant compounds from tart cherries (Prunus cerasus). J. Nat. Prod. 1999, 62, 86–88. [Google Scholar] [CrossRef]
- Micioni Di Bonaventura, M.V.; Martinelli, I.; Moruzzi, M.; Micioni Di Bonaventura, E.; Giusepponi, M.E.; Polidori, C.; Lupidi, G.; Tayebati, S.K.; Amenta, F.; Cifani, C.; et al. Brain alterations in high fat diet induced obesity: Effects of tart cherry seeds and juice. Nutrients 2020, 12, 623. [Google Scholar] [CrossRef]
- Martinelli, I.; Micioni Di Bonaventura, M.V.; Moruzzi, M.; Amantini, C.; Maggi, F.; Gabrielli, M.G.; Fruganti, A.; Marchegiani, A.; Dini, F.; Marini, C.; et al. Effects of Prunus cerasus L. seeds and juice on liver steatosis in an animal model of diet-induced obesity. Nutrients 2020, 12, 1308. [Google Scholar] [CrossRef]
- Cocci, P.; Moruzzi, M.; Martinelli, I.; Maggi, F.; Micioni Di Bonaventura, M.V.; Cifani, C.; Mosconi, G.; Tayebati, S.K.; Damiano, S.; Lupidi, G.; et al. Tart cherry (Prunus cerasus L.) dietary supplement modulates visceral adipose tissue CB1 mRNA levels along with other adipogenesis-related genes in rat models of diet-induced obesity. Eur. J. Nutr. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Moruzzi, M.; Kloting, N.; Blüher, M.; Martinelli, I.; Tayebati, S.K.; Gabrielli, M.G.; Roy, P.; Micioni Di Bonaventura, M.V.; Cifani, C.; Lupidi, G.; et al. Tart cherry juice and seeds affect pro-inflammatory markers in visceral adipose tissue of high-fat diet obese rats. Molecules 2021, 26, 1403. [Google Scholar] [CrossRef]
- Blando, F.; Gerardi, C.; Nicoletti, I. Sour Cherry (Prunus cerasus L.) Anthocyanins as ingredients for functional foods. J. Biomed. Biotechnol. 2004, 2004, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Tumbas, V.T.; Canadanović-Brunet, J.M.; Cetojević-Simin, D.D.; Cetković, G.S.; Ethilas, S.M.; Gille, L. Effect of rosehip (Rosa canina L.) phytochemicals on stable free radicals and human cancer cells. J. Sci. Food Agric. 2012, 92, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Pulcinelli, F.M.; Celestini, A.; Lenti, L.; Ghiselli, A.; Gazzaniga, P.P.; Violi, F. The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am. J. Clin. Nutr. 2000, 1150, 5. [Google Scholar] [CrossRef]
- Wu, T.; Guo, X.; Zhang, M.; Yang, L.; Liu, R.; Yin, J. Anthocyanins in black rice, soybean and purple corn increase fecal butyric acid and prevent liver inflammation in high fat diet-induced obese mic. Food Funct. 2017, 8, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Seymour, E.M.; Noon, K.R.; Llanes, D.E.U.; Kaufman, P.B.; Warber, S.L.; Bolling, S.F. Interactions of antioxidants isolated from tart cherry (Prunus cerasus) fruits. Food Chem. 2010, 122, 78–83. [Google Scholar] [CrossRef]
- Zorita, S.G.; Quintela, A.F.; Lasa, A.; Hijona, E.; Bujanda, L.; Portillo, M. Effects of resveratrol on obesity-related inflammation markers in adipose tissue of genetically obese rats. Nutrition 2013, 29, 1374–1380. [Google Scholar] [CrossRef]
- Sameer, S.K.; Carles, C. The molecular targets of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1114–1123. [Google Scholar]
- Park, E.J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta 2015, 1852, 1071–1113. [Google Scholar] [CrossRef]
- Wong, R.H.; Howe, P.R.; Buckley, J.D.; Coates, A.M.; Kunz, I.; Berry, N.M. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr. Metab. Cardiovasc. 2011, 21, 851–856. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; Weijer, T.V.D.; Goossens, G.H.; Hoeks, J.; Krieken, S.V.D.; Kersten, D.; Ryu, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Wong, R.H.; Berry, N.M.; Coates, A.M.; Buckley, J.D.; Bryan, J.; Kunz, I.; Howe, P.R. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J. Hypertens. 2013, 31, 819–1827. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cells 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jin, Y.; Choi, Y.; Park, T. Resveratrol exerts antiobesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem. Pharmacol. 2011, 81, 1343–1351. [Google Scholar] [CrossRef]
- Cho, S.J.; Jung, U.J.; Choi, M.S. Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice. Br. J. Nutr. 2012, 108, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, G.; Rodriguez, V.M.; Miranda, J.; Macarulla, M.T.; Churruca, I.; Portillo, M.P. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem. 2013, 141, 1530–1535. [Google Scholar] [CrossRef]
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) alpha1. Int. J. Obes. 2015, 39, 967–976. [Google Scholar] [CrossRef]
- Ku, C.R.; Cho, Y.H.; Hong, Z.Y.; Lee, H.; Lee, S.J.; Hong, S.S.; Lee, E.J. The effects of high fat diet and resveratrol on mitochondrial activity of brown adipocytes. Endocrinol. Metab. 2016, 31, 328–335. [Google Scholar] [CrossRef]
- Arias, N.; Pico, C.; Macarulla, M.T.; Oliver, P.; Miranda, J.; Palou, A.; Portillo, M.P. A combination of resveratrol and quercetin induces browning in white adipose tissue of rats fed an obesogenic diet. Obesity 2017, 25, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, J.; Li, X.; Zhou, Q.; Bai, J.; Shi, Y.; Le, G. Resveratrol prevents suppression of regulatory T-cell production, oxidative stress, and inflammation of mice prone or resistant to high-fat diet-induced obesity. Nutr. Res. 2013, 33, 971–981. [Google Scholar] [CrossRef]
- Jeon, B.T.; Jeong, E.A.; Shin, H.J.; Lee, Y.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 2012, 61, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gomez, Y.; Mattison, J.A.; Pearson, K.J.; Martin-Montalvo, A.; Palacios, H.H.; Sossong, A.M.; Ward, T.M.; Younts, C.M.; Lewis, K.; Allard, J.S.; et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013, 18, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.M.; Wang, Q.; Chen, Y.H.; Wang, S.H.; Huang, D.Q. Resveratrol attenuates inflammation and oxidative stress in epididymal white adipose tissue: Implications for its involvement in improving steroidogenesis in diet induced obese mice. Mol. Reprod. Dev. 2015, 82, 321–328. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2012, 821, 421–433. [Google Scholar]
- Kowalski, J.; Samojedni, A.; Paul, M.; Pietsz, G.; Wilczok, T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1 beta and tumor necrosis factor-alpha genes in J774.2 macrophages. Pharmacol. Rep. 2005, 390, 4. [Google Scholar]
- Ma, Z.H.; Ma, Q.Y.; Wang, L.C.; Sha, H.C.; Wu, S.L.; Zhang, M. Effect of resveratrol NF-kappaB activity in rat peritoneal macrophages. Am. J. Chin. Med. 2006, 34, 623–630. [Google Scholar] [CrossRef]
- Das, S.; Das, D.K. Anti-inflammatory responses of resveratrol. Inflamm. Allergy Drug Targets 2007, 6, 168–173. [Google Scholar] [CrossRef]
- Gonzalez, R.; Ballester, I.L.; Opez-Posadas, R.; Suarez, M.D.; Zarzuelo, A.; Martınez Augustin, O.; Sanchez de Madina, F. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Chuang, C.C.; Martinez, K.; Xie, G.; Kennedy, A.; Bumrungper, A.; Overman, A.; Jia, W.; McIntosh, M.K. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor-a–mediated inflammation and insulin resistance in primary human adipocytes. Am. J. Clin. Nutr. 2010, 92, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Mercader, J.; Palou, A.; Bonet, M.L. Resveratrol enhances fatty acid oxidation capacity and reduces resistin and Retinol-Binding Protein 4 expression in white adipocytes. J. Nutr. Biochem. 2011, 22, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Olholm, J.; Paulsen, S.K.; Cullberg, K.B.; Richelsen, B.; Peersen, S.B. Anti-inflammatory effect of resveratrol on adipokine expression and secretion inhuman adipose tissue explants. Int. J. Obes. 2010, 34, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.C.; McIntosh, M.K. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu. Rev. Nutr. 2011, 31, 155–176. [Google Scholar] [CrossRef]
- Terra, X.; Montagut, G.; Bustos, M.; Llopiz, N.; Ardevol, A.; Blade, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J. Nutr. Biochem. 2009, 20, 210–218. [Google Scholar] [CrossRef]
- Hogan, S.; Canning, C.; Sun, S.; Sun, X.; Kadouh, H.; Zhou, K. Dietary supplementation of grape skin extract improves glycemia and inflammation indiet-induced obese mice fed a Western high fat diet. J. Agric. Food Chem. 2011, 59, 3035–3041. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Pallares, V.; Ardevol, A.; Blad, C.F.; Andez-Larrea, J.; Pujadas, G.; Salvado, J.; Arola, L.; Blay, M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Cucciolla, V.; Borriello, A.; Oliva, A.; Galletti, P.; Zappia, V.; Della, R.F. Resveratrol: From basic science to the clinic. Cell Cycle 2007, 6, 2495–2510. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Singh, G.; Srivastava, R.K. Chemoprevention by resveratrol: Molecular mechanisms and therapeutic potential. Front. Biosci. 2007, 12, 4839–4854. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Back, J.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch. Biochem. Biophys. 2009, 486, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, S.; Holme, A.L. Resveratrol: Its biologic targets and functional activity. Antioxid. Redox Signal. 2009, 11, 2851–2897. [Google Scholar] [CrossRef]
- Packer, L.; Cadenas, E. Lipoic acid: Energy metabolism and redox regulation of transcription and cell signaling. J. Clin. Biochem. Nutr. 2011, 48, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Goraca, A.; Huk-Kolega, H.; Piechota, A.; Kleniewska, P.; Ciejka, E.; Skibska, B. Lipoic acid biological activity and therapeutic potential. Pharmacol. Rep. 2011, 63, 849–858. [Google Scholar] [CrossRef]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef]
- Szeląg, M.; Mikulski, D.; Molski, M. Quantum-chemical investigation of the structure and the antioxidant properties of α-lipoic acid and its metabolites. J. Mol. Model. 2012, 18, 2907–2916. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.B.; Negrato, C.A. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol. Metab. Syndr. 2014, 6, 80. [Google Scholar] [CrossRef]
- Reed, L.J. From lipoic acid to multi-enzyme complexes. Protein Sci. 1998, 7, 220–224. [Google Scholar] [CrossRef]
- Liu, J.; Head, E.; Gharib, A.M.; Yuan, W.; Ingersoll, R.T.; Hagen, T.M.; Cotman, C.W.; Ames, B.N. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: Partial reversal by feeding acetyl-L-carnitine and/or R-α-lipoic acid. Proc. Natl. Acad. Sci. USA 2002, 99, 2356–2361. [Google Scholar] [CrossRef]
- Suh, J.H.; Zhu, B.Z.; de Szoeke, E.; Frei, B.; Hagen, T.M. Dihydrolipoic acid lowers the redox activity of transition metal ions but does not remove them from the active site of enzymes. Redox Rep. 2004, 9, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.W.; Song, Y.M.; Kim, E.; Cho, H.S.; Cheon, K.A.; Kim, S.J.; Park, J.Y. Adjunctive α-lipoic acid reduces weight gain compared with placebo at 12 weeks in schizophrenic patients treated with atypical antipsychotics: A double-blind randomized placebo-controlled study. Int. Clin. Psychopharmacol. 2016, 31, 265–274. [Google Scholar] [CrossRef]
- Sun, H.; Yao, W.; Tang, Y.; Zhuang, W.; Wu, D.; Huang, S.; Sheng, H. Urinary exosomes as a novel biomarker for evaluation of α-lipoic acid’s protective effect in early diabetic nephropathy. J. Clin. Lab. Anal. 2017, 31, 22129. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, C.N.S.; da Silva Leite, C.M.G.; da Silva Medeiros, I.; Vasconcelos, L.C.; Cabral, L.M.; Patrocínio, C.F.V.; Patrocínio, M.L.V.; Mouaffak, F.; Kebir, O.; Macedo, D.; et al. Alpha-lipoic acid in the treatment of psychiatric and neurological disorders: A systematic review. Metab. Brain Dis. 2019, 34, 39–52. [Google Scholar] [CrossRef]
- Cakatay, U.; Telci, A.; Salman, S.; Satman, L.; Sivas, A. Oxidative protein damage in type I diabetic patients with and without complications. Endocr. Res. 2000, 26, 65–79. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S.; Richard, C.; Zeller, M.; Cottin, Y.; Vergely, C. Direct and indirect antioxidant properties of α-lipoic acid and therapeutic potential. Mol. Nutr. Food Res. 2013, 57, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Nowak, H.; Kempler, P.; Vargha, P.; Low, P.A. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: A meta-analysis. Diabet. Med. 2004, 21, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Huerta, A.E.; Navas-Carretero, S.; Prieto-Hontoria, P.L.; Martinez, J.A.; Moreno-Aliaga, M.J. Effects of alpha-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity 2015, 23, 313–321. [Google Scholar] [CrossRef]
- Prieto-Hontoria, P.L.; Perez-Matute, P.; Fernandez-Galilea, M.; Martinez, J.A.; Moreno-Aliaga, M.J. Effects of lipoic acid on AMPK and adiponectin in adipose tissue of low- and high-fat-fed rats. Eur. J. Nutr. 2013, 52, 779–787. [Google Scholar] [CrossRef]
- Carbonelli, M.G.; Renzo, L.D.; Bigioni, M.D.; Daniele, N.; Lorenzo, A.D.; Fusco, M.A. Alpha-lipoic acid supplementation: A tool for obesity therapy? Curr. Pharm. Des. 2010, 16, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.H.; Lee, W.J.; Lee, S.A.; Kim, E.H.; Cho, E.H.; Jeong, E.; Kim, D.W.; Kim, M.-S.; Park, J.-Y.; Park, K.-G.; et al. Effects of alpha-lipoic Acid on body weight in obese subjects. Am. J. Med. 2011, 124, 1–8. [Google Scholar] [CrossRef]
- Ansar, H.; Mazloom, Z.; Kazemi, F.; Hejazi, N. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med. J. 2011, 32, 584–588. [Google Scholar] [PubMed]
- McNeilly, A.M.; Davison, G.W.; Murphy, M.H.; Nadeem, N.; Trinick, T.; Duly, E.; Novials, A.; McEneny, J. Effect of alpha-lipoic acid and exercise training on cardiovascular disease risk in obesity with impaired glucose tolerance. Lipids Health Dis. 2011, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Kraemer, K.; Rimbach, G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001, 17, 888–895. [Google Scholar] [CrossRef]
- Brufani, M. Acido α-lipoico farmaco o integratore. Una panoramica sulla farmacocinetica, le formulazioni disponibili e le evidenze cliniche nelle complicanze del diabete. Prog. Nutr. 2014, 16, 62–74. [Google Scholar]
- Ou, P.; Tritschler, H.J.; Wolff, S.P. Thioctic (lipoic) acid: A therapeutic metal-chelating antioxidant? Biochem. Pharmacol. 1995, 50, 123–126. [Google Scholar] [CrossRef]
- Ziegler, D.; Ametov, A.; Barinov, A.; Dyck, J.P.; Gurieva, I.; Low, P.A.; Munzel, U.; Yakhno, N.; Raz, I.; Novosadova, M.; et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: The Sydney 2 trial. Diabetes Care 2006, 29, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.C.; Villagarcía, H.G.; Massa, M.L.; Francini, F. Alpha-lipoic acid and its protective role in fructose induced endocrine-metabolic disturbances. Food Funct. 2019, 10, 16–25. [Google Scholar] [CrossRef]
- Singh, U.; Jialal, I. Alpha-lipoic acid supplementation and diabetes. Nutr. Rev. 2008, 66, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Keith, D.J.; Butler, J.A.; Bemer, B.; Dixon, B.; Johnson, S.; Garrard, M.; Sudakin, D.L.; Christensen, J.M.; Pereira, C.; Hagen, T.M. Age and gender dependent bioavailability of R- and R,S-alpha-lipoic acid: A Pilot study. Pharmacol. Res. 2012, 66, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Solmonson, A.; De Berardinis, R.J. Lipoic acid metabolism and mitochondrial redox regulation. J. Biol. Chem. 2018, 293, 7522–7530. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D. Thioctic acid for patients with symptomatic diabetic polyneuropathy: A critical review. Treat. Endocrinol. 2004, 3, 173–189. [Google Scholar] [CrossRef]
- Henriksen, E.J. Exercise training and the antioxidant alpha-lipoic acid in the treatment of insulin resistance and type 2 diabetes. Free Radic. Biol. Med. 2006, 40, 3–12. [Google Scholar] [CrossRef]
- Ciftci, H.; Bakal, U. The effect of lipoic acid on macro and trace metal levels in living tissues exposed to oxidative stress. Anti Cancer Agents Med. Chem. 2009, 9, 560–568. [Google Scholar] [CrossRef]
- Akiba, S.; Matsugo, S.; Packer, L.; Konishi, T. Assay of protein-bound lipoic acid in tissues by a new enzymatic method. Anal. Biochem. 1998, 258, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Badran, M.; Laher, I. Diabetes and alpha lipoic Acid. Front. Pharmacol. 2011, 2, 69. [Google Scholar] [CrossRef]
- Smith, A.R.; Shenvi, S.V.; Widlansky, M.; Suh, J.H.; Hagen, T.M. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr. Med. Chem. 2004, 11, 1135–1146. [Google Scholar] [CrossRef]
- Han, D.; Sen, C.K.; Roy, S.; Kobayashi, M.S.; Tritschler, H.J.; Packer, L. Protection against glutamate-induced cytotoxicity in C6 glial cells by thiol antioxidants. Am. J. Physiol. Integr. Comp. Physiol. 1997, 273, 1771–1778. [Google Scholar] [CrossRef]
- Wray, D.W.; Nishiyama, S.K.; Harris, R.A.; Zhao, J.; McDaniel, J.; Fjeldstad, A.S.; Witman, M.A.; Ives, S.J.; Barrett-O’Keefe, Z.; Richardson, R.S. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 2012, 59, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef]
- Jin, T.; Song, Z.; Weng, J.; Fantus, I.G. Curcumin and other dietary polyphenols: Potential mechanisms of metabolic actions and therapy for diabetes and obesity. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E201–E205. [Google Scholar] [CrossRef]
- Mohammadi, A.; Sahebkar, M.; Iranshahi, M.; Amini, M.; Khojasteh, R.; Ghayour-Mobarhan, M.; Ferns, G.A. Effects of supplementation with curcuminoids on dyslipidemia in obese patients: A randomized crossover trial. Phytother. Res. PTR 2013, 27, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, S.; Sahebkar, A. Investigation of the effects of curcumin on serum cytokines in obese individuals: A randomized controlled trial. Sci. World J. 2014, 2014, 6. [Google Scholar] [CrossRef]
- Kim, C.Y.; Le, T.; Chen, C.; Cheng, J.X.; Kim, K.H. Curcumin inhibits adipocyte differentiation through modulation of mitotic clonal expansion. J. Nutr. Biochem. 2011, 22, 910–920. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Ha, T. Curcumin induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. Am. J. Physiol. Cell Physiol. 2010, 298, C1510–C1516. [Google Scholar] [CrossRef]
- Woo, H.M.; Kang, J.H.; Kawada, T.; Yoo, H.; Sung, M.K.; Yu, R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007, 80, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Miyazawa, T. Dietary curcuminoids prevent high-fat diet-induced lipid accumulation in rat liver and epididymal adipose tissue. J. Nutr. 2001, 131, 2932–2935. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid, M.N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef]
- Tian, L.; Song, Z.; Shao, W.; Du, W.W.; Zhao, L.R.; Zeng, K.; Yang, B.B.; Jin, T. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis. 2017, 8, e2559. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Ye, Z.; Xu, C.; Zhang, M.; Ruan, B.; Wei, M.; Jiang, Y.; Zhang, Y.; Wang, L.; et al. Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem. Biophys. Res. Commun. 2015, 466, 247–253. [Google Scholar] [CrossRef]
- Stevens, J.F.; Maier, C.S. The chemistry of gut microbial metabolism of polyphenols. Phytochem. Rev. 2016, 15, 425–444. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Leibel, P.R.; Tortoriello, D.V. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Yu, Z.; Chiang, Y.; Yang, Y.; Chai, T.; Foltz, W.; Lu, H.; Fantus, I.G.; Jin, T. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS ONE 2012, 7, 28784. [Google Scholar] [CrossRef]
- Liddle, M.; Hull, C.; Liu, C.; Powell, D. Contact urticaria from curcumin. Dermatitis 2006, 17, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Chand, S.; Hair, C.; Beswick, L. A rare case of turmeric-induced hepatotoxicity. Intern. Med. J. 2020, 50, 258–259. [Google Scholar] [CrossRef]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar] [PubMed]
- Boden, M.J.; Brandon, A.E.; Tid-Ang, J.D.; Preston, E.; Wilks, D.; Stuart, E.; Cleasby, M.E.; Turner, N.; Cooney, G.J.; Kraegen, E.W. Overexpression of manganese superoxide dismutase ameliorates high-fat diet-induced insulin resistance in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E798–E805. [Google Scholar] [CrossRef]
- Fu, S.; Watkins, S.M.; Hotamisligil, G.S. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012, 15, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Olefsky, J.M. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012, 15, 635–645. [Google Scholar] [CrossRef]
- Arruda, A.P.; Pers, B.M.; Parlakgül, G.; Güney, E.; Inouye, K.; Hotamisligil, G.S. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat. Med. 2014, 20, 1427–1435. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Turner, N. Mitochondrial dysfunction and insulin resistance: An update. Endocr. Connect. 2015, 4, R1–R15. [Google Scholar] [CrossRef]
- Haber, C.A.; Lam, T.K.; Yu, Z.; Gupta, N.; Goh, T.; Bogdanovic, E.; Giacca, A.; Fantus, I.G. N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: Possible role of oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E744–E753. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Dinh, T.C.; Thi, P.T.N.; Minh, L.B.; Minh, T.V.T.; Bac, N.D.; Van, T.N.; Pham, V.H.; Show, P.L.; Tao, Y.; Nhu, N.V.T. The effects of green tea on lipid metabolism and its potential applications for obesity and related metabolic disorders—An existing update. Diabetes Metab. Syndr. 2019, 13, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Noschang, C.G.; Krolow, R.; Pettenuzzo, L.F.; Avila, M.C.; Fachin, A.; Arcego, D.; von Pozzer Toigo, E.P.; Crema, L.M.; Diehl, L.A.; Vendite, D.; et al. Interactions between chronic stress and chronic consumption of caffeine on the enzymatic antioxidant system. Neurochem. Res. 2009, 34, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.V.; Silva-Oliveira, E.M.; Moraes, M.F.D.; Pereira, G.S.; Moraes-Santos, T. Chronic coffee and caffeine ingestion effects on the cognitive function and antioxidant system of rat brains. Pharmacol. Biochem. Behav. 2011, 99, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.G.; Mazen, N.F.; Mohamed, A.M. Caffeine intake decreases oxidative stress and inflammatory biomarkers in experimental liver diseases induced by thioacetamide: Biochemical and histological study. Int. J. Immunopathol. Pharmacol. 2017, 30, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.M.; Lima, D.R.A. Coffee consumption, obesity and type 2 diabetes: A mini review. Eur. J. Nutr. 2016, 55, 1345–1358. [Google Scholar] [CrossRef]
- Farias-Pereira, R.; Park, C.S.; Park, Y. Mechanisms of action of coffee bioactive components on lipid metabolism. Food Sci. Biotechnol. 2019, 2, 1287–1296. [Google Scholar] [CrossRef]
- Reto, M.; Figueira, M.E.; Filipe, H.M.; Almeida, C.M. Chemical composition of green tea (Camellia sinensis) infusions commercialized in Portugal. Plant. Foods Hum. Nutr. 2007, 62, 139–144. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Alschuler, L. Grean Tea: Healing tonic. Am. J. Nat. Med. 1998, 5, 28–31. [Google Scholar]
- Suzuki, T.; Miyoshi, N.; Hayakawa, S.; Imai, S.; Isemura, M.; Nakamura, Y. Health benefits of tea consumption. In Beverage Impacts on Health and Nutrition; Willson, T., Temple, N.J., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Serafini, M.; Ghiselli, A.; Ferro, L.A. In vivo antioxidant effect of green and black tea in man. Eur. J. Clin. Nutr. 1996, 50, 28–32. [Google Scholar] [PubMed]
- Erba, D.; Riso, P.; Colombo, A.; Testolin, G. Supplementation of Jurkat T cells with green tea extract decreases oxidative damage due to iron treatment. J. Nutr. 1999, 129, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Jigisha, A.; Nishant, R.; Navin, K. Green tea: A magical herb with miraculous outcomes. Int. Res. J. Pharm. 2012, 3, 139–148. [Google Scholar]
- Chatterjee, P.; Chandra, S.; Dey, P.; Bhattacharya, S. Evaluation of anti-inflammatory effects of green tea and black tea: A comparative in vitro study. J. Adv. Pharm. Technol. Res. 2012, 3, 136–138. [Google Scholar]
- Tsai, C.; Hsu, Y.; Ting, H.; Huang, C.; Yen, C. The in vivo antioxidant and antifbrotic properties of green tea (Camellia sinensis, Teaceae). Food Chem. 2013, 136, 1337–1344. [Google Scholar] [CrossRef]
- Newsome, B.J.; Petriello, M.C.; Han, S.G. Green tea diet decreases PCB 126-induced oxidative stress in mice by up-regulating antioxidant enzymes. J. Nutr. Biochem. 2014, 25, 126–135. [Google Scholar] [CrossRef]
- Gupta, D.A.; Bhaskar, D.J.; Gupta, R.K. Green tea: A review on its natural anti-oxidant therapy and cariostatic benefits. Biol. Sci. Pharm. Res. 2014, 2, 8–12. [Google Scholar]
- Ashihara, H.; Deng, W.W.; Mullen, W.; Crozier, A. Distribution and biosynthesis of flavan-3-ols in Camellia sinensis seedlings and expression of genes encoding biosynthetic enzymes. Phytochemistry 2010, 71, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Atomssa, T.; Cholap, A.V. Characterization and determination of catechins in green tea leaves using UV-visible spectrometer. J. Eng. Technol. Res. 2015, 7, 22–31. [Google Scholar]
- Katiyar, S.K.; Matsui, M.S.; Elmets, C.A.; Mukhtar, H. Polyphenolic antioxidant (-)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem. Photobiol. 1999, 69, 148–153. [Google Scholar] [CrossRef]
- Hofbauer, R.; Frass, M.; Gmeiner, B.; Handler, S.; Speiser, W.; Kapiotis, S. The green tea extract epigallocatechin gallate is able to reduce neutrophil transmigration through monolayers of endothelial cells. Wien. Klin. Wochenschr. 1999, 111, 278–282. [Google Scholar] [PubMed]
- Nomura, S.; Ichinose, T.; Jinde, M.; Kawashima, Y.; Tachiyashiki, K.; Imaizumi, K. Tea catechins enhance the mRNA expression of uncoupling protein 1 in rat brown adipose tissue. J. Nutr. Biochem. 2008, 19, 840–847. [Google Scholar] [CrossRef]
- Stohs, S.J.; Badmaev, V. A review of natural stimulant and non-stimulant thermogenic Agents. Phytother. Res. 2016, 30, 732–740. [Google Scholar] [CrossRef]
- Türközü, D.; Tek, N.A. A minireview of effects of green tea on energy expenditure. Crit. Rev. Food Sci. Nutr. 2017, 57, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Skrzydlewska, E.; Ostrowska, J.; Stankiewicz, A.; Farbiszewski, R. Green tea as a potent antioxidant in alcohol intoxication. Addict. Biol. 2002, 7, 307–314. [Google Scholar] [CrossRef]
- Yokozawa, T.; Nakagawa, T.; Kitani, K. Antioxidative activity of green tea polyphenol in cholesterol-fed rats. J. Agric. Food Chem. 2002, 50, 3549–3552. [Google Scholar] [CrossRef]
- Tadayon, M.; Movahedi, S.; Abedi, P.; Syahpoosh, A. Impact of green tea extract on serum lipid of postmenopausal women: A randomized controlled trial. J. Tradit. Complement. Med. 2017, 8, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Sabu, M.; Smitha, K.; Kuttan, R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J. Ethnopharmacol. 2002, 83, 109–116. [Google Scholar]
- Li, Y.; Zhao, S.; Zhang, W.; Zhao, P.; He, B.; Wu, N.; Han, P. Epigallocatechin-3-O-gallate (EGCG) attenuates FFAs-induced peripheral insulin resistance through AMPK pathway and insulin signaling pathway in vivo. Diabetes Res. Clin. Pract. 2011, 93, 205–214. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, B.; Liu, Z.; Liao, Z.; Zhong, Q.; Gu, L.; Wei, H.; Fang, X. Green tea polyphenols modulate colonic microbiota diversity and lipid metabolism in high-fat diet treated HFA mice. J. Food Sci. 2018, 83, 864–873. [Google Scholar] [CrossRef]
- Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003, 14, 326–332. [Google Scholar] [CrossRef]
- Crespy, V.; Williamson, G. A review of the health effects of green tea catechins in in vivo animal models. J. Nutr. 2004, 134, 3431S–3440S. [Google Scholar] [CrossRef]
- Wolfram, S.; Raederstorff, D.; Wang, Y.; Teixeira, S.R.; Elste, V.; Weber, P. Teavigotm (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass. Ann. Nutr. Metabol. 2005, 49, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, S.; Wang, Y.; Thielecke, F. Anti-obesity effects of green tea: From bedside to bench. Mol. Nutr. Food Res. 2006, 50, 176–187. [Google Scholar] [CrossRef]
- Garrow, J.S. Magic bullets now uncontrolled. BMJ 2010, 340, c1351. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Ye, J.; Zuberi, A.; Ribnicky, D.M.; Raskin, I.; Liu, Z.; Wang, Z.Q.; Brantley, P.J.; Howard, L.; Lefevre, M. Botanicals and the metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, S481–S487. [Google Scholar] [CrossRef]
- Czemplik, M.; Kulma, A.; Wang, Y.F.; Szopa, J. Therapeutic strategies of plant-derived compounds for diabetes via regulation of monocyte chemoattractant protein-1. Curr. Med. Chem. 2017, 24, 1453–1468. [Google Scholar] [CrossRef] [PubMed]
- Barzilay, J.I.; Abraham, L.; Heckbert, S.R.; Cushman, M.; Kuller, L.H.; Resnick, H.E.; Tracy, R.P. The relation of markers of inflammation to the development of glucose disorders in the elderly: The cardiovascular health study. Diabetes 2001, 50, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.M.; Lewis, S.K.; Urcuyo, L.D.E.; Tanone, I.I.; Kirakosyan, A.; Kaufman, P.B.; Bolling, S.F. Regular tart cherry intake alters abdominal adiposity, adipose gene transcription, and inflammation in obesity-prone rats fed a high fat diet. J. Med. Food 2009, 12, 935–942. [Google Scholar] [CrossRef]
- Prior, R.L.; Wilkes, S.; Rogers, T.; Khanal, R.C.; Wu, X.; Hager, T.J.; Hager, A.; Howard, L. Dietary black raspberry anthocyanins do not alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010, 58, 3977–3983. [Google Scholar] [CrossRef] [PubMed]
- DeFuria, J.; Bennett, G.; Strissel, K.J.; Perfield, J.W.; Milbury, P.E.; Greenberg, A.S.; Obin, M.S. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J. Nutr. 2009, 139, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.H.; Yoon, H.S.; Park, H.J.; Kim, M.Y.; Shin, H.K.; Park, K.Y.; Yang, J.O.; Sohn, M.S.; Do, M.S. Anti-obesity and antioxidative effects of purple sweet potato extract in 3t3-l1 adipocytes in vitro. J. Med. Food 2011, 14, 1097–1106. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, B.; Shen, J.; Wan, L.; Zhu, Y.; Yi, T.; Xiao, Z. The beneficial effects of quercetin, curcumin, and resveratrol in obesity. Oxid. Med. Cell Longev. 2017, 2017, 1459497. [Google Scholar] [CrossRef]
- Lasa, A.; Churruca, I.; Eseberri, I.; Andrés-Lacueva, C.; Portillo, M.P. Delipidating effect of resveratrol metabolites in 3T3-L1 adipocytes. Mol. Nutr. Food Res. 2012, 56, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Linus, P. Institute at Oregon State University, Micronutrient Information Center. Available online: http://lpi.oregonstate.edu/infocenter/contentnuts.html/ (accessed on 1 February 2021).
- Kim, J.; Jang, D.S.; Kim, H.; Kim, J.S. Anti-lipase and lipolytic activities of ursolic acid isolated from the roots of Actinidia arguta. Arch. Pharm. Res. 2009, 32, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, W.; Ma, X.; Ding, W. Evaluation of inhibition of fatty acid synthase by ursolic acid: Positive cooperation mechanism. Biochem. Biophys. Res. Commun. 2010, 392, 386–390. [Google Scholar] [CrossRef]
- Jia, Y.; Bhuiyan, M.J.; Jun, H.J.; Lee, J.H.; Hoang, M.H.; Lee, H.J.; Kim, N.; Lee, D.; Hwang, K.Y.; Hwang, B.Y. Ursolic acid is a PPAR-alpha agonist that regulates hepatic lipid metabolism. Bioorg. Med. Chem. 2011, 21, 5876–5880. [Google Scholar] [CrossRef]
- Kunkel, S.D.; Suneja, M.; Ebert, S.M.; Bongers, K.S.; Fox, D.K.; Malmberg, S.E.; Alipour, F.; Shields, R.K.; Adams, C.M. mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metab. 2011, 13, 627–638. [Google Scholar] [CrossRef]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. New developments and novel therapeutic perspectives for vitamin C. J. Nutr. 2007, 137, 2171–2184. [Google Scholar] [CrossRef]
- Harding, A.H.; Wareham, N.J.; Bingham, S.A.; Khaw, K.; Luben, R.; Welch, A.; Forouhi, N.G. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: The European prospective investigation of cancer—Norfolk prospective study. Arch. Intern. Med. 2008, 168, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Rotilio, G.; Calabrese, L.; Bossa, F.; Barra, D.; Agro, A.F.; Mondovi, B. Properties of the apoprotein and role of copper and zinc in protein conformation and enzyme activity of bovine superoxide dismutase. Biochemistry 1972, 11, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Gunasekara, P.; Hettiarachchi, M.; Liyanage, C.; Lekamwasam, S. Effects of zinc and multimineral vitamin supplementation on glycemic and lipid control in adult diabetes. Diabetes Metab. Syndr. Obes. 2011, 4, 53–60. [Google Scholar]
- Lands, B. Historical perspectives on the impact of n-3 and n-6 nutrients on health. Prog. Lipid Res. 2014, 55, 17–29. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Jenkins, D.J. Fish oil and Omega-3 fatty acids: Fact sheet. Can. Med. Assoc. J. 2008, 178, 150. [Google Scholar] [CrossRef][Green Version]
- Brenna, J.T.; Salem, N.; Sinclair, A.J.; Cunnane, S.C. Alpha- Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.A.; Garg, M.L. Dietary supplementation with long chain omega-3 polyunsaturated fatty acids and weight loss in obese adults. Obes. Res. Clin. Pract. 2013, 7, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Chien, Y.S.; Chen, Y.J.; Ajuwon, K.M.; Mersmann, H.M.; Ding, S.T. Role of n-3 Polyunsaturated fatty acids in ameliorating the obesity––Induced metabolic syndrome in animal models and humans. Int. J. Mol. Sci. 2016, 17, 1689. [Google Scholar] [CrossRef] [PubMed]
- Crane, F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Sohet, F.M.; Delzenne, N.M. Is there a place for coenzyme Q in the management of metabolic disorders associated with obesity? Nutr. Rev. 2012, 70, 631–641. [Google Scholar] [CrossRef] [PubMed]





| Antioxidant | Sources | Bioactive Dose of Antioxidant | Moderator | Metabolic Marker | Inflamatory Marker | Reference |
|---|---|---|---|---|---|---|
| Anthocyanine | Tart cherry powder | Cyanidin(3-sophoroside), cyanidin (3-glucosylrutinoside), cyanidin- glucose(3-glucoside) insulin, cyanidincholesterol (3-rutinoside), triglyceride (TG) peonidin (3-glucoside). | Zucker rats | Reduced glycemia and insulinemia as well as improved insulin resistance. | Decreased plasma levels of IL-6 and TNF-α. | [194] |
| Whole blueberry powder | Delphinidins, cyanidins, peonidins, malvidins. | C57BL/6 mice | Reduced levels of fasting glucose improved insulin tolerance test (ITT) | Reduced TNF-α expression in adipose tissue. | [195] | |
| Blueberry juice | Cyanidi(3-galactoside) cyanidi (3-arabinoside delphinidi(3-glucoside), delphinidinadiponectinpetunidin(3 arabinoside), malvidin (3-galactoside), malvidin(3-glucoside). Dose: 4.09 mg/mL | Mice | Reduced body weight, decreased the level of TG, leptin, and cholesterol, percentage of WAT. | Reduced TNF-α and IL-6 expression. | [196] | |
| Purple sweet potato | Cyanidnin(3-caffeylferulysophoroside-5-glucoside), peonidin(3caffeylferulysophoroside-5-glucoside). Dose: 4.28 to 12.84 µg/mL | Murine 3T3-L1 adipocytes | Decreased leptin and adipogenic factors. | Decreased COX-2, MCP-1, IL-6. | [197] | |
| Resveratrol | Red wine, acai, blueberry, cranberry, pomegranate, Japanese knotwood, Ziziphus. | Resveratrol-4′-O-glucuronide, resveratrol-3-O-glucuronide and resveratrol-3-O-sulfate. Dose: 15 mg/kg body weight/day (gavage). | Male Zucker rats | Decreased TG content, increased epinephrine-stimulated glycerol release, increaseD hormone-sensitive lipase (HSL) mRNA. | Reduced IL-6, TNF-α, IL-1β, and NF-κB. | [60,198] |
| Obese Zucker (fa/fa) rats | ||||||
| 1, 10, 25 µM resveratrol, resveratrol-4′-O-glucuronide, resveratrol-3-O glucuronide and resveratrol-3-O sulfate. | Murine 3T3-L1 adipocytes | Increased SIRT1 mRNA, increased TG content, increased peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) mRNA, increased adipose triglyceride lipase (ATGL) mRNA, increased HSL mRNA. | Reduced IL-6 and TNF-α. | [199] | ||
| Lipoic acid | Red meat, spinach, broccoli, tomatoes, peas, Brussels sprouts. | Oxoaciddehydrogenase, pyruvatedehydrogenase complex, 2-oxoglutarate dehydrogenase complex. | HFD-induced obesity | Improved glycemic control and lipid profile, decreased weight. | Reduced IL-6 and TNF-α. | [200] |
| Curcumine | Rhizome, or rootstalk of the turmeric plant. | 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione. | Primary human adipocytes and murine 3T3-L1 adipocytes | Suppressed the expression of adipogenic genes, PPARγ, and C/EBP α. | Reduced (MCP-1, a proinflammatory cytokine. | [137] |
| 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione | HFD-induced obesity and in genetic obesity (ob/ob mice). | Reduced body weight and energy metabolism, reduced epididymal adipose tissue, increased fatty acid β-oxidation. | Increased adiponectin production and reduced inflammation. | [144] | ||
| Catechin | Camellia sinensis leaves and buds, green tea. | (−)-EGCG | Obese Zucker (fa/fa) rats | Reduced deleterious effects, including hepatic injury. | Decreased TNF-α, IL-1β, COX-2, and matrix metallopeptidase 9 (MMP-9). | [165] |
| Caffeine | Coffee and tea. | 1,3,7-trimethylxanthine. Doses: 20 and 40 mg/kg per day; 37.5 mg/kg per day; 3–4 cups of coffee per day. | Male rats | Reduced lipogenesis, regulated lipid uptake and transport, increased fatty acid β-oxidation, increased lipolysis and reduced lipid digestion. Decreased lipid peroxidation and increased antioxidant enzyme activities. | Decreased serum levels of inflammatory cytokines TNF-α, IL-1β, and IL-6. | [157,158,159,160,161] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, P.; Tomassoni, D.; Traini, E.; Martinelli, I.; Micioni Di Bonaventura, M.V.; Cifani, C.; Amenta, F.; Tayebati, S.K. Natural Antioxidant Application on Fat Accumulation: Preclinical Evidence. Antioxidants 2021, 10, 858. https://doi.org/10.3390/antiox10060858
Roy P, Tomassoni D, Traini E, Martinelli I, Micioni Di Bonaventura MV, Cifani C, Amenta F, Tayebati SK. Natural Antioxidant Application on Fat Accumulation: Preclinical Evidence. Antioxidants. 2021; 10(6):858. https://doi.org/10.3390/antiox10060858
Chicago/Turabian StyleRoy, Proshanta, Daniele Tomassoni, Enea Traini, Ilenia Martinelli, Maria Vittoria Micioni Di Bonaventura, Carlo Cifani, Francesco Amenta, and Seyed Khosrow Tayebati. 2021. "Natural Antioxidant Application on Fat Accumulation: Preclinical Evidence" Antioxidants 10, no. 6: 858. https://doi.org/10.3390/antiox10060858
APA StyleRoy, P., Tomassoni, D., Traini, E., Martinelli, I., Micioni Di Bonaventura, M. V., Cifani, C., Amenta, F., & Tayebati, S. K. (2021). Natural Antioxidant Application on Fat Accumulation: Preclinical Evidence. Antioxidants, 10(6), 858. https://doi.org/10.3390/antiox10060858









