Distribution of Polyphenolic and Isoprenoid Compounds and Biological Activity Differences between in the Fruit Skin + Pulp, Seeds, and Leaves of New Biotypes of Elaeagnusmultiflora Thunb
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Identification and Quantification of Polyphenols
2.3. Determination of Procyanidins by the Phloroglucinolysis Method
2.4. Determination of Isoprenoids (Carotenoids, Tocopherols, and Chlorophylls)
2.5. Determination of Organic Acids
2.6. Analysis of Antioxidant Activity
2.7. Inhibitory of Biological Activity
2.8. Statistical Analysis
3. Results and Discussion
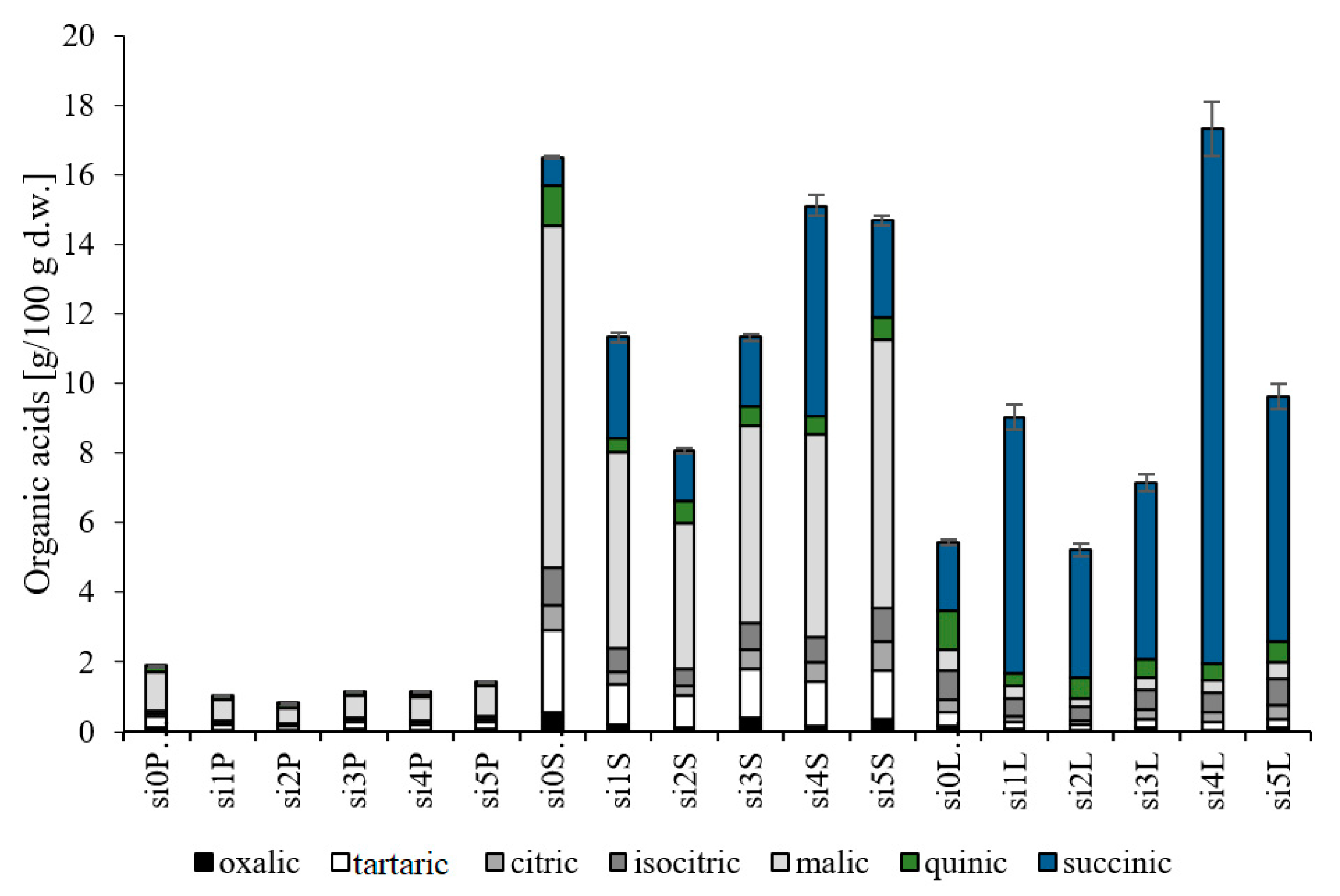
3.1. Evaluation of Organic Acids
3.2. Identification of Polyphenolic Compounds
3.2.1. Phenolic Acids
3.2.2. Flavonols
3.2.3. Hydrolysable Tannins
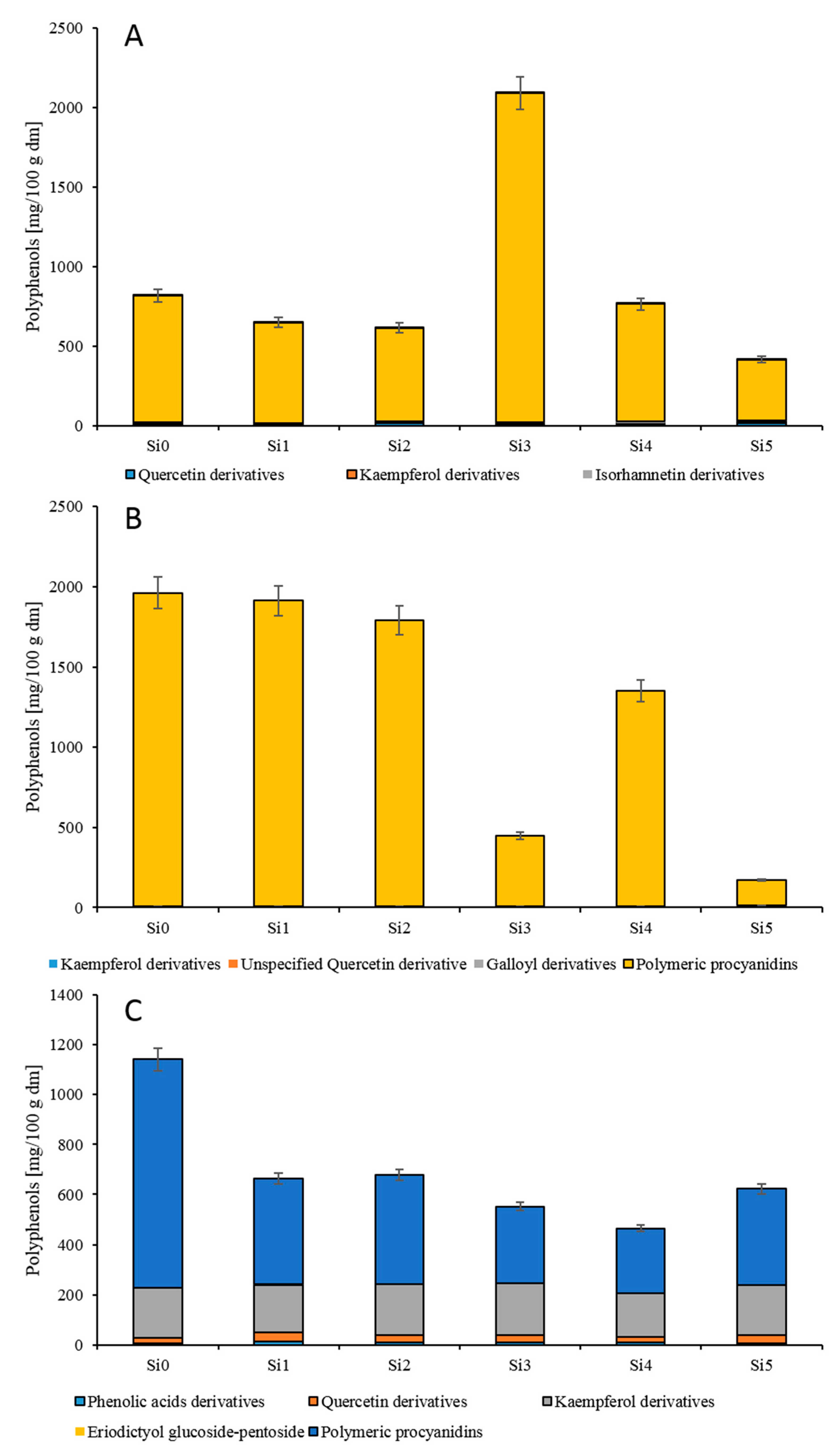
3.3. Quantification of Polyphenols
3.4. Evaluation of Carotenoids, Chlorophylls, and Tocopherols
3.5. Evaluation of Antioxidant Capacity
3.6. Evaluation of Inhibitory Biological Activity
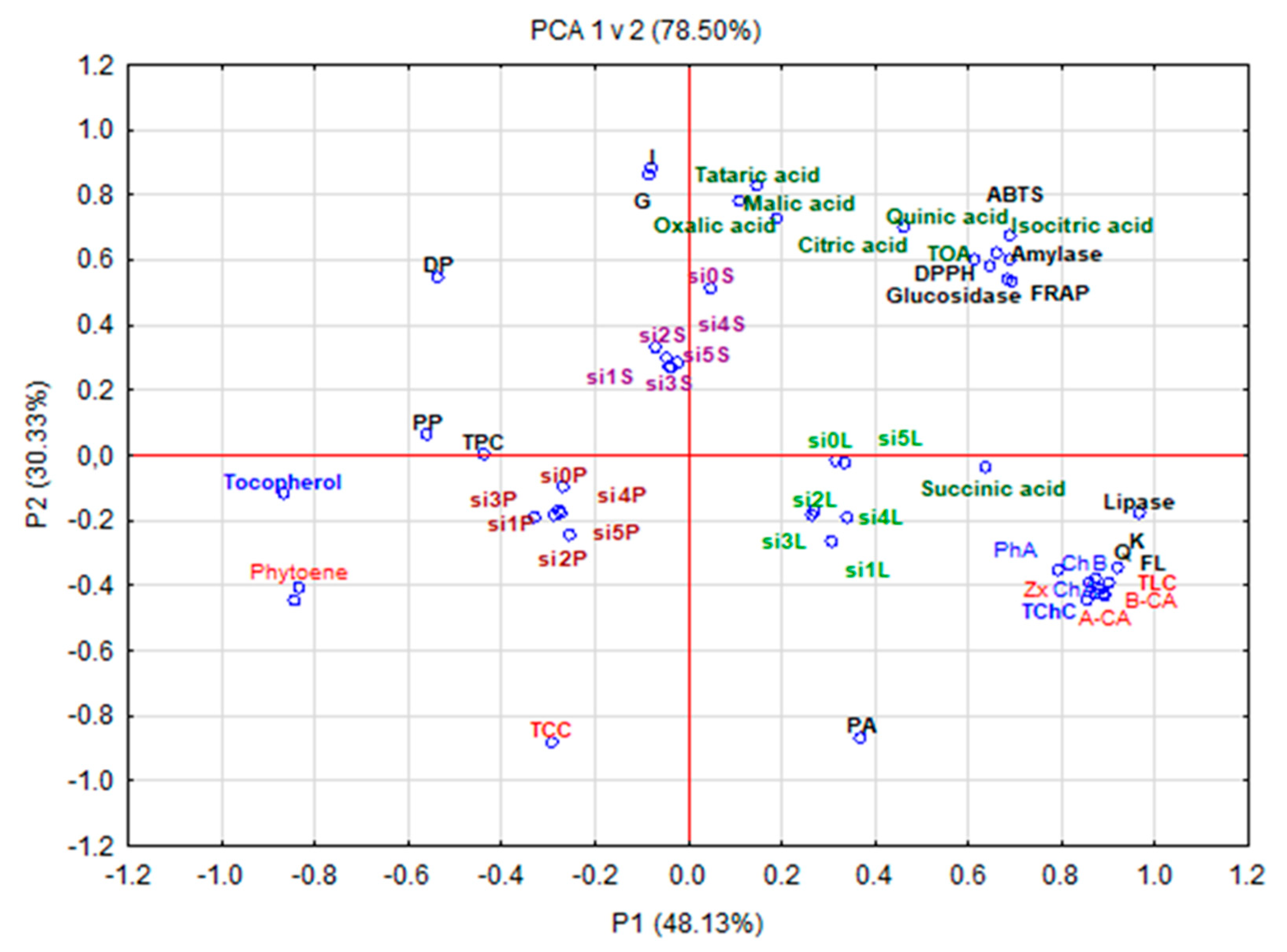
3.7. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bieniek, A.; Piłat, B.; Szałkiewicz, M.; Markuszewski, B.; Gojło, E. Evaluation of yield, morphology and Quality of fruits of cherry silverberry (Elaeagnus multiflora Thunb.) biotypes under conditions of north-eastern Poland. Pol. J. Nat. Sci. 2017, 32, 61–70. [Google Scholar]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, e1801045. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Seo, W.T.; Cho, K.M. Determination of phytochemical contents and biological activities from the fruits of Elaeagnus multiflora. J. Food Sci. Nutr. 2011, 16, 29–36. [Google Scholar] [CrossRef][Green Version]
- Lee, M.S.; Lee, Y.S.; Park, O.J. Cherry silverberry (Elaeagnus multiflora) extracts exert anti-inflammatory effects by inhibiting COX-2 and Akt signals in HT-29 clon cancer cells. Food Sci. Biotechnol. 2010, 19, 1673–1677. [Google Scholar] [CrossRef]
- Lachowicz, S.; Bieniek, A.; Gil, Z.; Bielska, N.; Markuszewski, B. Phytochemical parameters and antioxidant activity of new cherry silverberry biotypes (Elaeagnus multiflora Thunb.). Eur. Food Res. Technol. 2019, 245, 1997–2005. [Google Scholar] [CrossRef]
- Ahmadiani, A.; Hosseiny, J.; Semnanian, S.; Javan, M.; Saeedi, F.; Kamalinejad, M.; Saremi, S. Antinociceptive and antiinflammatory effects of Elaeagnus angustifolia fruit extract. J. Ethnopharmacol. 2000, 72, 287–292. [Google Scholar] [CrossRef]
- Lachowicz, S.; Kapusta, I.; Świeca, M.; Stinco, C.M.; Meléndez-Martínez, A.J.; Bieniek, A. In Vitro Biological Activities of Fruits and Leaves of Elaeagnus multiflora Thunb. and Their Isoprenoids and Polyphenolics Profile. Antioxidants 2020, 9, 436. [Google Scholar] [CrossRef]
- Dong, J.Z.; Wang, S.H.; Zhu, L.; Wang, Y. Analysis on the main active components of Lycium barbarum fruits and related environmental factors. J. Med. Plants Res. 2012, 6, 2276–2283. [Google Scholar]
- Meléndez-Martínez, A.J.; Böhm, V.; Borge, G.I.A.; Cano, M.P.; Fikselová, M.; Gruskiene, R.; O’Brien, N.M. Carotenoids: Considerations for Their Use in Functional Foods, Nutraceuticals, Nutricosmetics, Supplements, Botanicals, and Novel Foods in the Context of Sustainability, Circular Economy, and Climate Change. Annu. Rev. Food Sci. Technol. 2021, 12, 433–460. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, caffeine, and health outcomes: An umbrella review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef]
- Kapusta, I.; Cebulak, T.; Oszmiański, J. Characterization of polish wines produced from the interspecific hybrid grapes grown in south-east Poland. Eur. Food Res. Technol. 2018, 244, 441–455. [Google Scholar] [CrossRef]
- Stinco, C.M.; Fernández-Vázquez, R.; Escudero-Gilete, M.L.; Heredia, F.J.; Meléndez-Martínez, A.J.; Vicario, I.M. Effect of orange juice’s processing on the color, particle size, and bioaccessibility of carotenoids. J. Agric. Food Chem. 2012, 60, 1447–1455. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Podsedek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziołkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef]
- Nickavar, B.; Yousefian, N. Evaluation of α-amylase inhibitory activities of selected antidiabetic medicinal plants. J. Verbrauch. Lebensm. 2011, 6, 191–195. [Google Scholar] [CrossRef]
- Cho, K.M.; Joo, O.S. Quality and antioxidant charactistics of Elaeagnus multiflora wine through the thermal processing of juice. Korean J. Food Preserv. 2014, 21, 206–214. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Oszmiański, J. Characterization of phenolic compounds in different anatomical pear (Pyrus communis L.) parts by ultra-performance liquid chromatography photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS). Int. J. Mass Spectrom. 2015, 392, 154–163. [Google Scholar] [CrossRef]
- Figueirinha, A.; Paranhos, A.; Pérez-Alonso, J.J.; Santos-Buelga, C.; Batista, M.T. Cymbopogon citratus leaves: Characterization of flavonoids by HPLC–PDA–ESI/MS/MS and an approach to their potential as a source of bioactive polyphenols. Food Chem. 2008, 110, 718–728. [Google Scholar] [CrossRef]
- Ek, S.; Kartimo, H.; Mattila, S.; Tolonen, A. Characterization of phenolic compounds from lingonberry (Vaccinium vitis-idaea). J. Agric. Food Chem. 2006, 54, 9834–9842. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. LC-MS profiling and quantification of food phenolic components using a standard analytical approach for all plants. Food Sci. Technol. New Res. 2008, 60, 1–103. [Google Scholar]
- Ablajan, K.; Abliz, Z.; Shang, X.Y.; He, J.M.; Zhang, R.P.; Shi, J.G. Structural characterization of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2006, 41, 352–360. [Google Scholar] [CrossRef]
- Rehman, H.; Yasin, K.A.; Choudhary, M.A.; Khaliq, N.; Rahman, A.U.; Choudhary, M.I.; Malik, S. Studies on the chemical constituents of Phyllanthus emblica. Nat. Prod. Res. 2007, 21, 775–781. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, C.; Zhang, H. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl4-induced oxidative liver injury in mice. J. Food Drug Anal. 2015, 23, 310–317. [Google Scholar] [CrossRef]
- Carmona, M.; Sánchez, A.M.; Ferreres, F.; Zalacain, A.; Tomás-Barberán, F.; Alonso, G.L. Identification of the flavonoid fraction in saffron spice by LC/DAD/MS/MS: Comparative study of samples from different geographical origins. Food Chem. 2007, 100, 445–450. [Google Scholar] [CrossRef]
- Toker, G.; Aslan, M.; Yeşilada, E.; Memişoğlu, M.; Ito, S. Comparative evaluation of the flavonoid content in officinal Tiliae flos and Turkish lime species for quality assessment. J. Pharm. Biomed. Anal. 2001, 26, 111–121. [Google Scholar] [CrossRef]
- Püssa, T.; Pällin, R.; Raudsepp, P.; Soidla, R.; Rei, M. Inhibition of lipid oxidation and dynamics of polyphenol content in mechanically deboned meat supplemented with sea buckthorn (Hippophae rhamnoides) berry residues. Food Chem. 2008, 107, 714–721. [Google Scholar] [CrossRef]
- Aguirre-Hernández, E.; González-Trujano, M.E.; Martínez, A.L.; Moreno, J.; Kite, G.; Terrazas, T.; Soto-Hernández, M. HPLC/MS analysis and anxiolytic-like effect of quercetin and kaempferol flavonoids from Tilia americana var. mexicana. J. Ethnopharmacol. 2010, 127, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Olas, B.; Żuchowski, J.; Lis, B.; Skalski, B.; Kontek, B.; Grabarczyk, Ł.; Stochmal, A. Comparative chemical composition, antioxidant and anticoagulant properties of phenolic fraction (a rich in non-acylated and acylated flavonoids and non-polar compounds) and non-polar fraction from Elaeagnus rhamnoides (L.) A. Nelson fruits. Food Chem. 2018, 247, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.Y.; Barlow, P.J. Isolation and structure elucidation of phenolic compounds from longan (Dimocarpus longan Lour.) seed by high-performance liquid chromatography–electrospray ionization mass spectrometry. J. Chromatogr. A 2005, 1085, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.Y.; Hong, J.Y.; Shin, S.R. Analysis on the components of the Elaeagnus multiflora Thunb. leaves. Korean J. Food Preserv. 2007, 14, 639–644. [Google Scholar]
- Srinivasan, R.; Aruna, A.; Manigandan, K.; Pugazhendhi, A.; Kim, M.; Shivakumar, M.S.; Natarajan, D. The phytochemical, antioxidant, antimicrobial and antiproliferative potential of Elaeagnus indica. Biocatal. Agric. Biotechnol. 2019, 20, 101265. [Google Scholar] [CrossRef]
- Asofiei, I.; Calinescu, I.; Trifan, A.; David, I.G.; Gavrila, A.I. Microwave-assisted batch extraction of polyphenols from sea buckthorn leaves. Chem. Eng. Commun. 2016, 203, 1547–1553. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Lachowicz, S.; Gorzelany, J.; Matłok, N. Comparison of bioactive potential of cranberry fruit and fruit-based products versus leaves. J. Funct. Foods 2016, 22, 232–242. [Google Scholar] [CrossRef]
- Andreotti, C.; Costa, G.; Treutter, D. Composition of phenolic compounds in pear leaves as affected by genetics, ontogenesis and the environment. Sci. Hortic. 2006, 109, 130–137. [Google Scholar] [CrossRef]
- Lachowicz, S.; Seliga, Ł.; Pluta, S. Distribution of phytochemicals and antioxidative potency in fruit peel, flesh, and seeds of Saskatoon berry. Food Chem. 2020, 305, 125430. [Google Scholar] [CrossRef]
- Rebello, L.P.G.; Lago-Vanzela, E.S.; Barcia, M.T.; Ramos, A.M.; Stringheta, P.C.; Da-Silva, R.; Castillo-Muñoz, N.; Gomez-Alonso, S.; Hermosin-Gutierrez, I. Phenolic composition of the berry parts of hybrid grape cultivar BRS Violeta (BRS Rubea × IAC 1398-21) using HPLC–DAD–ESI-MS/MS. Food Res. Int. 2013, 54, 354–366. [Google Scholar] [CrossRef]
- Parus, A. Antioxidant and pharmacological properties of phenolic acids. Postępy Fitoter. 2013, 1, 48–53. [Google Scholar]
- Ribeiro, P.P.C.; da Silva Chaves, K.S.F.; de Veras, B.O.; de Oliveira, J.R.S.; de Menezes Lima, V.L.; de Assis, C.R.D.; Stamford, T.C.M. Chemical and biological activities of faveleira (Cnidoscolus quercifolius Pohl) seed oil for potential health applications. Food Chem. 2021, 337, 127771. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; O’Brien, N. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2020, 1–51. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Cao, L.; Lu, J. Phenolic compounds and antioxidant properties of different grape cultivars grown in China. Food Chem. 2010, 119, 1557–1565. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Zagorac, D.Č.D.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Nazir, N.; Zahoor, M.; Nisar, M.; Khan, I.; Karim, N.; Abdel-Halim, H.; Ali, A. Phytochemical analysis and antidiabetic potential of Elaeagnus umbellata (Thunb.) in streptozotocin-induced diabetic rats: Pharmacological and computational approach. BMC Complement. Altern. Med. 2018, 18, 332. [Google Scholar] [CrossRef] [PubMed]
- Saltan, F.Z.; Okutucu, B.; Canbay, H.S.; Ozel, D. In vitro α-Glucosidase and α-Amylase Enzyme Inhibitory Effects in Elaeagnus angustifolia Leaves Extracts. Eurasian J. Anal. Chem. 2017, 12, 117–126. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.D.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Varshneya, C.; Kaistha, K.; Tandon, T. In vitro evaluation of antidiabetic and antioxidant activity of Seabuckthorn (Hippophae rhamnoides L.) leaves. J. Med. Plants Res. 2015, 9, 929–932. [Google Scholar]
- Kim, J.S.; Kwon, Y.S.; Sa, Y.J.; Kim, M.J. Isolation and identification of sea buckthorn (Hippophae rhamnoides) phenolics with antioxidant activity and α-glucosidase inhibitory effect. J. Agric. Food Chem. 2011, 59, 138–144. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Chen, F.; Wang, X.; Jiang, Y.; Lin, S. Anti-diabetic activities of phenolic compounds in muscadine against alpha-glucosidase and pancreatic lipase. LWT Food Sci. Technol. 2012, 46, 164–168. [Google Scholar] [CrossRef]
- Zhang, L.; Hogan, S.; Li, J.; Sun, S.; Canning, C.; Zheng, S.J.; Zhou, K. Grape skin extract inhibits mammalian intestinal α-glucosidase activity and suppresses postprandial glycemic response in streptozocin-treated mice. Food Chem. 2011, 126, 466–471. [Google Scholar] [CrossRef]
| Peak No. | Tentative Identification | Rt (min) | [M-H]− (m/z) | [M-H]− MS/MS (m/z) | UV–VIS (nm) | Leaves | Seeds | Fruit Skin + Pulp | Literature |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Quinic acid | 1.05 | 191 | 172 | 262 | X | [7,11,20] | ||
| 2 | 3-p-Coumaroyloqunic acid | 1.33 | 337 | 191 | 276 | X | [7,11,20] | ||
| 3 | Methyl-quercetin 3-O-rhamnoside-pentoside | 1.94 | 609 | 463/331/299 | 317 | X | [7] | ||
| 4 | Quercetin glycoside-pentoside-glycoside | 2.53 | 757 | 595/463/301 | 255/352 | X | [7] | ||
| 5 | Kaempferol-tri-hexoside | 2.57 | 771 | 609/447/285 | 267/350 | X | [28] | ||
| 6 | Kaempferol 3-O-rutinoside-7-O-glucoside | 2.58 | 755 | 609/447/285 | 267/350 | X | [7,26,27] | ||
| 7 | Kaempferol-tri-hexoside-rhamnoside | 2.65 | 917 | 771/609/285 | 269/350 | X | [7] | ||
| 8 | Kaempferol di-rhamnoside-di-glucoside | 2.78 | 901 | 755/609/447/285 | 266/350 | X | [7] | ||
| 9 | Quercetin pentoside-rutinoside | 2.8 | 741 | 609/463/301 | 255/350 | X | [7] | ||
| 10 | Kaempferol 7-O-pentoside | 2.87 | 417 | 285 | 281/340 | X | [7,24,25] | ||
| 11 | Kaempferol 3-O-rhamnoside | 3.14 | 431 | 285 | 267/325 | X | [7,24,25] | ||
| 12 | Kaempferol glucoside-rutinoside | 3.17 | 755 | 431/285 | 266/319 | X | [7,26,27] | ||
| 13 | Kaempferol glucopyranoside-rhamnoside-deoxyhexose | 3.30 | 915 | 593/285 | 267/350 | X | [7] | ||
| 14 | Sinapic acid-O-hexoside | 3.36 | 385 | 223 | 325 | X | X | ||
| 15 | Kaempferol rhamnoside-dihexoside | 3.37 | 917 | 771/285 | 274/322 | X | [7] | ||
| 16 | Quercetin rhamnoside-pentoside-rutinoside | 3.39 | 887 | 609/579/301 | 255/352 | X | [7] | ||
| 17 | Kaempferol pentoside-rhamnoside-rutinoside | 3.43 | 887 | 755/609/447/285 | 264/338 | X | [7] | ||
| 18 | Kaempferol glucoside-di-rhamnoside | 3.43 | 755 | 593/431/285 | 266/347 | X | [7] | ||
| 19 | Quercetin 3-O-rutinoside | 3.44 | 609 | 463/301 | 255/352 | X | [30,31,32] | ||
| 20 | Quercetin rhamnoside-pentoside-rhamnoside | 5.45 | 887 | 741/595/433/301 | 255/355 | X | [7] | ||
| 21 | Quercetin 3-O-rhamnoside | 3.49 | 447 | 301 | 255/326 | X | [7,26,27] | ||
| 22 | Kaempferol rhamnoside-rutinoside | 3.53 | 739 | 593/447/285 | 265/326 | X | [30,31,32] | ||
| 23 | Trigalloyl-hexoside | 3.53 | 635 | 421/169 | 274 | X | [33] | ||
| 24 | Kaempferol rhamnoside-pentoside-rutinoside | 3.66 | 871 | 725/563/431/285 | 266/340 | X | [7] | ||
| 25 | Kaempferol pentoside-rutinoside | 3.67 | 725 | 579/417/285 | 267/350 | X | [7] | ||
| 26 | Trigalloyl-hexoside | 3.68 | 635 | 465/313 | 276 | X | [33] | ||
| 27 | Kaempferol pentoside-rutinoside | 3.71 | 725 | 579/417/285 | 265/345 | X | [7] | ||
| 28 | Kaempferol hexoside-pentoside-rhamnose | 3.76 | 725 | 563/431/285 | 267/350 | X | [7] | ||
| 29 | Kaempferol rhamnoside-pentoside | 3.79 | 563 | 417/285 | 265/328 | X | [7] | ||
| 30 | Kaempferol 3-O-rutinoside | 3.84 | 593 | 447/285 | 265/334 | X | [7,26,27] | ||
| 31 | Kaempferol glucopyranoside-dihexoside | 3.86 | 785 | 609/447/285 | 267/350 | X | [7] | ||
| 32 | Quercetin pentoside-rutinoside | 3.91 | 741 | 609/433 | 255/350 | X | [7] | ||
| 33 | Kaempferol di-rhamnoside-di-glycoside | 3.97 | 901 | 755/609/447/285 | 264/339 | X | [7] | ||
| 34 | Trigalloyl-hexahydroxydiphenoyl | 4.02 | 951 | 907/783 | 274 | X | [33] | ||
| 35 | Quercetin 3-O-rhamnoside-7-O-pentoside | 4.12 | 595 | 433/301 | 255/350 | X | [7] | ||
| 36 | Digalloyl-gallagyl-hexoside | 4.14 | 1085 | 765/633/451 | 272 | X | [33] | ||
| 37 | Quercetin-O-glucoside-O-pentoside | 4.16 | 595 | 463/301 | 255/345 | X | [7] | ||
| 38 | Kaempferol di-rhamnoside-glucoside | 4.19 | 739 | 593/447/285 | 264/345 | X | [7,26,27] | ||
| 39 | Tetragalloyl-hexoside | 4.22 | 787 | 635/617/301 | 272 | X | [33] | ||
| 40 | Kaempferol rhamnoside-rutinoside | 4.24 | 739 | 593/285 | 265/340 | X | [30,31,32] | ||
| 41 | Kaempferol-di-hexoside | 4.31 | 609 | 447/285 | 267/350 | X | [28] | ||
| 42 | Quercetin-tri-rhamnoside | 4.33 | 739 | 593/447/301 | 255/355 | X | [7] | ||
| 43 | Tetragalloyl-hexoside | 4.37 | 787 | 635/617/301 | 277 | X | [33] | ||
| 44 | Kaempferol di-rhamnoside-glucoside | 4.37 | 739 | 593/447/285 | 265/338 | X | [7,26,27] | ||
| 45 | Quercetin di-rhamnose | 4.47 | 593 | 447/301 | 255/347 | X | [7] | ||
| 46 | Quercetin-rhamnoside-glucopyranoside-rhamnoside | 4.48 | 769 | 593/447/301 | 255/352 | X | [30,31,32] | ||
| 47 | Unspecified quercetin derivative | 4.48 | 603 | 301 | 252/366 | X | |||
| 48 | Pentagalloyl-hexoside | 4.57 | 939 | 787/635/301 | 269 | X | [33] | ||
| 49 | Kaempferol pentoside-rhamnoside-glucuronide | 4.59 | 739 | 563/417/285 | 265/324 | X | [7] | ||
| 50 | Kaempferol di-rhamnoside-hexoside | 4.66 | 739 | 593/447/285 | 265/319 | X | [7] | ||
| 51 | Kaempferol pentoside-di-rhamnoside | 4.80 | 709 | 577/431/285 | 265/339 | X | [7] | ||
| 52 | Kaempferol di-rhamnose | 4.89 | 577 | 431/285 | 264/341 | X | [7] | ||
| 53 | Pentagalloyl-hexoside | 4.89 | 939 | 787/635/301 | 274 | X | [33] | ||
| 54 | Kaempferol-3-O-glucoside | 5.01 | 447 | 285 | 264/319 | X | [7,24,25] | ||
| 55 | Isorhamnetin-7-O-rutinoside | 5.09 | 623 | 477/315 | 253/358 | X | [7] | ||
| 56 | Kaempferol glucoside-glucuronide | 5.12 | 623 | 447/285 | 264/317 | X | [7] | ||
| 57 | Eriodictyol glucoside-pentoside | 5.19 | 581 | 285 | 265/315 | X | [18] | ||
| 58 | Isorhamnetin-3-O-glucoside | 5.20 | 477 | 315 | 256/380 | X | [7] | ||
| 59 | Kaempferol malonyl-glucuronide | 5.27 | 547 | 285 | 265/315 | X | [18] | ||
| 60 | Unknown derivative of Kaempferol | 5.63 | 891 | 285 | 269/325 | X | |||
| 61 | Isorhamnetin 3-O-(6”malonyl)-glucuronide-rhamnoside | 5.71 | 723 | 491/315 | 270/350 | X | [7] | ||
| 62 | Kaempferol 3-O-rhamnoside | 5.94 | 431 | 285 | 264/325 | X | [7,24,25] | ||
| 63 | Kaempferol 3-O-(6”-p-coumaryl)-galactoside | 6.87 | 593 | 447/285 | 267/312 | X | X | [29] | |
| 64 | Kaempferol 3-O-(6”-caffeoyl)-glucoside | 7.00 | 623 | 447/285 | 264/321 | X | X | [7] | |
| 65 | Kaempferol 3-O-(6”-p-coumaryl)-glucoside | 7.11 | 593 | 447/285 | 267/315 | X | X | [29] |
| No. | Compounds | Si0 | Si1 | Si2 | Si3 | Si4 | Si5 |
|---|---|---|---|---|---|---|---|
| Chlorophylls | |||||||
| 1 | Chlorophyll b | 127 ± 3a 1,2 | 161 ± 8b | 132 ± 1a | 139 ± 6a | 164 ± 1b | 139 ± 7a |
| 2 | Chlorophyll b-d | 17.18 ± 1.27a | 15.36 ± 1.03a | 8.38 ± 0.79b | 8.58 ± 0.97b | 8.36 ± 0.35b | 7.67 ± 0.43b |
| 3 | Chlorophyll a | 184 ± 7a | 297 ± 17bc | 156 ± 9a | 173 ± 12a | 263 ± 10b | 333 ± 22c |
| 4 | Pheophytin b | 4.21 ± 0.31a | 1.70 ± 0.04bc | 2.82 ± 0.18d | 3.11 ± 0.19d | 2.07 ± 0.17c | 1.24 ± 0.16b |
| Sum | 334 ± 8D | 477 ± 20B | 300 ± 11F | 325 ± 10E | 439 ± 9C | 482 ± 23A | |
| Carotenoids | |||||||
| 5 | Lutein | 30.71 ± 1.31a | 42.10 ± 0.42b | 35.40 ± 0.93c | 34.58 ± 1.06c | 42.61 ± 0.53b | 39.20 ± 1.19d |
| 6 | Zeaxanthin | 3.43 ± 0.32a | 2.11 ± 0.08b | 2.24 ± 0.09b | 2.20 ± 0.20b | 2.78 ± 0.12c | 2.18 ± 0.21b |
| 7 | α-Carotene | 10.19 ± 0.29a | 27.73 ± 0.28b | 18.37 ± 0.72c | 18.76 ± 1.06c | 23.97 ± 0.22d | 21.95 ± 0.92e |
| 8 | β-Carotene | 10.58 ± 0.58a | 20.89 ± 0.26b | 16.08 ± 0.56c | 19.90 ± 0.54b | 21.09 ± 0.22b | 19.61 ± 0.97b |
| 9 | 9Z β-carotene | 1.83 ± 0.05a | 3.46 ± 0.17b | 2.66 ± 0.15c | 4.16 ± 0.25d | 3.46 ± 0.04b | 3.30 ± 0.13b |
| Sum (mg/100 g d.w.) | 56.75 ± 2.52F | 96.28 ± 0.79A | 74.75 ± 1.75E | 79.60 ± 2.54D | 93.90 ± 0.98B | 86.23 ± 2.64C | |
| No. | Compounds | Si0 | Si1 | Si2 | Si3 | Si4 | Si5 |
|---|---|---|---|---|---|---|---|
| Tocopherol | |||||||
| 1 | α-Tocopherol | 2.48 ± 0.35ab 1,2 | 2.97 ± 0.33ab | 2.95 ± 0.22ab | 3.32 ± 0.25b | 2.00 ± 0.21a | 2.43 ± 0.60ab |
| Carotenoids | |||||||
| 2 | Phytoene | 0.06 ± 0.01a | 0.32 ± 0.06b | 0.05 ± 0.02a | 0.08 ± 0.01a | 0.07 ± 0.01a | 0.16 ± 0.01c |
| 3 | Lutein | 0.20 ± 0.03a | 0.30 ± 0.02a | 0.29 ± 0.02a | 0.24 ± 0.04a | 0.29 ± 0.14a | 0.23 ± 0.01a |
| 4 | β-Carotene | 0.39 ± 0.04a | 0.83 ± 0.05b | 0.78 ± 0.07b | 0.70 ± 0.10bc | 0.42 ± 0.03a | 0.63 ± 0.01c |
| 5 | 9Z β-carotene | 0.05 ± 0.01a | 0.10 ± 0.01b | 0.11 ± 0.02b | 0.11 ± 0.01b | 0.06 ± 0.01ac | 0.09 ± 0.01bc |
| 6 | di-Z lycopene | 0.04 ± 0.01a | 0.06 ± 0.02a | 0.06 ± 0.01a | 0.05 ± 0.01a | 0.04 ± 0.01a | 0.06 ± 0.01a |
| 7 | (15Z)-lycopene | 0.07 ± 0.01a | 0.08 ± 0.01ab | 0.09 ± 0.01ab | 0.09 ± 0.01ab | 0.08 ± 0.01ab | 0.10 ± 0.01b |
| 8 | (13Z)-lycopene | 0.26 ± 0.02a | 0.46 ± 0.03b | 0.36 ± 0.01cd | 0.33 ± 0.04ce | 0.29 ± 0.02ae | 0.40 ± 0.02bd |
| 9 | di-Z lycopene | 0.17 ± 0.01a | 0.26 ± 0.04b | 0.21 ± 0.03ab | 0.22 ± 0.03ab | 0.23 ± 0.03ab | 0.20 ± 0.01ab |
| 10 | (9Z)-lycopene | 0.15 ± 0.03a | 0.27 ± 0.09b | 0.18 ± 0.02ab | 0.12 ± 0.03a | 0.11 ± 0.01a | 0.15 ± 0.01a |
| 11 | (all-E)-lycopene | 5.15 ± 0.63a | 3.74 ± 0.41b | 3.73 ± 0.07b | 5.32 ± 0.31a | 4.03 ± 0.25b | 5.22 ± 0.21a |
| 12 | (5Z)-lycopene | 0.81 ± 0.06a | 1.89 ± 0.34b | 0.99 ± 0.14a | 0.94 ± 0.08a | 1.07 ± 0.13a | 0.95 ± 0.11a |
| 13 | ∑ Lycopene isomers | 6.66 ± 0.74ab | 6.77 ± 0.26ab | 5.63 ± 0.15c | 7.08 ± 0.32b | 5.85 ± 0.06ac | 7.08 ± 0.30b |
| Sum (mg/100 g d.w.) | 14.02 ± 1.56 | 15.08 ± 0.47 | 12.48 ± 0.30 | 15.30 ± 0.69 | 12.55 ± 0.13 | 15.26 ± 0.63 | |
| No. | Compounds | Si0 | Si1 | Si2 | Si3 | Si4 | Si5 |
|---|---|---|---|---|---|---|---|
| Tocopherol | |||||||
| 1 | α-Tocopherol | 9.79 ± 0.49a 1,2 | 9.93 ± 0.47a | 3.70 ± 0.20b | 7.07 ± 0.33c | 6.76 ± 0.54c | 3.31 ± 0.43b |
| Carotenoids | |||||||
| 2 | Phytoene | 0.81 ± 0.04a | 1.04 ± 0.06b | 1.08 ± 0.04b | 1.20 ± 0.05c | 0.44 ± 0.02d | 0.86 ± 0.04a |
| 3 | β-Carotene | 0.31 ± 0.02a | 0.29 ± 0.02ab | 0.30 ± 0.02 | 0.25 ± 0.01bc | 0.21 ± 0.01c | 0.30 ± 0.02a |
| 4 | di-Z lycopene | 0.33 ± 0.02a | 0.15 ± 0.01b | 0.21 ± 0.01c | 0.11 ± 0.01d | 0.19 ± 0.01c | 0.19 ± 0.01c |
| 5 | (15Z)-lycopene | 0.60 ± 0.02a | 0.66 ± 0.03a | 0.47 ± 0.03b | 0.70 ± 0.07a | 0.60 ± 0.04a | 1.01 ± 0.06c |
| 6 | (13Z)-lycopene | 9.09 ± 0.20a | 9.92 ± 0.39b | 6.15 ± 0.17c | 7.92 ± 0.15d | 6.35 ± 0.22c | 10.53 ± 0.42b |
| 7 | di-Z lycopene | 0.30 ± 0.02a | 0.39 ± 0.04a | 0.60 ± 0.03b | 0.68 ± 0.05b | 0.80 ± 0.05c | 1.68 ± 0.04d |
| 8 | (9Z)-lycopene | 1.09 ± 0.09a | 1.02 ± 0.08ab | 0.72 ± 0.06cd | 0.68 ± 0.05c | 0.87 ± 0.06bd | 1.17 ± 0.05a |
| 9 | (all-E)-lycopene | 110 ± 1a | 93.89 ± 1.83b | 108 ± 1a | 94.49 ± 0.96b | 80.31 ± 1.28c | 144 ± 1d |
| 10 | (5Z)-lycopene | 4.89 ± 0.27a | 13.75 ± 0.36b | 6.15 ± 0.11a | 6.10 ± 0.39a | 5.93 ± 0.42a | 10.21 ± 0.87d |
| 11 | ∑ Lycopene isomers | 126 ± 1a | 120 ± 2b | 123 ± 1ab | 111 ± 1c | 95.05 ± 1.65d | 169 ± 2e |
| Sum (mg/100 g d.w.) | 127 ± 2B | 121 ± 2D | 124 ± 1C | 112 ± 1E | 95.69 ± 1.66F | 170 ± 3A | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachowicz-Wiśniewska, S.; Kapusta, I.; Stinco, C.M.; Meléndez-Martínez, A.J.; Bieniek, A.; Ochmian, I.; Gil, Z. Distribution of Polyphenolic and Isoprenoid Compounds and Biological Activity Differences between in the Fruit Skin + Pulp, Seeds, and Leaves of New Biotypes of Elaeagnusmultiflora Thunb. Antioxidants 2021, 10, 849. https://doi.org/10.3390/antiox10060849
Lachowicz-Wiśniewska S, Kapusta I, Stinco CM, Meléndez-Martínez AJ, Bieniek A, Ochmian I, Gil Z. Distribution of Polyphenolic and Isoprenoid Compounds and Biological Activity Differences between in the Fruit Skin + Pulp, Seeds, and Leaves of New Biotypes of Elaeagnusmultiflora Thunb. Antioxidants. 2021; 10(6):849. https://doi.org/10.3390/antiox10060849
Chicago/Turabian StyleLachowicz-Wiśniewska, Sabina, Ireneusz Kapusta, Carla M. Stinco, Antonio J. Meléndez-Martínez, Anna Bieniek, Ireneusz Ochmian, and Zygmunt Gil. 2021. "Distribution of Polyphenolic and Isoprenoid Compounds and Biological Activity Differences between in the Fruit Skin + Pulp, Seeds, and Leaves of New Biotypes of Elaeagnusmultiflora Thunb" Antioxidants 10, no. 6: 849. https://doi.org/10.3390/antiox10060849
APA StyleLachowicz-Wiśniewska, S., Kapusta, I., Stinco, C. M., Meléndez-Martínez, A. J., Bieniek, A., Ochmian, I., & Gil, Z. (2021). Distribution of Polyphenolic and Isoprenoid Compounds and Biological Activity Differences between in the Fruit Skin + Pulp, Seeds, and Leaves of New Biotypes of Elaeagnusmultiflora Thunb. Antioxidants, 10(6), 849. https://doi.org/10.3390/antiox10060849







