Optimization of Glycerol–Water Extraction of Selected Bioactive Compounds from Peppermint and Common Nettle
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Water Extraction and Glycerol–Water Extraction
2.3. Ethanol Extraction and Ethanol–Water Extraction
2.4. Evaluation of Total Polyphenols, Flavonoids and Chlorophyll Contents
2.5. Evaluation of Total Antioxidant Capacity Based on Ferric Ion (III) Reduction (FRAP)
2.6. Optimal Parameters of the Extraction Mixture
2.7. Statistical Analysis
3. Results and Discussion
3.1. Total Polyphenols, Flavonoids and Chlorophyll Contents of Extracts from Peppermint and Common Nettle
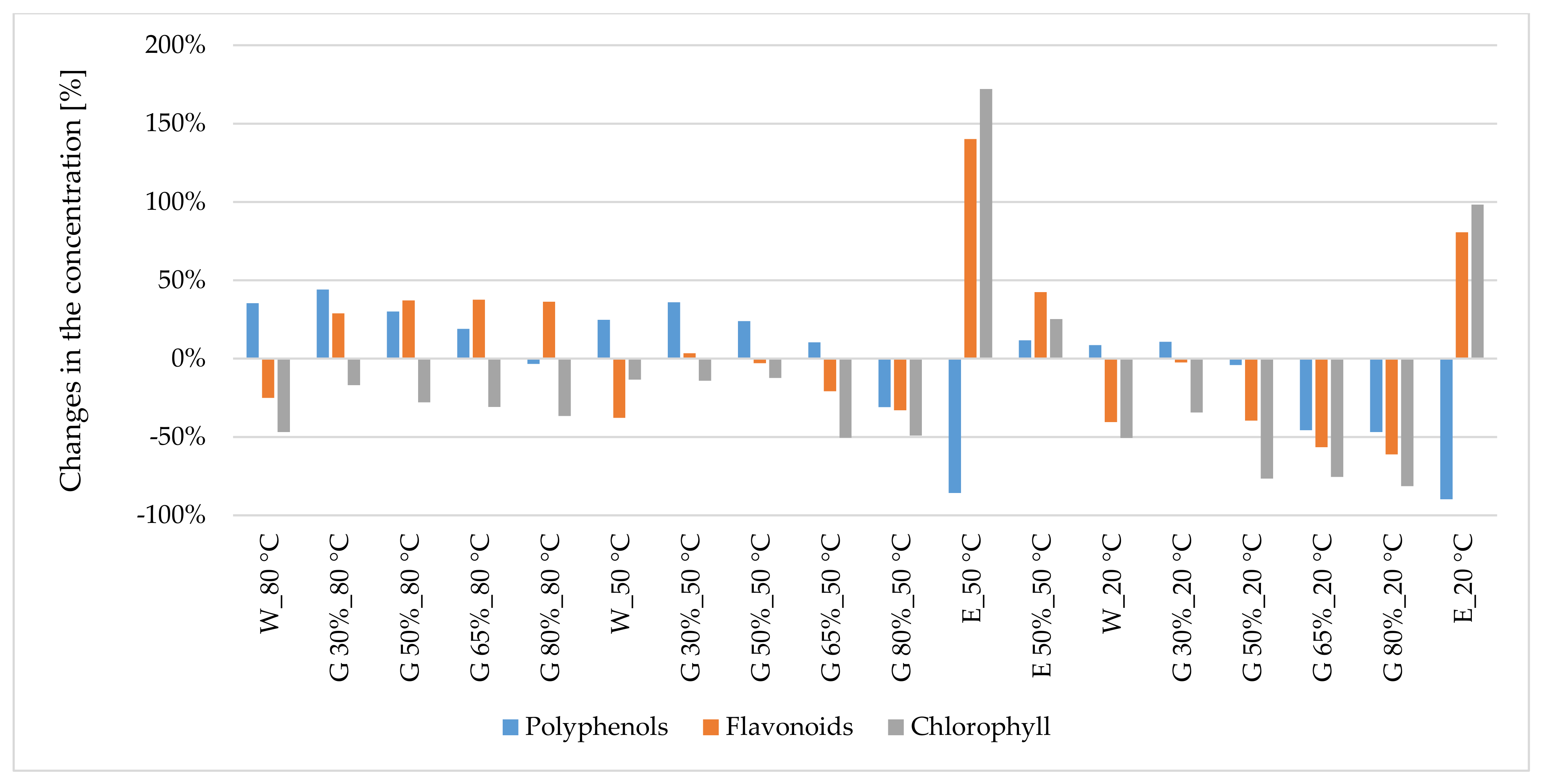
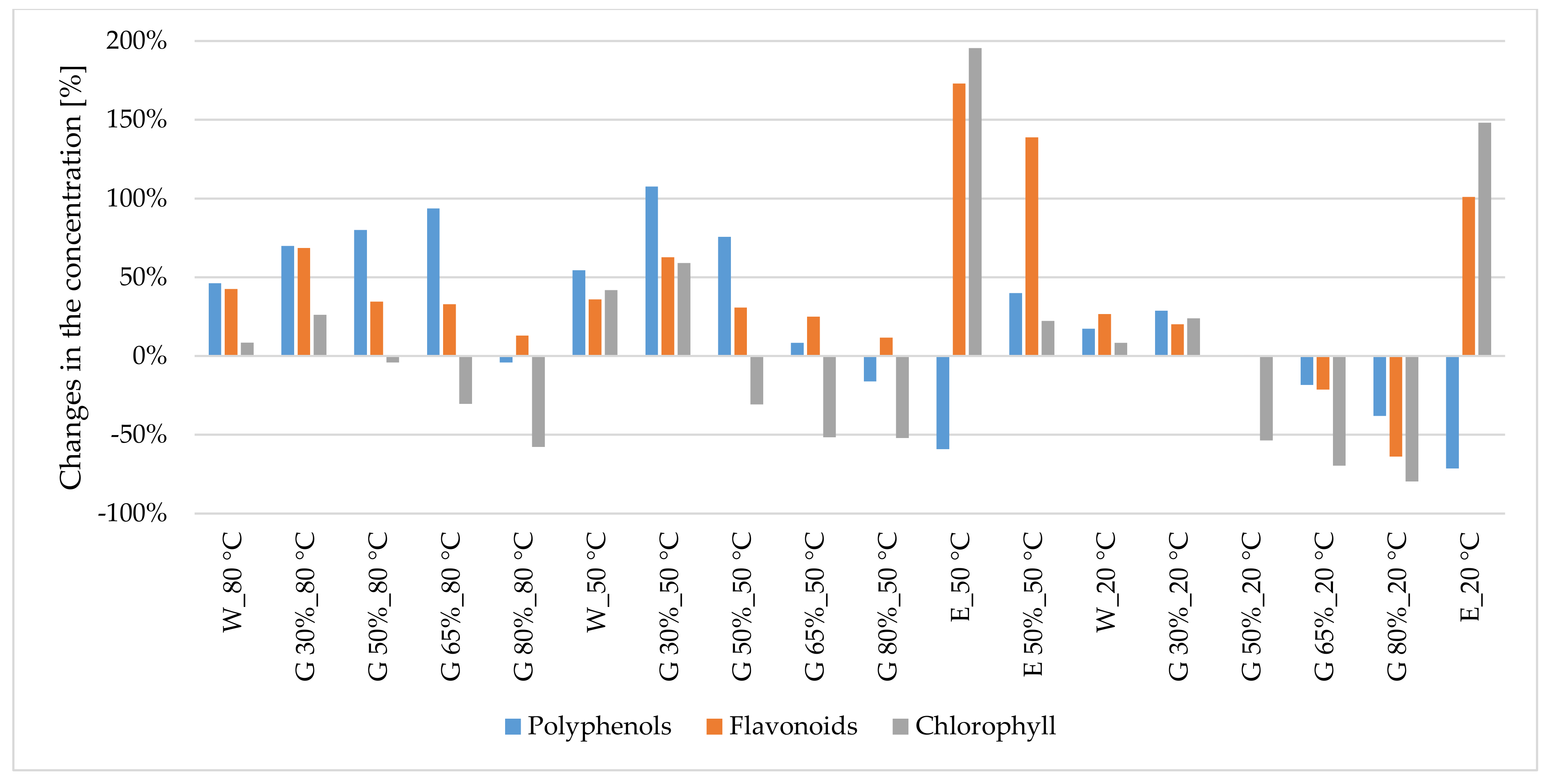
3.1.1. Polyphenols
3.1.2. Flavonoids
3.1.3. Chlorophyll
3.2. Antioxidant Properties of the Extracts
3.3. Determination of Optimum Conditions of Extraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Ramamurthy, P.; Santhiya, S.T.; Ramesh, A. Antioxidant activity measured in different solvent fractions obtained from Mentha spicata Linn.: An analysis by ABTS.+ decolorization assay. Asia Pac. J. Clin. Nutr. 2006, 15, 119–124. [Google Scholar] [PubMed]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Núñez, M.J. Effect of Solvent, Temperature, and Solvent-to-Solid Ratio on the Total Phenolic Content and Antiradical Activity of Extracts from Different Components of Grape Pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Nguyen, M.H.; Roach, P.D. Optimizing conditions for the extraction of catechins from green tea using hot water. J. Sep. Sci. 2011, 34, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Karaaslan, M.; Vardin, H. Optimization of extraction parameters on the isolation of phenolic compounds from sour cherry (Prunus cerasus L.) pomace. J. Food Sci. Technol. 2015, 52, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- He, G.-Q.; Xiong, H.-P.; Chen, Q.-H.; Ruan, H.; Wang, Z.-Y.; Traore, L. Optimization of conditions for supercritical fluid extraction of flavonoids from hops (Humulus lupulus L.). J. Zhejiang Univ. Sci. 2005, 6B, 999–1004. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Leblanc, J.; et al. Re-evaluation of glycerol (E 422) as a food additive. EFSA J. 2017, 15, 4720. [Google Scholar]
- Manousaki, A.; Jancheva, M.; Grigorakis, S.; Makris, D. Extraction of antioxidant phenolics from agri-food waste biomass using a newly designed glycerol-based natural low-transition temperature mixture: A comparison with conventional eco-friendly solvents. Recycling 2016, 1, 194. [Google Scholar] [CrossRef]
- Eyiz, V.; Tontul, I.; Turker, S. Optimization of green extraction of phytochemicals from red grape pomace by homogenizer assisted extraction. J. Food Meas. Charact. 2020, 14, 39–47. [Google Scholar] [CrossRef]
- Kurtulbaş, E.; Pekel, A.G.; Bilgin, M.; Makris, D.P.; Şahin, S. Citric acid-based deep eutectic solvent for the anthocyanin recovery from Hibiscus sabdariffa through microwave-assisted extraction. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Kowalska, G.; Wyrostek, J.; Kowalski, R.; Pankiewicz, U. Evaluation of glycerol usage for the extraction of anthocyanins from black chokeberry and elderberry fruits. J. Appl. Res. Med. Aromat. Plants 2021, 22, 100296. [Google Scholar]
- Apostolakis, A.; Grigorakis, S.; Makris, D.P. Optimisation and comparative kinetics study of polyphenol extraction from olive leaves (Olea europaea) using heated water/glycerol mixtures. Sep. Purif. Technol. 2014, 128, 89–95. [Google Scholar] [CrossRef]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimisation of polyphenol extraction from Hypericum perforatum (St. John’s Wort) using aqueous glycerol and response surface methodology. J. Appl. Res. Med. Aromat. Plants 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Michail, A.; Sigala, P.; Grigorakis, S.; Makris, D.P. Kinetics of Ultrasound-Assisted Polyphenol Extraction from Spent Filter Coffee Using Aqueous Glycerol. Chem. Eng. Commun. 2016, 203, 407–413. [Google Scholar] [CrossRef]
- Philippi, K.; Tsamandouras, N.; Grigorakis, S.; Makris, D.P. Ultrasound-assisted green extraction of eggplant peel (Solanum melongena) polyphenols using aqueous mixtures of glycerol and ethanol: Optimisation and kinetics. Environ. Process. 2016, 3, 369–386. [Google Scholar] [CrossRef]
- Tafrihi, M.; Imran, M.; Tufail, T.; Gondal, T.A.; Caruso, G.; Sharma, S.; Sharma, R.; Atanassova, M.; Atanassov, L.; Valere Tsouh Fokou, P.; et al. The wonderful activities of the genus Mentha: Not only antioxidant properties. Molecules 2021, 26, 1118. [Google Scholar] [CrossRef] [PubMed]
- Habs, M.; Binder, K.; Krauss, S.; Müller, K.; Ernst, B.; Valentini, L.; Koller, M. A Balanced Risk–Benefit Analysis to Determine Human Risks Associated with Pyrrolizidine Alkaloids (PA)—The Case of Tea and Herbal Infusions. Nutrients 2017, 9, 717. [Google Scholar] [CrossRef]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [PubMed]
- Łyczko, J.; Piotrowski, K.; Kolasa, K.; Galek, R.; Szumny, A. Mentha piperita L. Micropropagation and the Potential Influence of Plant Growth Regulators on Volatile Organic Compound Composition. Molecules 2020, 25, 2652. [Google Scholar] [CrossRef]
- Bodalska, A.; Kowalczyk, A.; Włodarczyk, M.; Fecka, I. Analysis of Polyphenolic Composition of a Herbal Medicinal Product—Peppermint Tincture. Molecules 2019, 25, 69. [Google Scholar] [CrossRef] [PubMed]
- Paulauskienė, A.; Tarasevičienė, Ž.; Laukagalis, V. Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica dioica L.). Plants 2021, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Bianco, A.; Russo, R.; Di Maro, A.; Isernia, C.; Pedone, P. Therapeutic Perspectives of Molecules from Urtica dioica Extracts for Cancer Treatment. Molecules 2019, 24, 2753. [Google Scholar] [CrossRef]
- Ożarowski, A.; Jaroniewski, W. Medicinal Plants and Its Practical Application; Instytut Wydawniczy Związków Zawodowych: Warsaw, Poland, 1987. [Google Scholar]
- Ożarowski, A. Phytotherapy—A Guide for Doctors; Państwowy Zakład Wydawnictw Lekarskich: Warsaw, Poland, 1982. [Google Scholar]
- Mancuso, M. The antibacterial activity of Mentha. In Herbs and Spices; IntechOpen: London, UK, 2020. [Google Scholar]
- Wolska, J.; Janda, K.; Szkyrpan, S.; Gutowska, I. Wpływ ekstraktów z pokrzywy zwyczajnej (Urtica dioica L.) na aktywność katalazy w monocytach/makrofagach THP1. Pomeranian J. Life Sci. 2016, 61, 315–318. [Google Scholar] [CrossRef]
- Bouchentouf, S.; Ghalem, S.; Nadia, K.; Soumaya, K. Identification of phenolic compounds from nettle as new candidate inhibitors of main enzymes responsible on type-II diabetes. Curr. Drug Discov. Technol. 2020, 17, 197–202. [Google Scholar]
- Berktas, S.; Cam, M. Peppermint leaves hydrodistillation by-products: Bioactive properties and incorporation into ice cream formulations. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Wyrostek, J.; Kowalski, R.; Pankiewicz, U.; Solarska, E. Estimation of the content of selected active substances in primary and secondary herbal brews by UV-VIS and GC-MS spectroscopic analyses. J. Anal. Methods Chem. 2020, 2020, 8891855. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Hrnčič, K.M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Ouedrhiri, W.; Balouiri, M.; Bouhdid, S.; Moja, S.; Chahdi, F.O.; Taleb, M.; Greche, H. Mixture design of Origanum compactum, Origanum majorana and Thymus serpyllum essential oils: Optimization of their antibacterial effect. Ind. Crop. Prod. 2016, 89, 1–9. [Google Scholar] [CrossRef]
- Baj, T.; Baryluk, A.; Sieniawska, E. Application of mixture design for optimum antioxidant activity of mixtures of essential oils from Ocimum basilicum L., Origanum majorana L. and Rosmarinus officinalis L. Ind. Crop. Prod. 2018, 115, 52–61. [Google Scholar] [CrossRef]
- Bezerra, F.I.C.; de Moraes, R.R.T.; Assunção, F.M.R.; Lira, S.L.A. Optimization Strategy for Extraction of Active Polyphenols from Leaves of Eugenia uniflora Linn. Food Anal. Methods 2020, 13, 735–750. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Polish Pharmacopoeia VIII; PTFarm; Polish Pharmaceutical Society: Warsaw, Poland, 2008.
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV–VIS Spectroscopy Current Protocols in Food Analytical Chemistry; John Wiley and Sons: New York, NY, USA, 2001. [Google Scholar]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of Pigment and Flavanol Content with Antioxidant Properties in Selected Aged Regional Wines from Greece. J. Food Compos. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Weisberg, S. Applied Linear Regression (Volume 528); John Wiley & Sons, Inc.: London, UK, 2005. [Google Scholar]
- Candioti, L.V.; De Zan, M.M.; Cámara, M.S.; Goicoechea, H.C. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef]
- Sivakumar, T.; Manavalan, R.; Valliappan, K. Global optimization using Derringer’s desirability function: Enantioselective determination of ketoprofen in formulations and in biological matrices. Acta Chromatogr. 2007, 19, 29–47. [Google Scholar]
- Uribe, E.; Marín, D.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Rodríguez, A. Assessment of vacuum-dried peppermint (Mentha piperita L.) as a source of natural antioxidants. Food Chem. 2016, 190, 559–565. [Google Scholar] [CrossRef]
- Zeljković, S.C.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic Compounds and Biological Activity of Selected Mentha Species. Plants 2021, 10, 550. [Google Scholar] [CrossRef]
- Pavlić, B.; Kaplan, M.; Bera, O.; Olgun, O.E.; Canli, O.; Milosavljević, N.; Antić, B.; Zeković, Z. Microwave-assisted extraction of peppermint polyphenols—Artificial neural networks approach. Food Bioprod. Process. 2019, 118, 258–269. [Google Scholar] [CrossRef]
- Pieszko, C.; Zaremba, A. Content of phenolic compounds in plant extracts. Bromatol. Toxicol. Chem. 2013, 46, 434–439. [Google Scholar]
- Najda, A. Chemical composition and antioxidant activity of extracts from Mentha x piperita L. Postępy Fitoter. 2017, 18, 251–258. [Google Scholar]
- Hudec, J.; Burdová, M.; Kobida, L.; Komora, L.; Macho, V.; Kogan, G.; Turianica, I.; Kochanová, R.; Ložek, O.; Habán, M.; et al. Antioxidant Capacity Changes and Phenolic Profile of Echinacea purpurea, Nettle (Urtica dioica L.), and Dandelion (Taraxacum officinale) after Application of Polyamine and Phenolic Biosynthesis Regulators. J. Agric. Food Chem. 2007, 55, 5689–5696. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.; Gharekhani, M.; Ghorbani, M.; Dargany, P. Effect of Extract of Aerial Parts of Urtica dioica (Urticaceae) on the Stability of Soybean Oil. Trop. J. Pharm. Res. 2015, 14, 125. [Google Scholar] [CrossRef]
- Sidaoui, F.; Igueld, S.B.; Barth, D.; Trabelsi-Ayadi, M.; Cherif, J.K. Study of tunisian nettle leaves (Urtica dioica L.): Mineral composition and antioxidant capacity of their extracts obtained by maceration and supercritical fluid extraction. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 707–713. [Google Scholar]
- Repajić, M.; Cegledi, E.; Zorić, Z.; Pedisić, S.; Elez Garofulić, I.; Radman, S.; Palčić, I.; Dragović-Uzelac, V. Bioactive Compounds in Wild Nettle (Urtica dioica L.) Leaves and Stalks: Polyphenols and Pigments upon Seasonal and Habitat Variations. Foods 2021, 10, 190. [Google Scholar] [CrossRef]
- Akyurt, B.; Başyiğit, B.; Çam, M. Phenolic compounds content, antioxidant and antidiabetic potentials of seven edible leaves. GIDA J. Food 2018, 43, 876–885. [Google Scholar]
- Ancuceanu, R.; Hovaneț, M.V.; Anghel, A.I.; Dinu, M.; Dune, A.; Ciolea, M.; Olaru, O.T.; Popescu, C. Variation of polyphenols and iron concentration in Mentha X piperita L. By development stage and soil type. Farmacia 2017, 65, 748–754. [Google Scholar]
- Kukric, Z.; Topalic-Trivunovic, L.; Kukavica, B.; Matos, S.; Pavicic, S.; Boroja, M.; Savic, A. Characterization of antioxidant and antimicrobial activities of nettle leaves (Urtica dioica L.). Acta Period. Technol. 2012, 257–272. [Google Scholar] [CrossRef]
- Straumite, E.; Kruma, Z.; Galoburda, R. Pigments in mint leaves and stems. Agron. Res. 2015, 13, 1104–1111. [Google Scholar]
- Rubinskienė, M.; Viškelis, P.; Dambrauskienė, E.; Viškelis, J.; Karklelienė, R. Effect of drying methods on the chemical composition and colour of peppermint (Mentha × piperita L.) leaves. Zemdirb. Agric. 2015, 102, 223–228. [Google Scholar] [CrossRef]
- Pisulewska, E.; Ciesielski, W.; Jackowska, M.; Gąstoł, M.; Oszczęda, Z.; Tomasik, P. Cultivation of peppermint (Mentha piperita rubescens) using water treated with low-pressure, low-temperature glow plasma of low frequency. Electron. J. Pol. Agric. Univ. 2018, 21. [Google Scholar] [CrossRef]
- Biesiada, A.; Woloszczak, E.; Sokol-Letowska, A.; Nawirska-Olszanska, A. The effect of nitrogen form and dose on yield, chemical composition and antioxidant activity of stinging nettle [Urtica dioica L.]. Herba Pol. 2009, 55, 84–93. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins, B.D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Rajurkar, N.; Hande, S. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146. [Google Scholar] [CrossRef]
- Brahmi, F.; Madani, K.; Dahmoune, F.; Rahmani, T.; Bousbaa, K.; Oukmanou, S.; Chibane, M. Optimisation of Solvent Extraction of Antioxidants (Phenolic Compounds) from Algerian Mint (Mentha spicata L.). Pharmacogn. Commun. 2012, 2, 72–86. [Google Scholar]
- Hayouni, E.; Abedrabba, M.; Bouix, M.; Hamdi, M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem. 2007, 105, 1126–1134. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef] [PubMed]
- Abaza, L.; Youssef, B.N.; Manai, H.; Haddada, M.F.; Methenni, K.; Zarrouk, M. Chétoui olive leaf extracts: Influence of the solvent type on phenolics and antioxidant activities. Grasas Aceites 2011, 62, 96–104. [Google Scholar] [CrossRef]
- Sathishkumar, T.; Baskar, R.; Shanmugam, S.; Rajasekaran, P.; Sadasivam, S.; Manikandan, V. Optimization of flavonoids extraction from the leaves of Tabernaemontana heyneana Wall. using L16 Orthogonal design. Nat. Sci. 2008, 6, 10–21. [Google Scholar]
- Luque de Castro, M.D.; Tena, M.T. Strategies for supercritical fluid extraction of polar and ionic compounds. TrAC Trends Anal. Chem. 1996, 15, 32–37. [Google Scholar] [CrossRef]
- Hojnik, M.; Škerget, M.; Knez, Ž. Isolation of chlorophylls from stinging nettle (Urtica dioica L.). Sep. Purif. Technol. 2007, 57, 37–46. [Google Scholar] [CrossRef]
- Azahar, N.F.; Gani, S.S.A.; Mokhtar, M.N.F. Optimization of phenolics and flavonoids extraction conditions of Curcuma Zedoaria leaves using response surface methodology. Chem. Cent. J. 2017, 11, 96. [Google Scholar] [CrossRef]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Mourtzinos, I.; Makris, D.P. Optimisation of organic solvent-free polyphenol extraction from Hypericum triquetrifolium Turra using Box–Behnken experimental design and kinetics. Int. J. Ind. Chem. 2015, 6, 85–92. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Anastasopoulou, E.; Petrou, A.; Grigorakis, S.; Makris, D.; Biliaderis, C.G. Optimization of a green extraction method for the recovery of polyphenols from olive leaf using cyclodextrins and glycerin as co-solvents. J. Food Sci. Technol. 2016, 53, 3939–3947. [Google Scholar] [CrossRef]
- Benchikh, Y.; Louailèche, H. Effects of extraction conditions on the recovery of phenolic compounds and in vitro antioxidant activity of carob (Ceratonia siliqua L.) pulp. Acta Bot. Gall. 2014, 161, 175–181. [Google Scholar] [CrossRef]
- Alide, T.; Wangila, P.; Kiprop, A. Effect of cooking temperature and time on total phenolic content, total flavonoid content and total in vitro antioxidant activity of garlic. BMC Res. Notes 2020, 13, 564. [Google Scholar] [CrossRef]
| Extraction System | The Solvent Content in the Extraction System [%] | |
|---|---|---|
| Glycerol (x1) | Water (x2) | |
| W | 0 | 100 |
| G 30 | 30 | 70 |
| G 50 | 50 | 50 |
| G 65 | 65 | 35 |
| G 80 | 80 | 20 |
| Temperature [°C] | Extraction System 1 | Polyphenols | Flavonoids | Chlorophyll | |||
|---|---|---|---|---|---|---|---|
| [mg GAE/L] 2 (Y1) | [mg GAE/g DM] 3 | [mg QE/L] (Y2) | [mg QE/g DM] | [mg/L] (Y3) | [mg/g DM] | ||
| Water and Glycerol–Water System | |||||||
| 80 | W | 387.7 ± 25.6 abc | 19.4 | 62.3 ± 3.6 ef | 3.1 | 54.0 ± 2.5 i | 2.7 |
| G 30 | 412.5 ± 32.7 a | 20.6 | 107.2 ± 5.6 c | 5.4 | 84.4 ± 4.1 e | 4.2 | |
| G 50 | 372.4 ± 22.0 bcd | 18.6 | 114.2 ± 2.4 c | 5.7 | 73.2 ± 3.4 f | 3.7 | |
| G 65 | 340.7 ± 22.9 cde | 17.0 | 114.5 ± 6.8 c | 5.7 | 70.2 ± 2.9 fg | 3.5 | |
| G 80 | 276.7 ± 21.8 hi | 13.8 | 113.4 ± 5.0 c | 5.7 | 64.3 ± 2.6 h | 3.2 | |
| 50 | W | 357.1 ± 23.8 bcd | 17.9 | 51.7 ± 2.8 f | 2.6 | 88.0 ± 4.1 e | 4.4 |
| G 30 | 389.1 ± 25.0 ab | 19.5 | 86.1 ± 4.4 d | 4.3 | 87.2 ±3.9 e | 4.4 | |
| G 50 | 355.0 ± 28.1 bcd | 17.8 | 80.8 ± 4.6 d | 4.0 | 89.0 ±4.7 e | 4.5 | |
| G 65 | 316.0 ± 24.7 efghi | 15.8 | 65.9 ± 3.7 e | 3.3 | 50.3 ±1.8 i | 2.5 | |
| G 80 | 197.6 ± 16.8 j | 9.9 | 55.8 ± 3.3 ef | 2.8 | 51.6 ±2.5 i | 2.6 | |
| 20 | W | 310.9 ± 21.4 fghi | 15.5 | 49.5 ± 3.2 f | 2.5 | 50.1 ± 1.4 i | 2.5 |
| G 30 | 316.9 ± 23.4 efg | 15.8 | 81.1 ± 4.5 d | 4.1 | 66.6 ± 3.1 gh | 3.3 | |
| G 50 | 274.6 ± 29.8 i | 13.7 | 50.3 ± 2.5 f | 2.5 | 23.8 ± 0.9 j | 1.2 | |
| G 65 | 155.2 ± 11.2 k | 7.8 | 36.2 ± 2.1 g | 1.8 | 24.8 ± 1.3 j | 1.2 | |
| G 80 | 152.1 ± 9.5 k | 7.6 | 32.3 ± 2.3 g | 1.6 | 18.9 ± 0.7 k | 0.9 | |
| Ethanol and Ethanol–Water System | |||||||
| 50 | E | 40.7 ± 2.9 l | 2.0 | 199.8 ± 12.7 a | 10.0 | 276.2 ± 14.3 a | 13.8 |
| E 50 | 319.6 ± 25.5 efg | 16.0 | 118.5 ± 6.7 c | 5.9 | 127.1 ± 6.6 c | 6.4 | |
| 20 | E | 29.5 ± 1.7 m | 1.5 | 150.3 ± 9.3 b | 7.5 | 201.3 ± 9.4 b | 10.1 |
| E 50 | 286.2 ± 21.6 ghi | 14.3 | 83.2 ± 4.5 d | 4.2 | 101.5 ± 4.3 d | 5.1 | |
| Temperature [°C] | Extraction System 1 | Polyphenols | Flavonoids | Chlorophyll | |||
|---|---|---|---|---|---|---|---|
| [mg GAE/L] 2 (Y1) | [mg GAE/g DM] 3 | [mg QE/L] (Y2) | [mg QE/g DM] | [mg/L] (Y3) | [mg/g DM] | ||
| Water and Glycerol–Water System | |||||||
| 80 | W | 113.9 ± 9.0 d | 5.7 | 53.6 ± 2.8 e | 2.7 | 130.3 ± 6.0 f | 6.5 |
| G 30 | 132.3 ± 10.5 bc | 6.6 | 63.4 ± 3.3 d | 3.2 | 151.4 ± 7.3 e | 7.6 | |
| G 50 | 140.3 ± 11.8 bc | 7.0 | 50.6 ± 2.4 e | 2.5 | 115.1 ± 5.0 g | 5.8 | |
| G 65 | 150.9 ± 12.1 ab | 7.5 | 50.0 ± 2.9 ef | 2.5 | 83.6 ± 3.9 h | 4.2 | |
| G 80 | 74.7 ± 5.5 gh | 3.7 | 42.5 ± 2.1 hi | 2.1 | 50.7 ± 2.1 j | 2.5 | |
| 50 | W | 120.3 ± 8.6 bcd | 6.0 | 51.1 ± 2.9 ef | 2.6 | 170.2 ± 8.1 d | 8.5 |
| G 30 | 161.8 ± 11.7 a | 8.1 | 61.2 ± 3.4 d | 3.1 | 190.9 ± 9.2 c | 9.5 | |
| G 50 | 136.9 ± 10.1 bc | 6.8 | 49.2 ± 2.7 ef | 2.5 | 83.2 ± 3.0 h | 4.2 | |
| G 65 | 84.5 ± 5.6 fg | 4.2 | 47.0 ± 2.2f g | 2.4 | 58.2 ± 2.1 i | 2.9 | |
| G 80 | 65.3 ± 4.5 hi | 3.3 | 42.0 ± 2.3 hi | 2.1 | 57.5 ± 2.9 i | 2.9 | |
| 20 | W | 91.4 ± 6.5 f | 4.6 | 47.6 ± 2.2 fg | 2.4 | 130.1 ± 6.6 f | 6.5 |
| G 30 | 100.3 ± 7.4 ef | 5.0 | 45.2 ± 1.9 gh | 2.3 | 148.7 ± 7.1 e | 7.4 | |
| G 50 | 78.3 ± 5.8 g | 3.9 | 37.8 ± 2.7 i | 1.9 | 55.8 ± 2.2 i | 2.8 | |
| G 65 | 63.6 ± 4.9 i | 3.2 | 29.6 ± 1.6 j | 1.5 | 36.5 ± 2.0 k | 1.8 | |
| G 80 | 48.3 ± 3.4 j | 2.4 | 13.6 ± 0.9 k | 0.7 | 24.5 ± 1.1 l | 1.2 | |
| Ethanol and Ethanol–Water System | |||||||
| 50 | E | 31.9 ± 2.1 k | 1.6 | 102.7 ± 5.4 a | 5.1 | 354.7 ± 19.9 a | 17.7 |
| E 50 | 109.0 ± 7.5 de | 5.5 | 89.8 ± 4.0 b | 4.5 | 146.8 ± 8.1 e | 7.3 | |
| 20 | E | 22.3 ± 1.6 l | 1.1 | 75.6 ± 4.1 c | 3.8 | 297.7 ± 13.4 b | 14.9 |
| E 50 | 77.9 ± 5.6 g | 3.9 | 37.6 ± 2.1 i | 1.9 | 120.0 ± 6.3 fg | 6.0 | |
| Temperature [°C] | Extraction System 1 | FRAP [μmol Fe2+/L] (Y4) | |
|---|---|---|---|
| Leaf of Peppermint | Leaf of Common Nettle | ||
| Water and Glycerol–Water System | |||
| 80 | W | 8494 ± 159 b | 4030 ± 82 e |
| G 30 | 10075 ± 228 a | 4587 ± 92 cd | |
| G 50 | 7270 ± 144 c | 4718 ± 93 c | |
| G 65 | 6635 ± 129 d | 5945 ± 119 b | |
| G 80 | 4222 ± 79 g | 3211 ± 61 g | |
| 50 | W | 7160 ± 148 c | 4460 ± 65 d |
| G 30 | 8625 ± 191 b | 6736 ± 63 a | |
| G 50 | 7208 ± 132 c | 4016 ± 86 e | |
| G 65 | 5760 ± 153 e | 3021 ± 76 hi | |
| G 80 | 4349 ± 161 g | 2388 ± 69 k | |
| 20 | W | 5165 ± 128 f | 3180 ± 104 gh |
| G 30 | 5761 ± 162 e | 3540 ± 91 f | |
| G 50 | 4373 ± 168 g | 2912 ± 88 ij | |
| G 65 | 3366 ± 55 h | 2827 ± 65 j | |
| G 80 | 2909 ± 49 i | 2493 ± 58 k | |
| Ethanol and Ethanol–Water System | |||
| 50 | E | 2283 ± 81 j | 1633 ± 38 l |
| E 50 | 5913 ± 143 e | 4164 ± 91 e | |
| 20 | E | 1600 ± 41 k | 1178 ± 29 m |
| E 50 | 4580 ± 149 g | 2838 ± 71 j | |
| Leaf of Peppermint | Leaf of Common Nettle | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Desirability | Li (0) | Mi (0.5) | Ui (1) | PV | PV [%] | Li (0) | Mi (0.5) | Ui (1) | PV | PV [%] |
| TPC 80 | 276.7 | 344.6 | 412.5 | 401.0 | 97.21 | 74.5 | 112.7 | 150.9 | 148.2 | 98.21 |
| TF 80 | 62.3 | 88.4 | 114.5 | 109.7 | 95.81 | 42.5 | 53.0 | 63.4 | 58.6 | 92.43 |
| CHLO 80 | 54.0 | 69.2 | 84.4 | 78.9 | 93.48 | 50.7 | 101.1 | 151.4 | 141.2 | 93.26 |
| FRAP 80 | 4222 | 7149 | 10075 | 9020 | 89.53 | 3211 | 4578 | 5945 | 5097 | 85.74 |
| TPC 50 | 197.6 | 293.6 | 389.1 | 396.1 | 101.80 | 65.3 | 113.6 | 161.8 | 150.5 | 93.02 |
| TF 50 | 51.7 | 6.8.9 | 86.1 | 81.9 | 95.12 | 42.0 | 51.6 | 61.2 | 56.3 | 91.99 |
| CHLO 50 | 50.3 | 69.7 | 89.0 | 88.6 | 99.55 | 55.7 | 123.3 | 190.9 | 160.6 | 84.13 |
| FRAP 50 | 4349 | 6487 | 8625 | 8202 | 95.10 | 2388 | 4562 | 6736 | 5563 | 82.59 |
| TPC 20 | 152.1 | 234.5 | 316.9 | 316.9 | 100.00 | 48.3 | 74.3 | 100.3 | 96.3 | 96.01 |
| TF 20 | 32.3 | 56.7 | 81.1 | 64.8 | 79.90 | 13.6 | 30.6 | 47.6 | 46.4 | 97.48 |
| CHLO 20 | 18.9 | 42.8 | 66.6 | 52.8 | 79.28 | 24.5 | 86.6 | 148.7 | 132.3 | 88.97 |
| FRAP 20 | 2493 | 3017 | 3540 | 3344 | 94.46 | 2493 | 3017 | 3540 | 3328 | 94.01 |
| Tested Raw Material | Extraction Temperature [°C] | Glycerol [%] | Water [%] | Desirability Level |
|---|---|---|---|---|
| Leaf of Peppermint | 80 | 37.4 | 62.6 | 0.86 |
| 50 | 30.5 | 69.5 | 0.94 | |
| 20 | 18.6 | 81.4 | 0.79 | |
| Leaf of Common Nettle | 80 | 31.8 | 68.3 | 0.82 |
| 50 | 21.6 | 78.4 | 0.78 | |
| 20 | 12.5 | 87.5 | 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, G.; Baj, T.; Kowalski, R.; Szymańska, J. Optimization of Glycerol–Water Extraction of Selected Bioactive Compounds from Peppermint and Common Nettle. Antioxidants 2021, 10, 817. https://doi.org/10.3390/antiox10050817
Kowalska G, Baj T, Kowalski R, Szymańska J. Optimization of Glycerol–Water Extraction of Selected Bioactive Compounds from Peppermint and Common Nettle. Antioxidants. 2021; 10(5):817. https://doi.org/10.3390/antiox10050817
Chicago/Turabian StyleKowalska, Grażyna, Tomasz Baj, Radosław Kowalski, and Jolanta Szymańska. 2021. "Optimization of Glycerol–Water Extraction of Selected Bioactive Compounds from Peppermint and Common Nettle" Antioxidants 10, no. 5: 817. https://doi.org/10.3390/antiox10050817
APA StyleKowalska, G., Baj, T., Kowalski, R., & Szymańska, J. (2021). Optimization of Glycerol–Water Extraction of Selected Bioactive Compounds from Peppermint and Common Nettle. Antioxidants, 10(5), 817. https://doi.org/10.3390/antiox10050817






