Protective Effect of Chlorogenic Acid on Human Sperm: In Vitro Studies and Frozen–Thawed Protocol
Abstract
1. Introduction
2. Materials and Methods
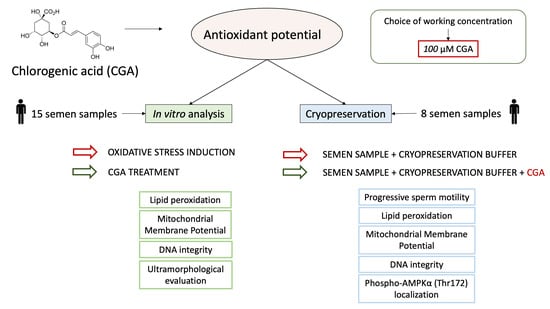
2.1. Study Design
- Step 1: an analysis of the protective action of CGA on OS induced in vitro with H2O2.
- Step 2: an applicative study of the effect of CGA during semen cryopreservation.
2.2. Semen Samples
2.3. Step 1: In Vitro Studies
2.3.1. Evaluation of CGA Effect on Sperm Motility
2.3.2. Swim-Up to Select Motile Sperm
- Untreated sperm as control;
- Sperm treated with 100 µM H2O2;
- Sperm treated with 100 µM CGA;
- Sperm treated with both 100 µM H2O2 and 100 µM CGA.
2.3.3. JC-1 to Evaluate Mitochondrial Membrane Potential (MMP)
2.3.4. Acridine Orange (AO) to Evaluate DNA Integrity
2.3.5. Malondialdehyde (MDA) Level Assessment
2.3.6. F2-isoprostane Determination
2.3.7. Transmission Electron Microscopy (TEM)
2.4. Step 2: Applicative Study
2.4.1. Cryopreservation
2.4.2. Immunocytochemistry
2.5. Statistical Analysis
3. Results
3.1. Effect of Chlorogenic Acid on Sperm Progressive Motility
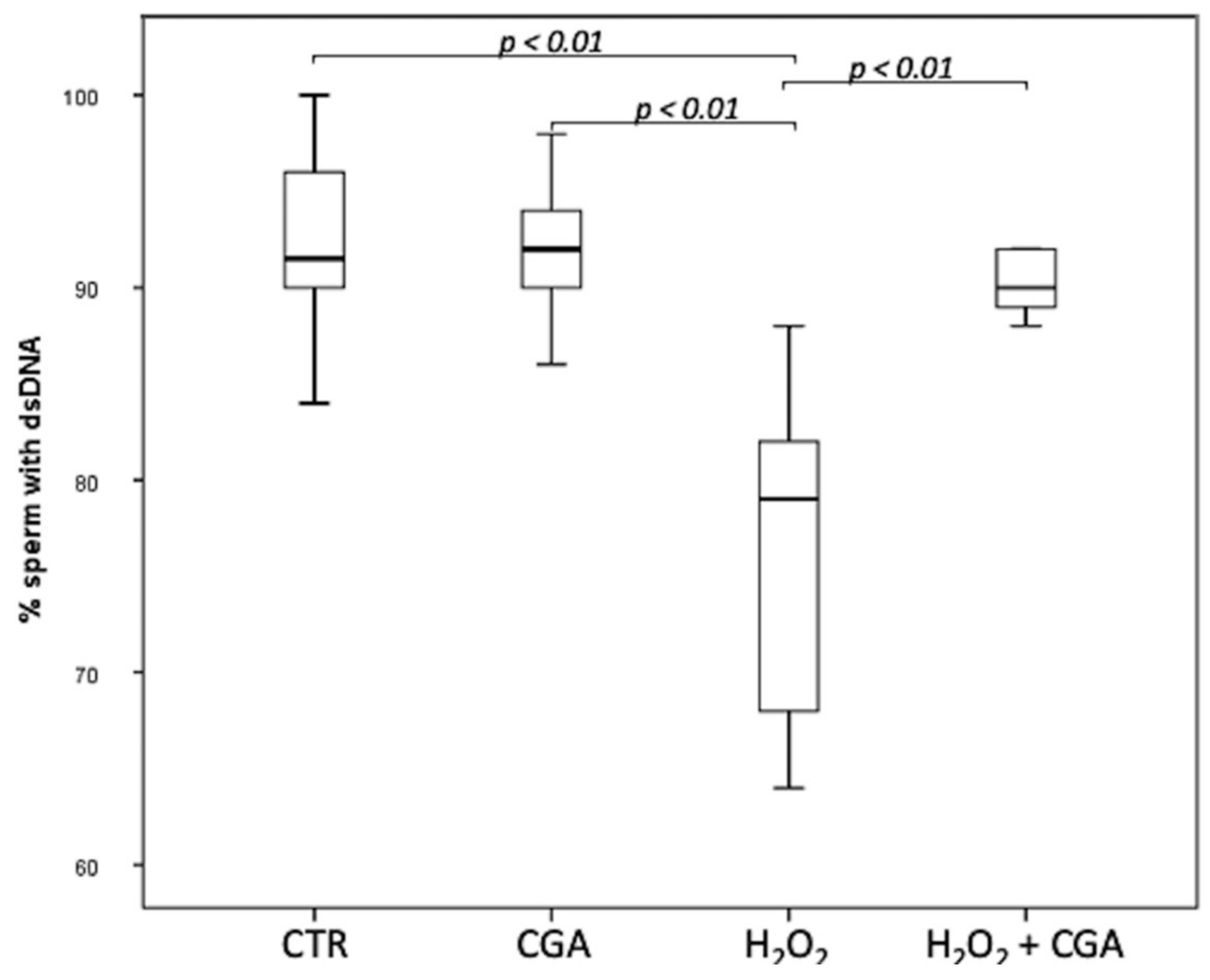
3.2. Effect of Chlorogenic Acid on Sperm DNA Integrity
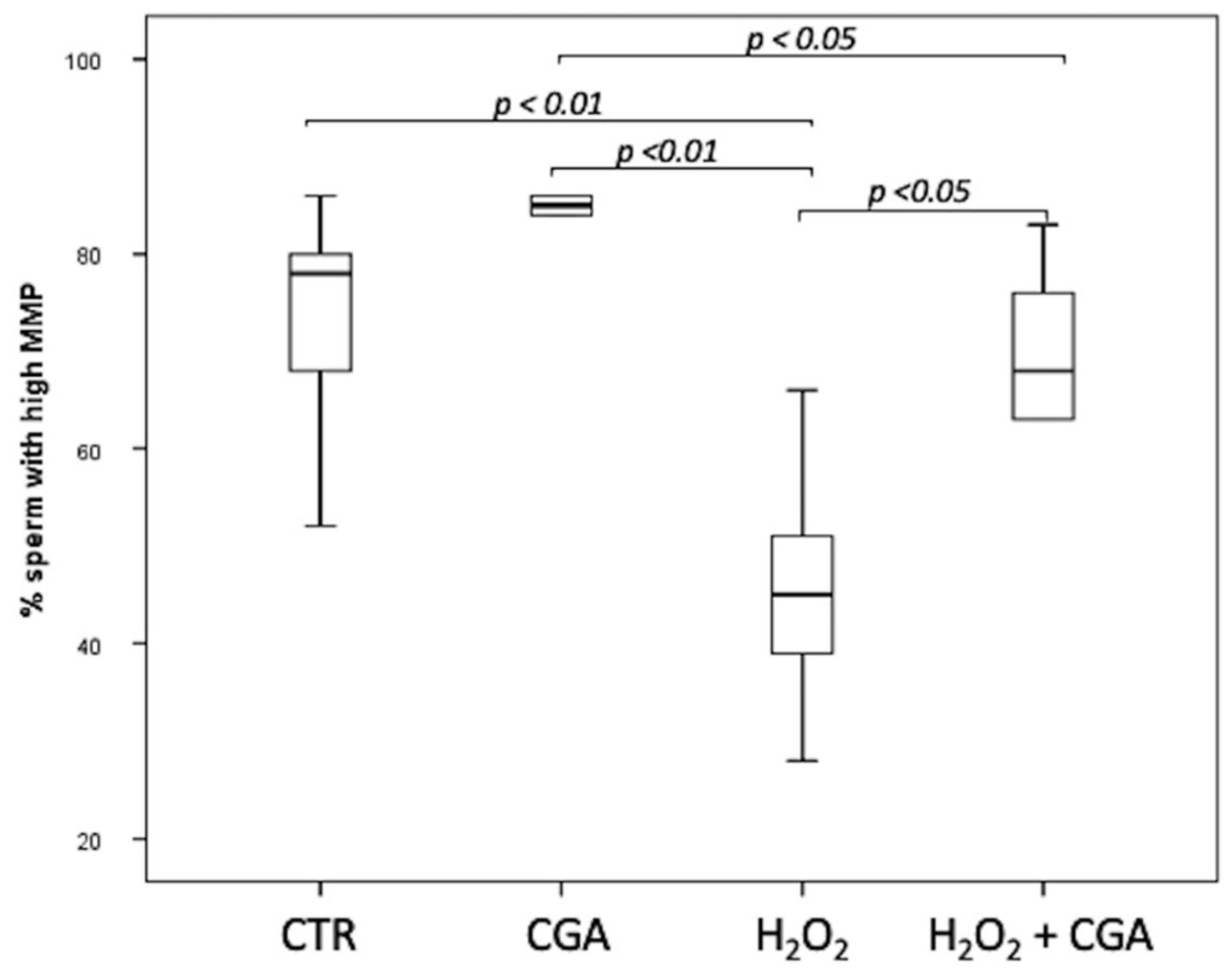
3.3. Effect of Chlorogenic Acid on Mitochondrial Membrane Potential (MMP)
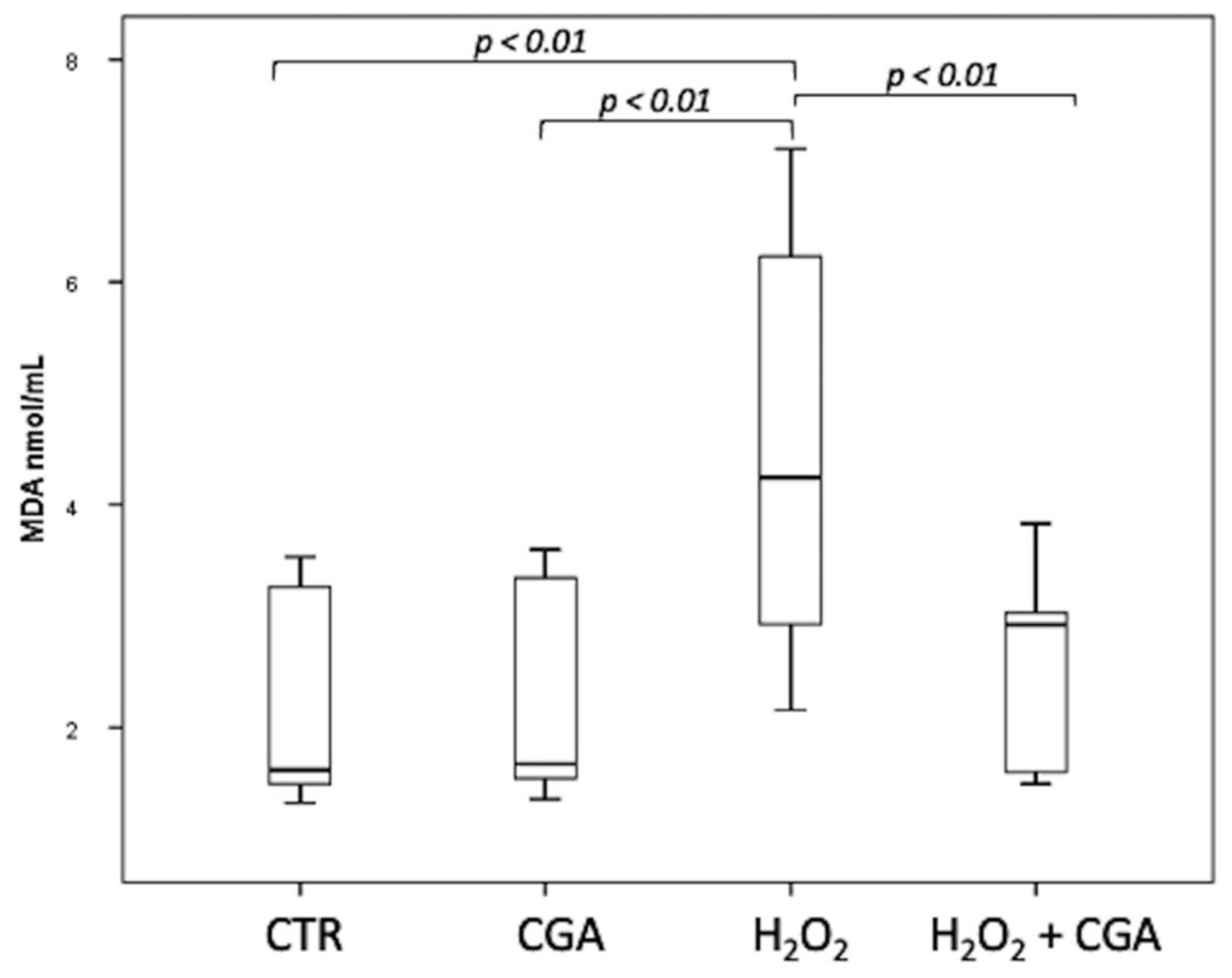
3.4. Effect of Chlorogenic Acid on Induced LPO in Sperm Samples: MDA Evaluation
3.5. F2-Isoprostane Determination
3.6. Effect of Chlorogenic Acid on Induced LPO in Sperm Samples: Ultramorphological Evaluation
3.7. Cryopreservation Experiments
3.8. Immunocytochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lampiao, F.; Strijdom, H.; du Plessis, S.S. Effects of sperm processing techniques involving centrifugation on nitric oxide, reactive oxygen species generation and sperm function. Open Androl. J. 2010, 2, 1–5. [Google Scholar] [CrossRef]
- Shahar, S.; Wiser, A.; Ickowicz, D.; Lubart, R.; Shulman, A.; Breitbart, H. Light-mediated activation reveals a key role for protein kinase A and sarcoma protein kinase in the development of sperm hyper-activated motility. Hum. Reprod. 2011, 26, 2274–2282. [Google Scholar] [CrossRef] [PubMed]
- Will, M.A.; Clark, N.A.; Swain, J.E. Biological pH buffers in IVF: Help or hindrance to success. J. Assist. Reprod. Genet. 2011, 28, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Said, T.M.; Bedaiwy, M.A.; Banerjee, J.; Alvarez, J.G. Oxidative stress in assisted reproductive techniques setting. Fertil. Steril. 2006, 86, 503–512. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhang, H.; Liu, B.; Wang, G.; Cao, M.; Fu, B.; Li, H.; Jiang, Q.; Yu, L.; et al. Importance and safety of autologous sperm cryopreservation for fertility preservation in young male patients with cancer. Medicine 2020, 99, e19589. [Google Scholar] [CrossRef]
- Ezzati, M.; Shanehbandi, D.; Hamdi, K.; Rahbar, S.; Pashaiasl, M. Influence of cryopreservation on structure and function of mammalian spermatozoa: An overview. Cell Tissue Bank 2020, 21, 1–15. [Google Scholar] [CrossRef]
- Valcarce, D.G.; Cartón-García, F.; Riesco, M.F.; Herráez, M.P.; Robles, V. Analysis of DNA damage after human sperm cryopreservation in genes crucial for fertilization and early embryo development. Andrology 2013, 1, 723–730. [Google Scholar] [CrossRef]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef]
- Zini, A.; Al-Hathal, N. Antioxidant therapy in male infertility: Fact or fiction? Asian J. Androl. 2011, 13, 374–381. [Google Scholar] [CrossRef]
- Aitken, R.J.; Smith, T.B.; Lord, T.; Kuczera, L.; Koppers, A.J.; Naumovski, N.; Connaughton, H.; Baker, M.A.; De Iuliis, G.N. On methods for the detection of reactive oxygen species generation by human spermatozoa: Analysis of the cellular responses to catechol oestrogen, lipid aldehyde, menadione and arachidonic acid. Andrology 2013, 1, 192–205. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Collodel, G.; Moretti, E.; Micheli, L.; Menchiari, A.; Moltoni, L.; Cerretani, D. Semen characteristics and malondialdehyde levels in men with different reproductive problems. Andrology 2015, 3, 280–286. [Google Scholar] [CrossRef]
- Signorini, C.; Moretti, E.; Collodel, G. Role of isoprostanes in human male infertility. Syst. Biol. Reprod. Med. 2020, 66, 291–299. [Google Scholar] [CrossRef]
- Collodel, G.; Moretti, E.; Noto, D.; Iacoponi, F.; Signorini, C. Fatty Acid Profile and Metabolism Are Related to Human Sperm Parameters and Are Relevant in Idiopathic Infertility and Varicocele. Mediat. Inflamm. 2020, 2020, 3640450. [Google Scholar] [CrossRef]
- Agarwal, A.; Durairajanayagam, D.; du Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence based review. Reprod. Biol. Endocrinol. 2014, 12, 112. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A. Role of Antioxidants in Assisted Reproductive Techniques. World J. Mens. Health 2017, 35, 77–93. [Google Scholar] [CrossRef]
- Garcez, M.E.; dos Santos Branco, C.; Lara, L.V.; Pasqualotto, F.F.; Salvador, M. Effects of resveratrol supplementation on cryopreservation medium of human semen. Fertil. Steril. 2010, 94, 2118–2121. [Google Scholar] [CrossRef]
- Collodel, G.; Federico, M.G.; Geminiani, M.; Martini, S.; Bonechi, C.; Rossi, C.; Figura, N.; Moretti, E. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod. Toxicol. 2011, 31, 239–246. [Google Scholar] [CrossRef]
- Moretti, E.; Mazzi, L.; Terzuoli, G.; Bonechi, C.; Iacoponi, F.; Martini, S.; Rossi, C.; Collodel, G. Effect of quercetin, rutin, naringenin and epicatechin on lipid peroxidation induced in human sperm. Reprod. Toxicol. 2012, 34, 651–657. [Google Scholar] [CrossRef]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The Role of Resveratrol in Mammalian Reproduction. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef]
- Hu, J.H.; Tian, W.Q.; Zhao, X.L.; Zan, L.S.; Wang, H.; Li, Q.W.; Xin, Y.P. The cryoprotective effects of ascorbic acid supplementation on bovine semen quality. Anim. Reprod. Sci. 2010, 121, 72–77. [Google Scholar] [CrossRef]
- Ben Abdallah, F.; Zribi, N.; Ammar-Keskes, L. Antioxidative potential of Quercetin against hydrogen peroxide induced oxidative stress in spermatozoa in vitro. Andrologia 2011, 43, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Malo, C.; Gil, L.; Cano, R.; Martínez, F.; Galé, I. Antioxidant effect of rosemary (Rosmarinus officinalis) on boar epididymal spermatozoa during cryopreservation. Theriogenology 2011, 75, 1735–1741. [Google Scholar] [CrossRef]
- Zhu, Z.; Fan, X.; Lv, Y.; Zhang, N.; Fan, C.; Zhang, P.; Zeng, W. Vitamin E Analogue Improves Rabbit Sperm Quality during the Process of Cryopreservation through Its Antioxidative Action. PLoS ONE 2015, 10, e0145383. [Google Scholar] [CrossRef]
- Longobardi, V.; Zullo, G.; Salzano, A.; De Canditiis, C.; Cammarano, A.; De Luise, L.; Puzio, M.V.; Neglia, G.; Gasparrini, B. Resveratrol prevents capacitation-like changes and improves in vitro fertilizing capability of buffalo frozen-thawed sperm. Theriogenology 2017, 88, 1–8. [Google Scholar] [CrossRef]
- Merati, Z.; Farshad, A. Ginger and echinacea extracts improve the quality and fertility potential of frozen-thawed ram epididymal spermatozoa. Cryobiology 2020, 92, 138–145. [Google Scholar] [CrossRef]
- Alamo, A.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Giacone, F.; Calabrese, V.; La Vignera, S.; Calogero, A.E. Environment and Male Fertility: Effects of Benzo-α-Pyrene and Resveratrol on Human Sperm Function In Vitro. J. Clin. Med. 2019, 8, 561. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic Acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Alternat. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Namula, Z.; Hirata, M.; Wittayarat, M.; Tanihara, F.; Thi Nguyen, N.; Hirano, T.; Nii, M.; Otoi, T. Effects of chlorogenic acid and caffeic acid on the quality of frozen-thawed boar sperm. Reprod. Domest. Anim. 2018, 53, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.A.; Chaves, B.R.; Teles, M.C.; Pontelo, T.P.; Oliveira, C.R.; de Souza, R.V.; Rodríguez-Gil, J.E.; Zangeronimo, M.G. Chlorogenic acid improves the quality of boar semen subjected to cooled storage at 15 °C. Andrologia 2018, 50, e12978. [Google Scholar] [CrossRef]
- Rabelo, S.S.; Resende, C.O.; Pontelo, T.P.; Chaves, B.R.; Pereira, B.A.; da Silva, W.E.; Peixoto, J.V.; Pereira, L.J.; Zangeronimo, M.G. Chlorogenic acid improves the quality of boar semen processed in Percoll. Anim. Reprod. 2020, 17, e20190021. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Tanihara, F.; Do, L.; Sato, Y.; Taniguchi, M.; Takagi, M.; Van Nguyen, T.; Otoi, T. Chlorogenic acid supplementation during in vitro maturation improves maturation, fertilization and developmental competence of porcine oocytes. Reprod. Domest. Anim. 2017, 52, 969–975. [Google Scholar] [CrossRef]
- Martin-Hidalgo, D.; Hurtado de Llera, A.; Calle-Guisado, V.; Gonzalez-Fernandez, L.; Garcia-Marin, L.; Bragado, M.J. AMPK Function in Mammalian Spermatozoa. Int. J. Mol. Sci. 2018, 19, 3293. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Moretti, E.; Mazzi, L.; Bonechi, C.; Salvatici, M.C.; Iacoponi, F.; Rossi, C.; Collodel, G. Effect of Quercetin-loaded liposomes on induced oxidative stress in human spermatozoa. Reprod. Toxicol. 2016, 60, 140–147. [Google Scholar] [CrossRef]
- Shara, M.A.; Dickson, P.H.; Bagchi, D.; Stohs, S.J. Excretion of formaldehyde, malondialdehyde, acetaldehyde and acetone in the urine of rats in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin, paraquat, endrin and carbon tetrachloride. J. Chromatogr. 1992, 576, 221–233. [Google Scholar] [CrossRef]
- Signorini, C.; Comporti, M.; Giorgi, G. Ion trap tandem mass spectrometric determination of F2-isoprostanes. J. Mass Spectrom. 2003, 38, 1067–1074. [Google Scholar] [CrossRef]
- Stefanello, N.; Spanevello, R.M.; Passamonti, S.; Porciúncula, L.; Bonan, C.D.; Olabiyi, A.A.; Teixeira da Rocha, J.B.; Assmann, C.E.; Morsch, V.M.; Schetinger, M.R.C. Coffee, caffeine, chlorogenic acid, and the purinergic system. Food Chem. Toxicol. 2019, 123, 298–313. [Google Scholar] [CrossRef]
- Gorodeski, G.I. Purinergic Signalling in the Reproductive System. Auton. Neurosci. 2015, 191, 82–101. [Google Scholar] [CrossRef]
- Kedechi, S.; Zribi, N.; Louati, N.; Menif, H.; Sellami, A.; Lassoued, S.; Ben Mansour, R.; Keskes, L.; Rebai, T.; Chakroun, N. Antioxidant effect of hydroxytyrosol on human sperm quality during in vitro incubation. Andrologia 2017, 49. [Google Scholar] [CrossRef]
- Tvrdá, E.; Tušimová, E.; Kováčik, A.; Paál, D.; Greifová, H.; Abdramanov, A.; Lukáč, N. Curcumin has protective and antioxidant properties on bull spermatozoa subjected to induced oxidative stress. Anim. Reprod. Sci. 2016, 172, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Sotler, R.; Poljšak, B.; Dahmane, R.; Jukić, T.; Pavan Jukić, D.; Rotim, C.; Trebše, P.; Starc, A. Prooxidant activities of antioxidants and their impact on health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Do, L.T.K.; Somfai, T.; Otoi, T.; Taniguchi, M.; Kikuchi, K. Presence of chlorogenic acid during in vitro maturation protects porcine oocytes from the negative effects of heat stress. Anim. Sci. J. 2019, 90, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.S. Organization of sperm DNA by the nuclear matrix. Am. J. Clin. Exp. Urol. 2018, 6, 87–92. [Google Scholar]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef]
- Thomson, L.K.; Fleming, S.D.; Aitken, R.J.; De Iuliis, G.N.; Zieschang, J.A.; Clark, A.M. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum. Reprod. 2009, 24, 2061–2070. [Google Scholar] [CrossRef]
- Nouri, H.; Shojaeian, K.; Samadian, F.; Lee, S.; Kohram, H.; Lee, J. Using Resveratrol and Epigallocatechin-3-Gallate to Improve Cryopreservation of Stallion Spermatozoa with Low Quality. J. Equine Vet. Sci. 2018, 70, 18–25. [Google Scholar] [CrossRef]
- Andersen, A.H.; Thinnesen, M.; Failing, K.; Goericke-Pesch, S. Effect of reduced glutathione (GSH) supplementation to Tris-egg yolk extender on chilled semen variables of dogs. Anim. Reprod. Sci. 2018, 198, 145–153. [Google Scholar] [CrossRef]
- Sun, L.; Fan, X.; Zeng, Y.; Wang, L.; Zhu, Z.; Li, R.; Tian, X.; Wang, Y.; Lin, Y.; Wu, D.; et al. Resveratrol protects boar sperm in vitro via its antioxidant capacity. Zygote 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Mohammadi, F.; Varanloo, N.; Heydari Nasrabadi, M.; Vatannejad, A.; Amjadi, F.S.; Javedani Masroor, M.; Bajelan, L.; Mehdizadeh, M.; Aflatoonian, R.; Zandieh, Z. Supplementation of sperm freezing medium with myoinositol improve human sperm parameters and protects it against DNA fragmentation and apoptosis. Cell Tissue Bank 2019, 20, 77–86. [Google Scholar] [CrossRef]
- Pariz, J.R.; Ranéa, C.; Monteiro, R.A.C.; Evenson, D.P.; Drevet, J.R.; Hallak, J. Melatonin and Caffeine Supplementation Used, Respectively, as Protective and Stimulating Agents in the Cryopreservation of Human Sperm Improves Survival, Viability, and Motility after Thawing compared to Traditional TEST-Yolk Buffer. Oxid. Med. Cell. Longev. 2019, 2019, 6472945. [Google Scholar] [CrossRef]
- Abdolsamadi, M.; Mohammadi, F.; Nashtaei, M.S.; Teimouri, M.; Sardar, R.; Dayani, M.; Haghighi, M.; Ghasemi, S.; Vatannejad, A.; Zandieh, Z. Does myoinositol supplement improve sperm parameters and DNA integrity in patients with oligoasthenoteratozoospermia after the freezing-thawing process? Cell Tissue Bank 2020, 21, 99–106. [Google Scholar] [CrossRef]
- Nijs, M.; Creemers, E.; Cox, A.; Janssen, M.; Vanheusden, E.; Castro-Sanchez, Y.; Thijs, H.; Ombelet, W. Influence of freeze-thawing on hyaluronic acid binding of human spermatozoa. Reprod. Biomed. Online 2009, 19, 202–206. [Google Scholar] [CrossRef]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Rodriguez-Gil, J.E.; Gil, M.C.; Garcia-Marin, L.J.; Bragado, M.J. AMP-activated kinase, AMPK, is involved in the maintenance of plasma membrane organization in boar spermatozoa. Biochim. Biophys. Acta 2013, 1828, 2143–2151. [Google Scholar] [CrossRef]
- Ke, R.; Xu, Q.; Li, C.; Luo, L.; Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 2018, 42, 384–392. [Google Scholar] [CrossRef]
- Lin, L.; Huang, S.; Zhu, Z.; Han, J.; Wang, Z.; Huang, W.; Huang, Z. P2X7 receptor regulates EMMPRIN and MMP-9 expression through AMPK/MAPK signaling in PMA-induced macrophages. Mol. Med. Rep. 2018, 18, 3027–3033. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Ma, G.; Bai, W.; Fan, X.; Lv, Y.; Luo, J.; Zeng, W. 5′-AMP-Activated Protein Kinase Regulates Goat Sperm Functions via Energy Metabolism In Vitro. Cell. Physiol. Biochem. 2018, 47, 2420–2431. [Google Scholar] [CrossRef]
- Thuwanut, P.; Comizzoli, P.; Pruksananonda, K.; Chatdarong, K.; Songsasen, N. Activation of adenosine monophosphate-activated protein kinase (AMPK) enhances energy metabolism, motility, and fertilizing ability of cryopreserved spermatozoa in domestic cat model. J. Assist. Reprod. Genet. 2019, 36, 1401–1412. [Google Scholar] [CrossRef]
- Feng, T.Y.; Lv, D.L.; Zhang, X.; Du, Y.Q.; Yuan, Y.T.; Chen, M.J.; Xi, H.M.; Li, Y.; Han, N.; Hu, J.H. Rosmarinic acid improves boar sperm quality, antioxidant capacity and energy metabolism at 17 °C via AMPK activation. Reprod. Domest. Anim. 2020, 55, 1714–1724. [Google Scholar] [CrossRef]

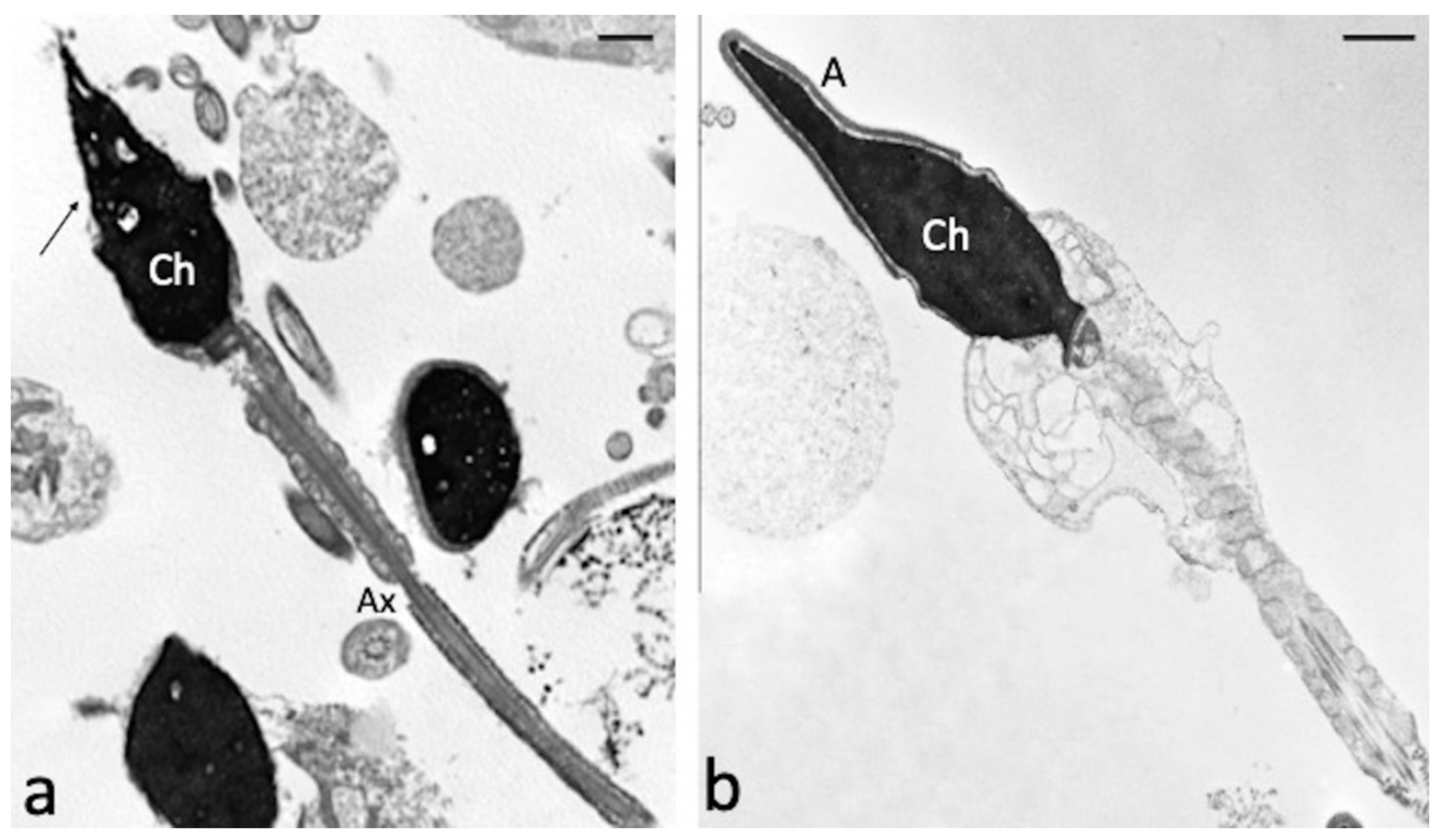
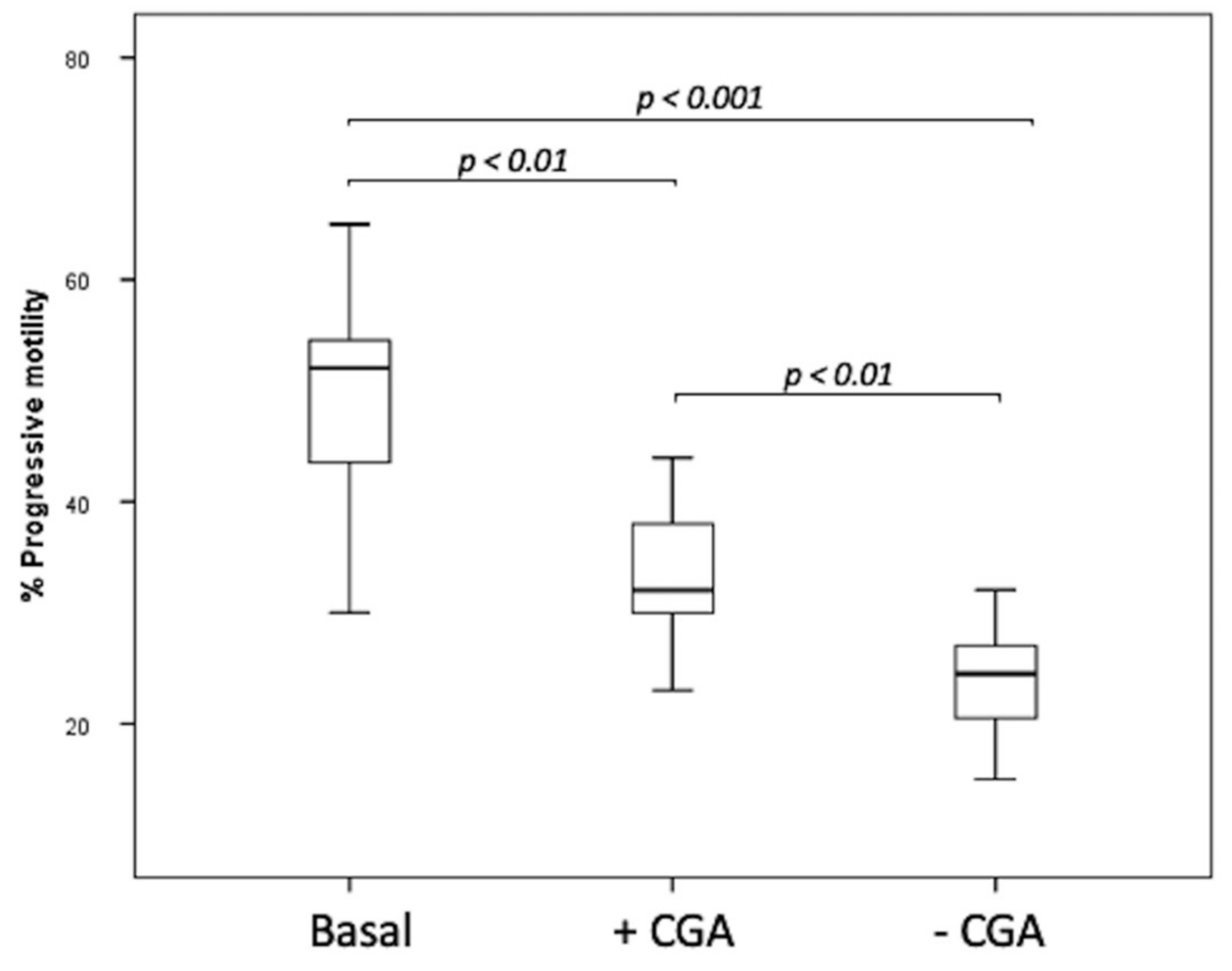
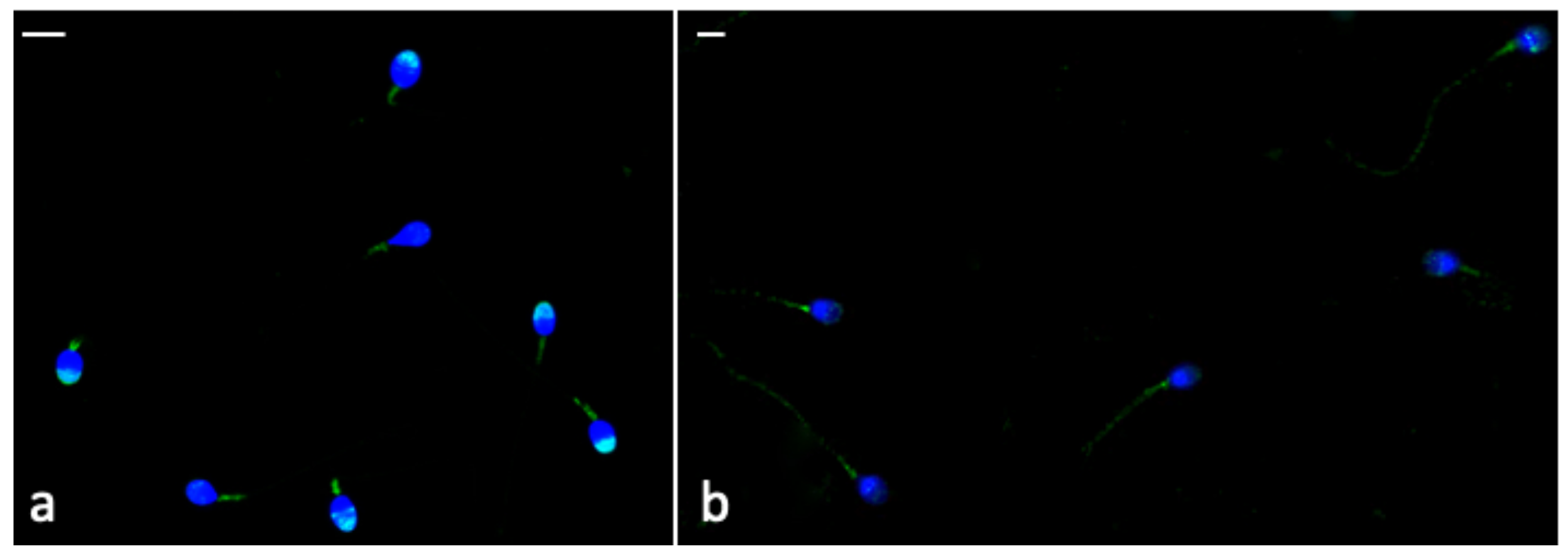
| Parameter | CTR | CGA 50 µM | CGA 100 µM | CGA 200 µM | CGA 500 µM |
|---|---|---|---|---|---|
| Progressive sperm motility % | 53.5 (49.5–65.0) | 55.0 (51.50–65.75) | 72.0 * (59.0–79.25) | 67.5 (56.25–77.75) | 59.5 (55.5–71.0) |
| % Altered Sperm Structures | H2O2 | H2O2 + CGA |
|---|---|---|
| Altered acrosome | 87.0 (76.75–92.50) | 29.0 (25.75–33.75) * |
| Altered chromatin | 44.5 (42.50–54.00) | 41.5 (39.50–45.25) |
| Altered axoneme | 50.0 (48.25–52.00) | 33.5 (30.00–45.75) * |
| Broken plasma membrane | 84.0 (79.50–87.25) | 30.0 (21.50–32.75) * |
| Frozen Samples | MDA Levels nmol/mL | Sperm with High MMP % | Sperm with dsDNA % |
|---|---|---|---|
| +CGA | 1.93 * (1.063–2.613) | 32.5 * (30.00–38.75) | 97.0 * (93.50–98.75) |
| −CGA | 3.238 (2.755–4.878) | 24.5 * (20.25–27.00) | 60.5 * (57.25–63.00) |
| Phospho-AMPKα Labelling | % Sperm Head | % Sperm Tail |
|---|---|---|
| +CGA | 56.00 * (49.50–60.50) | 16.00 * (12.75–20.50) |
| −CGA | 26.00 (23.50–28.25) | 55.50 (49.50–61.25) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noto, D.; Collodel, G.; Cerretani, D.; Signorini, C.; Gambera, L.; Menchiari, A.; Moretti, E. Protective Effect of Chlorogenic Acid on Human Sperm: In Vitro Studies and Frozen–Thawed Protocol. Antioxidants 2021, 10, 744. https://doi.org/10.3390/antiox10050744
Noto D, Collodel G, Cerretani D, Signorini C, Gambera L, Menchiari A, Moretti E. Protective Effect of Chlorogenic Acid on Human Sperm: In Vitro Studies and Frozen–Thawed Protocol. Antioxidants. 2021; 10(5):744. https://doi.org/10.3390/antiox10050744
Chicago/Turabian StyleNoto, Daria, Giulia Collodel, Daniela Cerretani, Cinzia Signorini, Laura Gambera, Andrea Menchiari, and Elena Moretti. 2021. "Protective Effect of Chlorogenic Acid on Human Sperm: In Vitro Studies and Frozen–Thawed Protocol" Antioxidants 10, no. 5: 744. https://doi.org/10.3390/antiox10050744
APA StyleNoto, D., Collodel, G., Cerretani, D., Signorini, C., Gambera, L., Menchiari, A., & Moretti, E. (2021). Protective Effect of Chlorogenic Acid on Human Sperm: In Vitro Studies and Frozen–Thawed Protocol. Antioxidants, 10(5), 744. https://doi.org/10.3390/antiox10050744