Salvadora persica: Nature’s Gift for Periodontal Health
Abstract
1. Introduction
2. Periodontitis and Periodontal Treatment
2.1. Periodontitis as a Worldwide Health Burden
2.2. Strategies for Periodontal Therapy
2.2.1. Mechanical Therapy
2.2.2. Chemotherapeutic Periodontal Therapy
Host Modulation Therapy (HMT)
Antimicrobial Therapy
Herbal Agents for Periodontal Therapy
3. Salvadora persica as a Therapeutic Agent
3.1. Historical and Cultural Importance of Salvadora persica
3.2. Plant Description and Classification
3.3. Chemical Composition of Salvadora persica
3.4. Toxicological Profile of Salvadora persica
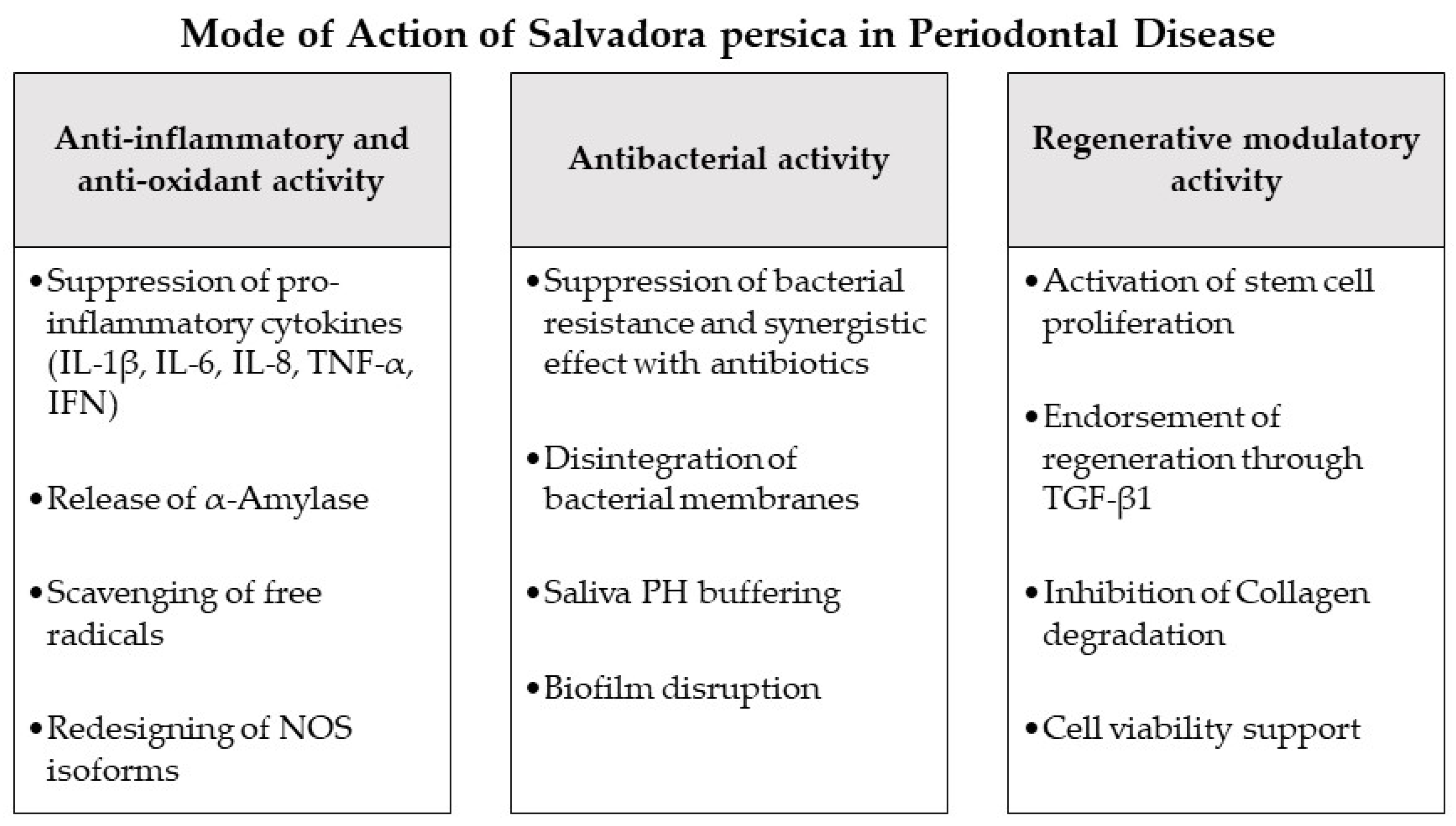
4. Modes of Action of Salvadora persica as Potential Adjuncts during Periodontal Therapy and in Periodontitis-Associated Settings
4.1. Antioxidant and Anti-Inflammatory Effects
4.2. Antibacterial Effects
4.3. Regenerative and Stem Cell Modulation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Attass, S.A.; Zahran, F.M.; Turkistany, S.A. Nigella sativa and its active constituent thymoquinone in oral health. Saudi Med. J. 2016, 37, 235–244. [Google Scholar] [CrossRef] [PubMed]
- WHO. Traditional Medicine Strategy, 2014–2023; World Health Organization: Geneva, Switzerland, 2013; p. 76. [Google Scholar]
- Sabbagh, H.J.; AlGhamdi, K.S.; Mujalled, H.T.; Bagher, S.M. The effect of brushing with Salvadora persica (miswak) sticks on salivary Streptococcus mutans and plaque levels in children: A clinical trial. BMC Complement. Med. Ther. 2020, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Khatak, M.; Khatak, S.; Siddqui, A.A.; Vasudeva, N.; Aggarwal, A.; Aggarwal, P. Salvadora persica. Pharmacogn. Rev. 2010, 4, 209–214. [Google Scholar] [CrossRef]
- Mansour, H.; Alsamadany, H.; Al-Hasawi, Z.M. Genetic diversity and genetic structure of Salvadora persica L., rare plant species in Rabigh province, Saudi Arabia: Implications for conservation. J. Taibah Univ. Sci. 2020, 14, 881–888. [Google Scholar] [CrossRef]
- Abhary, M.; Al-Hazmi, A.-A. Antibacterial activity of Miswak (Salvadora persica L.) extracts on oral hygiene. J. Taibah Univ. Sci. 2016, 10, 513–520. [Google Scholar] [CrossRef]
- Haque, M.M.; Alsareii, S.A. A review of the therapeutic effects of using miswak (Salvadora persica) on oral health. Saudi Med. J. 2015, 36, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Al Bratty, M.; Makeen, H.A.; Alhazmi, H.A.; Syame, S.M.; Abdalla, A.N.; Homeida, H.E.; Sultana, S.; Ahsan, W.; Khalid, A. Phytochemical, Cytotoxic, and Antimicrobial Evaluation of the Fruits of Miswak Plant. J. Chem. 2020, 2020, 4521951. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Khan, J.A. Antioxidant capacity of chewing stick miswak Salvadora persica. BMC Complement. Altern. Med. 2013, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Eid Abdelmagyd, H.A.; Ram Shetty, D.S.; Musa Musleh Al-Ahmari, D.M. Herbal medicine as adjunct in periodontal therapies—A review of clinical trials in past decade. J. Oral Biol. Craniofac. Res. 2019, 9, 212–217. [Google Scholar] [CrossRef]
- Gasner, N.S.; Schure, R.S. Periodontal Disease; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Fischer, R.G.; Lira Junior, R.; Retamal-Valdes, B.; Figueiredo, L.C.D.; Malheiros, Z.; Stewart, B.; Feres, M. Periodontal disease and its impact on general health in Latin America. Section V: Treatment of periodontitis. Braz. Oral Res. 2020, 34. [Google Scholar] [CrossRef]
- Herrera, D.; Matesanz, P.; Martín, C.; Oud, V.; Feres, M.; Teughels, W. Adjunctive effect of locally delivered antimicrobials in periodontitis therapy: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Heta, S.; Robo, I. The Side Effects of the Most Commonly Used Group of Antibiotics in Periodontal Treatments. Med. Sci. 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hu, J.; Zhao, L. Adjunctive subgingival application of Chlorhexidine gel in nonsurgical periodontal treatment for chronic periodontitis: A systematic review and meta-analysis. BMC Oral Health 2020, 20, 34. [Google Scholar] [CrossRef]
- De Moraes Mello Boccolini, P.; Siqueira Boccolini, C. Prevalence of complementary and alternative medicine (CAM) use in Brazil. BMC Complement. Med. Ther. 2020, 20, 51. [Google Scholar] [CrossRef]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (Black Cumin): A Promising Natural Remedy for Wide Range of Illnesses. Evid. Based Complement. Alternat. Med. 2019, 2019, 1528635. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Billings, M.; Holtfreter, B.; Papapanou, P.N.; Mitnik, G.L.; Kocher, T.; Dye, B.A. Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S130–S148. [Google Scholar] [CrossRef] [PubMed]
- James, S. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Sanz, M.; Marco del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef]
- Komine-Aizawa, S.; Aizawa, S.; Hayakawa, S. Periodontal diseases and adverse pregnancy outcomes. J. Obstet. Gynaecol. Res. 2019, 45, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Righolt, A.J.; Jevdjevic, M.; Marcenes, W.; Listl, S. Global-, Regional-, and Country-Level Economic Impacts of Dental Diseases in 2015. J. Dent. Res. 2018, 97, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Iqbal, Z.; Ali, J.; Baboota, S.; Talegaonkar, S.; Ahmad, Z.; Sahni, J.K. Treatment modalities and evaluation models for periodontitis. Int. J. Pharm. Investig. 2012, 2, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontology 2017, 75, 152–188. [Google Scholar] [CrossRef]
- Patil, V.; Mali, R.; Mali, A. Systemic anti-microbial agents used in periodontal therapy. J. Indian Soc. Periodontol. 2013, 17, 162–168. [Google Scholar] [CrossRef]
- Puri, K.; Puri, N. Local drug delivery agents as adjuncts to endodontic and periodontal therapy. J. Med. Life 2013, 6, 414–419. [Google Scholar]
- Mekhemar, M.; Hassan, Y.; Dörfer, C. Nigella sativa and Thymoquinone: A Natural Blessing for Periodontal Therapy. Antioxidants 2020, 9, 1260. [Google Scholar] [CrossRef]
- Chiniforush, N.; Pourhajibagher, M.; Parker, S.; Benedicenti, S.; Bahador, A.; Sălăgean, T.; Bordea, I.R. The Effect of Antimicrobial Photodynamic Therapy Using Chlorophyllin–Phycocyanin Mixture on Enterococcus faecalis: The Influence of Different Light Sources. Appl. Sci. 2020, 10, 4290. [Google Scholar] [CrossRef]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernández, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Anand, V.; Govila, V.; Jain, N. Host modulation therapy: An indispensable part of perioceutics. J. Indian Soc. Periodontol. 2014, 18, 282–288. [Google Scholar] [CrossRef]
- Spasovski, S.; Belazelkoska, Z.; Popovska, M.; Atanasovska-Stojanovska, A.; Radojkova-Nikolovska, V.; Muratovska, I.; Toseska-Spasova, N.; Dzipunova, B.; Nikolovski, B. Clinical Therapeutic Effects of the Application of Doxycycline in the Treatment of Periodontal Disease. Open Access Maced. J. Med. Sci. 2016, 4, 152–157. [Google Scholar] [CrossRef]
- Polak, D.; Martin, C.; Sanz-Sánchez, I.; Beyth, N.; Shapira, L. Are anti-inflammatory agents effective in treating gingivitis as solo or adjunct therapies? A systematic review. J. Clin. Periodontol. 2015, 42, S139–S151. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.; Byakod, G.; Pudakalkatti, P. Growth Factors in Periodontal Regeneration. J. Adv. Oral Res. 2014, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Seshima, F.; Aoki, H.; Takeuchi, T.; Suzuki, E.; Irokawa, D.; Makino-Oi, A.; Sugito, H.; Tomita, S.; Saito, A. Periodontal regenerative therapy with enamel matrix derivative in the treatment of intrabony defects: A prospective 2-year study. BMC Res. Notes 2017, 10, 256. [Google Scholar] [CrossRef]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 2018, 76, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijden, G.A. Use of antimicrobial agents in periodontology. Ned. Tijdschr. Tandheelkd. 2019, 126, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Souto, M.L.S.; Rovai, E.S.; Ganhito, J.A.; Holzhausen, M.; Chambrone, L.; Pannuti, C.M. Efficacy of systemic antibiotics in nonsurgical periodontal therapy for diabetic subjects: A systematic review and meta-analysis. Int. Dent. J. 2018, 68, 207–220. [Google Scholar] [CrossRef]
- Feres, M.; Figueiredo, L.C.; Soares, G.M.; Faveri, M. Systemic antibiotics in the treatment of periodontitis. Periodontol. 2015, 67, 131–186. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.M.; Torres, T.C.; Pereira, S.L.; Mota, O.M.; Carlos, M.X. Effect of a dentifrice containing Aloe vera on plaque and gingivitis control. A double-blind clinical study in humans. J. Appl. Oral Sci. 2008, 16, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Solderer, A.; Kaufmann, M.; Hofer, D.; Wiedemeier, D.; Attin, T.; Schmidlin, P.R. Efficacy of chlorhexidine rinses after periodontal or implant surgery: A systematic review. Clin. Oral Investig. 2019, 23, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Amjed, S.; Junaid, K.; Jafar, J.; Amjad, T.; Maqsood, W.; Mukhtar, N.; Tariq, K.; Sharif, M.; Awan, S.J.; Ansari, F. Detection of antibacterial activities of Miswak, Kalonji and Aloe vera against oral pathogens & anti-proliferative activity against cancer cell line. BMC Complement. Altern. Med. 2017, 17, 265. [Google Scholar] [CrossRef]
- Nordin, A.; Bin Saim, A.; Ramli, R.; Abdul Hamid, A.; Mohd Nasri, N.W.; Bt Hj Idrus, R. Miswak and oral health: An evidence-based review. Saudi J. Biol. Sci. 2020, 27, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Aumeeruddy, M.Z.; Zengin, G.; Mahomoodally, M.F. A review of the traditional and modern uses of Salvadora persica L. (Miswak): Toothbrush tree of Prophet Muhammad. J. Ethnopharmacol. 2018, 213, 409–444. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sharma, P.K. Traditional Use, Phytochemicals and Pharmacological Activity of Salvadora persica: A Review. Curr. Nutr. Food Sci. 2021, 17, 302–309. [Google Scholar] [CrossRef]
- Hyson, J.M., Jr. History of the toothbrush. J. Hist. Dent. 2003, 51, 73–80. [Google Scholar] [PubMed]
- Aboul-Enein, B.H. The miswak (Salvadora persica L.) chewing stick: Cultural implications in oral health promotion. Saudi J. Dental. Res. 2014, 5, 9–13. [Google Scholar] [CrossRef]
- Faruk, E.M.; Nafea, O.E.; Fouad, H.; Ebrahim, U.F.A.; Hasan, R.A.A. Possible healing effects of Salvadora persica extract (MISWAK) and laser therapy in a rabbit model of a caustic-induced tongue ulcers: Histological, immunohistochemical and biochemical study. J. Mol. Histol. 2020, 51, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Al-Ayed, M.S.; Asaad, A.M.; Qureshi, M.A.; Attia, H.G.; AlMarrani, A.H. Antibacterial Activity of Salvadora persica L. (Miswak) Extracts against Multidrug Resistant Bacterial Clinical Isolates. Evid. Based Complement. Alternat. Med. 2016, 2016, 7083964. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Shakour, Z.T.; Lübken, T.; Frolov, A.; Wessjohann, L.A.; Mahrous, E. Unraveling the metabolome composition and its implication for Salvadora persica L. use as dental brush via a multiplex approach of NMR and LC–MS metabolomics. J. Pharm. Biomed. Anal. 2021, 193, 113727. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.; Abdel-Mageed, W.M.; Basudan, O.; El-Gamal, A. Persicaline, A New Antioxidant Sulphur-Containing Imidazoline Alkaloid from Salvadora persica Roots. Molecules 2018, 23, 483. [Google Scholar] [CrossRef]
- Akhtar, J.; Siddique, K.M.; Bi, S.; Mujeeb, M. A review on phytochemical and pharmacological investigations of miswak (Salvadora persica Linn). J. Pharm. Bioallied Sci. 2011, 3, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, K.; Kasai, R.; Yamasaki, K.; Tanaka, O.; Kamel, M.S.; Assaf, M.H.; El-Shanawani, M.A.; Ali, A.A. Lignan glycosides from stems of Salvadora persica. Phytochemistry 1992, 31, 2469–2471. [Google Scholar] [CrossRef]
- Alali, F.; Hudaib, M.; Aburjai, T.; Khairallah, K.; Al-Hadidi, N. GC-MS Analysis and Antimicrobial Activity of the Essential Oil from the Stem of the Jordanian Toothbrush Tree Salvadora persica. Pharm. Biol. 2005, 42, 577–580. [Google Scholar] [CrossRef][Green Version]
- Khalil, A.T. Benzylamides fromSalvadora persica. Arch. Pharmacal. Res. 2006, 29, 952. [Google Scholar] [CrossRef]
- Ahmad, H.; Rajagopal, K. Salvadora persica L. (Meswak) in dental hygiene. Saudi J. Dent. Res. 2014, 5, 130–134. [Google Scholar] [CrossRef]
- Abeer, Y.I.; Souad, E.E.-G. Safety Profile of Meswak Root Extract on Liver, Kidney, Sexual Hormones and Hematological Parameters of Rats. Not. Sci. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Darmani, H.; Al-Hiyasat, A.S.; Elbetieha, A.M.; Alkofahi, A. The effect of an extract of Salvadora persica (Meswak, chewing stick) on fertility of male and female mice. Phytomedicine 2003, 10, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, F.S.; Moezizadeh, M.; Javand, F. Effects of extracts of Salvadora persica on proliferation and viability of human dental pulp stem cells. J. Conserv. Dent. 2015, 18, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Balto, H.A.G.; Halawany, H.S.; Jacob, V.; Abraham, N.B. The efficacy of Salvadora persica extracts in preserving the viability of human foreskin fibroblasts. Saudi Dent. J. 2015, 27, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Imran, H.; Yaqeen, Z.; Rehman, Z.; Rahman, A.; Fatima, N.; Sohail, T. Pharmacological profile of Salvadora persica. Pak. J. Pharm. Sci. 2011, 24, 323–330. [Google Scholar] [PubMed]
- Marchesan, J.T.; Girnary, M.S.; Moss, K.; Monaghan, E.T.; Egnatz, G.J.; Jiao, Y.; Zhang, S.; Beck, J.; Swanson, K.V. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontology 2020, 82, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Al Sahli, A.A.A.; Alaraidh, I.A.; Al-Homaidan, A.A.; Mostafa, E.M.; El-Gaaly, G.A. Assessment of antioxidant activities in roots of Miswak (Salvadora persica) plants grown at two different locations in Saudi Arabia. Saudi J. Biol. Sci. 2015, 22, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Lebda, M.A.; El-Far, A.H.; Noreldin, A.E.; Elewa, Y.H.A.; Al Jaouni, S.K.; Mousa, S.A. Protective Effects of Miswak (Salvadora persica) against Experimentally Induced Gastric Ulcers in Rats. Oxid. Med. Cell. Longev. 2018, 2018, 6703296. [Google Scholar] [CrossRef] [PubMed]
- Mariod, A.A.; Matthäus, B.; Hussein, I.H. Chemical Characterization of the Seed and Antioxidant Activity of Various Parts of Salvadora persica. J. Am. Oil Chem. Soc. 2009, 86, 857–865. [Google Scholar] [CrossRef]
- Nomani, M.; Hosseini, M.J.; Vazirian, M.; Nomani, A.; Monsef-Esfahani, H.R. Evaluation of anti-inflammatory effect of Salvadora persica in IBD-induced rat. Res. J. Pharmacogn. 2017, 4, 27. [Google Scholar]
- Al-Quraishy, S.; Thagfan, F.A.; Al-Shaebi, E.M.; Qasem, M.; Abdel-Gaber, R.; Dkhil, M.A.M. Salvadora persica protects mouse intestine from eimeriosis. Rev. Bras. Parasitol. Vet. 2019, 28, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Sardari, F.; Kazemi Arababadi, M.; Heiranizade, M.; Mosadeghi, M. Anti-inflammatory and cytotoxicity effects of Salvadora persica (meswak) extracts on jurkat t-cells. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 379–382. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Almulaiky, Y.Q.; Ahmed, Y.M.; Al-Bar, O.A.M.; Ibrahim, I.H. Purification and characterization of α-Amylase from Miswak Salvadora persica. BMC Complement. Altern. Med. 2014, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, E.; Lloyd, C.; Teixeira, S.R.; Conlan, R.S.; Whitaker, I.S. Clinical applications of amylase: Novel perspectives. Surgery 2016, 160, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Seo, E.-J.; Efferth, T. Effects of anti-inflammatory and adaptogenic herbal extracts on gene expression of eicosanoids signaling pathways in isolated brain cells. Phytomedicine 2019, 60, 152881. [Google Scholar] [CrossRef] [PubMed]
- Abdulbaqi, H.R.; Himratul-Aznita, W.H.; Baharuddin, N.A. Evaluation of Salvadora persica L. and green tea anti-plaque effect: A randomized controlled crossover clinical trial. BMC Complement. Altern. Med. 2016, 16, 493. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.V.; Shruthi, S.; Kumar, S. Clinical effect of miswak as an adjunct to tooth brushing on gingivitis. J. Indian Soc. Periodontol. 2012, 16, 84–88. [Google Scholar] [CrossRef]
- Aspalli, S.; Shetty, V.S.; Devarathnamma, M.V.; Nagappa, G.; Archana, D.; Parab, P. Evaluation of antiplaque and antigingivitis effect of herbal mouthwash in treatment of plaque induced gingivitis: A randomized, clinical trial. J. Indian Soc. Periodontol. 2014, 18, 48–52. [Google Scholar] [CrossRef]
- Bahrololoomi, Z.; Sadat-Hashemi, A.; Hassan-Akhavan-Karbassi, M.; Khaksar, Y. Evaluating the additive effect of Persica and chlorhexidine mouthwashes on oral health status of children receiving chemotherapy for their hematomalignancy: A randomized clinical trial. J. Clin. Exp. Dent. 2020, 12, e574–e580. [Google Scholar] [CrossRef]
- Bhate, D.; Jain, S.; Kale, R.; Muglikar, S. The comparative effects of 0.12% chlorhexidine and herbal oral rinse on dental plaque-induced gingivitis: A randomized clinical trial. J. Indian Soc. Periodontol. 2015, 19, 393–395. [Google Scholar] [CrossRef]
- Gupta, P.; Agarwal, N.; Anup, N.; Manujunath, B.C.; Bhalla, A. Evaluating the anti-plaque efficacy of meswak (Salvadora persica) containing dentifrice: A triple blind controlled trial. J. Pharm. Bioallied Sci. 2012, 4, 282–285. [Google Scholar] [CrossRef]
- Manjiri Abhay Deshmukh, A.S.D. Gundabaktha Karibasappa, Mahesh Ravindra Khairnar, Rahul Gaybarao Naik, Harish Chaitram Jadhav. Comparative Evaluation of the Efficacy of Probiotic, Herbal and Chlorhexidine Mouthwash on Gingival Health: A Randomized Clinical Trial. J. Clin. Diagn. Res. 2017, 11, ZC13–ZC16. [Google Scholar] [CrossRef]
- Prasad, K.A.; John, S.; Deepika, V.; Dwijendra, K.S.; Reddy, B.R.; Chincholi, S. Anti-Plaque Efficacy of Herbal and 0.2% Chlorhexidine Gluconate Mouthwash: A Comparative Study. J. Int. Oral Health 2015, 7, 98–102. [Google Scholar]
- Rezaei, S.; Rezaei, K.; Mahboubi, M.; Jarahzadeh, M.; Momeni, E.; Bagherinasab, M.; Targhi, M.; Memarzadeh, M. Comparison the efficacy of herbal mouthwash with chlorhexidine on gingival index of intubated patients in Intensive Care Unit. J. Indian Soc. Periodontol. 2016, 20, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Mohammad, S.; Saha, S.; Samadi, F. Efficiency of traditional chewing stick (miswak) as an oral hygiene aid among Muslim school children in Lucknow: A cross-sectional study. J. Oral Biol. Craniofac. Res. 2012, 2, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Tadikonda, A.; Pentapati, K.C.; Urala, A.S.; Acharya, S. Anti-plaque and anti-gingivitis effect of Papain, Bromelain, Miswak and Neem containing dentifrice: A randomized controlled trial. J. Clin. Exp. Dent. 2017, 9, e649–e653. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.R.; Sherif, H.; Serafi, A.; Fanas, S.A.; Desai, V.; Abuhijleh, E.; Al Radaidah, A. The Antiplaque Efficacy of Two Herbal-Based Toothpastes: A Clinical Intervention. J. Int. Soc. Prev. Community Dent. 2018, 8, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Albabtain, R.; Azeem, M.; Wondimu, Z.; Lindberg, T.; Borg-Karlson, A.K.; Gustafsson, A. Investigations of a Possible Chemical Effect of Salvadora persica Chewing Sticks. Evid. Based Complement. Alternat. Med. 2017, 2017, 2576548. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Jacob, S.; Suthanthiran, T.; Dhasthaheer, S.; Vikraman, S.; Kaliappan, K. Characterization and formulation of miswak film for the treatment of chronic periodontitis: An in vitro study. J. Pharm. Bioallied Sci. 2020, 12, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Maiorani, C.; Molino, D.; Chiesa, A.; Preda, C.; Esposito, F.; Scribante, A. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Bartold, P.M. Periodontal health. J. Periodontol. 2018, 89, S9–S16. [Google Scholar] [CrossRef]
- Al-sieni, A.I. The antibacterial activity of traditionally used Salvadora persica L. (miswak) and Commiphora gileadensis (palsam) in Saudi Arabia. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 23–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saquib, S.A.; AlQahtani, N.A.; Ahmad, I.; Kader, M.A.; Al Shahrani, S.S.; Asiri, E.A. Evaluation and Comparison of Antibacterial Efficacy of Herbal Extracts in Combination with Antibiotics on Periodontal pathobionts: An in vitro Microbiological Study. Antibiotics 2019, 8, 89. [Google Scholar] [CrossRef]
- Pathan, M.; Bhat, K.; Joshi, V. Comparative evaluation of the efficacy of a herbal mouthwash and chlorhexidine mouthwash on select periodontal pathogens: An in vitro and ex vivo study. J. Indian Soc. Periodontol. 2017, 21, 270–275. [Google Scholar] [CrossRef]
- Salman Siddeeqh, A.P.; Maji, J.; Vidya, P. Estimation of Antimicrobial Properties of Aqueous and Alcoholic Extracts of Salvadora Persica (Miswak) on Oral Microbial Pathogens—An Invitro Study. J. Clin. Diagn. Res. 2016, 10, FC13–FC16. [Google Scholar] [CrossRef] [PubMed]
- Jelvehgaran Esfahani, Z.; Kadkhoda, Z.; Eshraghi, S.S.; Salehi Surmaghi, M.H. Antibacterial effect of an herbal product persica on porphyromonas gingivalis and aggregatibacter actinomycetemcomitans: An in-vitro study. J. Dent. 2014, 11, 464–472. [Google Scholar]
- Sofrata, A.; Santangelo, E.M.; Azeem, M.; Borg-Karlson, A.-K.; Gustafsson, A.; Pütsep, K. Benzyl Isothiocyanate, a Major Component from the Roots of Salvadora Persica Is Highly Active against Gram-Negative Bacteria. PLoS ONE 2011, 6, e23045. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Griffen, A.L.; Beall, C.J.; Campbell, J.H.; Firestone, N.D.; Kumar, P.S.; Yang, Z.K.; Podar, M.; Leys, E.J. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. Isme J. 2012, 6, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014, 162, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Vartoukian, S.R.; Palmer, R.M.; Wade, W.G. Diversity and morphology of members of the phylum "synergistetes" in periodontal health and disease. Appl. Environ. Microbiol. 2009, 75, 3777–3786. [Google Scholar] [CrossRef]
- Matarazzo, F.; Ribeiro, A.C.; Feres, M.; Faveri, M.; Mayer, M.P. Diversity and quantitative analysis of Archaea in aggressive periodontitis and periodontally healthy subjects. J. Clin. Periodontol. 2011, 38, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Lepp, P.W.; Brinig, M.M.; Ouverney, C.C.; Palm, K.; Armitage, G.C.; Relman, D.A. Methanogenic Archaea and human periodontal disease. Proc. Natl. Acad. Sci. USA 2004, 101, 6176–6181. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.M.; Bedoya-García, J.A. Antimicrobial resistance of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythia in periodontitis patients. J. Glob. Antimicrob. Resist. 2020, 22, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.A.; El-Sabbagh, M.S.; El Naggar, E.B.; El-Erian, R.H. Antibacterial activity of Salvadora persica against oral pathogenic bacterial isolates. Niger. J. Clin. Pract. 2019, 22, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Arshad, H.; Sami, M.A.; Sadaf, S.; Hassan, U. Salvadora persica mediated synthesis of silver nanoparticles and their antimicrobial efficacy. Sci. Rep. 2021, 11, 5996. [Google Scholar] [CrossRef]
- Khan, M.; Alkhathlan, H.Z.; Khan, S.T. Antibiotic and Antibiofilm Activities of Salvadora persica L. Essential Oils against Streptococcus mutans: A Detailed Comparative Study with Chlorhexidine Digluconate. Pathogens 2020, 9, 66. [Google Scholar] [CrossRef]
- Sukkarwalla, A.; Ali, S.M.; Lundberg, P.; Tanwir, F. Efficacy of miswak on oral pathogens. Dent. Res. J. 2013, 10, 314–320. [Google Scholar] [CrossRef]
- Mouwakeh, A.; Telbisz, Á.; Spengler, G.; Mohácsi-Farkas, C.; Kiskó, G. Antibacterial and Resistance Modifying Activities of Nigella sativa Essential Oil and its Active Compounds Against Listeria monocytogenes. In Vivo 2018, 32, 737–743. [Google Scholar] [CrossRef]
- Rafiei, M.; Kiani, F.; Sayehmiri, F.; Sayehmiri, K.; Sheikhi, A.; Zamanian Azodi, M. Study of Porphyromonas gingivalis in periodontal diseases: A systematic review and meta-analysis. Med. J. Islam. Repub. Iran 2017, 31, 62. [Google Scholar] [CrossRef]
- Auer, G.K.; Weibel, D.B. Bacterial Cell Mechanics. Biochemistry 2017, 56, 3710–3724. [Google Scholar] [CrossRef]
- Sculean, A.; Chapple, I.L.; Giannobile, W.V. Wound models for periodontal and bone regeneration: The role of biologic research. Periodontology 2015, 68, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Akazawa, K.; Nagata, M.; Komaki, M.; Peng, Y.; Umeda, M.; Watabe, T.; Morita, I. Angiogenic Effects of Secreted Factors from Periodontal Ligament Stem Cells. Dent. J. 2021, 9, 9. [Google Scholar] [CrossRef]
- Vaquette, C.; Pilipchuk, S.P.; Bartold, P.M.; Hutmacher, D.W.; Giannobile, W.V.; Ivanovski, S. Tissue Engineered Constructs for Periodontal Regeneration: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, e1800457. [Google Scholar] [CrossRef]
- Bartold, P.M.; Shi, S.; Gronthos, S. Stem cells and periodontal regeneration. Periodontol. 2006, 40, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Y.; Li, X.; Wang, J.; He, X.-T.; Sun, H.-H.; Chen, F.-M. Concise Review: Periodontal Tissue Regeneration Using Stem Cells: Strategies and Translational Considerations. Stem Cells Transl. Med. 2019, 8, 392–403. [Google Scholar] [CrossRef]
- Udalamaththa, V.L.; Jayasinghe, C.D.; Udagama, P.V. Potential role of herbal remedies in stem cell therapy: Proliferation and differentiation of human mesenchymal stromal cells. Stem Cell. Res. Ther. 2016, 7, 110. [Google Scholar] [CrossRef]
- Johnson, T.C.; Siegel, D. Directing Stem Cell Fate: The Synthetic Natural Product Connection. Chem. Rev. 2017, 117, 12052–12086. [Google Scholar] [CrossRef]
- Fouda, A.-M.; Youssef, A.R. Antiosteoporotic activity of Salvadora persica sticks extract in an estrogen deficient model of osteoporosis. Osteoporos. Sarcopenia 2017, 3, 132–137. [Google Scholar] [CrossRef]
- Khunkar, S.; Hariri, I.; Alsayed, E.; Linjawi, A.; Khunkar, S.; Islam, S.; Bakhsh, T.A.; Nakashima, S. Inhibitory effect of Salvadora persica extract (Miswak) on collagen degradation in demineralized dentin: In vitro study. J. Dent. Sci. 2021, 16, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, L.; Yuan, Q.; Zhen, G.; Crane, J.L.; Zhou, X.; Cao, X. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. 2018, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Checchi, V.; Maravic, T.; Bellini, P.; Generali, L.; Consolo, U.; Breschi, L.; Mazzoni, A. The Role of Matrix Metalloproteinases in Periodontal Disease. Int. J. Environ. Res. Public Health 2020, 17, 4923. [Google Scholar] [CrossRef] [PubMed]
| Kingdom | Plantae |
| Phylum | Magnoliphyta |
| Class | Magnoliopsida |
| Order | Brassicales |
| Family | Salvadoraceae |
| Genus | Salvadora |
| Species | Persica oleoides |
| Binomial classification | Salvadora persica |
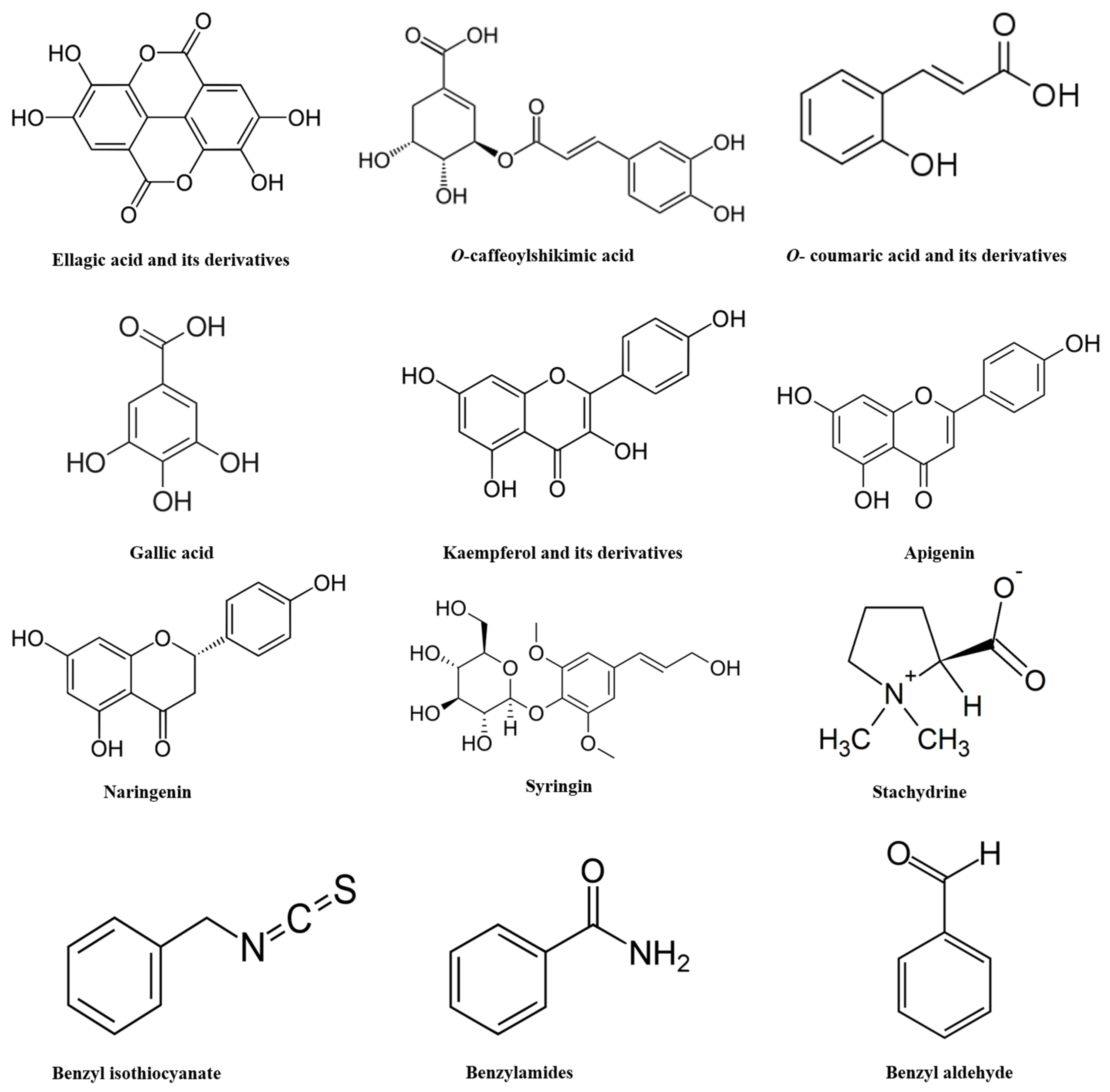
| Phytoconstituent | Plant Part | Reported Chemical/Biological Activity | Reference |
|---|---|---|---|
| Persicaline (Sulphur-containing imidazoline alkaloid) | Roots | Strong antioxidant properties | [54] |
| Salvodourea, m-anisic acid and benzyl isothiocyanate | Roots | Antiviral activity | [55] |
| Salvadoside, salvadoraside, syringin, and liriodendrin (lignin glycosides) | Stem | - | [56] |
| Essential oil components (1,8-cineole (eucalyptol), αβ-caryophellene, β-pinene, and 9-epi-(E)-caryophellene) | Stem | Anti-microbial activity | [57] |
| Benzylamides | Stem | Human collagen-induced platelet aggregation and antibacterial activity | [58] |
| Trimethylamine and salvadorine | Roots | Antibacterial, antiphlogistic and gingiva-stimulating effects | [59] |
| Benzaldehyde, benzyl nitrile and benzyl Isothiocyanate | Roots and twigs | Antimicrobial effects | [7] |
| Potential Salvadora persica Related Toxicity | Reported Effects | Reference |
|---|---|---|
| Sex hormone imbalance | Reduced testosterone and increased estrogen secretion in male rats with decreased progesterone levels in female rats | [60] |
| Reduced fertility | Adverse effects on male and female mice reproductive systems and fertility | [61] |
| Cytotoxic effects | Cell toxicity with dental pulp stem cells and gingival fibroblasts | [62,63] |
| Natural Compound | Study Type | Sample Studied, n | Adminitration (Dosage, Frecuency and Duration) | Main Effects | Reference |
|---|---|---|---|---|---|
| Combination of SP and CS extracts | RCT | Systemic healthy male and female aged 25–40 years without periodontitis, n = 14. | Combination of extracts of CS (0.25 g) + SP (7.82 mg). 15 mL rinse twice daily from baseline with follow up after 24 h. | Significant decrease in PI | [75] |
| SP | RCT | Systemic healthy males aged 8–10 years, n = 94. | Usage of SP sticks in combination with rolling brush technique 3 times per day for 3 weeks. From baseline with follow up after 3 weeks, 1- and 3 months. | Significant decrease in PI | [3] |
| SP | RCT | Systemic healthy participants aged 18–35 years with mild to moderate generalized marginal gingivitis, a PPD of 3 mm or less and with GI and PI higher than 1, n = 30 | Usage of toothbrush without toothpaste versus usage of toothbrush without toothpaste in combination with SP sticks versus usage of SP sticks only. Each group performed the procedure 3 times per day. From baseline with follow up after 2, 4, 6 and 8 weeks. | Significant improvement of PI and GI after using SP adjunctive to tooth brushing | [76] |
| Combination of SP and AV extracts | RCT | Intubated patients aged 18–64 years hospitalized in Intensive Care Unit, n = 67 | Mouth irrigation with herbal mouthwash (10 mg/mL SP and 940 mg/mL AV) or Chlorhexidine (0.2%) for 30 s before and after brushing the teeth every 2–3 h. From baseline with follow up after 4 days. | Significant improvement of GI after using herbal mouthwash compared to Chlorhexidine | [83] |
| SP | RCT | Participants aged 13–54 years with baseline PI of more than 1. | Toothbrushing with test dentifrice (SP dentrifice versus fluoride dentrifice) for 2 min twice a day. From baseline with follow up after 2 and 4 weeks. | Significant improvement of PI with SP dentrifice | [80] |
| Combination of extracts of SP, Bibhitaka, Nagavalli, Gandhapura taila, Ela, Peppermint satva, and Yavani satva | RCT | Systemic healthy participants aged 20–45 years with mild to moderate gingivitis and bleeding on probing, n = 100. | Herbal mouthwash (HiOra) along with scaling versus scaling only. 15 mL mouthwash for 30 s twice daily after food. PI and GI taken at baseline and follow at day 21. | Significant improvement of PI and GI with herbal mouthwash | [77] |
| Combination of extracts of SP, Bibhitaka, Nagavalli, Gandhapura taila, Ela, Peppermint satva, and Yavani satva | RCT | Systemic healthy participants aged 18–21 years, n = 45. | Herbal mouthwash (HiOra) 15 mL for 60 s versus Chlorhexidine (0.2%) 10 mL for 60 s versus probiotic solution 20 mL for 60 s each twice a day 30 min after toothbrushing for 14 days. PI and GI were taken at baseline with follow up at day 7 and day 14. | Significant improvement of PI and GI with herbal mouthwash | [81] |
| Combination of extracts of SP, Papain, Bromelain, and Neem | RCT | Systemic healthy participants aged over 18 years and undergoing fixed orthodontic treatment, n = 52. | Usage of toothpaste containing herbal mixture and fluoride versus standard fluoridated toothpaste only 2–3 min twice a day for 30 days. PI and GI were taken at baseline with follow up after 30 days. | Significant improvement of PI and GI with herbal toothpaste compared to the standard fluoridated toothpaste | [85] |
| SP | RCT | Children in chemotherapy treatment aged 6 to 12 years, n = 44 | Additive to Chlorhexidine mouthwash SP oral drops (10 drops in 15 mL water) or normal saline (15 mL) twice a day for 2 weeks. Oral assessment guide index was recorded at baseline with follow up at day 8 and 15. | Significant improvement of plaque and gingival status with SP oral drops | [78] |
| Combination of extracts of SP, Bibhitaka, Nagavalli, Gandhapura taila, Ela, Peppermint satva, and Yavani satva | RCT | Dental college students, n = 150 | Herbal mouthwash (HiOra) (5 mL) versus 0.2% Chlorhexidine (10 mL) versus saline (5 ml), each for 30 s twice daily. PI and GI recorded at baseline and follow up after 5 days. | Significant improvement of PI and GI with herbal mouthwash, similar to Chlorhexidine | [82] |
| SP extract and tea tree oil | RCT | Systemically healthy male and female participants aged 20–40 years, not using SP or tea tree oil- based toothpaste and with grade 2 or 3 PI on at least one of the Oral Hygiene Index teeth, n = 25 | SP toothpaste vs. tea tree oil toothpaste. PI recorded at baseline and follow up after 24 h. | SP toothpaste showed a significant higher reduction of plaque compared to tea tree oil-based toothpaste | [86] |
| Combination of extracts of SP, Bibhitaka, Nagavalli, Gandhapura taila, Ela, Peppermint satva, and Yavani satva | RCT | Systemically healthy participants aged 20–50 years, n = 152 | Herbal mouthwash (HiOra) (15 mL) versus 0.12% Chlorhexidine (15 mL) for 30 s twice daily. PI and GI recorded at baseline and follow up after 21 days. | Significantly higher improvement of plaque and gingival status with Chlorhexidine | [79] |
| SP | In Vitro | Periodontal pathogens (FN) and other oral bacteria | 100 mg/mL extracts were added to cultured bacteria and incubated for 24 h at 37 °C. | Moderate to high inhibitory activity on pathogenic bacteria with no toxicity. Methanol extract was more effective compared to water extract. | [91] |
| SP | In Vitro | Periodontal pathogens (PG, TD, TF, AA) | SP extract (2 mg/mL) was added to cultured bacteria and incubated for 24 h. Antibiotic discs were impregnated with SP extract added to cultured bacteria and incubated for 24 h at 37 °C. | Ethanolic extract of SP showed a significant inhibitory effect on all periodontal pathogens with synergistic antibacterial effect when SP was combined with antibiotics. | [92] |
| Combination of extracts of SP, Bibhitaka, Nagavalli, Gandhapura taila, Ela, Peppermint satva, and Yavani satva | In Vitro and Ex Vivo | Periodontal pathogens (PG, FN, AA) and oral bacteria in supragingival plaque samples of male and female participants aged over 18 years and periodontally healthy | In Vitro: Test mouthwash was added in different solutions (20–200 μg/mL) to cultured bacteria and incubated as appropriate for the species. Ex Vivo: Test mouthwash was added to bacteria cultured from supra gingival plaque samples with incubation for 5–7 days | Significant inhibitory effect of the herbal mouthwash on the tested bacteria | [93] |
| SP | In Vitro | Periodontal pathogens (PTI) and other oral bacteria | Alcoholic and water extracts of SP (200 μg/mL and 400 μg/mL) were incubated with cultivated samples for 24 h at 37 °C. The inhibitory zone was measured after 24 h. | Alcoholic extract of SP showed antimicrobial effect against all tested microbial pathogens. | [94] |
| Combination of SP, mint and yarrow extracts | In Vitro | Periodontal pathogens (PG, AA) isolated from 50 patients with moderate to severe periodontitis | Herbal solution (6%) versus sterile distilled water versus chlorhexidine were incubated with the cultured bacteria for 48 h at 37 °C. After 48 h the zone of inhibition was measured. | Herbal solution showed significant inhibitory effect against PG with weaker effect against AA | [95] |
| SP | In Vivo/In Vitro | Adults with good oral health, n = 12. Antibacterial effect of SP essential oil on oral bacteria and periodontal pathogens (PG, AA) | Usage of fresh cut SP root versus one time-, two time- and four time used twig. Saliva sample taken before and after brushing with the SP sticks and follow up 5-, 10- and 30 min after brushing. | Highest concentration of active compounds was detected in saliva immediately after brushing with fresh SP. Bacteria growth was inhibited by SP with PG being the most sensitive. | [87] |
| SP | In Vitro | Periodontal pathogens (PG) and herpes simplex virus-1 | SP films (100 µg per 2 cm2) formulated and the SP inhibitory effect tested on the cultured microorganisms | Significant inhibitory effect of the SP films against PG and the herpes simplex virus-1 | [88] |
| SP | In Vitro | Periodontal pathogens (PG, AA) and other oral bacteria | Essential oil of SP in concentration 1%, 0.1%, 0.05%, 0.02%, 0.01%, 0.001% was incubated with cultured test bacteria for 90 min at 37 °C. Medium-pressure liquid chromatography, Thin-layer chromatography, Gas chromatography-mass spectrometry and Transmission electron microscopy were performed | SP extract and its active constituent benzyl isothiocyanate exhibited rapid and strong bactericidal effects against all Gram-negative bacteria. | [96] |
| SP | Cross-Sectional | 287 male school children aged 12–15 years. | Participants were assigned in group I: SP stick users, group II: toothpaste/toothbrush users and group III: SP stick and toothbrush users. All individuals were interviewed regarding their oral hygiene habits. Oral Hygiene, PI and GI were recorded. | Statistically significant differences of GI was observed among SP and toothbrush & toothpaste users as SP users had lower GI scores. PI was lowest among combined users of toothbrush and miswak. | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekhemar, M.; Geib, M.; Kumar, M.; Radha; Hassan, Y.; Dörfer, C. Salvadora persica: Nature’s Gift for Periodontal Health. Antioxidants 2021, 10, 712. https://doi.org/10.3390/antiox10050712
Mekhemar M, Geib M, Kumar M, Radha, Hassan Y, Dörfer C. Salvadora persica: Nature’s Gift for Periodontal Health. Antioxidants. 2021; 10(5):712. https://doi.org/10.3390/antiox10050712
Chicago/Turabian StyleMekhemar, Mohamed, Mathias Geib, Manoj Kumar, Radha, Yasmine Hassan, and Christof Dörfer. 2021. "Salvadora persica: Nature’s Gift for Periodontal Health" Antioxidants 10, no. 5: 712. https://doi.org/10.3390/antiox10050712
APA StyleMekhemar, M., Geib, M., Kumar, M., Radha, Hassan, Y., & Dörfer, C. (2021). Salvadora persica: Nature’s Gift for Periodontal Health. Antioxidants, 10(5), 712. https://doi.org/10.3390/antiox10050712









