Abstract
Asphodelus tenuifolius Cav. (A. tenuifolius) is a medicinal plant with a long history of traditional use to treat ailments. In this study, total phenolic and flavonoid content evaluation using LC-ESI/MS analysis and various biological activities (antioxidant, antibacterial, antifungal, antiviral and cytotoxicity) of organic extracts from the aerial parts of A. tenuifolius were analyzed. ADME tools were used to predict the potential of the identified compounds from the most potent extract as specific drugs. As shown, LC-ESI/MS results of chloroformic extract allowed the tentative identification of 12 compounds. Chloroformic extract was rich in polyphenols and flavonoids and exhibited the highest antioxidant activity given by DPPH (IC50 = 25 µg/mL) as compared to the BHT standard (11.5 µg/mL) and β-carotene bleaching assays (IC50 = 95.692 µg/mL). Antibacterial activity results showed that chloroformic extract has a highest activity against Gram-positive and -negative bacteria, especially against Salmonella Typhimurium DT104 (IZ = 19.3 mm, MIC = 18.75 mg/mL, MBC = 37.5 mg/mL). The MBC/MIC ratio was evaluated to interpret the activity that was bacteriostatic rather than bactericidal. Conversely, weaker antifungal activity was registered, and no antiviral activity was observed for all extracts against Herpes Simplex Virus type 2 and Coxsakievirus B-3 viruses. Cytotoxic activity on VERO cell line results revealed that butanol extract was not toxic, with CC50 value of 1430 µg/mL, while chloroformic extract showed moderate cytotoxicity. Additionally, in silico studies performed proved promising pharmacokinetic and drug-likeness properties of the main compounds from the chloroformic extract. Taken together, this work highlights the potent bioactivity and acceptable drug-likeness of this plant, which supports its further preclinical development.
1. Introduction
The Liliaceae family is a rich source of natural products displaying a vast range of structural diversity. It includes approximately 289 genera and 4000 grown species, with many important medicinal, edible and ornamental plants [1]. Among these genera, eighteen species of Asphodelus have been widely cultivated throughout the Mediterranean area, North Africa and Southeast Asia. It reaches its maximum diversity in the Iberian Peninsula and Northwest Africa [2]. Asphodelus species were found to contain several secondary metabolites such as flavonoids [3,4], anthraquinones [5,6], phenolic acids [7,8], triterpenes [9,10], fatty acids [11] and naphthalene derivatives [12]. Different ethnomedical uses were ascribed to Asphodelus species, including Asphodelus tenuifolius Cav., known as onion weed [13], native to the Mediterranean region, which is used traditionally not only as a vegetable but also for colds, hemorrhoids, rheumatic pain, diuretic agent and wound healing [14]. As recently overviewed [15], organic extracts from the whole plant, fruits, roots, seeds and leaves were studied for samples collected from different harvest regions in the world [13,16,17,18,19,20,21] and also specifically from Algerian regions [14,22]. The diversity of results is mainly due to the harsh climatic conditions, which stimulate the biosynthesis of secondary metabolites, different plant growing conditions, collecting regions and different extraction procedures applied [23,24].
In the present study, we set out to provide more information on the A. tenuifolius phytochemical composition by LC-ESI/MS analysis. In addition, evaluation of total phenolic and flavonoids contents of chloroformic, ethyl acetate and butanol extracts was performed, investigating their antioxidant, antibacterial, antifungal, cytotoxic and antiviral activities. To the best of our knowledge, this work is the first report on chemical composition study based on RP-HPLC-ESI/MS measurements of A. tenuifolius plant extracts. Additionally, ADME/pharmacokinetics and drug-likeness properties of the identified metabolites were carried out through in silico SwissADME online program.
2. Materials and Methods
2.1. Chemical and Reagents
Folin-Ciocalteu reagent, sodium carbonate anhydrous (Na2CO3), gallic acid, sodium nitrite solution (NaNO2), sodium hydroxide (NaOH), sodium chloride (NaCl), aluminum chloride hexahydrate solution (AlCl3-6H2O), 2,2-Diphenyl-1 picrylhydrazyl (DPPH), catechin and β-carotene were from Fluka (Buchs, Switzerland). Linoleic acid and Tween 20 were purchased from Sigma-Aldrich (GmbH, Sternheim, Germany). Sulfuric acid (H2SO4) was obtained from Merck (Darmstadt, Germany). All analytical grade solvents were purchased from Sigma-Aldrich (Milan, Italy), with the exception of solvents for LC–MS analysis, which were HPLC–MS grade.
2.2. Plant Material and Extraction Procedure
The whole plant of A. tenuifolius Cav. was collected in May 2012 from southwest Algeria and identified by an expert botanist M. Mohamed Ben Abd-elhakem (Ex-Director of the National Agency of Preservation of Natural Resources, Bechar, Algeria). An authenticated voucher specimen with the identification number (AS10TEN) was deposited at the herbarium of the VARENBIOMOL research unit, University Mentouri Constantine. A total of 1250 g of the whole plant was dried, powdered and extracted with 80% ethanol aqueous solution at room temperature (each extraction lasting about 48 h). After filtration and concentration under vacuum at about 40 °C, the combined concentrated ethanol extract was suspended in distilled water. Each resulting solution was extracted successively using chloroform, ethyl acetate and butanol. The organic phases were filtered and concentrated in a vacuum at 38 °C to obtain dry extracts: chloroform (CHE, 3.60 g), ethyl acetate (EAE, 4.05 g) and butanol (BE, 6.40 g).
2.3. Total Phenolic Content (TPC)
Total phenolic content (TPC) was determined using Folin-Ciocalteu method according to Dewanto et al. [25] using Gallic acid as a standard. The reference range was prepared with Gallic acid in different concentrations from 50 to 500 µg/mL. Total phenolic contents are expressed as milligram Gallic acid equivalents per gram of dry residue (mg GAE/g DR). All samples were analyzed in three replicates.
2.4. Total Flavonoids Content (TFC)
Total flavonoids content (TFC) was measured using the colorimetric method introduced by Dewanto et al. [25] with few modifications. A calibration curve was constructed using catechin standard solution in different concentrations from 50 to 500 µg/mL. Total flavonoids contents were calculated as catechin equivalents per gram of plant dry residue (mg CE/g DR). Measurements were performed at least in triplicate.
2.5. LC-ESI/MS Analysis
LC-ESI/MS profiles were performed using a Hewlett–Packard (Palo Alto, CA, USA) Model 1100 Series liquid chromatography coupled to a Photo Diode Array detector (Agilent, Palo Alto, CA, USA) 1100 Series, and to an Esquire LC-ion trap mass spectrometer (Bruker Daltonics, Billerica, MA, USA) equipped with an electrospray ionization (ESI) interface. Separation was achieved on a Phenomenex Luna C18 analytical column (250 mm × 4.6 mm i.d.; 5 μm particle diameter, end-capped). The mobile phase consisted of water (eluent A) and acetonitrile (eluent B) at a flow rate of 1 mL/min. The injection volume was 2 µL. Gradient elution was carried out using the following timetable: 20% A and 80% B in 30 min, then 100% B in 40 min. The resulting total run time was 70 min. The Photo Diode Array detector was set at a range of 200–700 nm for all peaks. The chromatogram was recorded at 215 nm, 254 nm, 300 nm and 330 nm.
2.6. NMR Analysis
NMR experiments were performed on: (1) Bruker DRX-600 spectrometer (Bruker BioSpin, Rheinstetten, Germany) equipped with a Bruker 5 mm TCI CryoProbe at 300 K. (2) Bruker-Avance 400 spectrometer by using a 5 mm BBI probe. NMR spectra were acquired in CDCl3, DMSO-d6, CD3COCD3 and CD3OD in the phase-sensitive mode with the transmitter set at the solvent resonance and time-proportional phase increment (TPPI) used to achieve frequency discrimination in the ω1 dimension. The standard pulse sequence and phase cycling were used for HSQC and HMBC experiments.
2.7. Evaluation of Antioxidant Activity
DPPH Radical-Scavenging Activity and β-Carotene Bleaching Assay
The effect of the various tested extracts on DPPH-degradation was estimated according to the method described by Espín et al. [26] while β-carotene linoleic acid bleaching assay was done according to Condelli et al. [27].
where A0 is the absorbance of the blank, and A1 is the absorbance of the sample.
DPPH scavenging effect (%) = [(A0 − A1) × 100]/A0;
Results were expressed as percentage of β-carotene bleaching inhibition (AA%) and calculated as follows (Equation (2)):
where A0 and A1 have the same meaning as in Equation (1). The results are expressed as IC50 values (µg/mL).
AA% = (A β-carotene after 180 min/A initial β-carotene) × 100;
2.8. Antibacterial and Antifungal Activities
2.8.1. Disk Diffusion Assay
The antibacterial and antifungal activities of A. tenuifolius organic extracts were evaluated by the agar disk diffusion method described by Rios and Recio [28] and Snoussi et al. [29]. Eight strains generally recognized as the most important pathogens affecting food dishes (Escherichia coli ATCC 35218, Vibrio parahaemolyticus ATCC 17802, Staphylococcus aureus ATCC 25923, Salmonella typhimurium DT 104, Staphylococcus epidermidis CIP 106510, Salmonella typhimurium ATCC 1408, Bacillus cereus ATCC 11778, Listeria monocytogenes ATCC 19115) were tested in the present study. On other hand, the antifungal activity effect was tested against four Candida strains (C. tropicalis 06-85, C. parapsilosis ATCC 22019, C. krusei ATCC 6258 and C. albicans ATCC 2019). The same technique was used to evaluate the antifungal activity. Ampicillin (10 mg/mL) and Amphotericin B (10 mg/mL) were used as a positive control [30]. The antibacterial activities were evaluated by measuring the diameter of the growth inhibition zone (IZ) around the discs using a flat rule. All tests were performed in triplicate, and the mean diameter of IZ was calculated. The results were expressed in terms of IZ of growth around each disc in millimeters, considered as low activity (IZ from 1 mm to 6 mm), moderate activity (7 mm to 10 mm), high activity (11 mm to 15 mm) and very high activity (16 mm to 20 mm) [31].
2.8.2. Microdilution Assay: MICs and MBCs Determinations
Minimal inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs) values were determined according to the method described by Gormez et al., Boulaaba et al. [32,33] and Snoussi et al. [29].
2.9. Cytotoxic and Antiviral Activities
The cytotoxic activity was evaluated according to the method described by Snoussi et al. [30] on VERO (African green monkey kidney) cells line. The 50% Cytotoxic Concentration (CC50), defined as the concentration of the extract able to reduce of 50% the cell viability, was determined by regression analysis in comparison to negative control. The extracts that demonstrated activity at or below 100 µg/mL were categorized as having strong cytotoxic activity. Consequently, CC50 values between 100 µg/mL and 500 µg/mL were categorized as having moderate cytotoxicity, the extracts that had CC50 values between 500 µg/mL and 1000 µg/mL were considered to have weak cytotoxic activity [34] and the extracts had CC50 values more than 1000 µg/mL were considered to be nontoxic [35]. Antiviral activity was also evaluated according to the method reported by Snoussi et al. [30] on two viruses, Herpes Simplex Virus type 2 (HSV-2) and Coxsakievirus B-3 (CVB-3).
2.10. In Silico ADME Profiles
The pharmacokinetics and drug-likeness properties of identified compounds from CHE of A. tenuifolius were estimated using ADME (absorption, distribution, metabolism and excretion) descriptors through SwissADME online server (http://www.swissadme.ch/ (accessed on May 2020)) by entering chemical structure followed by SMILES [36,37].
2.11. Statistical Analysis
The results were given as the average ± SD for three replicates. The IC50 of DPPH, the CC50 and the antiviral IC50 were calculated by linear regression analysis. The β-carotene bleaching method values, the total secondary metabolite contents and the inhibition zone determination were performed using Microsoft Excel. The data were subjected to Duncan’s multiple range tests. The statistical analyses were determined with the SPSS statistical software program (SPSS v.16), and p values < 0.05 were regarded as significant.
3. Results and Discussion
3.1. Phytochemical Contents
The chloroformic extract was rich in phenolic constituents (40.99 mg GAE/g DR) compared to the rest of the studied A. tenuifolius extracts (Table 1). This can be attributed to the higher solubility of constituents containing phenolic rings in this extract, whereas butanol extract was the one with the lowest phenolic concentration (10.54 mg GAE/g DR), suggesting their weaker solubility in this extract. In fact, Mahboub et al. [22] reported different results obtained from lyophilized samples of A. tenuifolius harvested from septentrional Algerian Sahara (101.82 µg GAE/g DR). In the same way, Munir et al. [38] and Al-Laith et al. [39] reported other data on A. tenuifolius from Pakistan and Bahrain, respectively, with total phenolic contents ranging within 53.40 to 76.23 mg GAE/g DR and 139.66 to 442.44 mg GAE/g DR, respectively, for various solvent extracts and harvested sites.

Table 1.
Total phenolic and flavonoid contents expressed as gallic acid and catechin equivalents, in mg/g dry sample, respectively, DPPH radical-scavenging activity and β-carotene bleaching capacity.
Results of total flavonoid contents are shown in Table 1. Chloroformic extract displayed also the highest flavonoid contents (213.07 mg CE/g DR), followed by EAE (202.89 mg CE/g DR). Previously, Mahboub et al. [22] found a good level of flavonoid contents marked in lyophilized dried extracts with a value of 16.10 µg QE/g DR. Similarly to our results, Munir and colleagues [38] also found flavonoid contents in the range of 165.82 to 312.12 mg QE/g DR for all studied extracts. The presence of phenolics and flavonoids is very important to assess the antioxidant potency, especially due to their chemical structure affecting redox properties, which play a vital role in absorbing and neutralizing free radicals [40,41].
In summary, the lowest polar solvent (CHE) displayed the highest phenolic and flavonoid contents, and the higher polar solvent (BE) showed the lowest amount, indicating the richness of A. tenuifolius extract in low polar phenolic compounds.
3.2. HPLC-DAD-ESI/MS Analysis
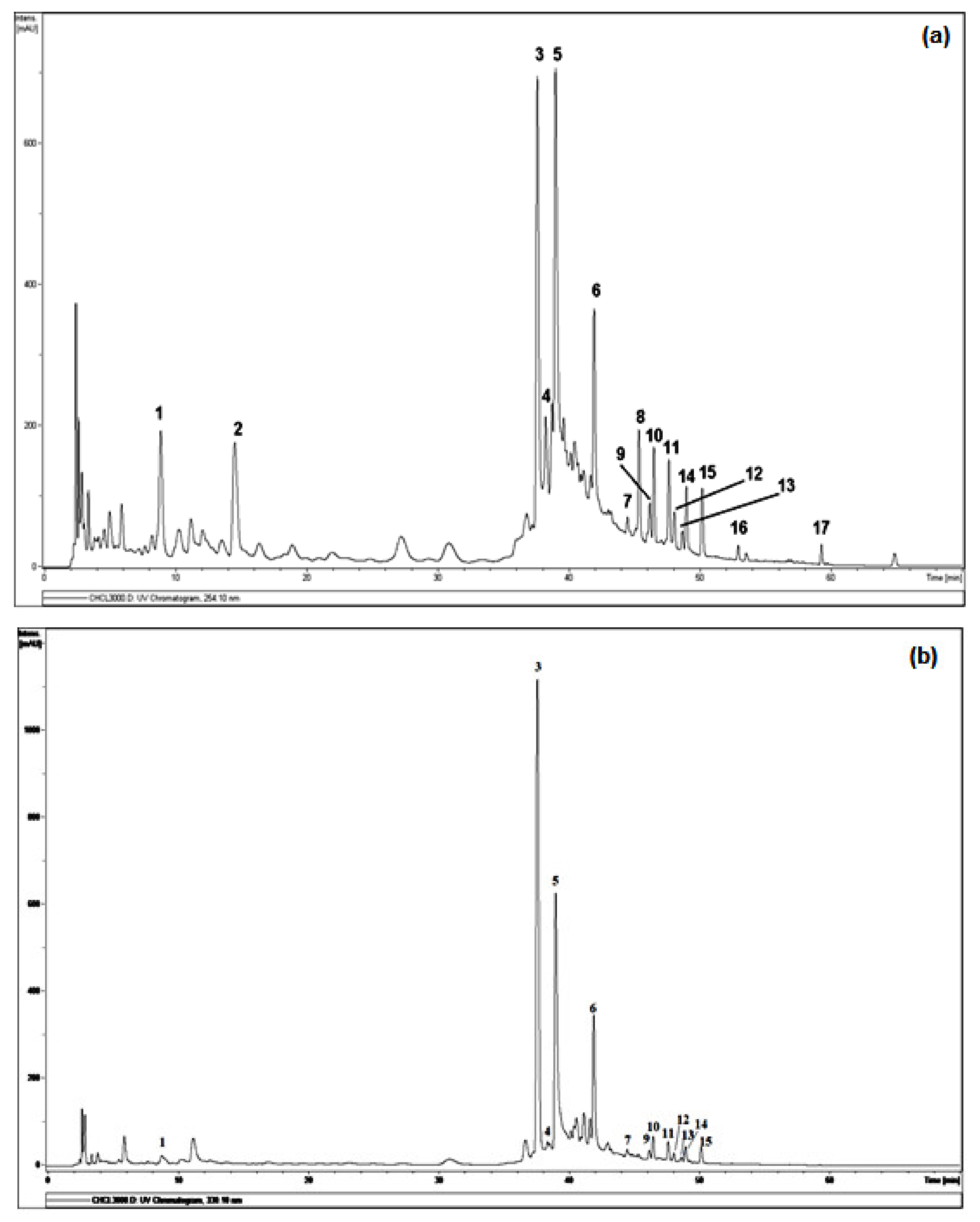
A qualitative analysis of constituents present in chloroformic extract was performed by LC-ESI/MS. As shown in Figure 1, the full scan using negative electrospray ionization mode revealed the presence of several phenolic compounds, based on the over 17 major peaks detected within 70 min of elution. Table 2 summarizes retention time (Rt), m/z, molecular weight and formula of compounds identified or deduced based on data reported in the literature. All tentatively identified compounds are already known from A. tenuifolius plant and Asphodelus genus, while some minor metabolites are still unidentified.
Figure 1.
DAD-HPLC profile detected at 254 nm (a) and 330 nm (b) of LC–MS analysis on CHE. Legends: (1): Apigenin-7-O-glucoside, (3): Tamgermanetin, (5): Luteolin, (6): Apigenin, (7): Ramosin, (8): Aloe-emodin, (10): (P,10′S)-oxanthrone-10′-β-glucopyranosyl asphodelin, (12): (M,10′S)-oxanthrone-10′-β-glucopyranosyl asphodelin, (13): (10′R)-oxanthrone-10′-β-D-xylopyranoside asphodelin, (14): (10′S)-oxanthrone-10′-β-D-xylopyranoside asphodelin, (15): (10′S)-oxanthrone-10′-β-L-arabinopyranoside asphodelin, (17): Asphodeline. Peaks 2, 4, 9, 11 and 16 are still unidentified.

Table 2.
Phenolic profile by LC-ESI/MS analysis in negative ion mode of compounds 1–17 from CHE. Rt, retention time of the peaks detected in the chromatogram reported in Figure 1. NI = not identified.
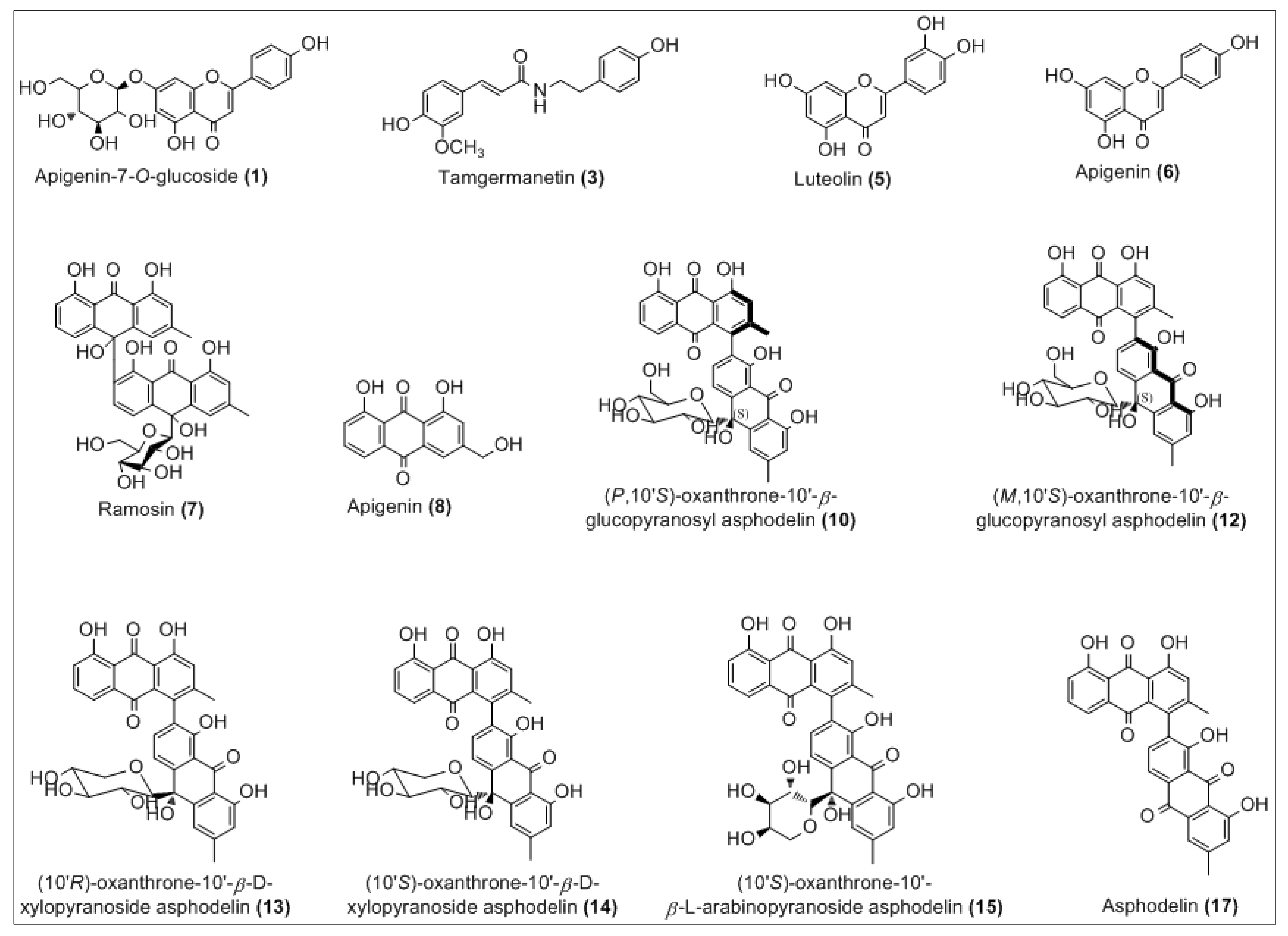
The analysis of the chloroformic extract leads to the tentative identification (Figure 2) of 12 compounds of 17 detected by comparison with the respective reported literature data, both full and fragmentation ESI-MS ion peaks [M-H]− and supported by 1D and 2D NMR analysis.
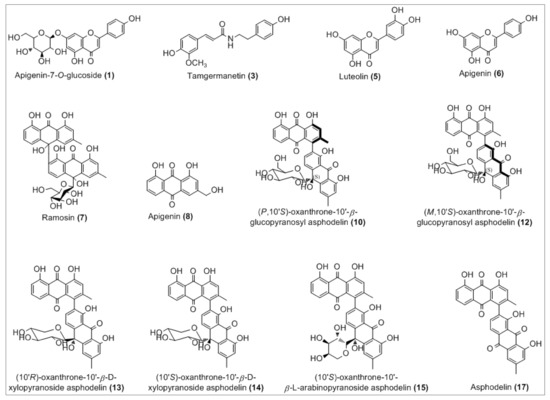
Figure 2.
Chemical structures of the tentatively identified compounds.
The complete list of the identified phytoconstituents is as follows: (1) apigenin-7-O-glucosyl (m/z 431.3), which was never reported in A. tenuifolius but reported previously from Asphodelus ramosus by Reynaud et al. [42]; (3) tamgermanetin (m/z 312.2) reported by Faidi et al. [17] from A. tenuifolius; (5) luteolin (m/z 284.9) reported in many plants of Asphodelus genus [4,8,17,43]; (6) apigenin (m/z 269.0) found in A. tenuifolius by Faidi et al. and Di Petrillo et al., from Asphodelus microcarpus [4,17]; (7) Ramosin (m/z 671.0) reported only one time by Adinolfi et al. from A. ramosus [44]; (8) Aloe-emodin (m/z 269.0) described by Hammouda et al. from Asphodelus fistulosus and van Oudtshoorn from Asphodelus albus [45,46]. Recently, Khalfaoui et al. [47] found both compounds (10) P,10′S-oxanthrone-(10′)-β-glucopyranosyl asphodelin (m/z 668.9) and (12) M,10′S-oxanthrone-(10′)-β-glucopyranosyl asphodelin (m/z 668.9) from A. tenuifolius. Compounds (13) 10′R-oxanthrone-(10′)-β-D-xylopyranoside asphodelin (m/z 638.9), (14) 10′S-oxanthrone-(10′)-β-D-xylopyranoside asphodelin (m/z 639.0) and (15) 10′S-oxanthrone-(10′)-β-L-arabinopyranoside asphodelin (m/z 639.0) reported previously by Ghoneim et al.from A. microcarpus [48]. Finally, (17) asphodelin (m/z 505.0) reported in many studies from A. acaulis, A. albus, A. fistulosus and A. microcarpus [12,43,45,49]. However, compounds corresponding to peaks 2, 4, 9, 11 and 16 are still unidentified.
In detail, the Mass spectrum (MS) recorded in negative ion mode showed a molecular deprotonated ion at m/z 431.3, it fragmented giving as base peak the radical aglycone ion at m/z 269, corresponding to the loss of a glucose moiety and assigned to apigenin glycoside (1). For peak (3), the MS base peak at m/z 312.2 also showed two peaks (m/z 178.1 and 135.1) which are characteristic of the tamgermanetin. Compound eluted at peaks (8) showed a molecular deprotonated ion at m/z 269.2 corresponding to an Aloe-emodin with a characteristic fragment at m/z 240 [M-H-CHO]−. Results showed also molecular deprotonated ion at m/z 668.9 for four peaks (9, 10, 11 and 12) observed in different retention times with the same fragment at m/z 506.0 due to the loss of a hexoside moiety [M-H-162]−. Regarding Khalfaoui et al. [47], the peak (10) corresponds to (P,10′S)-oxanthrone-10′-β-glucopyranosyl asphodelin and (12) to (M,10′S)-oxanthrone-10′-β-glucopyranosyl asphodelin. However, both peaks 9 and 11 are still unidentified, and the exact structure cannot be assigned; only planar structure can be assigned on the basis of all available spectral data similarly to peaks 10 and 12 as (10′S)-oxanthrone-10′-β-glucopyranosyl asphodelin. The same value for the molecular deprotonated ion at m/z 639.0 was observed for peaks 13, 14 and 15 with the common fragment at m/z 506.1 due to the loss of xylose unit corresponding to (10′R)-oxanthrone-10′-β-D-xylopyranoside asphodelin (13), (10′S)-oxanthrone-10′-β-D-xylopyranoside asphodelin (14) and loss of arabinose unit corresponding to (10′S)-oxanthrone-10′-β-L-arabinopyranoside asphodelin (15) [48].
Although many phytochemical studies were previously reported on A. tenuifolius collected from many regions [13,14,16,17,18,19,20,21,22], but no LC-ESI/MS analysis was performed, and it is reported here in this study for the first time.
3.3. Antioxidant Activities
Antioxidant activity of different Algerian A. tenuifolius extracts was evaluated using two different tests: DDPH radical scavenging activity and β-carotene bleaching. The results presented in Table 1 were expressed as IC50 values and compared to the positive control BHT. As shown, all extracts displayed potent free radical scavenging activity on DPPH with IC50 values ranging from 25 to 92 µg/mL, clearly less important than thatpositive control BHT (11.5 ± 0.01 µg/mL). CHE had the strongest DPPH radical scavenging activity (25 ± 4.36 µg/mL) followed by EAE (45 ± 2.88 µg/mL), and BE (92 ± 4.05 µg/mL). In our results, the extract rich in flavonoids and phenolics (CHE) was found to be the most significant scavenger of DPPH radical, which is supported by the good correlation between phenolic and flavonoid contents and DPPH outcomes. Polyphenolic compounds are usually the major antioxidants in plant extracts [50]. Based on LC-ESI/MS results, chloroformic extract showed an important richness, especially in apigenin-7-O-glucoside, apigenin, luteolin, anthraquinones and their derivatives formerly known for their antioxidant roles [51,52,53,54,55].
Previous studies on methanol, ethanol and petroleum ether extracts of Algerian A. tenuifolius displayed an important antioxidant activity, with IC50 values ranging between 28.34 and 75.91 µg/mL [14]. Similarly, Al-Laith et al. [39] reported that, for extracts of the plant collected from two different harvested sites from Bahrain, IC50 values of antioxidant and antiradical activity varied between 18.37 and 37.24 mg/mL. Kalim et al. [18], also reported DDPH radical scavenging results from Indian A. tenuifolius with an IC50 = 2.00 µg/mL of 50% methanolic extract. This difference could also be due to the variable plant growing conditions, collection regions and extraction procedures [56,57].
Table 1 depicts the inhibition of β-carotene bleaching by A. tenuifolius extracts. It is possible to note that EAE had a significant activity, with an IC50 value of 73.581 µg/mL, close to BHTreported a study on a whole Indian A. tenuifolius methanol extract against two Gram-positive bacteria (S. aureus and B. cereus), with IZ diameters of 9 and 13 mm, respectively, while acetone extract showed only activity against a Gram-negative bacteria K. pneumoniae (IZ = 17 mm). Additionally, Panghal et al. [20] outlined an antibacterial study of Indian A. tenuifolius fruits using six organic solvents for extraction, in which no activities were observed for petroleum ether and aqueous extracts against all tested bacterial strains, specifically against E. coli for the aqueous extract (IZ = 13.67 ± 0.5). The same study revealed that benzene extract exhibited very good susceptibility to K. pneumoniae, P. aeruginosa (IZ = 10.33 ± 0.5) and A. fumigatus (IZ = 10.66 ± 0.5). In the same way, all other extracts displayed antibacterial activity against overall tested bacterial strains. Moreover, a good antibacterial activity was observed by Dangi et al. and Menghani et al. [16,19] against the number of selected bacteria. From Indian A. tenuifolius, various extracts were found to be active against almost all the tested bacteria. Eddine et al. and Mahboub et al. [14,22] reported studies from Algerian A. tenuifolius, collected from the south-east and septentrional Sahara of Algeria, respectively. In detail, the results on the antibacterial effect of methanol, ethanol and petroleum ether extracts reported by Eddine et al. [14] showed a marked activity against S. aureus and P. putida (IZ = 11 mm), B. cereus, P. aerigunosa, A. tumefaciens and E. coli (IZ = 10 mm), and S. Arizona (IZ = 9 mm). Mahboub et al. [22] found a remarkable inhibition against E. coli and P. aeruginosa for the lyophilized sample, unlike our results on chloroformic, ethyl acetate and butanol extracts.
Table 3 shows that antibacterial effect of the extracts was more important than the antifungal one, suggesting that yeast strains are more resistant to bacteria. These results are consistent with the ones previously reported, indicating that the inhibitory activity is pathogen-specific and depends on a number of factors, including the solvents, concentration of the crude drug, temperature, plant parts used for the extraction of secondary metabolites and rate of diffusion [58]. Both MICs and MBCs of A. tenuifolius organic extracts are summarized in Table 3. MICs ranged from 0.58 to 37.5 mg/mL, from 0.39 to 25 mg/mL, from 0.78 to 50 mg/mL for CHE, EAE and BE, respectively, for all bacterial and fungal strains tested. Regarding MBCs, high concentrations were needed to eliminate the growth of all tested bacterial and fungal strains, with values ranging from 2.34 to >100 mg/mL. Results indicated that CHE was the most effective compared to data obtained for EAE and BE. Our values of MICs and MBCs parameters are different from those obtained in numerous studies, specifically by Soliman et al. [59], in which MICs ranged from 25 to 50 µg/mL; Faidi et al. [17], found values from 0.15 to 4.1 mg/mL; Dangi et al. [16], with MICs results in range from 8 to 32 µg/mL; and Panghal et al. [20], reporting values in the range from 31 to 500 µg/mL. This behavior could be related mainly to the difference in the phenolic composition of each studied extract, plant origin, tested microorganisms and the size of the inoculum [60].

Table 3.
Antibacterial and antifungal activities (expressed as diameter of IZ ± SD, on mm), MIC and MBC values (mg/mL) of A. tenuifolius organic extracts.
The inhibitory effect of A. tenuifolius extracts was evaluated against four yeasts. The results revealed weaker antifungal potency as compared with the standard, and Amphotericin B. EAE seems to be the most effective, especially against Candida albicans, followed by BE and CHE. In fact, C. albicans ATCC 2019 was found to be the most sensitive to A. tenuifolius extracts, while C. krusei ATCC 6258 was the most resistant yeast. In addition, the MIC values related to the extraction solvents on the four Candida species are similar (ranging from 12.5 to 37.5 mg/mL). Moreover, concentrations ranging from 50 to >75 mg/mL for almost all tested Candida strains were sufficient to reproduce a fungicidal effect. Amphotericin B was more efficient on the four tested Candida species in comparison with the three types of extract tested with low MIC values, ranging from 0.024 to 0.195 mg/mL, and low MFC values (0.39 to 6.25 mg/mL). Previous results have shown a moderate antifungal activity of A. ramosus L., and A. tenuifolius L. tested on C. albicans, C. dubliniensis, C. glabrata and C. krusei, with a diameter of growth inhibition zone ranging between 10 and 16 mm [61]. Recently, Soliman et al. [59] reported that the ethanolic extract of A. tenuifolius (100 μg/mL) inhibits the growth of C. albicans on Lauria Bertani agar with GIZ = 16 ± 0.5 mm. Additionally, Salhi et al. [62] reported that the A. tenuifolius aqueous extract (at 20%) was able to inhibit mycelial growth in Fusarium graminearum with a percentage of about (60.34%).
According to reported data [33,63], an extract has bacteriostatic effect when the ratio MBC/MIC is more than 4 and a bactericidal effect if the ratio MBC/MIC is less than 4. In our study, and based on the obtained results, the effect of CHE was bacteriostatic rather than bactericidal.
3.4. Cytotoxic and Antiviral Activities
Our results showed that only BE was safe and non-toxic, with CC50 value of about 1340 µg/mL, while chloroformic extract and ethyl acetate extract exhibited moderate cytotoxicity on VERO cell line, with CC50 values of 400 and 333 µg/mL, respectively, indicating the presence of some cytotoxic compounds responsible for the observed toxicological activity [47]. Consistent with our results, Soliman et al. [59], testing different A. tenuifolius extracts at different concentrations by using fresh human erythrocytes, reported that all extracts are safe and not toxic.
The antiviral activity was evaluated for the first time on A. tenuifolius extracts against Herpes Simplex Virus type 2 (HSV-2) and Coxsackievirus B-3 (CVB-3), but results showed that no extracts were active, despite the high number of polyphenols reported in the literature to possess inhibitory activity against viruses [64].
3.5. ADME Predictions
Pharmacokinetic and drug-likeness properties were evaluated using the SwissADME online program to predict whether the identified bioactive molecules become a starting scaffold or lead compounds toward future synthetic drug discovery. As shown (Table 4), out of the present compounds, only 3–6 and 8 were estimated to have high absorption in the gastrointestinal tract, which makes them a successful drug. Another advantage is that they are not P-gp substrate (except 1), which makes them a good candidate against multidrug-resistant cancer cells, overexpressing this drug transporter. They were predicted to not be blood–brain-barrier (BBB)-permeant, meaning that they are not able to cross the blood–brain barrier into the brain, where it binds to specific receptors. The prediction of putative drug–drug interaction through Cytochromes P450 (CYPs) inhibition, which affects the metabolism of numerous xenobiotics, demonstrates that only compounds 5, 6 and 7 were expected to be inhibitors of CYP1A2. The compound 17 affected CYP2C19; those 7 and 10, 12–15 and 17 affected CYP2C9; and 3, 5 and 6 affected CYP2D6. These results suggest that all compounds may be metabolized by more than one enzyme, which in turn can minimize the risk of drug–drug interaction. In addition, eight of the twelve compounds were not inhibitors of the isoenzyme CYP3A4, which is largely implicated in the metabolism and elimination of the majority of clinically used drugs. As for the skin sensitivity prediction given by their logKp values, all tested compounds displayed negative values ranging from −8.95 to −5.24 cm/s, meaning that they are not accessible through the skin. Regarding their drug-likeness properties, more than half of the compounds considered were expected to have a good bioavailability score (0.55) and obeyed Lipinski’s rule of five.

Table 4.
Physicochemical properties, pharmacokinetics and drug-likeness of identified compounds according to SwissADME software.
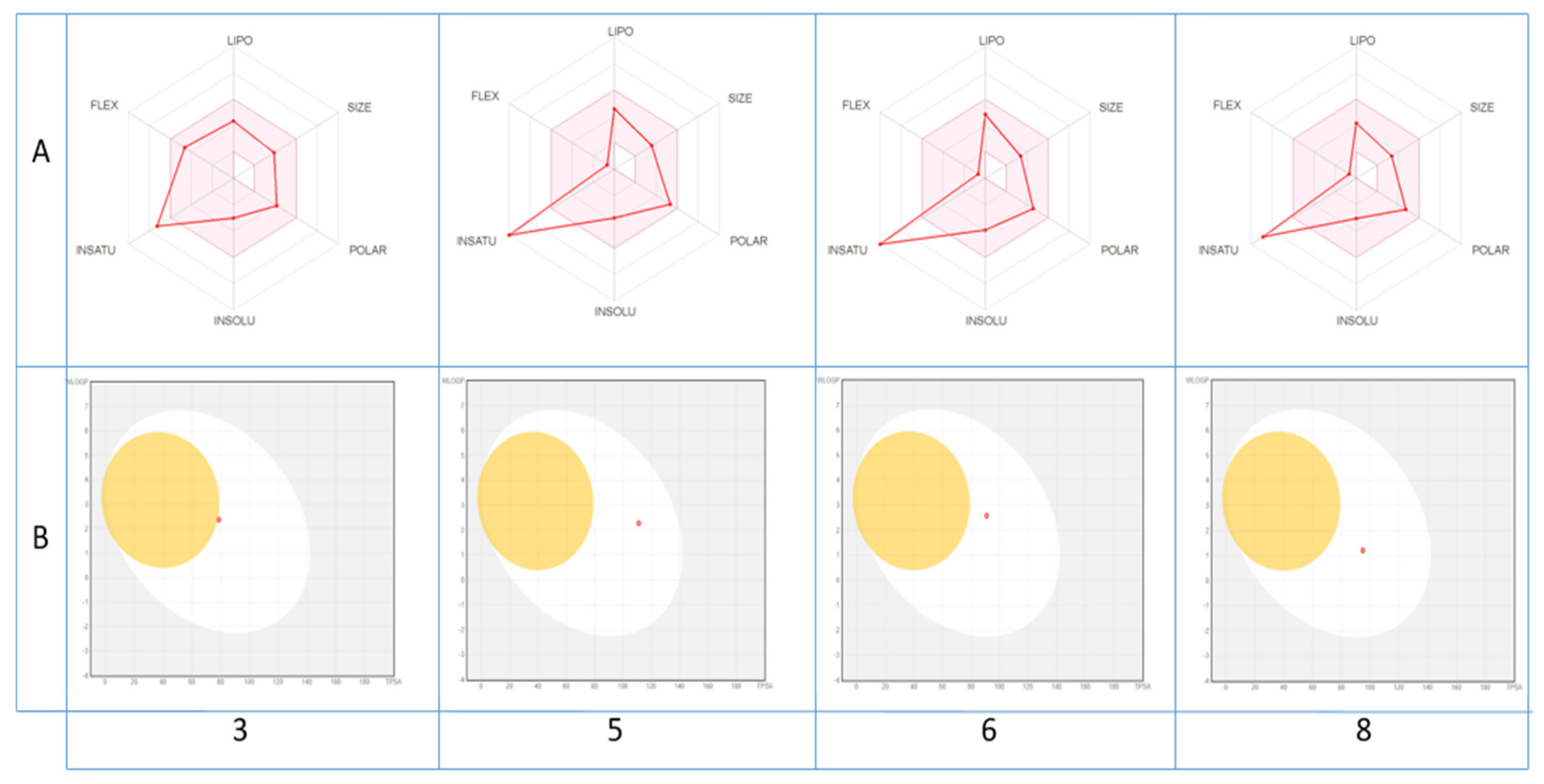
The druglikeness of the identified molecules can be estimated also through a visualization of their bioavailability radar (Figure 3A), with the pink area representing the optimal range for each property (lipophilicity, size, polarity, solubility, unsaturation and flexibility).
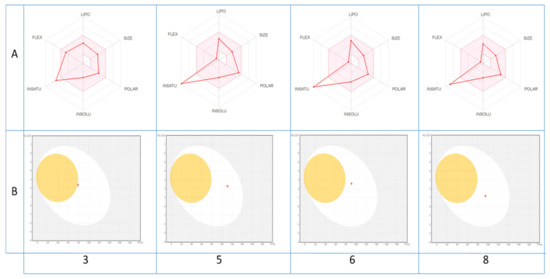
Figure 3.
Bioavailability radar of (A) the top drug like phytoconstituents based on their suitable physicochemical indices ideal for oral bioavailability and boiled-egg model, (B) top bioavailable phytoconstituents using Swiss ADME predictor. LIPO, Lipophilicity: −0.7 < XLOGP3 < þ5; SIZE, Molecular size: 150 g/mol < mol. wt. < 500 g/mol; POLAR, Polarity: 20 Å2 < TPSA < 130 Å2; INSOLU, Insolubility: 0 < Log S (ESOL) < 6; INSATU, Instauration: 0.25 < Fraction Csp3 < 1; FLEX, Flexibility: 0 < Number of rotatable bonds < 9. The colored zone is the suitable physicochemical space for oral bioavailability.
As shown, all compounds fall entirely in the pink area (except for unsaturation fraction), suggesting their better drug-like properties. In contrast, their pharmacokinetic properties may be also predicted via the passive gastrointestinal absorption (HIA) and brain penetration (BBB) of the top bioavailable compounds as a function of the position of the molecules in the WLOGP-versus-TPSA referential. The depicted results of the BOILED-Egg model (Figure 3B) indicate clearly that only compounds 5, 6, 8 and to lesser degree 3 with a red point in the white ellipse have a high probability of being passively absorbed by the gastrointestinal tract and are non-substrate of the P-gp.
4. Conclusions
The aerial parts of Algerian A. tenuifolius were subjected to successive solvent fractionation, and chloroform extract was targeted. To the best of our knowledge, this work represents the first attempt to study the chemical composition by LC-ESI/MS analysis which led to the tentative identification of 12 compounds out of 17 detected, as well to study the biological activities, especially antibacterial, cytotoxic and antiviral, of the various solvent extracts. In this context, A. tenuifolius chloroformic extract gave interesting results in terms of both antioxidant and antibacterial activities. Our findings confirm the interesting potential of this plant as a valuable source of natural bioactive molecules that can be used in the food industry. The ADME of some isolated compounds from chloroformic extract demonstrate their good bioavailability and drug-likeness properties, especially tamgermanetin, luteolin, apigenin and aloe-emodin, which suggest, in parallel with in vivo and preclinical assays, a future new drug candidate.
Author Contributions
Conceptualization, A.K. (Ayoub Khalfaoui), A.D. and I.M.; Formal analysis, A.K. (Ayoub Khalfaoui), E.N., S.B., K.A. and M.S.; Funding acquisition, M.A.K. and I.M.; Investigation, S.B.; Methodology, A.K. (Ayoub Khalfaoui), E.N., S.B., B.L., M.A. and M.S.; Project administration, E.N., A.D. and I.M.; Resources, A.K. (Ayoub Khalfaoui) and M.A.K.; Software, K.A., B.L. and M.A.; Supervision, A.D. and A.K. (Adel Kadri); Visualization, M.A.; Writing—original draft, A.D., A.K. (Adel Kadri), M.S. and I.M.; Writing—review and editing, A.D., A.K. (Adel Kadri), M.S. and I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Acknowledgments
The authors are grateful to the Algerian Ministry of Higher Education and Scientific Research (MESRS) for the financial support. The authors would like also to thank all of the colleagues who contributed to this study, in particular, Adriano Sterni, from the University of Trento for the LC–MS and mass spectra recording.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ADME | Adsorption: distribution, metabolism and extraction |
| BBB | Blood–brain barrier |
| BE | Butanol extract |
| BHT | Butylated hydroxytoluene |
| CC | Cytotoxic conentration |
| CE | Catechin equivalent |
| CHE | Chloroform extract |
| CVB-3 | Coxsackievirus B-3 |
| CYP | Cytochrome P450 |
| DR | Dry residue |
| EAE | Ethyl acetate extract |
| GAE | Gallic acid equivalent |
| GI | Gastrointestinal |
| HIA | Human intestinal adsorption |
| HSV-2 | Herpes simplex type 2 |
| IZ | Inhibition zone |
| MIC | Minimal inhibitory concentration |
| MBC | Minimal bactericidal concentration |
| NI | Not identified |
| NMR | Nuclear magnetic resonance |
| P-gp | P glycoprotein |
| TPSA | Total prostate specific antigen |
References
- Ahmad, B. Antioxidant activity and phenolic compounds from Colchicum luteum Baker (Liliaceae). Afr. J. Biotechnol. 2010, 9, 5762–5766. [Google Scholar]
- Díaz Linfante, Z.; Asphodelus, L.; Talavera, S.; Andrés, C.; Arista, M.; Piedra, M.; Rico, E.; Crespo, M.; Quintanar, A.; Herrero, A. Flora Ibérica. Consejo Superior de Investigaciones Científicas (CSIC); Real Jardin Botänico: Madrid, Spain, 2013. [Google Scholar]
- Di Petrillo, A.; Fais, A.; Pintus, F.; Santos-Buelga, C.; González-Paramás, A.M.; Piras, V.; Orrù, G.; Mameli, A.; Tramontano, E.; Frau, A. Broad-range potential of Asphodelus microcarpus leaves extract for drug development. BMC Microbiol. 2017, 17, 159. [Google Scholar] [CrossRef] [PubMed]
- Di Petrillo, A.; González-Paramás, A.M.; Era, B.; Medda, R.; Pintus, F.; Santos-Buelga, C.; Fais, A. Tyrosinase inhibition and antioxidant properties of Asphodelus microcarpus extracts. BMC Complement. Altern. Med. 2016, 16, 453. [Google Scholar] [CrossRef] [PubMed]
- Çalış, I.; Birincioğlu, S.S.; Kırmızıbekmez, H.; Pfeiffer, B.; Heilmann, J. Secondary Metabolites from Asphodelus aestivus. Z. Nat. B. 2006, 61, 1304–1310. [Google Scholar] [CrossRef]
- El-Ghaly, E.-S.M. Phytochemical and biological activities of Asphodelus microcarpus leaves. J. Pharmacogn. Phytochem. 2017, 6, 259–264. [Google Scholar]
- Adawia, K. Comparison of the total phenol, flavonoid contents and antioxidant activity of methanolic root extracts of As-phodelus microcarpus and Asphodeline lutea growing in Syria. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 159–164. [Google Scholar]
- Chimona, C.; Karioti, A.; Skaltsa, H.; Rhizopoulou, S. Occurrence of secondary metabolites in tepals of Asphodelus ramosus L. Plant. Biosyst. Int. J. Deal. Asp. Plant. Biol. 2013, 148, 31–34. [Google Scholar]
- Abdel-Mogib, M.; Basaif, S.A. Two new naphthalene and anthraquinone derivatives from Asphodelus tenuifolius. Die Pharm. 2002, 57, 286–287. [Google Scholar]
- Zellagui, A.; Gherraf, N.; Rhouati, S. A Germacrene–D, characteristic essential oil from A. microcarpus Salzm and Viv. flowers growing in Algeria. Glob. J. Biodivers. Sci. Manag. 2013, 3, 108–110. [Google Scholar]
- Fafal, T.; Yilmaz, F.F.; Birincioğlu, S.S.; Hoşgör-Limoncu, M.; Kivçak, B. Fatty acid composition and antimicrobial activity of Asphodelus aestivus seeds. Hum. Vet. Med. 2016, 8, 103–107. [Google Scholar]
- Ghoneim, M.M.; Ma, G.; El-Hela, A.A.; Mohammad, A.-E.I.; Kottob, S.; El-Ghaly, S.; Cutler, S.J.; Ross, S.A. Biologically Active Secondary Metabolites from Asphodelus Microcarpus. Nat. Prod. Commun. 2013, 8, 1117–1119. [Google Scholar] [CrossRef]
- Aslam, N.; Janbaz, K.H.; Jabeen, Q. Hypotensive and diuretic activities of aqueous-ethanol extract of Asphodelus tenuifolius. Bangladesh J. Pharmacol. 2016, 11, 830–837. [Google Scholar] [CrossRef]
- Eddine, L.S.; Segni, L.; Ridha, O.M. In vitro assays of the antibacterial and antioxidant properties of extracts from Asphodelus tenuifolius Cav and its main constituents: A comparative study. Int. J. Pharm. Clin. Res. 2015, 7, 119–125. [Google Scholar]
- Malmir, M.; Serrano, R.; Caniça, M.; Silva-Lima, B.; Silva, O. A Comprehensive Review on the Medicinal Plants from the Genus Asphodelus. Plants 2018, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Dangi, A.; Aparna, M.S.; Yadav, J.; Arora, D.; Chaudhary, U. Antimicrobial potential of Asphodelus tunifolius (CAV). J. Evol. Med. Dent. Sci. 2013, 2, 5663–5667. [Google Scholar]
- Faidi, K.; Hammami, S.; Salem, A.B.; El Mokni, R.; Mastouri, M.; Gorcii, M.; Ayedi, M. Polyphenol derivatives from bioactive butanol phase of the Tunisian narrow-leaved asphodel (Asphodelus tenuifolius Cav., Asphodelaceae). J. Med. Plant. Res. 2014, 8, 550–557. [Google Scholar]
- Kalim, M.D.; Bhattacharyya, D.; Banerjee, A.; Chattopadhyay, S. Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complement. Altern. Med. 2010, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Menghani, E.; Bhatnagar, K.; Saraswat, P.; Soni, M. Isolation and characterization of bioactives from arid zone plants. Int. J. Pharm. Res. Dev. 2012, 4, 113–118. [Google Scholar]
- Panghal, M.; Kaushal, V.; Yadav, J.P. In vitro antimicrobial activity of ten medicinal plants against clinical isolates of oral cancer cases. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 21. [Google Scholar] [CrossRef]
- Vaghasiya, Y.; Chanda, S. Screening of methanol and acetone extracts of fourteen Indian medicinal plants for antimicrobial activity. Turk. J. Biol. 2007, 31, 243–248. [Google Scholar]
- Mahboub, N.; Slimani, N.; Hechifa, D.; Merad, K.; Khelil, A. Study of the effect of drying methods on biochemical determi-nation of some spontaneous plants character medicinales in the Northen Algerian Sahara. Adv. Environ. Biol. 2016, 10, 131–140. [Google Scholar]
- Falleh, H.; Ksouri, R.; Chaieb, K.; Karray-Bouraoui, N.; Trabelsi, N.; Boulaaba, M.; Abdelly, C. Phenolic composition of Cynara cardunculus L. organs, and their biological activities. Comptes Rendus Biol. 2008, 331, 372–379. [Google Scholar] [CrossRef]
- Ncube, B.; Finnie, J.; Van Staden, J. Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S. Afr. J. Bot. 2012, 82, 11–20. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the Total Free Radical Scavenger Capacity of Vegetable Oils and Oil Fractions Using 2,2-Diphenyl-1-picrylhydrazyl Radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Condelli, N.; Caruso, M.C.; Galgano, F.; Russo, D.; Milella, L.; Favati, F. Prediction of the antioxidant activity of extra virgin olive oils produced in the Mediterranean area. Food Chem. 2015, 177, 233–239. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Snoussi, M.; Noumi, E.; Trabelsi, N.; Flamini, G.; Papetti, A.; De Feo, V. Mentha spicata Essential Oil: Chemical Composition, Antioxidant and Antibacterial Activities against Planktonic and Biofilm Cultures of Vibrio spp. Strains. Molecules 2015, 20, 14402–14424. [Google Scholar] [CrossRef]
- Snoussi, M.; Trabelsi, N.; Dehmeni, A.; Benzekri, R.; Bouslama, L.; Hajlaoui, B.; Al-Sieni, A.; Papetti, A. Phytochemical analysis, antimicrobial and antioxidant activities of Allium roseum var. odoratissimum (Desf.) Coss extracts. Ind. Crop. Prod. 2016, 89, 533–542. [Google Scholar] [CrossRef]
- Parveen, M.; Ghalib, R.M.; Khanam, Z.; Mehdi, S.H.; Ali, M. A novel antimicrobial agent from the leaves of Peltophorum vogelianum (Benth.). Nat. Prod. Res. 2010, 24, 1268–1273. [Google Scholar] [CrossRef]
- Gormez, A.; Bozari, S.; Yanmis, D.; Gulluce, M.; Sahin, F.; Agar, G. Chemical composition and antibacterial activity of essential oils of two species of Lamiaceae against phytopathogenic bacteria. Pol. J. Microbiol. 2015, 64, 121–127. [Google Scholar] [CrossRef]
- Boulaaba, M.; Snoussi, M.; Saada, M.; Mkadmini, K.; Smaoui, A.; Abdelly, C.; Ksouri, R. Antimicrobial activities and phy-tochemical analysis of Tamarix gallica extracts. Ind. Crop. Prod. 2015, 76, 1114–1122. [Google Scholar] [CrossRef]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Oketch-Rabah, H.; Dossaji, S.; Mberu, E. Antimalarial Activity of Some Kenyan Medicinal Plants. Pharm. Biol. 1999, 37, 329–334. [Google Scholar] [CrossRef]
- Kadri, A.; Aouadi, K. In vitro antimicrobial and α-glucosidase inhibitory potential of enantiopure cycloalkylglycine deriv-atives: Insights into their in silico pharmacokinetic, druglikeness, and medicinal chemistry properties. J. Appl. Pharm. Sci. 2020, 10, 107–115. [Google Scholar]
- Othman, I.M.; Gad-Elkareem, M.A.; Snoussi, M.; Aouadi, K.; Kadri, A. Novel fused pyridine derivatives containing pyrimidine moiety as prospective tyrosyl-tRNA synthetase inhibitors: Design, synthesis, pharmacokinetics and molecular docking studies. J. Mol. Struct. 2020, 1219, 128651. [Google Scholar] [CrossRef]
- Munir, H.; Sarfraz, R.; Hussain, A.; Shahid, M.; Sultana, B. Antioxidant and antimicrobial activities of different solvent extracts of Asphodilus tenifolius. Oxid. Commun. 2014, 37, 741–754. [Google Scholar]
- Al-Laith, A.A.; Alkhuzai, J.; Freije, A. Assessment of antioxidant activities of three wild medicinal plants from Bahrain. Arab. J. Chem. 2019, 12, 2365–2371. [Google Scholar] [CrossRef]
- Bakari, S.; Ncir, M.; Felhi, S.; Hajlaoui, H.; Saoudi, M.; Gharsallah, N.; Kadri, A. Chemical composition and in vitro evaluation of total phenolic, flavonoid, and antioxydant properties of essential oil and solvent extract from the aerial parts of Teucrium polium grown in Tunisia. Food Sci. Biotechnol. 2015, 24, 1943–1949. [Google Scholar] [CrossRef]
- Bakari, S.; Daoud, A.; Felhi, S.; Smaoui, S.; Gharsallah, N.; Kadri, A. Proximate analysis, mineral composition, phytochemical contents, antioxidant and antimicrobial activities and GC-MS investigation of various solvent extracts of cactus cladode. Food Sci. Technol. 2017, 37, 286–293. [Google Scholar] [CrossRef]
- Reynaud, J.; Lussignol, M.; Flament, M.M.; Becchi, M. Flavonoid content of Asphodelus ramosus (Liliaceae). Can. J. Bot. 1997, 75, 2105–2107. [Google Scholar] [CrossRef]
- El-Fattah, H.A. Chemistry of Asphodelus fistulosus. Int. J. Pharmacogn. 1997, 35, 274–277. [Google Scholar] [CrossRef]
- Adinolfi, M.; Corsaro, M.M.; Lanzetta, R.; Parrilli, M.; Scopa, A. A bianthrone C-glycoside from Asphodelus ramosus tubers. Phytochemistry 1989, 28, 284–288. [Google Scholar] [CrossRef]
- Hammouda, F.; Rizk, A.; El-Nasr, M.S.; Asr, E.-N. Anthraquinones of Certain Egyptian Asphodelus Species. Z. Nat. C 1974, 29, 351–354. [Google Scholar] [CrossRef]
- Oudtshoorn, M.V.R.V. Chemotaxonomic investigations in asphodeleae and aloineae (liliaceae). Phytochemistry 1964, 3, 383–390. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Chini, M.G.; Bouheroum, M.; Belaabed, S.; Lauro, G.; Terracciano, S.; Vaccaro, M.C.; Bruno, I.; Benayache, S.; Mancini, I. Glucopyranosylbianthrones from the Algerian Asphodelus tenuifolius: Structural insights and biological evalua-tion on melanoma cancer cells. J. Nat. Prod. 2018, 81, 1786–1794. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; Elokely, K.M.; El-Hela, A.A.; Mohammad, A.-E.I.; Jacob, M.; Radwan, M.M.; Doerksen, R.J.; Cutler, S.J.; Ross, S.A. Asphodosides A-E, anti-MRSA metabolites from Asphodelus microcarpus. Phytochemistry 2014, 105, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E.; Yenesew, A.; Dagne, E. Chemotaxonomic significance of anthraquinones in the roots of asphodeloideae (asphodelaceae). Biochem. Syst. Ecol. 1995, 23, 277–281. [Google Scholar] [CrossRef]
- Beara, I.N.; Lesjak, M.M.; Četojević-Simin, D.D.; Orčić, D.Z.; Janković, T.; Anačkov, G.T.; Mimica-Dukić, N.M. Phenolic profile, antioxidant, anti-inflammatory and cytotoxic activities of endemic Plantago reniformis G. Beck. Food Res. Int. 2012, 49, 501–507. [Google Scholar] [CrossRef]
- Dave, H.; Ledwani, L. A review on anthraquinones isolated from Cassia species and their applications. Indian J. Nat. Prod. Resour. 2012, 3, 291–319. [Google Scholar]
- De Martino, L.; Mencherini, T.; Mancini, E.; Aquino, R.P.; De Almeida, L.F.R.; De Feo, V. In vitro phytotoxicity and antioxidant activity of selected flavonoids. Int. J. Mol. Sci. 2012, 13, 5406–5419. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Miri, R.; Tavakkoli, M.; Saso, L. Antioxidant Therapy: Current Status and Future Prospects. Curr. Med. Chem. 2011, 18, 3871–3888. [Google Scholar] [CrossRef]
- Mellado, M.; Madrid, A.; Pena-Cortes, H.; López, R.; Jara, C.; Espinoza, L. Antioxidant activity of anthraquinones isolated from leaves of Muehlenbeckia hastulata (je sm.) johnst.(polygonaceae). J. Chil. Chem. Soc. 2013, 58, 1767–1770. [Google Scholar] [CrossRef]
- Zargar, B.A.; Masoodi, M.H.; Ahmed, B.; Ganie, S.A. Phytoconstituents and therapeutic uses of Rheum emodi wall. ex Meissn. Food Chem. 2011, 128, 585–589. [Google Scholar] [CrossRef]
- Felhi, S.; Hajlaoui, H.; Ncir, M.; Bakari, S.; Ktari, N.; Saoudi, M.; Gharsallah, N.; Kadri, A. Nutritional, phytochemical and antioxidant evaluation and FT-IR analysis of freeze dried extracts of Ecballium elaterium fruit juice from three localities. Food Sci. Technol. 2016, 36, 646–655. [Google Scholar] [CrossRef]
- Felhi, S.; Saoudi, M.; Daoud, A.; Hajlaoui, H.; Ncir, M.; Chaabane, R.; El Feki, A.; Gharsallah, N.; Kadri, A. Investigation of phytochemical contents, in vitro antioxidant and antibacterial behavior and in vivo anti-inflammatory potential of Ecballium elaterium methanol fruits extract. Food Sci. Technol. 2017, 37, 558–563. [Google Scholar] [CrossRef]
- Moorthy, K.K.; Subramaniam, P.; Senguttuvan, J. In vitro antifungal activity of various extracts of leaf and stem parts of Solena amplexicaulis (Lam.) Gandhi. Int. J. Pharm. Pharm. Sci. 2013, 5, 745–747. [Google Scholar]
- Soliman, S.S.M.; Semreen, M.H.; El-Keblawy, A.A.; Abdullah, A.; Uppuluri, P.; Ibrahim, A.S. Assessment of herbal drugs for promising anti-Candida activity. BMC Complement. Altern. Med. 2017, 17, 257. [Google Scholar] [CrossRef]
- Dziri, S.; Hassen, I.; Fatnassi, S.; Mrabet, Y.; Casabianca, H.; Hanchi, B.; Hosni, K. Phenolic constituents, antioxidant and an-timicrobial activities of rosy garlic (Allium roseum var. odoratissimum). J. Funct. Foods 2012, 4, 423–432. [Google Scholar] [CrossRef]
- Asdadi, A.; Gharby, S.; Hamdouch, A.; Moutaj, R.; Chebli, B.; Hassani, L.M.I. Screening for antifungal activity of medicinal and aromatic plants is another way to valuing the Moroccan Arganeraie. J. Chem. Pharm. Res. 2016, 8, 590–595. [Google Scholar]
- Salhi, N.; Saghir, S.A.M.; Terzi, V.; Brahmi, I.; Ghedairi, N.; Bissati, S. Antifungal Activity of Aqueous Extracts of Some Dominant Algerian Medicinal Plants. BioMed Res. Int. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Marmonier, A. Introduction aux techniques d’étude des antibiotiques. In Bactériologie médicale techniques usuelles; Doin: Paris, France, 1990; pp. 227–236. [Google Scholar]
- Selway, J.W. Antiviral activity of flavones and flavans. Prog. Clin. Boil. Res. 1986, 213, 521–536. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).