Natural Chain-Breaking Antioxidants and Their Synthetic Analogs as Modulators of Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. Chemical Synthesis
2.2.1. General Procedure for the Synthesis of Compounds M4, M6 and M7
2.2.2. General Procedure for the Synthesis of Compounds D4 and D6
2.2.3. 3,3′-Dimethoxy-5,5′-di((E)-prop-1-en-1-yl)-[1,1′-biphenyl]-2,2′-diol DisoEu
2.2.4. 5,5′-Diallyl-[1,1′-biphenyl]-2,2′,3,3′-tetraol DHCh
2.2.5. (2E,2′E)-3,3′-(5,5′,6,6′-Tetrahydroxy-[1,1′-biphenyl]-3,3′-diyl) diacrylic acid DCA
2.2.6. 3,3′-(5,5′,6,6′-Tetrahydroxy-[1,1′-biphenyl]-3,3′-diyl) dipropanoic acid DHCA
2.3. Lipid Autoxidation
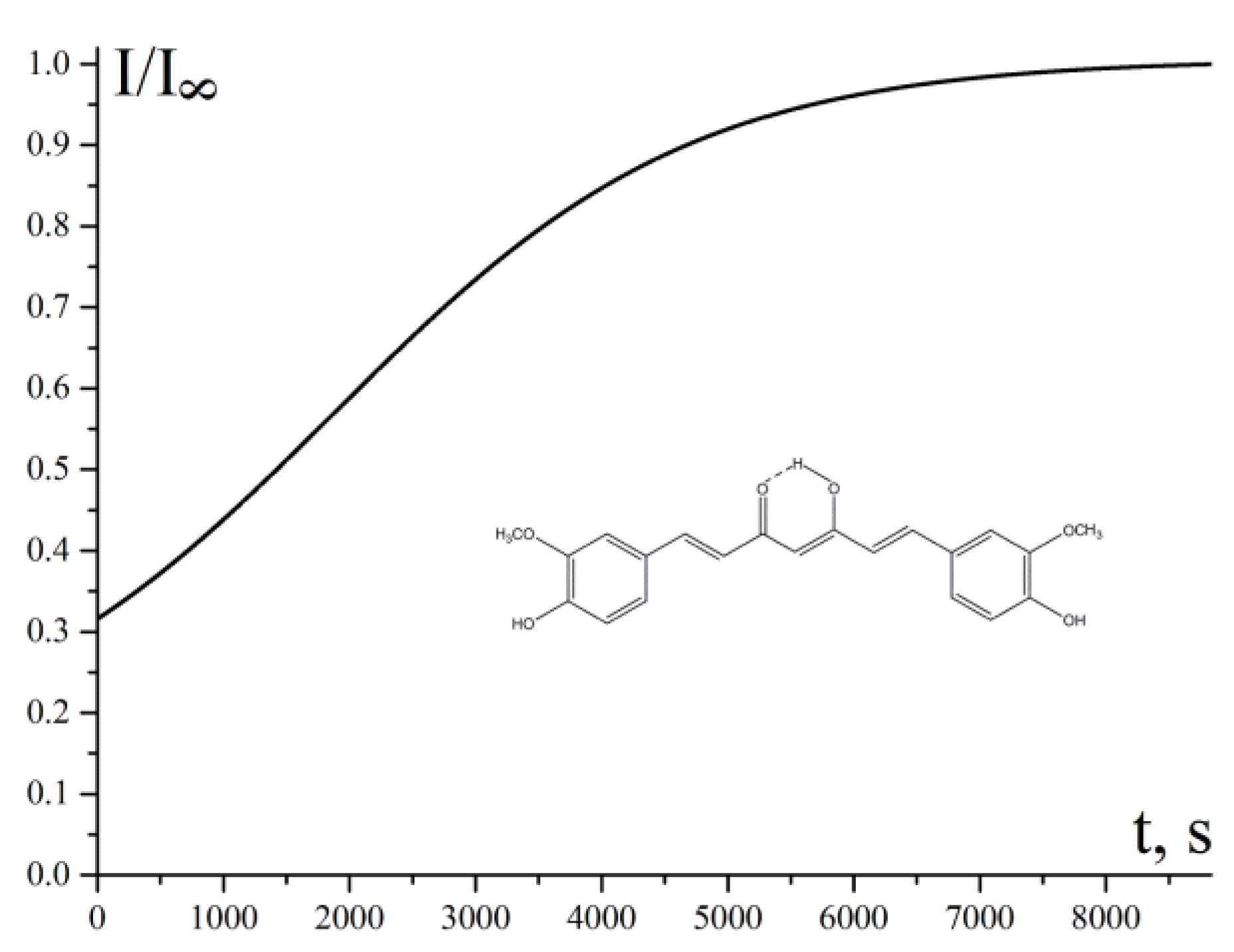
2.3.1. Determination of the Main Kinetic Parameters of the Studied Compounds (Details Are in Supporting Information)
2.3.2. Statistical Analysis
2.4. Estimation of Rate Constant of Antioxidant Reaction with Peroxyl Radicals (kA) Using Kinetic Chemiluminescence (CL) Method (Details Are Provided in Supporting Information)
2.5. Computational Studies
2.5.1. DFT Computational Details
2.5.2. In Silico Prediction of ADME/Tox Properties
- Prediction of various ADME/Tox properties using models implemented in ACD/Percepta software [47];
- Prediction of CNS (central nervous system) access based on estimated brain/plasma equilibration rate, and steady-state brain/plasma distribution ratio.
- Prediction of interactions with P-glycoprotein and Cytochrome P450 based on the respective models in the software platform.
- Toxicity predictions;
- Toxicity prediction for multiple endpoints in a number of mammalian species was carried out using Derek Nexus knowledge-based expert system v.6.1.0 [48]. The system identifies alerts with a particular level of likelihood to exert a given toxic effect. The predictions in Derek Nexus are provided with the following levels of likelihood from highest to lowest order: certain, probable, plausible, equivocal, doubted, improbable, and impossible. In this study, a threshold for the level of likelihood “plausible” was applied meaning “the weight of evidence supports the proposition” [49].
- Toxicity prediction using ACD/Percepta models with probabilistic categorization that is based on calculated of the effect occurrence and the reliability index of the prediction” [47]. The following toxicity endpoints were estimated: mutagenicity based on the AMES test model, acute toxicity, endocrine system disruption, based on estimated compound’s binding affinity to ERα (estrogen receptor alpha).
3. Results and Discussion
3.1. Synthesis of Compounds M4, D4, M6, D6, M7, DisoEu, DHCh, DCA, DHCA
3.2. Experimental Studies of the Antioxidant Activity
3.2.1. Chain-Breaking Antioxidant Activity during Bulk Lipid Autoxidation
- side chain substitution;
- concentrations used (0.1 mm and 1.0 mM);
- presence of biphenyl structure (dimers vs. monomers);
- phenol ring substitution (catechol/guaiacol structure).
- ⮚
- Effect of the substituents in the side chain
- ⮚
- Effect of concentration
- ⮚
- Effect of biphenyl structure
- ⮚
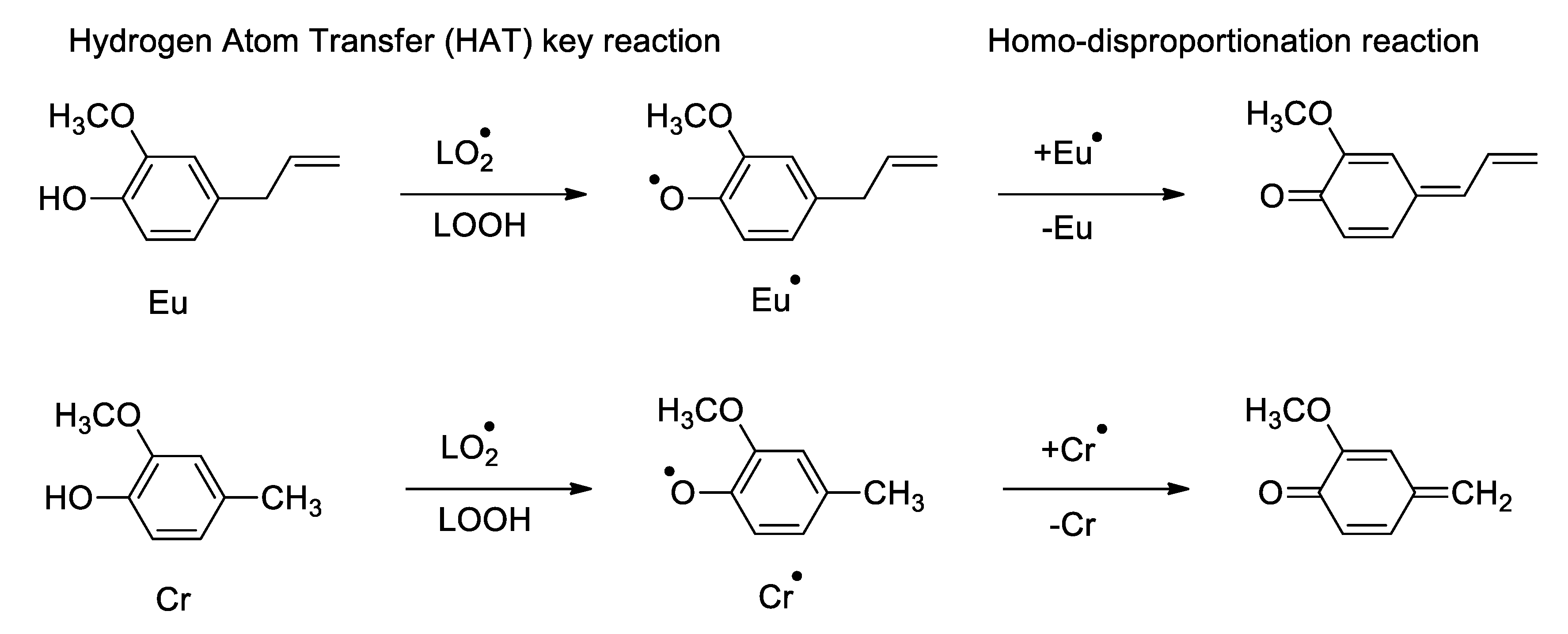
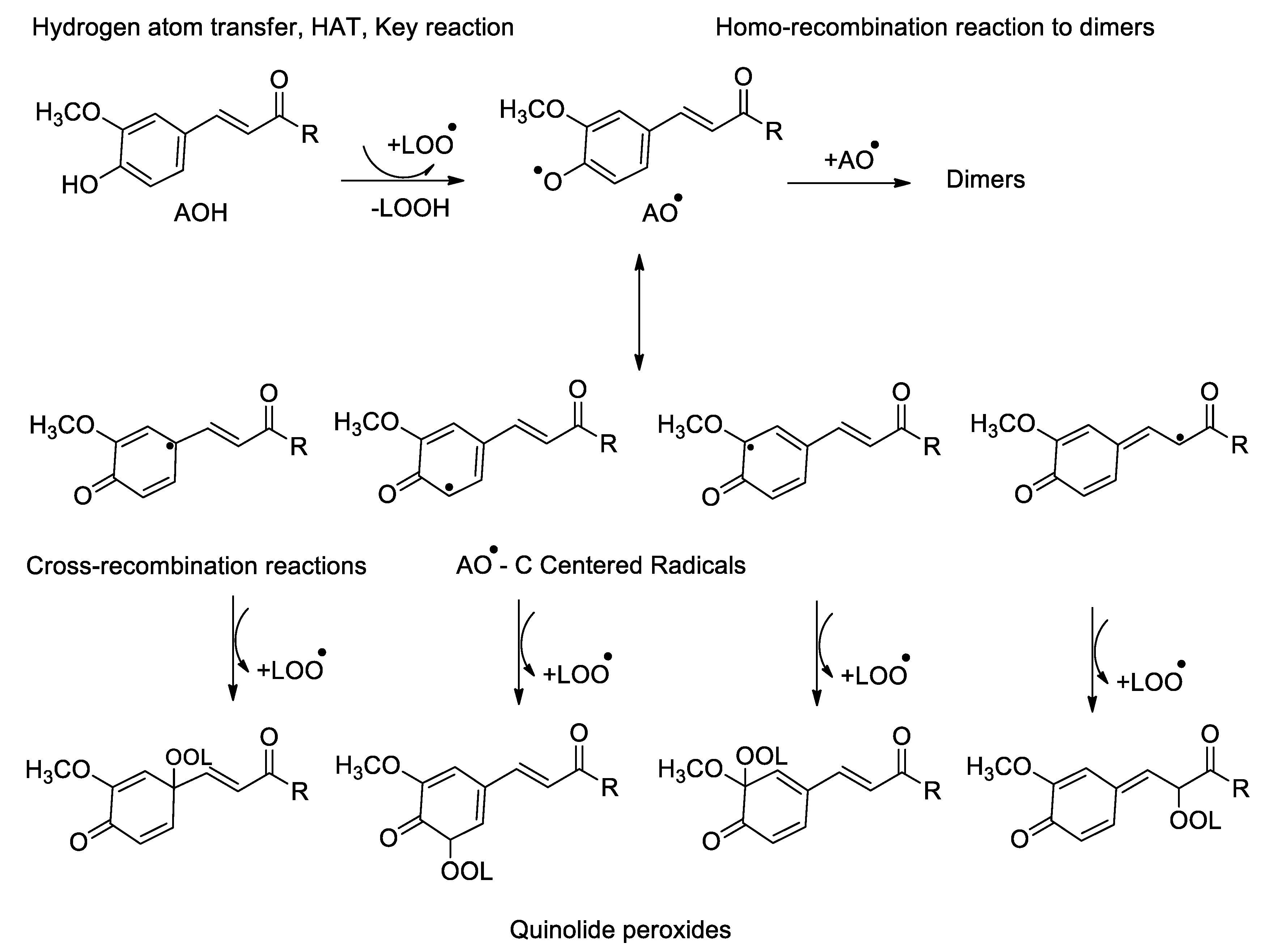
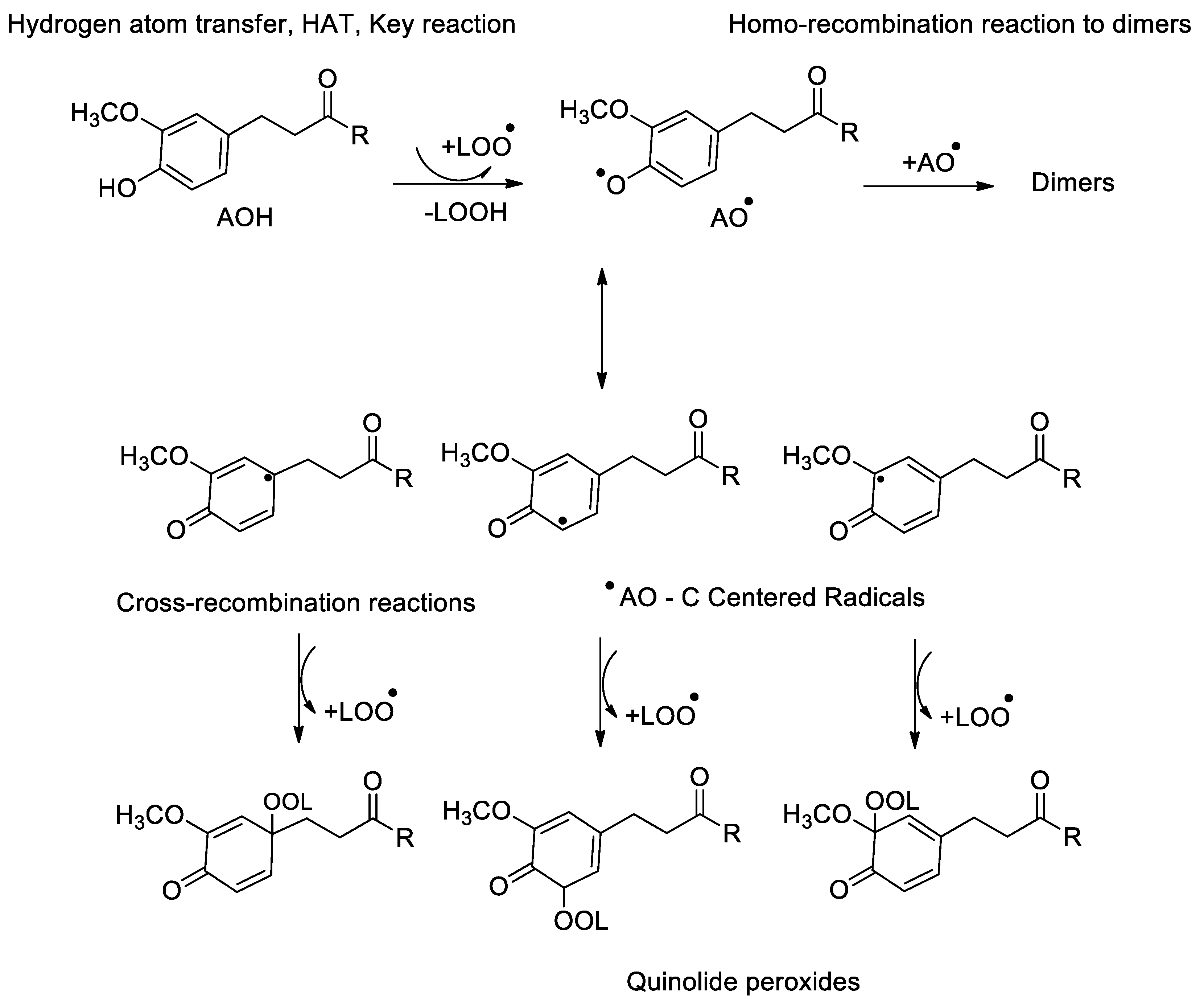
- Reaction mechanism for monophenolic antioxidants
- ⮚
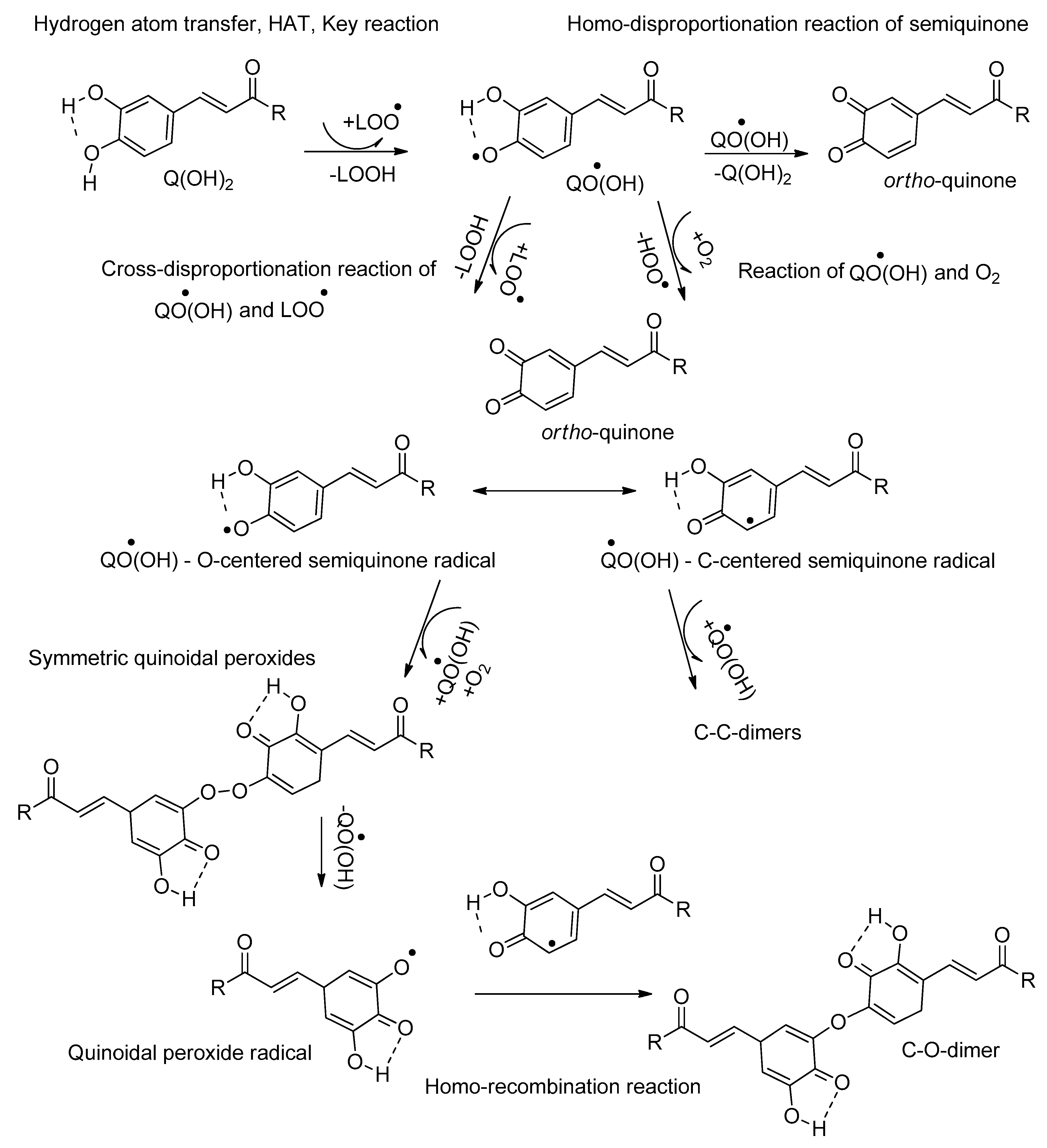
- Activity of ortho-hydroxyphenols (monomers and dimers)
- ⮚
- Reaction mechanism of ortho-hydroxyphenols
- ⮚
- Effect of catechol/guaiacol structures of the examined antioxidants
3.2.2. Chain-Breaking Antioxidant Activity during Initiated Oxidation
3.3. Computational Studies
3.3.1. DFT Calculations
3.3.2. In Silico Predictions of ADME/Tox Properties
3.4. Comparison between Experimentally Obtained and Theoretically Predicted Antioxidant and Biological Activity
4. Conclusions
5. Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Kancheva, V.D. Oxidative Stress and Lipid Oxidation. In Antioxidants—Prevention and Healthy Aging; Ribarova, F., Ed.; Simel Press: Sofia, Bulgaria, 2015; pp. 233–238. [Google Scholar]
- Fedorova, G.F.; Kancheva, V.D.; Menshov, V.A.; Naumov, V.V.; Vasil’ev, R.F.; Veprintsev, T.L.; Trofimov, A.V.; Tsaplev, Y.B.; Yablonskaya, O.I. Chapter 11—Exogenous and Endogenous Mediators of Oxygen Metabolism: Alternatives for Chemical and Biological Activity. In Studies in Natural Products Chemistry; Atta-ur-Rahman, B.T., Ed.; Elsevier Science: Oxford, UK, 2016; Volume 47, pp. 357–385. [Google Scholar] [CrossRef]
- Kancheva, V.D.; Kasaikina, O.T. Bio-antioxidants—A chemical base of their antioxidant activity and beneficial effect on human health. Curr. Med. Chem. 2013, 20, 4784–4805. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, V.D. Oxidative Stress and Lipid Oxidation—Non-Inhibited and Inhibited. In Reactive Oxygen Species, Lipid Peroxidation and Protein Oxidation; Catala, A., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2010; pp. 1–41. [Google Scholar]
- Burlakova, E.B. Bioantioxidants. Molecular cell biophysics. Russ. Chem. J. 2007, 51, 3–12. [Google Scholar]
- Nikolova, G. Oxidative stress and Parkinson disease. Trakia J. Sci. 2013, 10, 92–100. [Google Scholar]
- Kancheva, V.D. Phenolic Antioxidants of Natural Origin—Structure Activity Relationship and their Beneficial Effect on Human Health. In Phytochemicals and Human Health: Pharmacological and Molecular Aspects; Faraooqui, A.A., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2012; pp. 1–45. [Google Scholar]
- Tichonov, I.; Roginsky, V.; Pliss, E. Natural polyphenols as chain-breaking antioxidants during methyl linoleate peroxidation. Eur. J. Lipid Sci. Technol. 2010, 112, 887–893. [Google Scholar] [CrossRef]
- Kancheva, V.D.; Kasaikina, O.T. Lipid Oxidation in Homogeneous and Micro-heterogeneous Media in Presence of Prooxidants, Antioxidants and Surfactants. In Lipid Peroxidation Inhibition, Effects and Mechanisms; Catala, A., Ed.; InTech Open: London, UK, 2012; pp. 31–62. [Google Scholar]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Bors, W.; Kazazic, S.P.; Michel, C.; Kortenska, V.D.; Stettmaier, K.; Klasinc, L. Methoxyphenols—Antioxidant principles in food plants and spices: Pulse radiolysis, EPR spectroscopy, and density functional theory calculations*. Int. J. Quantum Chem. 2002, 90, 969–979. [Google Scholar] [CrossRef]
- Slavova-Kazakova, A.K.; Angelova, S.E.; Veprintsev, T.L.; Denev, P.; Fabbri, D.; Dettori, M.A.; Kratchanova, M.; Naumov, V.V.; Trofimov, A.V.; Vasil’ev, R.F.; et al. Antioxidant potential of curcumin-related compounds studied by chemiluminescence kinetics, chain-breaking efficiencies, scavenging activity (ORAC) and DFT calculations. Beilstein J. Org. Chem. 2015. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its derivatives: Their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrue 2018, 10, 855. [Google Scholar] [CrossRef]
- Tsaplev, Y.B.; Lapina, V.A.; Trofimov, A.V. Curcumin in dimethyl sulfoxide: Stability, spectral, luminescent and acid-base properties. Dyes Pigments 2020, 177, 108327. [Google Scholar] [CrossRef]
- Kancheva, V.; Slavova-Kazakova, A.; Fabbri, D.; Dettori, M.A.; Delogu, G.; Janiak, M.; Amarowicz, R. Protective effects of equimolar mixtures of monomer and dimer of dehydrozingerone with α-tocopherol and/or ascorbyl palmitate during bulk lipid autoxidation. Food Chem. 2014, 157, 263–274. [Google Scholar] [CrossRef]
- Katsori, A.-M.; Chatzopoulou, M.; Dimas, K.; Kontogiorgis, C.; Patsilinakos, A.; Trangas, T.; Hadjipavlou-Litina, D. Curcumin analogues as possible anti-proliferative & anti-inflammatory agents. Eur. J. Med. Chem. 2011, 46, 2722–2735. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Liang, G.; Yang, S.; Zhou, H.; Shao, L.; Huang, K.; Xiao, J.; Huang, Z.; Li, X. Synthesis, crystal structure and anti-inflammatory properties of curcumin analogues. Eur. J. Med. Chem. 2009, 44, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Chen, Y.; Li, Y.-P.; Chen, S.-H.; Tan, J.-H.; Ou, T.-M.; Gu, L.Q.; Huang, Z.S. Design, synthesis, and biological evaluation of curcumin analogues as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2011, 19, 5596–5604. [Google Scholar] [CrossRef] [PubMed]
- Roginsky, V.; Lissi, E.A. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005, 92, 235–254. [Google Scholar] [CrossRef]
- Kancheva, V.D. Phenolic antioxidants—Radical-scavenging and chain-breaking activity: A comparative study. Eur. J. Lipid Sci. Technol. 2009, 111, 1072–1089. [Google Scholar] [CrossRef]
- Slavova-Kazakova, A.; Angelova, S.; Fabbri, D.; Antonietta Dettori, M.; Kancheva, V.D.; Delogu, G. Antioxidant properties of novel curcumin analogues: A combined experimental and computational study. J. Food Biochem. 2021, 45, e13584. [Google Scholar] [CrossRef]
- Foti, M.C.; Slavova-Kazakova, A.; Rocco, C.; Kancheva, V.D. Kinetics of curcumin oxidation by 2,2-diphenyl-1-picrylhydrazyl (DPPH˙): An interesting case of separated coupled proton–electron transfer. Org. Biomol. Chem. 2016, 14, 8331–8337. [Google Scholar] [CrossRef]
- Ekins, S.; Rose, J. In silico ADME/Tox: The state of the art. J. Mol. Graph. Model. 2002, 20, 305–309. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The application of in silico drug-likeness predictions in pharmaceutical research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef]
- van Mourik, T.; Bühl, M.; Gaigeot, M.-P. Density functional theory across chemistry, physics and biology. Philos. Trans. A Math. Phys. Eng. Sci. 2014, 372, 20120488. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, F.; Zeng, Z.; Wen, L.; Xiao, Z.; Bu, H.; Lv, F.; Xu, Z.; Lin, Q. DFT-based quantitative structure–activity relationship studies for antioxidant peptides. Struct. Chem. 2015, 26, 739–747. [Google Scholar] [CrossRef]
- Koleva, L.; Angelova, S.; Dettori, M.A.; Fabbri, D.; Delogu, G.; Kancheva, V.D. Antioxidant activity of selected o-Antioxidant activity of selected o-methoxyphenols and biphenols: Theoretical and experimental studies. Bulg. Chem. Commun. 2018, 50, 238–246. [Google Scholar]
- Marchiani, A.; Mammi, S.; Siligardi, G.; Hussain, R.; Tessari, I.; Bubacco, L.; Delogu, G.; Fabbri, D.; Dettori, M.A.; Sanna, D.; et al. Small molecules interacting with α-synuclein: Antiaggregating and cytoprotective properties. Amino Acids 2013, 45, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Scobbie, L.; Chesson, A. Structural modification of phenylpropanoid-derived compounds and the effects on their participation in redox processes. Bioorg. Med. Chem. 2005, 13, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Wenskowsky, L.; Wagner, M.; Reusch, J.; Schreuder, H.; Matter, H.; Opatz, T.; Petry, S.M. Resolving Binding Events on the Multifunctional Human Serum Albumin. ChemMedChem 2020, 15, 738–743. [Google Scholar] [CrossRef]
- Dettori, M.A.; Pisano, M.; Rozzo, C.; Delogu, G.; Fabbri, D. Synthesis of Hydroxylated Biphenyl Derivatives Bearing an α,β-Unsaturated Ketone as a Lead Structure for the Development of Drug Candidates against Malignant Melanoma. ChemMedChem 2021, 16, 1022–1033. [Google Scholar] [CrossRef]
- Nishiwaki, K.; Ohigashi, K.; Deguchi, T.; Murata, K.; Nakamura, S.; Matsuda, H.; Nakamura, S.; Matsuda, H.; Nakanishi, I. Structure–Activity Relationships and Docking Studies of Hydroxychavicol and Its Analogs as Xanthine Oxidase Inhibitors. Chem. Pharm. Bull. 2018, 66, 741–747. [Google Scholar] [CrossRef]
- Aung, H.T.; Furukawa, T.; Nikai, T.; Niwa, M.; Takaya, Y. Contribution of cinnamic acid analogues in rosmarinic acid to inhibition of snake venom induced hemorrhage. Bioorg. Med. Chem. 2011, 19, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, G.F.; Menshov, V.A.; Trofimov, A.V.; Vasil’ev, R.F. Facile chemiluminescence assay for antioxidative properties of vegetable lipids: Fundamentals and illustrative examples. Analyst 2009, 134, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision d. 01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Hehre, W.J.; Lathan, W.A. Self-Consistent Molecular Orbital Methods. XIV. An Extended Gaussian-Type Basis for Molecular Orbital Studies of Organic Molecules. Inclusion of Second Row Elements. J. Chem. Phys. 1972, 56, 5255–5257. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Anouar, E.; Košinová, P.; Kozlowski, D.; Mokrini, R.; Duroux, J.L.; Trouillas, P. New aspects of the antioxidant properties of phenolic acids: A combined theoretical and experimental approach. Phys. Chem. Chem. Phys. 2009, 11, 7659–7668. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System; Version 1.7.6.6; Schrödinger, LLC: New York, NY, USA, 2015.
- Avdeef, A. Absorption and Drug Development: Solubility, Permeability, and Charge State, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Diukendjieva, A.; Alov, P.; Tsakovska, I.; Pencheva, T.; Richarz, A.; Kren, V.; Cronin, M.T.; Pajeva, I. In vitro and in silico studies of the membrane permeability of natural flavonoids from Silybum marianum (L.) Gaertn. and their derivatives. Phytomedicine 2019, 53, 79–85. [Google Scholar] [CrossRef]
- ACD/Percepta; Advanced Chemistry Development, Inc.: Toronto, Japan, 2021; Available online: www.acdlabs.com (accessed on 29 March 2021).
- Marchant, C.A.; Briggs, K.A.; Long, A. In Silico Tools for Sharing Data and Knowledge on Toxicity and Metabolism: Derek for Windows, Meteor, and Vitic. Toxicol. Mech. Methods 2008, 18, 177–187. [Google Scholar] [CrossRef]
- Judson, P.N.; Stalford, S.A.; Vessey, J. Assessing confidence in predictions made by knowledge-based systems. Toxicol. Res. 2013, 2, 70–79. [Google Scholar] [CrossRef]
- Feng, J.-Y.; Liu, Z.-Q. Feruloylacetone as the model compound of half-curcumin: Synthesis and antioxidant properties. Eur. J. Med. Chem. 2011, 46, 1198–1206. [Google Scholar] [CrossRef]
- Baschieri, A.; Pulvirenti, L.; Muccilli, V.; Amorati, R.; Tringali, C. Chain-breaking antioxidant activity of hydroxylated and methoxylated magnolol derivatives: The role of H-bonds. Org. Biomol. Chem. 2017, 15, 6177–6184. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Shin, S.H.; Kim, H.O.; Rim, K.T. Globally Harmonized System of Classification and Labelling of Chemicals (GHS). J. Appl. Biol. Chem. 2019, 62, 355–359. [Google Scholar] [CrossRef]
- Oufensou, S.; Casalini, S.; Balmas, V.; Carta, P.; Chtioui, W.; Dettori, M.A.; Fabbri, D.; Migheli, Q.; Delogu, G. Prenylated Trans-Cinnamic Esters and Ethers against Clinical Fusarium spp.: Repositioning of Natural Compounds in Antimicrobial Discovery. Molecules 2021, 26, 658. [Google Scholar] [CrossRef] [PubMed]
- Pani, G.; Scherm, B.; Azara, E.; Balmas, V.; Jahanshiri, Z.; Carta, P.; Fabbri, D.; Dettori, M.A.; Fadda, A.; Dessì, A.; et al. Natural and Natural-like Phenolic Inhibitors of Type B Trichothecene in Vitro Production by the Wheat (Triticum sp.) Pathogen Fusarium culmorum. J. Agric. Food Chem. 2014, 62, 4969–4978. [Google Scholar] [CrossRef]
- Hirose, M.; Fukushima, S.; Shirai, T.; Hasegawa, R.; Kato, T.; Tanaka, H.; Asakawa, E.; Ito, N. Stomach Carcinogenicity of Caffeic Acid, Sesamol and Catechol in Rats and Mice. Jpn. J. Cancer Res. 1990, 81, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Takesada, Y.; Tanaka, H.; Tamano, S.; Kato, T.; Shirai, T. Carcinogenicity of antioxidants BHA, caffeic acid, sesamol, 4-methoxyphenol and catechol at low doses, either alone or in combination, and modulation of their effects in a rat medium-term multi-organ carcinogenesis model. Carcinogenesis 1998, 19, 207–212. [Google Scholar] [CrossRef]
- Ito, N.; Hirose, M.; Shirai, T. Carcinogenicity and Modification of Carcinogenic Response by Plant Phenols. In Phenolic Compounds in Food and Their Effects on Health II; AACS Simposium Series; Oxford University Press: Oxford, UK, 1992; Volume 507, pp. 20–269. [Google Scholar] [CrossRef]
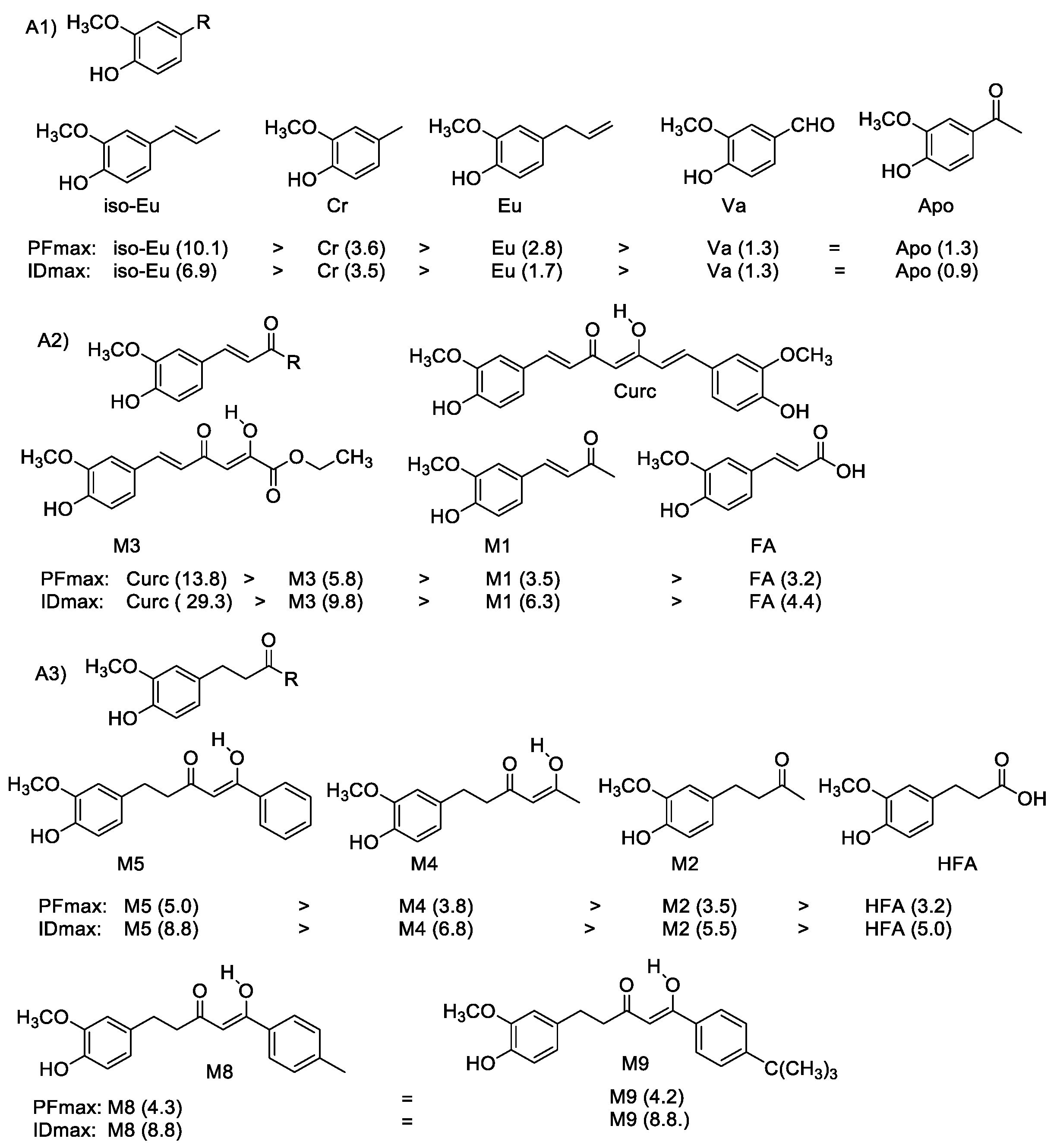
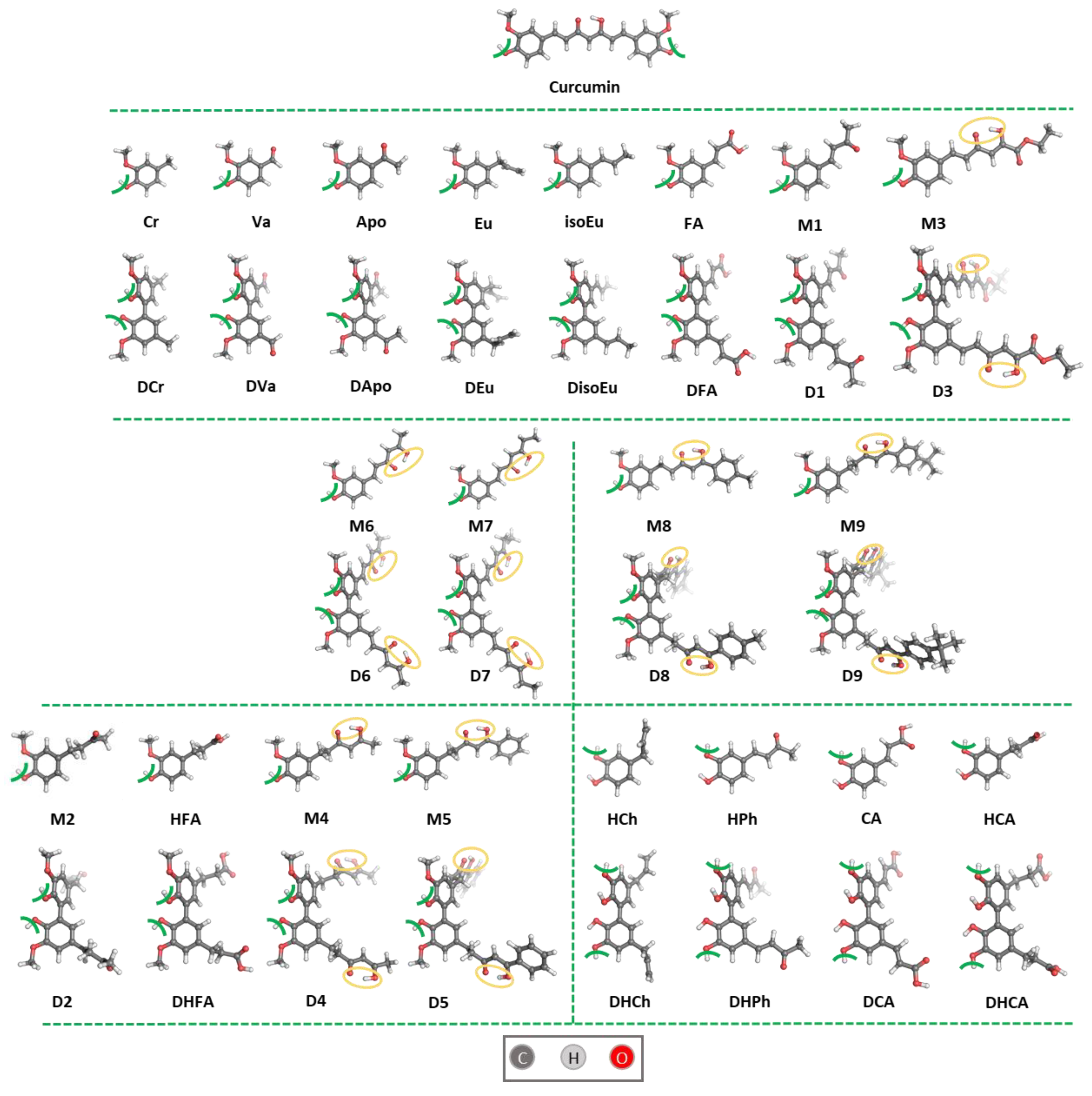

| (A) Ortho-methoxyphenols | ||
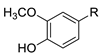 | R |  |
| (A1) | ||
| Cr | –CH3 | DCr |
| Va | –CHO | DVa |
| Apo | –COCH3 | DApo |
| Eu | 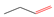 | DEu |
| IsoEu |  | DisoEu |
| (A2) | ||
| FA | 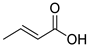 | DFA |
| M1 |  | D1 |
| M6 | 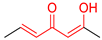 | D6 |
| M7 | 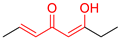 | - |
| M3 | 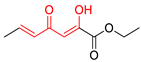 | D3 |
| Curc | 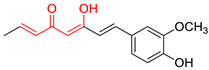 | - |
| (A3) | ||
| HFA | 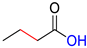 | DHFA |
| M2 | 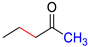 | D2 |
| M4 | 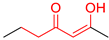 | D4 |
| M5 |  | D5 |
| M8 | 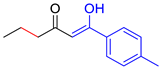 | D8 |
| M9 | 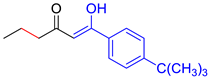 | D9 |
| (B) Ortho-hydroxyphenols | ||
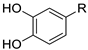 | R | 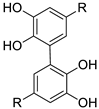 |
| (B1) | ||
| HCh |  | DHCh |
| (B2) | ||
| HPh | 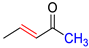 | DHPh |
| CA | 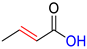 | DCA |
| (B3) | ||
| HCA | 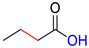 | DHCA |
| Abbr. | PF 0.1 mM | PF 1.0 mM | Effect | ID 0.1 mM | ID 1.0 mM | Effect | References |
|---|---|---|---|---|---|---|---|
| Monomers | |||||||
| (A1) | |||||||
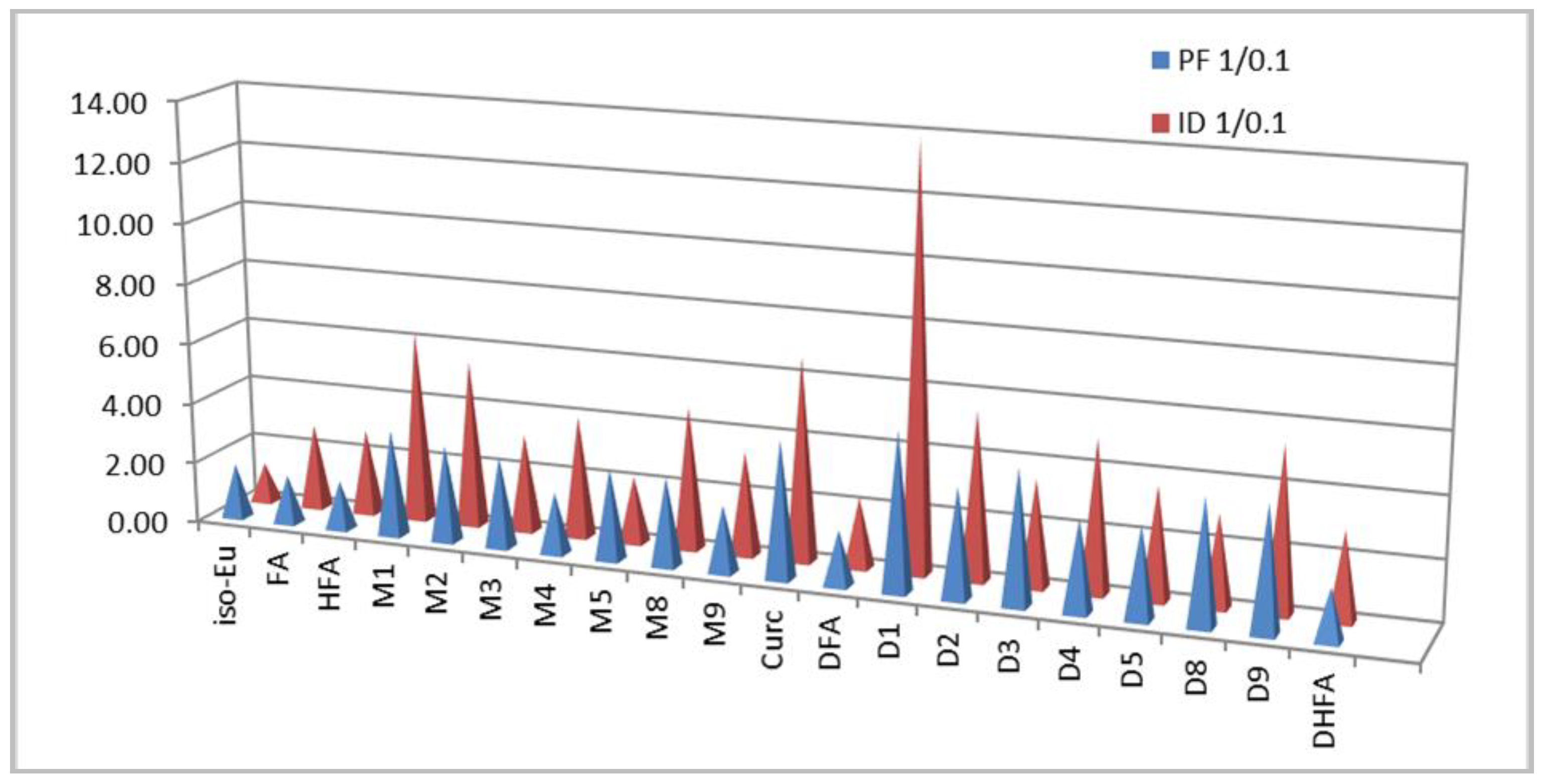
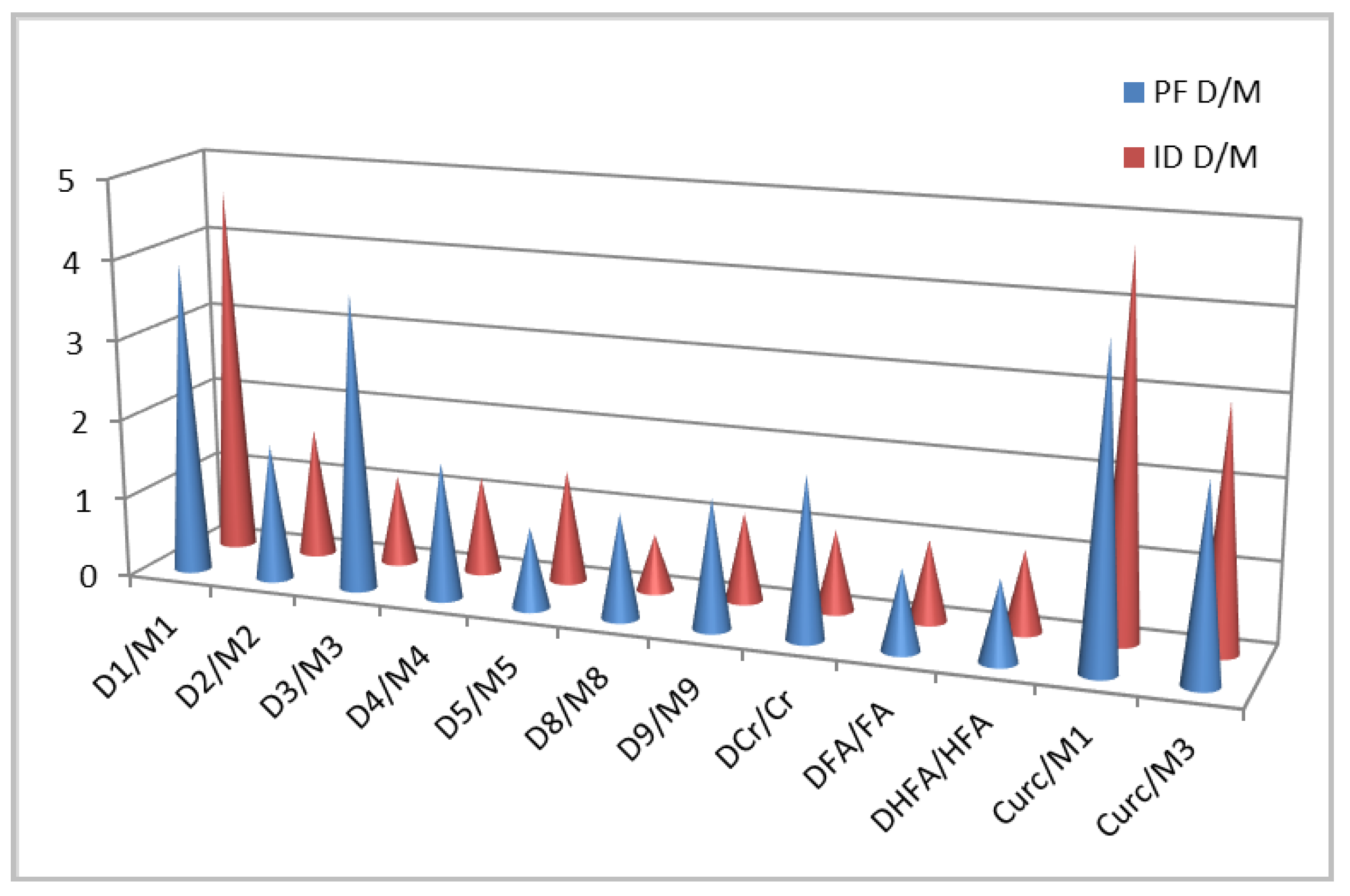
| Cr | 2.7 | 3.6 | Moderate | 3.3 | 3.5 | Moderate | [31] |
| Va | 1.1 | 1.3 | Weak | 1.3 | 1.3 | Weak | [31] |
| Apo | 1.1 | 1.3 | Weak | 0.7 | 0.9 | Weak | [31] |
| Eu | 2.5 | 3.2 | Moderate | 2.4 | 2.9 | Weak | [31] |
| iso-Eu | 5.6 | 10.1 | Strongest | 5.2 | 6.9 | Strong | [31] |
| (A2) | |||||||
| FA | 1.5 | 3.2 | Moderate | 1.6 | 4.4 | Moderate | [13,24] |
| M1 | 1.0 | 3.5 | Moderate | 1.0 | 6.3 | Strong | [13] |
| M3 | 1.9 | 5.8 | Strong | 3.1 | 9.8 | Strongest | [13] |
| Curc | 3.1 | 13.8 | Strongest | 4.4 | 29.3 | Strongest | [13] |
| (A3) | |||||||
| HFA | 1.3 | 3.2 | Moderate | 1.3 | 5.0 | Moderate | Tw |
| M2 | 1.1 | 3.5 | Moderate | 1.0 | 5.5 | Strong | [13] |
| M4 | 1.9 | 3.8 | Moderate | 1.5 | 6.8 | Strong | Tw |
| M5 | 1.7 | 5.0 | Strong | 4.0 | 8,8 | Strong | [25] |
| M8 | 1.5 | 4.3 | Moderate | 2.7 | 8.8 | Strong | [25] |
| M9 | 1.9 | 4.2 | Moderate | 2.6 | 8.8 | Strong | [25] |
| Dimers | |||||||
| DCr | 5.2 | 7.2 | Strong | 2.1 | 3.2 | Moderate | [31] |
| DFA | 1.8 | 3.3 | Moderate | 1.8 | 4.2 | Moderate | [13] |
| D1 | 2.6 | 13.5 | Strongest | 2.1 | 29.3 | Strongest | [13] |
| D3 | 2.9 | 12.8 | Strongest | 3.1 | 11.0 | Strongest | [13] |
| DHFA | 1.6 | 3.8 | Moderate | 1.6 | 4.8 | Moderate | Tw |
| D2 | 1.6 | 5.8 | Strong | 1.6 | 8.8 | Strong | [13] |
| D4 | 2.1 | 6.4 | Strong | 1.7 | 8.0 | Strong | Tw |
| D5 | 1.6 | 4.9 | Moderate | 1.7 | 6.3 | Strong | [25] |
| D8 | 1.4 | 5.8 | Strong | 2.0 | 5.9 | Strong | [25] |
| D9 | 1.7 | 6.9 | Strong | 1.8 | 9.8 | Strongest | [25] |
| Abbr. | PF 0.1 mM | PF 1.0 mM | Activity | ID 0.1 mM | ID 1.0 mM | Activity | Refs. |
|---|---|---|---|---|---|---|---|
| CA | 6.7 | 33.1 | Strongest | 9.3 | 28.0 | Strongest | [23] |
| HPh | 14.6 | 46.2 | Strongest | 43.8 | 58.7 | Strongest | tw |
| HCh | 8.2 | 15.2 | Strongest | n.d. | n.d. | - | tw |
| HCA | 6.7 | 20.7 | Strongest | 13.7 | 36.7 | Strongest | tw |
| DHCh | 12.5 | 15.2 | Strongest | n.d. | n.d. | - | tw |
| DCA | n.s. | n.s. | - | n.s. | n.s. | - | tw |
| DHPh | n.s. | n.s. | - | n.s. | n.s. | - | tw |
| DHCA | n.s. | n.s. | - | n.s. | n.s. | - | tw |
| Abbr. | Compound | kA (M−1s−1) |
|---|---|---|
| Curc | 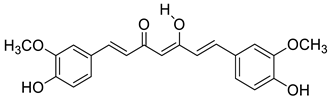 | (4.3 ± 0.3) × 104 |
| FA | 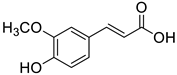 | (1.6 ± 0.1) × 104 |
| DFA | 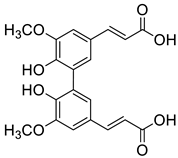 | (4.1 ± 0.3) × 104 |
| M1 | 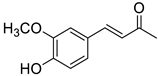 | (1.7 ± 0.1) × 104 |
| D1 | 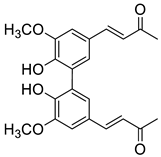 | (4.2 ± 0.3) × 104 |
| M2 | 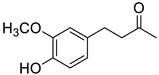 | (2.6 ± 0.3) × 104 |
| D2 | 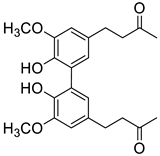 | (5.1 ± 0.3) × 104 |
| M3 | 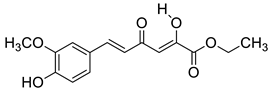 | (2.4 ± 0.3) × 104 |
| D3 | 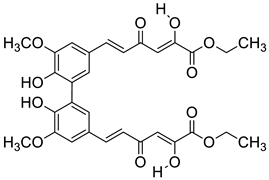 | (3.7 ± 0.3) × 104 |
| Compound | logP | logS | MAD (%HIA) | BBB | P-gp | Mammal Hepato-Toxicity | Mammal Carcino-Genicity | Hazard Category |
|---|---|---|---|---|---|---|---|---|
| A1 | ||||||||
| Cr | 1.83 | −1.70 | 27.7 (28%) | P | 0.05 | A | IV | |
| DCr | 2.57 | −3.31 | 0.3 (0%) | P | 0.11 * | A | IV | |
| Va | 1.29 | −1.41 | 11.5 (11%) | W | 0.04 | IV | ||
| DVa | 1.53 | −2.20 | 1.0 (1%) | N | 0.08 | V | ||
| Apo | 1.4 | −1.70 | 13.8 (14%) | P | 0.06 | IV | ||
| DApo | 1.83 | −3.13 | 0.2 (0%) | N | 0.22 | IV | ||
| Eu | 2.48 | −2.08 | 11.3 (11%) | P | 0.06 | A | IV | |
| DEu | 3.43 | −4.27 | 0.0 (0%) | P | 0.16 * | A | V | |
| iso-Eu | 2.55 | −2.30 | 9.3 (9%) | P | 0.05 | A | IV | |
| Diso-Eu | 3.60 | −4.56 | 0.0 (0%) | P | 0.13 | A | V | |
| A2 | ||||||||
| FA | 1.68 | 0.71 | 139.9 (100%) | N | 0.02 | V | ||
| DFA | 1.86 | 0.41 | 35.0 (35%) * | N | 0.02 * | V | ||
| M1 | 1.69 | −2.02 | 14.9 (15%) | P | 0.10 | IV | ||
| D1 | 2.13 | −3.97 | 0.1 (0%) | P | 0.57 * | V | ||
| M6 | 1.62 | −2.35 | 2.9 (3%) | P | 0.09 * | IV | ||
| D6 | 2.32 | −4.74 | 0.0 (0%) | W | 0.60 * | V | ||
| M7 | 1.98 | −2.67 | 1.8 (2%) | P | 0.10 * | IV | ||
| M3 | 1.03 | −2.56 | 1.2 (1%) | P | 0.14 * | IV | ||
| D3 | 1.75 | −5.01 | 0.0 (0%) | N | 0.70 * | V | ||
| Curc | 2.64 | −3.63 | 0.4 (0%) | P | 0.40 | V | ||
| A3 | ||||||||
| HFA | 1.27 | 0.71 | 529.1 (100%) | N | 0.03 | A, B | V | |
| DHFA | 1.17 | 0.41 | 34.3 (34%) * | N | 0.02 | A, B | V | |
| M2 | 1.1 | −1.44 | 55.6 (56%) | P | 0.07 | A | V | |
| D2 | 1.06 | −2.96 | 1.9 (2%) | W | 0.21 * | A | V | |
| M4 | 1.18 | −2.30 | 11.3 (11%) | P | 0.08 | A | IV | |
| D4 | 1.83 | −4.80 | 0.2 (0%) | W | 0.39 * | A | V | |
| M5 | 2.41 | −3.81 | 3.7 (4%) | P | 0.19 * | A | IV | |
| D5 | 4.16 | −7.26 | 0.0 (0%) * | N | 0.76 * | A | IV | |
| M8 | 2.86 | −3.89 | 1.4 (1%) | P | 0.22 * | A | IV | |
| D8 | 4.76 | −7.69 | 0.0 (0%) * | N | 0.84 * | A | IV | |
| M9 | 3.83 | −4.64 | 0.4 (0%) | P | 0.42 * | A | V | |
| D9 | 7.2 | -8.95 | 0.0 (0%) * | N | 0.94 * | A | V | |
| B1 | ||||||||
| HCh | 1.9 | −1.00 | 9.7 (10%) | P | 0.04 | A | C | IV |
| DHCh | 2.92 | −2.85 | 0.1 (0%) | P | 0.06 | A | IV | |
| B2 | ||||||||
| HPh | 1.68 | −1.03 | 14.4 (14%) | P | 0.04 | C | IV | |
| DHPh | 1.92 | −2.86 | 0.3 (0%) | P | 0.12 * | V | ||
| CA | 1.35 | 0.74 | 124.7 (100%) | W | 0.01 | C | V | |
| DCA | 1.32 | 0.45 | 10.8 (11%) * | N | 0.00 | V | ||
| B3 | ||||||||
| HCA | 0.83 | 0.74 | 476.3 (100%) | N | 0.02 | A, B | C | IV |
| DHCA | 0.46 | 0.44 | 10.7 (11%) * | N | 0.01 | A, B | V | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kancheva, V.D.; Dettori, M.A.; Fabbri, D.; Alov, P.; Angelova, S.E.; Slavova-Kazakova, A.K.; Carta, P.; Menshov, V.A.; Yablonskaya, O.I.; Trofimov, A.V.; et al. Natural Chain-Breaking Antioxidants and Their Synthetic Analogs as Modulators of Oxidative Stress. Antioxidants 2021, 10, 624. https://doi.org/10.3390/antiox10040624
Kancheva VD, Dettori MA, Fabbri D, Alov P, Angelova SE, Slavova-Kazakova AK, Carta P, Menshov VA, Yablonskaya OI, Trofimov AV, et al. Natural Chain-Breaking Antioxidants and Their Synthetic Analogs as Modulators of Oxidative Stress. Antioxidants. 2021; 10(4):624. https://doi.org/10.3390/antiox10040624
Chicago/Turabian StyleKancheva, Vessela D., Maria Antonietta Dettori, Davide Fabbri, Petko Alov, Silvia E. Angelova, Adriana K. Slavova-Kazakova, Paola Carta, Valerii A. Menshov, Olga I. Yablonskaya, Aleksei V. Trofimov, and et al. 2021. "Natural Chain-Breaking Antioxidants and Their Synthetic Analogs as Modulators of Oxidative Stress" Antioxidants 10, no. 4: 624. https://doi.org/10.3390/antiox10040624
APA StyleKancheva, V. D., Dettori, M. A., Fabbri, D., Alov, P., Angelova, S. E., Slavova-Kazakova, A. K., Carta, P., Menshov, V. A., Yablonskaya, O. I., Trofimov, A. V., Tsakovska, I., & Saso, L. (2021). Natural Chain-Breaking Antioxidants and Their Synthetic Analogs as Modulators of Oxidative Stress. Antioxidants, 10(4), 624. https://doi.org/10.3390/antiox10040624