Abstract
Coenzyme Q10 (CoQ10) is classically viewed as an important endogenous antioxidant and key component of the mitochondrial respiratory chain. For this second function, CoQ molecules seem to be dynamically segmented in a pool attached and engulfed by the super-complexes I + III, and a free pool available for complex II or any other mitochondrial enzyme that uses CoQ as a cofactor. This CoQ-free pool is, therefore, used by enzymes that link the mitochondrial respiratory chain to other pathways, such as the pyrimidine de novo biosynthesis, fatty acid β-oxidation and amino acid catabolism, glycine metabolism, proline, glyoxylate and arginine metabolism, and sulfide oxidation metabolism. Some of these mitochondrial pathways are also connected to metabolic pathways in other compartments of the cell and, consequently, CoQ could indirectly modulate metabolic pathways located outside the mitochondria. Thus, we review the most relevant findings in all these metabolic functions of CoQ and their relations with the pathomechanisms of some metabolic diseases, highlighting some future perspectives and potential therapeutic implications.
1. Introduction
Ubiquinone or coenzyme Q (CoQ) is an endogenously synthesized vital molecule that is present in many unicellular and pluricellular organisms, the latter being mostly localized in the mitochondria. In mammalian cells, at least 14 proteins are needed to synthetize CoQ, and its biosynthesis starts with the formation of a 4-hydroxybenzoic acid (4-HB) head group and a lipophilic polyisoprenoid tail. Whereas the 4-HB is derived from tyrosine or phenylalanine, the polyisoprenoid tail is produced by addition of isopentenyl diphosphate molecules, derived from the mevalonate pathway, to farnesyl diphosphate or geranylgeranyl diphosphate in multiple steps catalyzed by the polyprenil diphosphate synthase. In human and mice, this enzyme is a heterotetramer and the two subunits are encoded by PDSS1 and PDSS2. Another enzyme, encoded by COQ2, mediates the conjugation of the benzoquinone ring to the side chain; while four others, encoded by COQ3, COQ5, COQ6 and COQ7, reside in the mitochondrial inner membrane and modify the benzoquinone ring of CoQ by reactions of methylation, decarboxylation and hydroxylation [1]. Moreover, four other proteins, encoded by COQ4, COQ8A, COQ8B and COQ9, seem to have different regulatory functions over other CoQ biosynthetic proteins [1]. In particular, COQ9 physically interacts with COQ7 and has the ability to bind some CoQ biosynthetic intermediates, including demethoxyubiquinone (DMQ), the substrate for COQ7 [2,3]. Furthermore, some other proteins (ADCK1, ADCK2, ADCK5 and PTC7p), may have the capacity to increase/reduce the CoQ biosynthetic rate by phosphorylating/dephosphorylating some CoQ biosynthetic proteins, e.g., COQ7, or by other unknown mechanisms [1]. However, the CoQ biosynthesis in mitochondria is not fully understood. Interestingly, Golgi has the COQ2 homolog UBIAD1 [4], which also supports the biosynthesis of CoQ also in this organelle.
Mutations in any of the genes involved in CoQ10 biosynthesis cause primary CoQ10 deficiency, a mitochondrial syndrome associated with clinically heterogeneous diseases. Specifically, five major phenotypes have been described: (1) encephalomyopathy (recurrent myoglobinuria, encephalopathy and mitochondrial myopathy), (2) cerebellar ataxia (cerebellar atrophy associated with other neurologic manifestations and, occasionally, endocrine dysfunctions), (3) infantile multisystemic form, (4) isolated myopathy, characterized by muscle weakness, myoglobinuria, exercise intolerance and elevated creatine kinase (CK), and (5) nephropathy. Hypertrophic cardiomyopathy, retinopathy or optic atrophy, and sensorineural hearing loss have been also reported in some patients [5]. Additionally, CoQ10 deficiency has been identified as a secondary consequence of other medical conditions. This category includes patients with mutations in the aprataxin (APTX) gene, causing ataxia and oculomotor apraxia, with mutations in the electron-transferring flavoprotein dehydrogenase gene (ETFDH), causing isolated myopathy, and with mutations in Serine/threonine-protein kinase B-raf (BRAF), causing cardiofaciocutaneous (CFC) syndrome [6]. Moreover, CoQ10 deficiency has been reported in association with pathogenic mitochondrial DNA (mtDNA) depletion, deletions, or point mutations [6]. Also, a decrease in the levels of CoQ biosynthetic proteins has been related to secondary CoQ deficiency in various mouse models of mitochondrial diseases [7], as well as in muscle and adipose tissue of patients and a mouse model with insulin resistance [8]. The wide clinical spectrum of CoQ10 deficiencies must be related to its structure, characteristics and multiple metabolic functions.
Structurally, CoQ is differentiated into a benzoquinone ring, which confers the redox properties of the molecule, and a polyprenoid tail, which is responsible for its lipophilicity [1,9,10]. The benzoquinone ring may exist in three redox states, fully oxidized (producing ubiquinone), semiquinone radical (producing ubisemiquinone) and fully reduced (producing ubiquinol). Also, the polyprenoid tail may be different in size, which is a characteristic of the different CoQ forms present in different organisms. This size seems to be determined by the homolog of the human PDSS1 protein in different species. For instance, the polyprenoid tail of the CoQ in Saccharomyces cerevisiae has six isoprenoid units, leading to the production of Coenzyme Q6 (CoQ6), mouse has a major form of nine isoprenoid units and a minor form of ten isoprenoid units, leading to the production of Coenzyme Q9 (CoQ9) and Coenzyme Q10 (CoQ10) respectively, and in human, CoQ10 is the major form, and CoQ9 is the minor one [1]. The distribution of CoQ9 and CoQ10 in mouse and human varies between tissues and cell types, but specific functional differences between both CoQ forms have not been described as yet [1].
The lipophilicity and the redox capability provide CoQ with the characteristics to perform most of its functions. Generally, CoQ is considered as one of the most important endogenous antioxidants, being especially effective in reducing/preventing lipid peroxidation. This antioxidant role of CoQ has been confirmed by (1) studies of characterization of in vitro and in vivo models of CoQ deficiency, which show increased generation of reactive oxygen species (ROS) and oxidative damage, pointing out CoQ’s role in ROS generation and scavenging, (2) studies of CoQ10 supplementation in the same models of CoQ deficiency, showing that the rescue of biochemical and clinical phenotypes and survival correlate with improvement of oxidative stress, demonstrating the antioxidant effects of CoQ10, and (3) studies in different models of increased oxidative stress in which CoQ10 is able to reduce markers of oxidative damage [2,11,12,13,14,15,16]. The antioxidant capacity of CoQ is due to its capability to directly reduce ROS, but also to regenerate other antioxidants, e.g., tocopherol and ascorbate [17]. To work as an antioxidant, CoQ must be able to reduce itself after being oxidized. This can be done by different NAD(P)H oxidoreductases localized in the plasma membrane, such as NADH-cytochrome b5 reductase, NAD(P)H:quinone oxidoreductase 1, or NADPH-CoQ reductase [17]. For all these functions in cell membranes, CoQ must be distributed among them and that distribution seems to be regulated by specific proteins [18,19].
In addition to this antioxidant capacity and redox regulation, which have been extensively reviewed in the literature, CoQ has other key metabolic functions in mitochondria, where its main biosynthetic process mostly occurs. Thus, this review is focused in describing the functional metabolic roles of CoQ in mitochondria and how, through those functions, CoQ can also influence other metabolic pathways outside of mitochondria. Particularly, this review provides an in-depth look at the metabolic pathways linked to a functional structure called the CoQ-junction (Figure 1). As we will emphasize, all these processes have important physiological and pathophysiological implications.
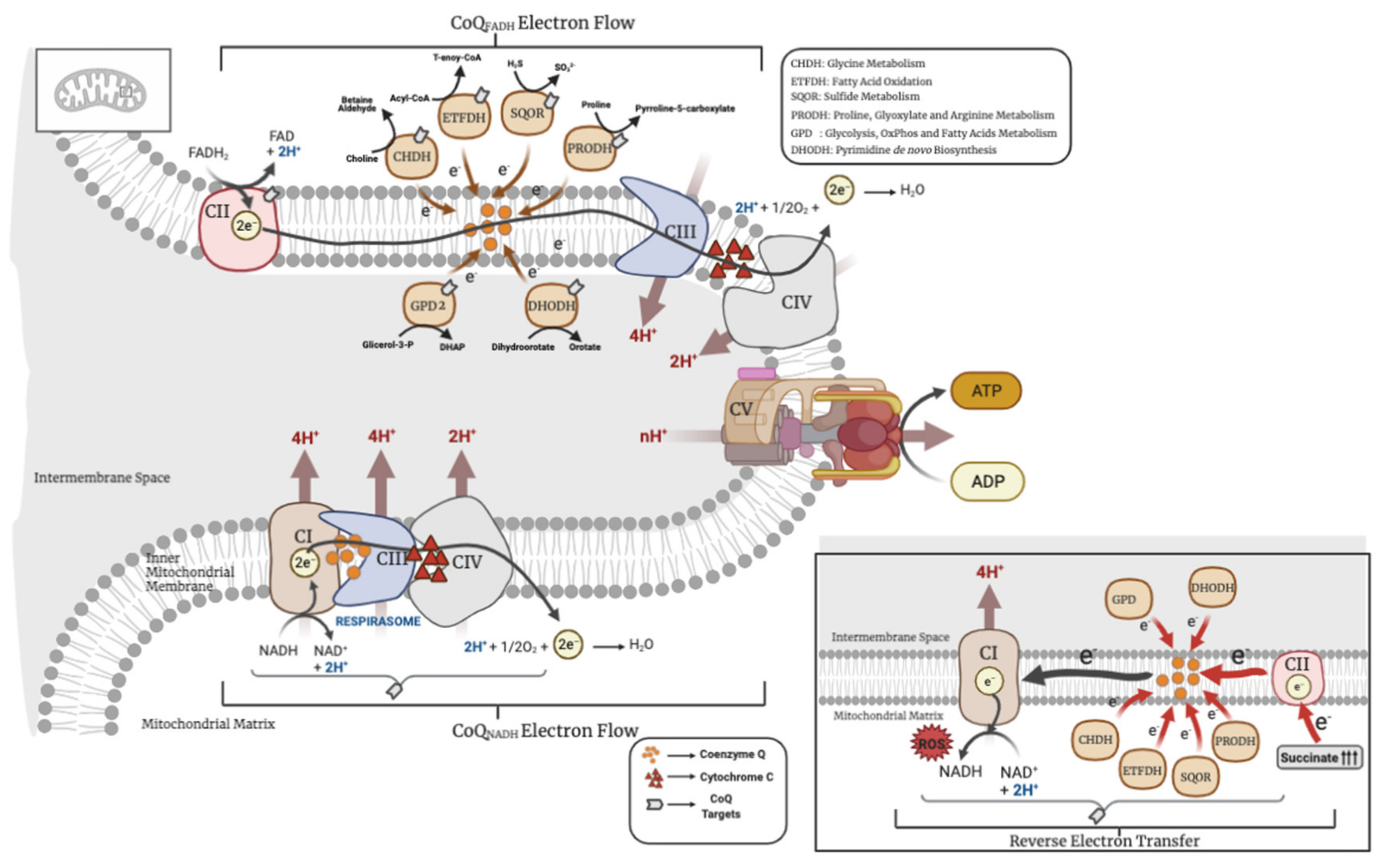
Figure 1.
Metabolic uses of coenzyme Q (CoQ) in mitochondria. CHDH = choline dehydrogenase; ETFDH = electron transfer flavoprotein dehydrogenase; SQOR = sulfide:quinone oxidoreductase; PRODH = proline dehydrogenase; GPD2 = glycerol phosphate dehydrogenase; DHODH = dihydroorotate dehydrogenase. The above image represents the forward electron transport and the image below, the reverse electron transport (RET).
2. CoQ in the OxPhos System
CoQ was first isolated by Professor Frederick Crane in 1957 in beef heart, describing the presence and function of CoQ in the mitochondrial respiratory chain. Since then, its proprieties as a mobile electron carrier in the mitochondrial respiratory chain and as a molecule with redox capabilities in the cell have been widely studied [20]. In mitochondria, CoQ is mainly localized in the inner mitochondrial membrane, where it accepts electrons from NADH through NADH ubiquinone oxidoreductase (CI), and/or from FADH2 through succinate dehydrogenase (CII). Those electrons are then transferred to cytochrome c through CoQH2-cytochrome c reductase (CIII), and the cytochrome c transfers the electrons to the oxygen through cytochrome c oxidase (CIV). The transfer of electrons among the complexes is accompanied by the pumping of protons to the intermembrane space, generating a proton motive force that is used by the ATP synthase (CV) to produce the ATP. The transfer of electrons in the mitochondrial respiratory complexes is favored by the formation of super-complexes, a supramolecular organization that joins the mitochondrial individual complexes in one single structure, in which CoQ is an essential component [21]. Therefore, the complexes can be found individually or, varying in the number of complexes, organized in super-complexes, which encompass complexes I, III and IV. CII, however, has not been proven to be in association with any other complex [22,23]. Both individual complexes and super-complexes are functional, indicating that the organization in super-complexes has to provide some advantages over the individual complexes, i.e., better stability, lower ROS production, or a better mobilization of the electron transfer from the mobile carriers, CoQ or cytochrome c, to their targets [21]. For years, CoQ was thought to be free in the mitochondrial membranes in a homogeneous pool ready to be used by any enzyme that needed it [24]. However, more recently, it has been demonstrated that the supramolecular organization of complexes in super-complexes dynamically segments the CoQ molecules in: (1) a pool attached and engulfed by the super-complexes I + III, exclusively dedicated to the oxidation of NADH (CoQNADH pool), and (2) a free pool, available for CII or any other enzyme that uses CoQ as a cofactor (CoQFADH pool) [25]. However, the two pools can interact with each other. For example, fasting leads to an energy shift from glucose to fatty acids, which results in a decrease in the NADH/FADH2 ratio. In that situation, the CoQFADH pool can be over-reduced and the electron flux could be reversed in order to reduce NAD+ through the CoQNADH pool, thus restoring the oxidized CoQ [25]. This reverse electron transport (RET) from CoQ to NAD+ enhances the scape of electrons and produces an accumulation of ROS in the form of anion superoxide at the level of CI, resulting in CI-derived oxidative damage [25,26]. The CI-derived oxidative damage leads to the degradation of CI with the subsequent release of CIII and CoQNADH pool from the super-complexes. This partial release of the CoQNADH pool is then used to restore the oversaturated CoQFADH pool by the shift in the metabolism from carbohydrates to fats [25]. In addition, the generation of the RET could be enhanced by the electrons that other enzymes transfer to CoQ, such as glycerol-3-phosphate dehydrogenase or the electron-transferring flavoprotein, also leading to ROS production [16,26]. Therefore, both the direction of the flow of electrons in the mitochondrial respiration and the formation of mitochondrial complexes/super-complexes are modulated by the CoQH2/CoQ ratio, which serves as a sensor to the metabolic status of the mitochondria [27]. This key role of the CoQH2/CoQ ratio has been experimentally demonstrated with the uses of the alternative oxidase (AOX), which accepts electrons from CoQ and, therefore, contributes to decrease the CoQH2/CoQ ratio, resulting in a decline of RET and ROS production [28]. However, it is important to highlight that the ROS derived from RET may provide some beneficial effects since they can work as cellular signalers. The toxic or beneficial effect of ROS depends on their amount, localization and type of ROS [29]. In fact, ROS production via RET at CI, controlled by the CoQH2/CoQ ratio, has been associated with different physiological processes, such as: (1) the in vitro differentiation of myoblasts into myotubes [30], (2) the metabolic shift from carbohydrates to fatty acids [27], (3) the macrophages’ reprograming and activation under bacterial stimuli [31], (4) the sensing of oxygen levels by the specialized cells in the carotid body [32], (5) the increase of the lifespan in Drosophila melanogaster [33] or (6) in the oxidative damage produced by the reperfusion of heart or brain after infarction or stroke, since the interruption of electron flow induces the accumulation of succinate in the ischemic phase of these conditions, follow by a rapid use of that succinate in the reperfusion phase, leading to an increase of the CoQH2/CoQ ratio and RET [34,35]. This underlies the importance of the CoQH2/CoQ ratio and RET in both physiological and pathophysiological contexts.
From a different perspective, the importance of CoQ in the mitochondrial respiratory chain is evidenced by the OxPhos defect and decreased ATP production in tissues from patients with primary CoQ10 deficiency, as well as in cells and animal models of CoQ deficiency [5]. For instance, decreased activities of CoQ-dependent mitochondrial complexes have been described in muscle and/or skin fibroblasts from the first patients with identified molecular defects in the CoQ biosynthetic pathway, i.e., COQ2, PDSS2, PDSS1 or COQ9 [36,37,38,39,40]. Curiously, the severity of the OxPhos defect inversely correlates with ROS production and oxidative damage in skin fibroblasts from patients with primary CoQ10 deficiency [12,13]. This correlates with the low mitochondrial ROS production in the cardiac mitochondria from the Coq7 conditional Knockout (KO) mice, which contains only 10% of the normal CoQ levels [41]. The connection between CoQ levels and bioenergetics defects has also been demonstrated in conditions of pharmacological inhibition of CoQ biosynthesis in human skin fibroblasts and neurons [15]. Also, mouse embryonic fibroblasts (MEFs) from a Coq7 knockout mouse model or a Coq9 knock-in mouse model (Coq9R239X), generated by independent groups, show a reduction in mitochondrial respiration and a decrease in ATP production [42,43,44,45]. COQ7 is a hydroxylase that uses demethoxyubiquinone (DMQ) as substrate in the CoQ biosynthetic pathway, and it needs COQ9 for its stability and function [2,3,44]. Consequently, Coq7 knockout mice and Coq9R239X mice accumulate DMQ, which could compete with CoQ and inhibit the CI + III activity [46]. Additionally, the mouse model of CoQ deficiency due to a spontaneous mutation in Pdss2 (Pdss2kd/kd) shows decreased activities of complex I and II + III activities in kidneys, the most clinically affected tissue [47]. Interestingly, OxPhos defects in cell and animal models of CoQ deficiency are not always rescued by CoQ10 supplementation, and their role in the pathogenesis of the disease seems to be variable, probably depending on the molecular defect and/or tissue specificity. In vitro, CoQ10 supplementation for 7 days normalizes the ATP levels and ATP/ADP ratio in skin fibroblast from patients with mutations in PDSS2, COQ2 and COQ9 [48]. However, other short-tail ubiquinone analogs are not able to restore the ATP synthesis, demonstrating the importance of the CoQ structure in the function of the OxPhos system [48]. In vivo, the low absorption and bioavailability of the exogenous CoQ10 limits its bioenergetic effects in some tissues. In Pdss2kd/kd mice, CoQ10 supplementation does not rescue the CoQ-dependent complexes activities in kidneys, although therapeutic benefits were reported, presumably due to other CoQ10 functions [47,49]. In Coq9R239X mice, ubiquinol-10 (CoQ10H2), but not ubiquinone-10 (CoQ10), is able to partially increase CI + III activity in the brain due to its superior absorption, bioavailability and mitochondrial uptake [50], leading to an increase in survival [51]. Since Coq9R239X mice accumulates DMQ, the reduction in the levels of DMQ achieved by the treatment with beta-resorcylic acid (β-RA) is able to increase the bioenergetics and obtain therapeutic outcomes that are superior to those obtained under CoQ10H2 supplementation [51]. The decrease on the levels of DMQ, and its subsequent therapeutic benefits, is also achieved by b-RA in the conditional knockout Coq7 mouse model, as well as in skin fibroblasts from patients with mutations in COQ7 or COQ9 [41,52,53], pointing out the structural specificity of CoQ in its function in the mitochondrial respiratory chain. The importance of CoQ in the OxPhos system has also been revealed in cases of secondary CoQ deficiencies, although, in those cases, it is unclear the contribution of the different functions of CoQ to the pathophysiologic characteristics of the disease.
3. The Role of CoQ in the Regulation of Sulfide Metabolism and Others Linked Pathways
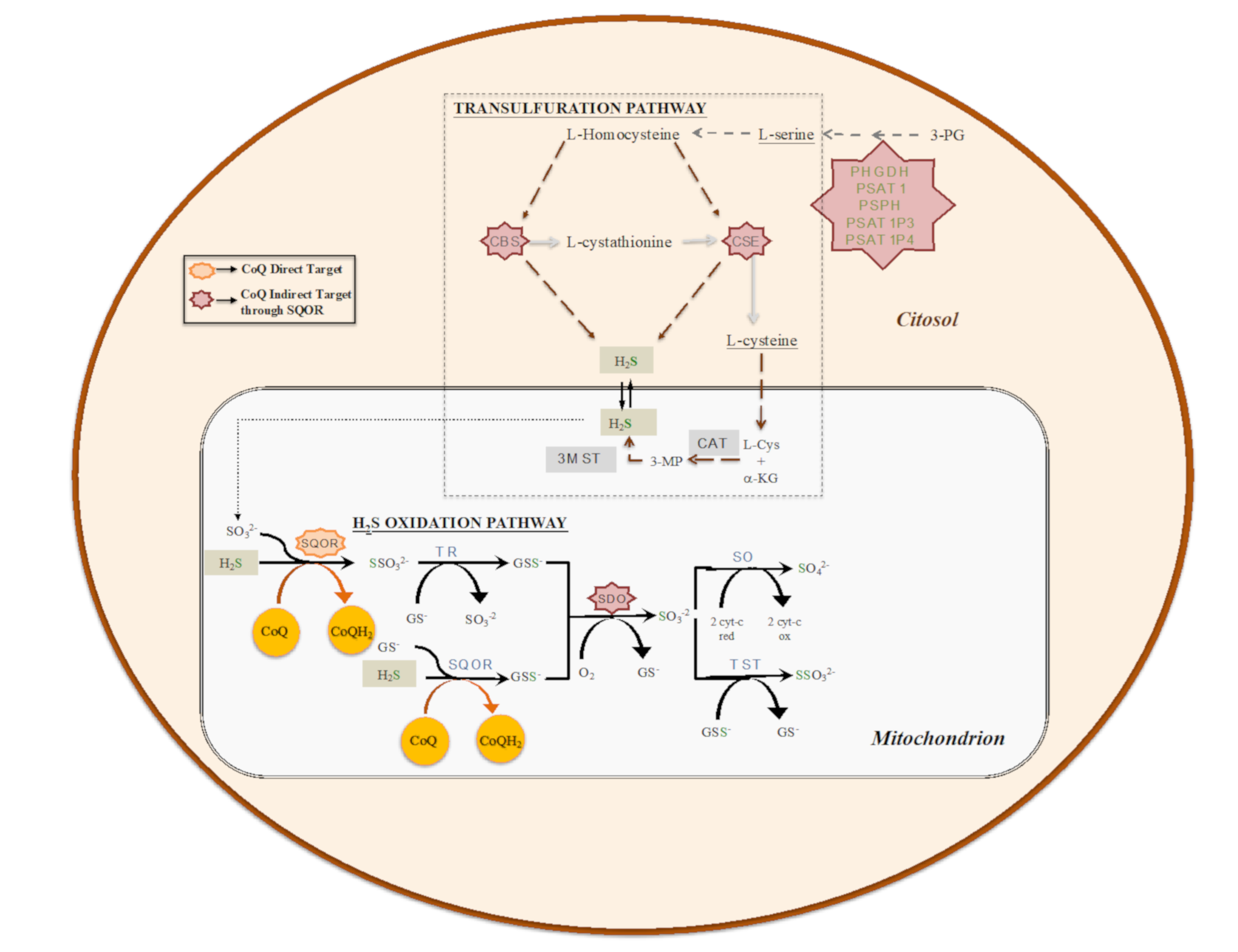
Given its central role in the mitochondrial respiratory chain, CoQ links the mitochondrial respiratory chain to other mitochondrial enzymes and pathways in the functional structure called the CoQ-junction. One of these enzymes is the sulfide:quinone oxidoreductase (SQOR), which catalyzes the first step in the mitochondrial sulfide oxidation pathway. SQOR couples H2S oxidation to CoQ reduction, forming a protein-bound persulfide (Figure 2). The SQOR-bound persulfide is transferred to a sulfane sulfur acceptor, e.g., such as glutathione (GSH) or sulfite, resulting in the generation of GSH persulfide (GSSH) or thiosulfate, respectively. The persulfide group from GSSH is further oxidized to sulfite by an iron-dependent sulfur dioxygenase (SDO) (also known as ETHE1 or persulfide dioxygenase). Sulfite can then either be oxidized to sulfate by sulfite oxidase (SO) or converted to thiosulfate via addition of a persulfide catalyzed by the thiosulfate sulfurtransferase or rhodanese (TST). The sulfane sulfur from thiosulfate can be remobilized by another sulfurtransferase called thiosulfate reductase (TR) (Figure 2) [54,55,56,57]. The H2S used as a substrate by the SQOR is produced by at least three enzymes, of which two are in the transsulfuration pathway (Figure 2), localized in the cytosol. These two enzymes are Cystathionine β-synthase (CBS), which produces H2S primarily by condensation of cysteine and homocysteine, and cystathionine γ-lyase (CSE), which produces H2S primarily by α- and β-elimination of cysteine, generating pyruvate and ammonia. A third enzyme, 3-mercaptopyruvate sulfurtransferase (3MST) [56], produces H2S from 3-mercaptopyruvate (3MP), an achiral α-keto acid generated by cysteine aminotransferase (CAT) from L-cysteine and α-ketoglutarate (α-KG). The CAT/3MST pathway has also been detected in mitochondria, which opens the possibility of an intramitochondrial production of H2S (Figure 2) [58].
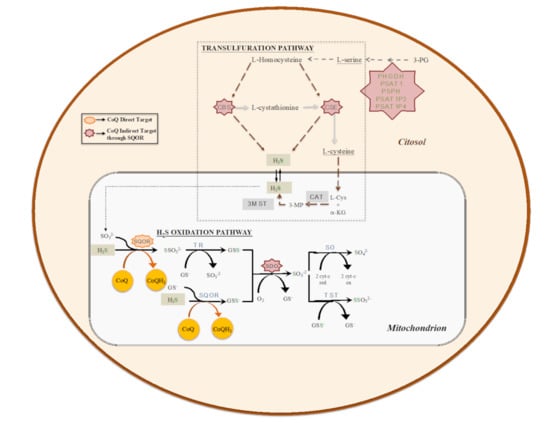
Figure 2.
The biosynthetic (transsulfuration) and catabolic (oxidation) pathways of H2S. Transsulfuration pathway involves the enzymes cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE) and pyridoxal-5′-phosphate (PLP)-independent 3-mercaptopyruvate sulfurtransferase (3MST). Mitochondrial H2S oxidation pathway involves the enzymes sulfide:quinone oxidoreductase (SQOR), sulfur dioxygenase (SDO; also known as ETHE1 or persulfide dioxygenase), sulfite oxidase (SO), thiosulfate sulfurtransferase or rhodanese (TST) and thiosulfate reductase (TR).
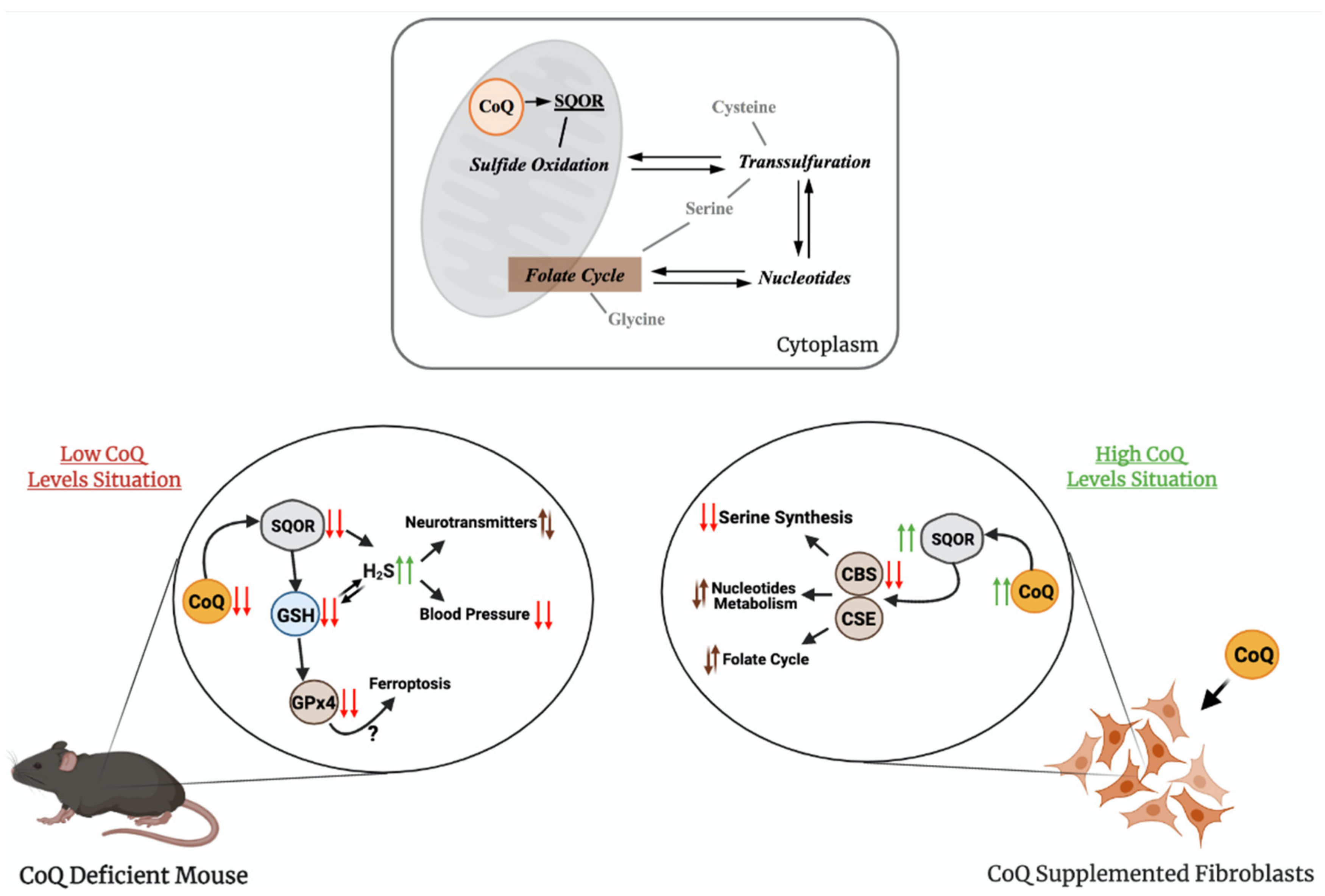
The regulation of production and the use of H2S occurs at the level of expression and activity of the biosynthetic and catabolic enzymes. For example, tonic inhibition of CBS is relieved during hypoxia, leading to elevated H2S production [59], the restriction in sulfur aminoacids (SAAs) upregulates the transsulfuration pathway, increasing H2S production [60,61], or the levels of SQOR are increased under hypoxia and restriction in SAAs [62,63]. However, an integration of regulatory aspects in both the biogenesis and the catabolism of H2S has not been clearly formulated. Recently, our group has demonstrated that CoQ10 supplementation leads to an upregulation of SQOR, together with a downregulation of CBS and CSE, in vitro (Figure 3) [64].
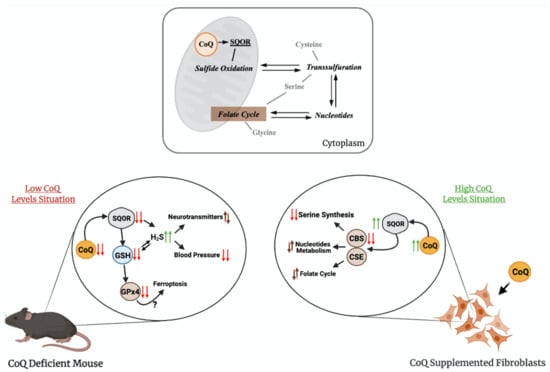
Figure 3.
The relation of CoQ with sulfide metabolism and other linked pathways. The upper panel shows how CoQ is connected to sulfide metabolism and one-carbon metabolism. The bottom panel shows the main finding on these pathways in conditions of supraphysiological levels of CoQ10 or deficiency in CoQ10. SQOR = sulfide:quinone oxidoreductase; CBS = cystathionine-β-synthase; CSE = cystathionine γ-lyase; GSH = glutathione; H2S = hydrogen sulfide; GPx4 = glutathione peroxidase 4.
Therefore, CoQ10 regulates both pathways in opposite directions by modifying the gene expression and protein levels of SQOR, CBS and CSE. Although the mechanisms of this regulation have not been identified yet, these results may have relevant therapeutic implications for diseases with disrupted H2S metabolism. For instance, the reduction in the H2S levels, as a consequence of the effects of CoQ10 supplementation, could be a therapeutic approach in genetic diseases with defects in sulfide metabolism enzymes, i.e., the ethylmalonic encephalopathy caused by mutations in ETHE1 [65], or the Leigh syndrome caused by mutations in SQOR [66]. In both cases, the accumulation of high levels of H2S induces mitochondrial toxicity and, consequently, the repression of the CBS and CSE, together with the stimulation of SQOR, may limit that toxicity by reducing the levels of H2S. CoQ10 supplementation may also provide therapeutic benefits in more common diseases, where cell-specific accumulation of H2S has been reported. For example, a decrease in the enzymes involved in the H2S oxidation pathway has been reported in the gut of mouse models and patients with Crohn’s Disease, together with a relative increase in the abundance of H2S microbial producers, resulting in pathological accumulation of H2S that contributes to the disease [67], and the increase in CBS has been reported in human biopsies of precancerous adenomatous polyps [68].
The transsulfuration pathway, and specifically, mRNA levels, protein levels and activities of CSE or CBS, has been linked to alterations in other metabolic pathways, such as serine and nucleotides biosynthesis or folate cycle [7,69,70]. Several evidences have demonstrated the contribution of these alterations to pathophysiological states related to mitochondrial dysfunction, such as: (1) the inhibition of CI in human cells causes an upregulation of the mRNA levels of CBS and CSE, together with the upregulation of PSAT1, SHMT2 and PHGDH, which are involved in serine/glycine biosynthesis and folate cycle [71], (2) the levels of the MTHF2 protein, involved in the folate cycle, are increased in the Deletor mouse model, a mouse model with accumulation of mtDNA deletions, resulting in modifications in one-carbon metabolism [72], (3) the levels of MTHF2 and SHTM2 proteins are increased in other mouse models of mitochondrial diseases caused by defects in mtDNA [7], (4) CBS, CSE, PHGDGH, PSPH and PSAT1 mRNAs are upregulated in other human cell models of pharmacologically induced mtDNA depletion [73] and (5) MTHFD1L, MTHFD2, PSAT1 and PHGDH are upregulated in patients with mitochondrial myopathy caused by mtDNA deletion [70]. Thus, the regulatory effects of CoQ over sulfide metabolism could indirectly modulate these pathways and, consequently, provide therapeutic benefits on these pathophysiological states (Figure 3). In fact, the serine/glycine ratio is increased by the supplementation with CoQ10 in vitro, together with a metabolic adaptation of nucleotides biosynthesis and folate cycle [64]. Therefore, CoQ10 supplementation could be beneficial in pathologies with alterations in sulfide metabolism, serine biosynthesis, folate cycle or nucleotide metabolism by repressing CBS, CSE, PHGDH, PSAT1 and MTHDF1L, among others [64].
The role of CoQ in sulfide metabolism and related pathways has also been demonstrated in models of primary CoQ deficiency. Two independent studies showed that either in vitro, in skin fibroblasts from patients with primary CoQ10 deficiency, or in vivo, in two different mouse models of primary CoQ deficiency, the deficiency in CoQ leads to reduced activity and levels of SQOR [74,75]. The consequence of this reduction is an increase in the amount of H2S and a reduction in the levels of glutathione (GSH), an antioxidant composed by glutamate, cysteine and glycine, the last two linked to H2S metabolism [74,75]. In one study, two mouse models of CoQ deficiency caused by different mutations in Coq9 were used [75]. The Coq9Q95X mouse model, which has a mild reduction of CoQ levels and shows mild signs of late-onset myopathy, has reduced levels and activity of SQOR in the skeletal muscle. The Coq9R239X mouse model, which has a severe reduction in CoQ levels and suffers a fatal mitochondrial encephalopathy, has severe reduction in the levels and activity of SQOR in the brain, kidneys and skeletal muscle [75], and, as a consequence, the CBS levels are increased [64]. Besides, the levels of SQOR downstream enzymes, Sulfite Oxidase (SO) and Thiosulfate Sulfurtransferase (TST), but not ETHE1, are increased, most likely as a compensatory mechanism [75]. Moreover, the most clinically affected tissue in Coq9R239X mice, the brain, shows a reduction in the levels of total GSH, together with a decrease in the levels and activities of the glutathione reductase (GRd) and glutathione peroxidase 4 (GPx4) enzymes [75]. Interestingly, CoQ and GPx4, together with the apoptosis-inducing factor mitochondria-associated 2 (AIFM2), have been related to the suppression of ferroptosis, a cellular process that has not been evaluated in CoQ deficiency [76,77]. Furthermore, the levels of specific metabolites of the serotonin biosynthesis pathway and the levels of tyrosine are increased. This may suggest that sulfide metabolism can interact and alter the neurotransmitter biosynthesis. Accordingly, similar alterations in the levels of L-glutamate, dopamine and 5-hydroxyindoleacetic acid are detected in wild-type animals treated with H2S donors [75]. Furthermore, the effects of H2S, a well-known vasodilator [78], cause low blood pressure in Coq9R239X mice. These abnormalities are rescued by the treatment with b-RA [51], which, as previously mentioned, rescues CoQ deficiency due to mutations in Coq7 or Coq9 [41,51,52,53].
Another study used the Pdss2kd/kd mice, which suffer from nephrotic syndrome [74]. Pdss2kd/kd mice show severe reduction in the levels of SQOR in the kidneys. In this model, however, contrary to what was observed in Coq9 mutant mice and human CoQ-deficient fibroblasts, also, SQOR downstream enzymes TST, SO and ETHE1 are downregulated, indicating genotype- and/or tissue-specific mechanisms. As a consequence, H2S levels are increased, and GSH levels are decreased in the kidneys of Pdss2 mice [74]. Moreover, urine and plasma thiosulfates are decreased, and short-chain acylcarnitine is increased in plasma of these mice. The altered acylcarnitine profile might result from inhibition of short-chain Acyl-CoA dehydrogenase (SCAD) by H2S, or might be the consequence of low levels of CoQ on lipid metabolism, as discussed in the next section of this review. In any case, chronic administration of oral CoQ10 increases SQOR and the other enzymes of the H2S oxidation, and GSH levels, normalizes plasma acylcarnitine profile and prevents renal failure in Pdss2kd/kd mice [48]. Therefore, the disruption in sulfide metabolism is one of the pathomechanisms of primary CoQ deficiency and it should be considered for the development of new treatments [47,51,74,75].
4. Other CoQ-Linked Reactions in Mitochondria
CoQ receives electrons from other enzymes of different metabolic pathways in the CoQ-junction (Figure 1). It receives electrons from dihydroorotate dehydrogenase (DHODH), which catalyzes the conversion of dihydroorotate to orotate, the fourth reaction step within pyrimidine de novo biosynthesis [79]. Therefore, the pyrimidine de novo biosynthesis is directly connected to CoQ and, consequently, low levels of CoQ may impair uridine-5′-triphosphate (UTP), cytidine 5′-triphosphate (CTP) and deoxythymidine triphosphate (dTMP) synthesis, as well as RNA and DNA synthesis. In fact, supplementation with uridine, the precursor of UTP, CTP and dTMP in the pyrimidine salvage pathway [80], increases the growth rate in CoQ10-deficient fibroblasts, but not in wild-type fibroblasts [36]. Moreover, secondary CoQ10 deficiency has been reported in patients with mitochondrial DNA depletion due to mutations in DGUOK (encoding mitochondrial deoxyguanosine kinase, which is involved in the purine nucleotide salvage pathway), SUCLA2 (encoding the β subunit of succinyl-CoA synthase, which is involved in the Krebs cycle), MPV17 (involved in mitochondrial deoxynucleoside triphosphates pool homeostasis) or from unknown etiology [81,82]. The CoQ-dependent DHODH seems to also be important in tumorigenesis since the pyrimidine biosynthesis required in mouse breast cancer cells need a functional CoQ redox-cycling and DHODH activity [83].
CoQ also receives electrons from the electron transfer flavoprotein-dehydrogenase (ETFDH), which serves as a short electron transfer pathway to conduct electrons from nine different mitochondrial flavin adenine dinucleotide (FAD)-containing acyl-CoA dehydrogenases of fatty acid β-oxidation and amino acid catabolism to the ubiquinone pool [84]. Curiously, some patients with mitochondrial myopathy due to mutations in ETFDH show CoQ10 deficiency, although the precise mechanisms for this association are unknown [85,86]. Importantly, a trend toward decreased levels of short- and medium-length acylcarnitines are showed in the liver and kidneys of Pdss2kd/kd, while the levels of acylcarnitines C4, C5 and C6 are increased in plasma of the same mice, indicating a defective oxidation of fatty acids [74].
A third type of enzymes that use CoQ as electron acceptors are proline dehydrogenase and proline dehydrogenase 2 (PRODH and PRODH2) [87,88], which are related with proline, glyoxylate and arginine metabolism. Interestingly, PRODH2 has been proposed as a molecular target for treating primary hyperoxaluria, and some CoQ analogs seem to stimulate PRODH2 activity [87]. The remaining mitochondrial enzymes that use CoQ are glycerol 3-phosphate dehydrogenase (GPD2) [89], which connects glycolysis, OxPhos and fatty acids metabolism, and choline dehydrogenase (CHDH) [90], which is involved in the glycine metabolism. Remarkably, the behavior of these CoQ-linked proteins in CoQ deficiency seems to be different from SQOR, since kidneys of Coq9R239X mice show an increase in the levels of ETFDH, CHDH, DHODH, PRODH and PRODH2 [3] (Table 1), most likely due to a compensatory mechanism, although the functional evaluation of theses enzymes has not been assessed in CoQ deficiency.

Table 1.
Levels of the CoQ-linked proteins in Coq9R239X mice compared to Coq9+/+ mice. The values are expressed as Log2 (Fold-Change (Coq9R239X/Coq9+/+)). Data obtained from Reference [3].
Other mitochondrial components susceptible to be regulated by redox reactions are the UCPs. Controversial results have been reported on the involvement of CoQ in the regulation of the UCPs. By using bacterial overexpressed UCP1, 2 and 3 in liposomes, Echtay and colleagues [91,92] showed that CoQ, but not CoQH2, activated the H+ transport through the UCPs. These studies suggested that CoQ acts as a non-covalent UCP cofactor in cooperation with other well-known UCPs modulators, such as free fatty acids or retinoids. Later on, Jaburek and Garlid [93] optimized the conditions of isolation and refolding of bacterially expressed UCPs and evaluated the effect of CoQ in the same model. Contrary to what was reported by Echtay and colleagues [91,92], they found that CoQ had no effect on the proton transport catalyzed by any of the UCPs [93]. Similar conclusions were documented by Esteves and collaborators using a CoQ-deficient yeast model [94]. Nevertheless, a more recent study showed that CoQ, through its redox state, is a regulator of the inhibition, by purine nucleotides, of free fatty acid-activated UCP1 homologues under phosphorylating respiration conditions [95]. Therefore, additional studies are required to elucidate the role of CoQ in the modulation of UCPs activities, especially under physiological conditions and taking into account potential type- and tissue-specific differences. Also, some reports have shown that CoQ10, as well as other short-tail CoQ analogs, can function as modulators of apoptosis by the regulation of the mitochondrial permeability transition pore (PTP) [96,97,98]. As it happens with other CoQ functions, the size of the polyprenoid tail has functional implications, i.e., CoQ10 inhibits PTP opening induced by H2O2, CoQ5 does not produce any effect on the PTP opening induced by H2O2, while CoQ0 induces PTP opening and H2O2 production [99]. However, cell- and tissue-specific differences have been observed [99,100], and, consequently, additional studies in physiological and pathophysiological conditions are required to better understand the role of CoQ10 and other short-tail analogs in the regulation of the mitochondrial PTP opening.
5. Conclusions and Perspectives
From the first isolation of CoQ, its role in the mitochondrial respiratory chain and energy production has been clearly demonstrated. The generation and characterization of different models of CoQ deficiency and studies of CoQ10 supplementation in the same models have confirmed it. Also, recent studies have unveiled the importance of the redox state of CoQ in the regulation of super-complexes’ formation and, consequently, in the use of the reducing equivalents by the respiratory chain, as well as in the production of ROS. These data could open the possibility to modulate/modify the energy metabolism through physiological or pharmacological interventions that target the redox state of CoQ. Besides its implication in the mitochondria respiratory chain, CoQ is also a key component in the reactions mediated by other mitochondrial enzymes and, therefore, CoQ links the energy production to other metabolic pathways of the cell. The contribution of these CoQ-linked enzymes to RET must be better defined in different physiological and pathophysiological conditions. Also, whether all these metabolic pathways related to the CoQ-linked enzymes are influenced by CoQ levels, including the cases of primary and secondary CoQ deficiencies, or CoQ redox state, remains to be evaluated. However, recent studies indicate that modifying the levels of CoQ and/or its redox state indeed affects some of these pathways, i.e., sulfide metabolism, in the mitochondrial and cytosolic compartments. In any case, the link between CoQ, sulfide metabolism, one-carbon metabolism, glutathione and ferroptosis shown in recent studies must be validated and further investigated in different models. Furthermore, those pathways could be influenced by an alteration in the distribution of CoQ among the different compartments of the cell. However, our knowledge about the mechanisms of CoQ distribution in the cell is still very limited, and it is unknown if the cell responds with a redistribution in the CoQ pool under CoQ deficiency. Together, the results exposed above prove the relevance of CoQ10 beyond its classical function as an electron carrier in the mitochondrial respiratory chain and, consequently, they may have significant implications in the use of CoQ10 supplementation in patients with different metabolic diseases. Nevertheless, additional research studies are required in models of CoQ deficiency, models of CoQ10 supplementation and models with different redox states of CoQ, paying close attention to the bioavailability of the exogenous CoQ10 and the potential cell- and tissue-specific differences.
Author Contributions
Writing—original draft preparation, A.H.-G. and L.C.L.; writing—review and editing, A.H.-G., C.M.Q. and L.C.L.; writing—review, P.G.-G., M.E.D.-C., E.B.-C. and S.L.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Ministerio de Ciencia e Innovación, Spain, and the ERDF (RTI2018-093503-B-100), the Muscular Dystrophy Association (MDA-602322). C.M.Q. is supported by the Department of Defense (DOD) grant PR190511. A.H.-G. and P.G.-G. are ‘FPU fellows’ from the Ministerio de Universidades, Spain. S.L.-H. is supported by the “becas de colaboración” from the Ministerio de Universidades, Spain. E.B.-C. is supported by the Consejería de Salud, Junta de Andalucía, Spain.
Acknowledgments
We thank Stacy Kelly Aguirre for the English editing. Figures created with BioRender.com.
Conflicts of interest
The other authors have declared that no conflict of interest exists.
References
- Kawamukai, M. Biosynthesis of coenzyme Q in eukaryotes. Biosci. Biotechnol. Biochem. 2016, 80, 23–33. [Google Scholar] [CrossRef]
- Garcia-Corzo, L.; Luna-Sanchez, M.; Doerrier, C.; Garcia, J.A.; Guaras, A.; Acin-Perez, R.; Bullejos-Peregrin, J.; Lopez, A.; Escames, G.; Enriquez, J.A.; et al. Dysfunctional Coq9 protein causes predominant encephalomyopathy associated with CoQ deficiency. Hum. Mol. Genet. 2013, 22, 1233–1248. [Google Scholar] [CrossRef] [PubMed]
- Lohman, D.C.; Forouhar, F.; Beebe, E.T.; Stefely, M.S.; Minogue, C.E.; Ulbrich, A.; Stefely, J.A.; Sukumar, S.; Luna-Sanchez, M.; Jochem, A.; et al. Mitochondrial COQ9 is a lipid-binding protein that associates with COQ7 to enable coenzyme Q biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, E4697–E4705. [Google Scholar] [CrossRef]
- Mugoni, V.; Postel, R.; Catanzaro, V.; De Luca, E.; Turco, E.; Digilio, G.; Silengo, L.; Murphy, M.P.; Medana, C.; Stainier, D.Y.; et al. Ubiad1 is an antioxidant enzyme that regulates eNOS activity by CoQ10 synthesis. Cell 2013, 152, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Fabra, M.; Trevisson, E.; Brea-Calvo, G. Clinical syndromes associated with Coenzyme Q10 deficiency. Essays Biochem. 2018, 62, 377–398. [Google Scholar] [CrossRef] [PubMed]
- Desbats, M.A.; Lunardi, G.; Doimo, M.; Trevisson, E.; Salviati, L. Genetic bases and clinical manifestations of coenzyme Q10 (CoQ 10) deficiency. J. Inherit. Metab. Dis. 2015, 38, 145–156. [Google Scholar] [CrossRef]
- Kuhl, I.; Miranda, M.; Atanassov, I.; Kuznetsova, I.; Hinze, Y.; Mourier, A.; Filipovska, A.; Larsson, N.G. Transcriptomic and proteomic landscape of mitochondrial dysfunction reveals secondary coenzyme Q deficiency in mammals. Elife 2017, 6, e30952. [Google Scholar] [CrossRef]
- Fazakerley, D.J.; Chaudhuri, R.; Yang, P.; Maghzal, G.J.; Thomas, K.C.; Krycer, J.R.; Humphrey, S.J.; Parker, B.L.; Fisher-Wellman, K.H.; Meoli, C.C.; et al. Mitochondrial CoQ deficiency is a common driver of mitochondrial oxidants and insulin resistance. Elife 2018, 7. [Google Scholar] [CrossRef]
- Awad, A.M.; Bradley, M.C.; Fernandez-Del-Rio, L.; Nag, A.; Tsui, H.S.; Clarke, C.F. Coenzyme Q10 deficiencies: Pathways in yeast and humans. Essays Biochem. 2018. [Google Scholar] [CrossRef]
- Wang, Y.; Hekimi, S. The Complexity of Making Ubiquinone. Trends Endocrinol. Metab. 2019, 30, 929–943. [Google Scholar] [CrossRef]
- Diaz-Casado, M.E.; Quiles, J.L.; Barriocanal-Casado, E.; Gonzalez-Garcia, P.; Battino, M.; Lopez, L.C.; Varela-Lopez, A. The Paradox of Coenzyme Q10 in Aging. Nutrients 2019, 11, 2221. [Google Scholar] [CrossRef] [PubMed]
- Quinzii, C.M.; Lopez, L.C.; Von-Moltke, J.; Naini, A.; Krishna, S.; Schuelke, M.; Salviati, L.; Navas, P.; DiMauro, S.; Hirano, M. Respiratory chain dysfunction and oxidative stress correlate with severity of primary CoQ10 deficiency. FASEB J. 2008, 22, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
- Quinzii, C.M.; Lopez, L.C.; Gilkerson, R.W.; Dorado, B.; Coku, J.; Naini, A.B.; Lagier-Tourenne, C.; Schuelke, M.; Salviati, L.; Carrozzo, R.; et al. Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency. FASEB J. 2010, 24, 3733–3743. [Google Scholar] [CrossRef] [PubMed]
- Quinzii, C.M.; Garone, C.; Emmanuele, V.; Tadesse, S.; Krishna, S.; Dorado, B.; Hirano, M. Tissue-specific oxidative stress and loss of mitochondria in CoQ-deficient Pdss2 mutant mice. FASEB J. 2013, 27, 612–621. [Google Scholar] [CrossRef]
- Duberley, K.E.; Abramov, A.Y.; Chalasani, A.; Heales, S.J.; Rahman, S.; Hargreaves, I.P. Human neuronal coenzyme Q(10) deficiency results in global loss of mitochondrial respiratory chain activity, increased mitochondrial oxidative stress and reversal of ATP synthase activity: Implications for pathogenesis and treatment. J. Inherit. Metab. Dis. 2013, 36, 63–73. [Google Scholar] [CrossRef]
- Wang, Y.; Hekimi, S. Understanding Ubiquinone. Trends Cell Biol. 2016, 26, 367–378. [Google Scholar] [CrossRef]
- Navas, P.; Villalba, J.M.; Lenaz, G. Coenzyme Q-dependent functions of plasma membrane in the aging process. Age 2005, 27, 139–146. [Google Scholar] [CrossRef]
- Barros, M.H.; Johnson, A.; Gin, P.; Marbois, B.N.; Clarke, C.F.; Tzagoloff, A. The Saccharomyces cerevisiae COQ10 gene encodes a START domain protein required for function of coenzyme Q in respiration. J. Biol. Chem. 2005, 280, 42627–42635. [Google Scholar] [CrossRef]
- Kemmerer, Z.A.; Robinson, K.P.; Schmitz, J.M.; Paulson, B.R.; Jochem, A.; Hutchins, P.D.; Coon, J.J.; Pagliarini, D.J. UbiB proteins regulate cellular CoQ distribution. bioRxiv 2020. [Google Scholar] [CrossRef]
- Alcazar-Fabra, M.; Navas, P.; Brea-Calvo, G. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim. Biophys. Acta 2016, 1857, 1073–1078. [Google Scholar] [CrossRef]
- Acin-Perez, R.; Fernandez-Silva, P.; Peleato, M.L.; Perez-Martos, A.; Enriquez, J.A. Respiratory active mitochondrial supercomplexes. Mol Cell 2008, 32, 529–539. [Google Scholar] [CrossRef]
- Schagger, H. Blue-native gels to isolate protein complexes from mitochondria. Methods Cell Biol. 2001, 65, 231–244. [Google Scholar]
- Sousa, P.M.; Silva, S.T.; Hood, B.L.; Charro, N.; Carita, J.N.; Vaz, F.; Penque, D.; Conrads, T.P.; Melo, A.M. Supramolecular organizations in the aerobic respiratory chain of Escherichia coli. Biochimie 2011, 93, 418–425. [Google Scholar] [CrossRef]
- Lenaz, G.; Genova, M.L. Kinetics of integrated electron transfer in the mitochondrial respiratory chain: Random collisions vs. solid state electron channeling. Am. J. Physiol. Cell Physiol. 2007, 292, C1221–C1239. [Google Scholar] [CrossRef] [PubMed]
- Lapuente-Brun, E.; Moreno-Loshuertos, R.; Acin-Perez, R.; Latorre-Pellicer, A.; Colas, C.; Balsa, E.; Perales-Clemente, E.; Quiros, P.M.; Calvo, E.; Rodriguez-Hernandez, M.A.; et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 2013, 340, 1567–1570. [Google Scholar] [CrossRef]
- Scialo, F.; Fernandez-Ayala, D.J.; Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Guaras, A.; Perales-Clemente, E.; Calvo, E.; Acin-Perez, R.; Loureiro-Lopez, M.; Pujol, C.; Martinez-Carrascoso, I.; Nunez, E.; Garcia-Marques, F.; Rodriguez-Hernandez, M.A.; et al. The CoQH2/CoQ Ratio Serves as a Sensor of Respiratory Chain Efficiency. Cell Rep. 2016, 15, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Szibor, M.; Gainutdinov, T.; Fernandez-Vizarra, E.; Dufour, E.; Gizatullina, Z.; Debska-Vielhaber, G.; Heidler, J.; Wittig, I.; Viscomi, C.; Gellerich, F.; et al. Bioenergetic consequences from xenotopic expression of a tunicate AOX in mouse mitochondria: Switch from RET and ROS to FET. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148137. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef]
- Lee, S.; Tak, E.; Lee, J.; Rashid, M.A.; Murphy, M.P.; Ha, J.; Kim, S.S. Mitochondrial H2O2 generated from electron transport chain complex I stimulates muscle differentiation. Cell Res. 2011, 21, 817–834. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Dabritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.e413. [Google Scholar] [CrossRef]
- Fernandez-Aguera, M.C.; Gao, L.; Gonzalez-Rodriguez, P.; Pintado, C.O.; Arias-Mayenco, I.; Garcia-Flores, P.; Garcia-Perganeda, A.; Pascual, A.; Ortega-Saenz, P.; Lopez-Barneo, J. Oxygen Sensing by Arterial Chemoreceptors Depends on Mitochondrial Complex I Signaling. Cell Metab. 2015, 22, 825–837. [Google Scholar] [CrossRef]
- Scialo, F.; Sriram, A.; Fernandez-Ayala, D.; Gubina, N.; Lohmus, M.; Nelson, G.; Logan, A.; Cooper, H.M.; Navas, P.; Enriquez, J.A.; et al. Mitochondrial ROS Produced via Reverse Electron Transport Extend Animal Lifespan. Cell Metab. 2016, 23, 725–734. [Google Scholar] [CrossRef]
- Milliken, A.S.; Kulkarni, C.A.; Brookes, P.S. Acid enhancement of ROS generation by complex-I reverse electron transport is balanced by acid inhibition of complex-II: Relevance for tissue reperfusion injury. Redox Biol. 2020, 37, 101733. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijevic, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Lopez-Martin, J.M.; Salviati, L.; Trevisson, E.; Montini, G.; DiMauro, S.; Quinzii, C.; Hirano, M.; Rodriguez-Hernandez, A.; Cordero, M.D.; Sanchez-Alcazar, J.A.; et al. Missense mutation of the COQ2 gene causes defects of bioenergetics and de novo pyrimidine synthesis. Hum. Mol. Genet. 2007, 16, 1091–1097. [Google Scholar] [CrossRef]
- Duncan, A.J.; Bitner-Glindzicz, M.; Meunier, B.; Costello, H.; Hargreaves, I.P.; Lopez, L.C.; Hirano, M.; Quinzii, C.M.; Sadowski, M.I.; Hardy, J.; et al. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: A potentially treatable form of mitochondrial disease. Am. J. Hum. Genet. 2009, 84, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.C.; Schuelke, M.; Quinzii, C.M.; Kanki, T.; Rodenburg, R.J.; Naini, A.; Dimauro, S.; Hirano, M. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am. J. Hum. Genet. 2006, 79, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Quinzii, C.; Naini, A.; Salviati, L.; Trevisson, E.; Navas, P.; Dimauro, S.; Hirano, M. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am. J. Hum. Genet. 2006, 78, 345–349. [Google Scholar] [CrossRef]
- Mollet, J.; Giurgea, I.; Schlemmer, D.; Dallner, G.; Chretien, D.; Delahodde, A.; Bacq, D.; de Lonlay, P.; Munnich, A.; Rotig, A. Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J. Clin. Investig. 2007, 117, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Oxer, D.; Hekimi, S. Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis. Nat. Commun. 2015, 6, 6393. [Google Scholar] [CrossRef]
- Levavasseur, F.; Miyadera, H.; Sirois, J.; Tremblay, M.L.; Kita, K.; Shoubridge, E.; Hekimi, S. Ubiquinone is necessary for mouse embryonic development but is not essential for mitochondrial respiration. J. Biol. Chem. 2001, 276, 46160–46164. [Google Scholar] [CrossRef]
- Nakai, D.; Yuasa, S.; Takahashi, M.; Shimizu, T.; Asaumi, S.; Isono, K.; Takao, T.; Suzuki, Y.; Kuroyanagi, H.; Hirokawa, K.; et al. Mouse homologue of coq7/clk-1, longevity gene in Caenorhabditis elegans, is essential for coenzyme Q synthesis, maintenance of mitochondrial integrity, and neurogenesis. Biochem. Biophys. Res. Commun. 2001, 289, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, P.; Grunler, J.; Mattsson, J.; Sindelar, P.J.; Nordlund, P.; Berthold, D.A. A new member of the family of di-iron carboxylate proteins. Coq7 (clk-1), a membrane-bound hydroxylase involved in ubiquinone biosynthesis. J. Biol. Chem. 2001, 276, 33297–33300. [Google Scholar] [CrossRef] [PubMed]
- Barriocanal-Casado, E.; Cueto-Urena, C.; Benabdellah, K.; Gutierrez-Guerrero, A.; Cobo, M.; Hidalgo-Gutierrez, A.; Rodriguez-Sevilla, J.J.; Martin, F.; Lopez, L.C. Gene Therapy Corrects Mitochondrial Dysfunction in Hematopoietic Progenitor Cells and Fibroblasts from Coq9R239X Mice. PLoS ONE 2016, 11, e0158344. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Vasta, V.; Hahn, S.; Gangoiti, J.A.; Opheim, E.; Sedensky, M.M.; Morgan, P.G. The role of DMQ(9) in the long-lived mutant clk-1. Mech. Ageing Dev. 2011, 132, 331–339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kleiner, G.; Barca, E.; Ziosi, M.; Emmanuele, V.; Xu, Y.; Hidalgo-Gutierrez, A.; Qiao, C.; Tadesse, S.; Area-Gomez, E.; Lopez, L.C.; et al. CoQ10 supplementation rescues nephrotic syndrome through normalization of H2S oxidation pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3708–3722. [Google Scholar] [CrossRef]
- Lopez, L.C.; Quinzii, C.M.; Area, E.; Naini, A.; Rahman, S.; Schuelke, M.; Salviati, L.; DiMauro, S.; Hirano, M. Treatment of CoQ(10) deficient fibroblasts with ubiquinone, CoQ analogs, and vitamin C: Time- and compound-dependent effects. PLoS ONE 2010, 5, e11897. [Google Scholar] [CrossRef]
- Saiki, R.; Lunceford, A.L.; Shi, Y.; Marbois, B.; King, R.; Pachuski, J.; Kawamukai, M.; Gasser, D.L.; Clarke, C.F. Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2. Am. J. Physiol. Renal Physiol. 2008, 295, F1535–F1544. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Corzo, L.; Luna-Sanchez, M.; Doerrier, C.; Ortiz, F.; Escames, G.; Acuna-Castroviejo, D.; Lopez, L.C. Ubiquinol-10 ameliorates mitochondrial encephalopathy associated with CoQ deficiency. Biochim. Biophys. Acta 2014, 1842, 893–901. [Google Scholar] [CrossRef]
- Hidalgo-Gutierrez, A.; Barriocanal-Casado, E.; Bakkali, M.; Diaz-Casado, M.E.; Sanchez-Maldonado, L.; Romero, M.; Sayed, R.K.; Prehn, C.; Escames, G.; Duarte, J.; et al. beta-RA reduces DMQ/CoQ ratio and rescues the encephalopathic phenotype in Coq9 (R239X) mice. EMBO Mol. Med. 2019, 11. [Google Scholar] [CrossRef]
- Luna-Sanchez, M.; Diaz-Casado, E.; Barca, E.; Tejada, M.A.; Montilla-Garcia, A.; Cobos, E.J.; Escames, G.; Acuna-Castroviejo, D.; Quinzii, C.M.; Lopez, L.C. The clinical heterogeneity of coenzyme Q10 deficiency results from genotypic differences in the Coq9 gene. EMBO Mol. Med. 2015, 7, 670–687. [Google Scholar] [CrossRef]
- Wang, Y.; Smith, C.; Parboosingh, J.S.; Khan, A.; Innes, M.; Hekimi, S. Pathogenicity of two COQ7 mutations and responses to 2,4-dihydroxybenzoate bypass treatment. J. Cell Mol. Med. 2017, 21, 2329–2343. [Google Scholar] [CrossRef]
- Di Meo, I.; Lamperti, C.; Tiranti, V. Mitochondrial diseases caused by toxic compound accumulation: From etiopathology to therapeutic approaches. EMBO Mol. Med. 2015, 7, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Grieshaber, M.K. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008, 275, 3352–3361. [Google Scholar] [CrossRef]
- Kabil, O.; Vitvitsky, V.; Banerjee, R. Sulfur as a signaling nutrient through hydrogen sulfide. Annu. Rev. Nutr. 2014, 34, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Libiad, M.; Yadav, P.K.; Vitvitsky, V.; Martinov, M.; Banerjee, R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J. Biol. Chem. 2014, 289, 30901–30910. [Google Scholar] [CrossRef] [PubMed]
- Modis, K.; Coletta, C.; Erdelyi, K.; Papapetropoulos, A.; Szabo, C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013, 27, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. H2S: A Novel Gasotransmitter that Signals by Sulfhydration. Trends Biochem. Sci. 2015, 40, 687–700. [Google Scholar] [CrossRef]
- Kabil, H.; Kabil, O.; Banerjee, R.; Harshman, L.G.; Pletcher, S.D. Increased transsulfuration mediates longevity and dietary restriction in Drosophila. Proc. Natl. Acad. Sci. USA 2011, 108, 16831–16836. [Google Scholar] [CrossRef]
- Hine, C.; Mitchell, J.R. Calorie restriction and methionine restriction in control of endogenous hydrogen sulfide production by the transsulfuration pathway. Exp. Gerontol. 2015, 68, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yao, Q.; Lu, L.; Li, Y.; Chen, P.J.; Duan, C. Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Rep. 2014, 6, 1110–1121. [Google Scholar] [CrossRef]
- Hine, C.; Harputlugil, E.; Zhang, Y.; Ruckenstuhl, C.; Lee, B.C.; Brace, L.; Longchamp, A.; Trevino-Villarreal, J.H.; Mejia, P.; Ozaki, C.K.; et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 2015, 160, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, P.; Hidalgo-Gutierrez, A.; Mascaraque, C.; Barriocanal-Casado, E.; Bakkali, M.; Ziosi, M.; Abdihankyzy, U.B.; Sanchez-Hernandez, S.; Escames, G.; Prokisch, H.; et al. Coenzyme Q10 modulates sulfide metabolism and links the mitochondrial respiratory chain to pathways associated to one carbon metabolism. Hum. Mol. Genet. 2020, 29, 3296–3311. [Google Scholar] [CrossRef]
- Tiranti, V.; Viscomi, C.; Hildebrandt, T.; Di Meo, I.; Mineri, R.; Tiveron, C.; Levitt, M.D.; Prelle, A.; Fagiolari, G.; Rimoldi, M.; et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat. Med. 2009, 15, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Friederich, M.W.; Elias, A.F.; Kuster, A.; Laugwitz, L.; Larson, A.A.; Landry, A.P.; Ellwood-Digel, L.; Mirsky, D.M.; Dimmock, D.; Haven, J.; et al. Pathogenic variants in SQOR encoding sulfide:quinone oxidoreductase are a potentially treatable cause of Leigh disease. J. Inherit. Metab. Dis. 2020, 43, 1024–1036. [Google Scholar] [CrossRef]
- Mottawea, W.; Chiang, C.K.; Muhlbauer, M.; Starr, A.E.; Butcher, J.; Abujamel, T.; Deeke, S.A.; Brandel, A.; Zhou, H.; Shokralla, S.; et al. Altered intestinal microbiota-host mitochondria crosstalk in new onset Crohn’s disease. Nat. Commun. 2016, 7, 13419. [Google Scholar] [CrossRef]
- Phillips, C.M.; Zatarain, J.R.; Nicholls, M.E.; Porter, C.; Widen, S.G.; Thanki, K.; Johnson, P.; Jawad, M.U.; Moyer, M.P.; Randall, J.W.; et al. Upregulation of Cystathionine-beta-Synthase in Colonic Epithelia Reprograms Metabolism and Promotes Carcinogenesis. Cancer Res. 2017, 77, 5741–5754. [Google Scholar] [CrossRef]
- Nikkanen, J.; Forsstrom, S.; Euro, L.; Paetau, I.; Kohnz, R.A.; Wang, L.; Chilov, D.; Viinamaki, J.; Roivainen, A.; Marjamaki, P.; et al. Mitochondrial DNA Replication Defects Disturb Cellular dNTP Pools and Remodel One-Carbon Metabolism. Cell Metab. 2016, 23, 635–648. [Google Scholar] [CrossRef]
- Forsstrom, S.; Jackson, C.B.; Carroll, C.J.; Kuronen, M.; Pirinen, E.; Pradhan, S.; Marmyleva, A.; Auranen, M.; Kleine, I.M.; Khan, N.A.; et al. Fibroblast Growth Factor 21 Drives Dynamics of Local and Systemic Stress Responses in Mitochondrial Myopathy with mtDNA Deletions. Cell Metab. 2019, 30, 1040–1054.e1047. [Google Scholar] [CrossRef]
- Krug, A.K.; Gutbier, S.; Zhao, L.; Poltl, D.; Kullmann, C.; Ivanova, V.; Forster, S.; Jagtap, S.; Meiser, J.; Leparc, G.; et al. Transcriptional and metabolic adaptation of human neurons to the mitochondrial toxicant MPP+. Cell Death Dis. 2014, 5, e1222. [Google Scholar] [CrossRef]
- Tyynismaa, H.; Carroll, C.J.; Raimundo, N.; Ahola-Erkkila, S.; Wenz, T.; Ruhanen, H.; Guse, K.; Hemminki, A.; Peltola-Mjosund, K.E.; Tulkki, V.; et al. Mitochondrial myopathy induces a starvation-like response. Hum. Mol. Genet. 2010, 19, 3948–3958. [Google Scholar] [CrossRef]
- Bao, X.R.; Ong, S.E.; Goldberger, O.; Peng, J.; Sharma, R.; Thompson, D.A.; Vafai, S.B.; Cox, A.G.; Marutani, E.; Ichinose, F.; et al. Mitochondrial dysfunction remodels one-carbon metabolism in human cells. Elife 2016, 5. [Google Scholar] [CrossRef]
- Ziosi, M.; Di Meo, I.; Kleiner, G.; Gao, X.H.; Barca, E.; Sanchez-Quintero, M.J.; Tadesse, S.; Jiang, H.; Qiao, C.; Rodenburg, R.J.; et al. Coenzyme Q deficiency causes impairment of the sulfide oxidation pathway. EMBO Mol. Med. 2017, 9, 96–111. [Google Scholar] [CrossRef]
- Luna-Sanchez, M.; Hidalgo-Gutierrez, A.; Hildebrandt, T.M.; Chaves-Serrano, J.; Barriocanal-Casado, E.; Santos-Fandila, A.; Romero, M.; Sayed, R.K.; Duarte, J.; Prokisch, H.; et al. CoQ deficiency causes disruption of mitochondrial sulfide oxidation, a new pathomechanism associated with this syndrome. EMBO Mol. Med. 2017, 9, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; da Silva, T.N.X.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Hosoki, R.; Matsuki, N.; Kimura, H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef]
- Evans, D.R.; Guy, H.I. Mammalian pyrimidine biosynthesis: Fresh insights into an ancient pathway. J. Biol. Chem. 2004, 279, 33035–33038. [Google Scholar] [CrossRef]
- Rosenfeldt, F.L. Metabolic supplementation with orotic acid and magnesium orotate. Cardiovasc. Drugs Ther. 1998, 12 (Suppl. s2), 147–152. [Google Scholar] [CrossRef]
- Montero, R.; Sanchez-Alcazar, J.A.; Briones, P.; Navarro-Sastre, A.; Gallardo, E.; Bornstein, B.; Herrero-Martin, D.; Rivera, H.; Martin, M.A.; Marti, R.; et al. Coenzyme Q10 deficiency associated with a mitochondrial DNA depletion syndrome: A case report. Clin. Biochem. 2009, 42, 742–745. [Google Scholar] [CrossRef]
- Montero, R.; Grazina, M.; Lopez-Gallardo, E.; Montoya, J.; Briones, P.; Navarro-Sastre, A.; Land, J.M.; Hargreaves, I.P.; Artuch, R.; Coenzyme, Q.D.S.G. Coenzyme Q10 deficiency in mitochondrial DNA depletion syndromes. Mitochondrion 2013, 13, 337–341. [Google Scholar] [CrossRef]
- Bajzikova, M.; Kovarova, J.; Coelho, A.R.; Boukalova, S.; Oh, S.; Rohlenova, K.; Svec, D.; Hubackova, S.; Endaya, B.; Judasova, K.; et al. Reactivation of Dihydroorotate Dehydrogenase-Driven Pyrimidine Biosynthesis Restores Tumor Growth of Respiration-Deficient Cancer Cells. Cell Metab. 2019, 29, 399–416.e310. [Google Scholar] [CrossRef] [PubMed]
- Watmough, N.J.; Frerman, F.E. The electron transfer flavoprotein: Ubiquinone oxidoreductases. Biochim. Biophys. Acta 2010, 1797, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Gempel, K.; Topaloglu, H.; Talim, B.; Schneiderat, P.; Schoser, B.G.; Hans, V.H.; Palmafy, B.; Kale, G.; Tokatli, A.; Quinzii, C.; et al. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase (ETFDH) gene. Brain 2007, 130, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.C.; Ohkuma, A.; Hayashi, Y.K.; Lopez, L.C.; Hirano, M.; Nonaka, I.; Noguchi, S.; Chen, L.H.; Jong, Y.J.; Nishino, I. ETFDH mutations, CoQ10 levels, and respiratory chain activities in patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Neuromuscul. Disord. 2009, 19, 212–216. [Google Scholar] [CrossRef]
- Summitt, C.B.; Johnson, L.C.; Jonsson, T.J.; Parsonage, D.; Holmes, R.P.; Lowther, W.T. Proline dehydrogenase 2 (PRODH2) is a hydroxyproline dehydrogenase (HYPDH) and molecular target for treating primary hyperoxaluria. Biochem. J. 2015, 466, 273–281. [Google Scholar] [CrossRef]
- Moxley, M.A.; Tanner, J.J.; Becker, D.F. Steady-state kinetic mechanism of the proline:ubiquinone oxidoreductase activity of proline utilization A (PutA) from Escherichia coli. Arch. Biochem. Biophys. 2011, 516, 113–120. [Google Scholar] [CrossRef]
- Rauchova, H.; Battino, M.; Fato, R.; Lenaz, G.; Drahota, Z. Coenzyme Q-pool function in glycerol-3-phosphate oxidation in hamster brown adipose tissue mitochondria. J. Bioenerg. Biomembr. 1992, 24, 235–241. [Google Scholar] [CrossRef]
- Barrett, M.C.; Dawson, A.P. The reaction of choline dehydrogenase with some electron acceptors. Biochem. J. 1975, 151, 677–683. [Google Scholar] [CrossRef]
- Echtay, K.S.; Winkler, E.; Frischmuth, K.; Klingenberg, M. Uncoupling proteins 2 and 3 are highly active H+ transporters and highly nucleotide sensitive when activated by coenzyme Q (ubiquinone). Proc. Natl. Acad. Sci. USA 2001, 98, 1416–1421. [Google Scholar] [CrossRef]
- Echtay, K.S.; Winkler, E.; Klingenberg, M. Coenzyme Q is an obligatory cofactor for uncoupling protein function. Nature 2000, 408, 609–613. [Google Scholar] [CrossRef]
- Jaburek, M.; Garlid, K.D. Reconstitution of recombinant uncoupling proteins: UCP1, -2, and -3 have similar affinities for ATP and are unaffected by coenzyme Q10. J. Biol. Chem. 2003, 278, 25825–25831. [Google Scholar] [CrossRef]
- Esteves, T.C.; Echtay, K.S.; Jonassen, T.; Clarke, C.F.; Brand, M.D. Ubiquinone is not required for proton conductance by uncoupling protein 1 in yeast mitochondria. Biochem. J. 2004, 379, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Sluse, F.E.; Jarmuszkiewicz, W.; Navet, R.; Douette, P.; Mathy, G.; Sluse-Goffart, C.M. Mitochondrial UCPs: New insights into regulation and impact. Biochim. Biophys. Acta 2006, 1757, 480–485. [Google Scholar] [CrossRef]
- Walter, L.; Miyoshi, H.; Leverve, X.; Bernard, P.; Fontaine, E. Regulation of the mitochondrial permeability transition pore by ubiquinone analogs. A progress report. Free Radic. Res. 2002, 36, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, E.; Bernardi, P. Progress on the mitochondrial permeability transition pore: Regulation by complex I and ubiquinone analogs. J. Bioenerg. Biomembr. 1999, 31, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, E.; Ichas, F.; Bernardi, P. A ubiquinone-binding site regulates the mitochondrial permeability transition pore. J. Biol. Chem. 1998, 273, 25734–25740. [Google Scholar] [CrossRef]
- Belliere, J.; Devun, F.; Cottet-Rousselle, C.; Batandier, C.; Leverve, X.; Fontaine, E. Prerequisites for ubiquinone analogs to prevent mitochondrial permeability transition-induced cell death. J. Bioenerg. Biomembr. 2012, 44, 207–212. [Google Scholar] [CrossRef]
- Devun, F.; Walter, L.; Belliere, J.; Cottet-Rousselle, C.; Leverve, X.; Fontaine, E. Ubiquinone analogs: A mitochondrial permeability transition pore-dependent pathway to selective cell death. PLoS ONE 2010, 5, e11792. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).