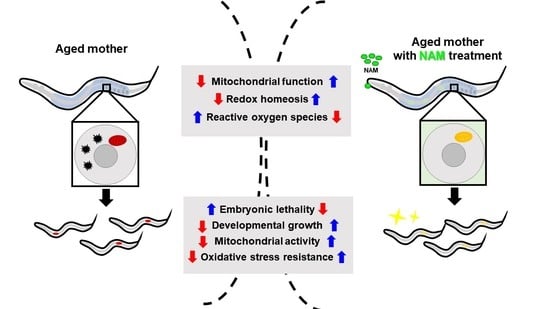
Nicotinamide Supplementation Improves Oocyte Quality and Offspring Development by Modulating Mitochondrial Function in an Aged Caenorhabditis elegans Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Caenorhabditis elegans Strains and Nicotinamide (NAM) Supplementation
2.2. Analysis of Embryonic Lethality, Unfertilized Oocytes, Small Embryos, and Percent Larval Development
2.3. DNA Staining in Oocytes
2.4. Immunofluorescence Analysis
2.5. Western Blot Analysis
2.6. Germ Cell Apoptosis Assay
2.7. Analysis of Mitochondrial Activity and Mitochondrial Membrane Potential (MMP)
2.8. Analysis of Mitochondrial Reactive Oxygen Species (ROS)
2.9. Live Image Observation of Fluorescence-Tagged Transgenic Animals
2.10. Locomotion Behavior Assay
2.11. Survival Assay under Paraquat-Induced Oxidative Stress
2.12. Statistical Analysis
3. Results
3.1. Nicotinamide (NAM) Supplementation Improves Fertility of Aged Oocytes
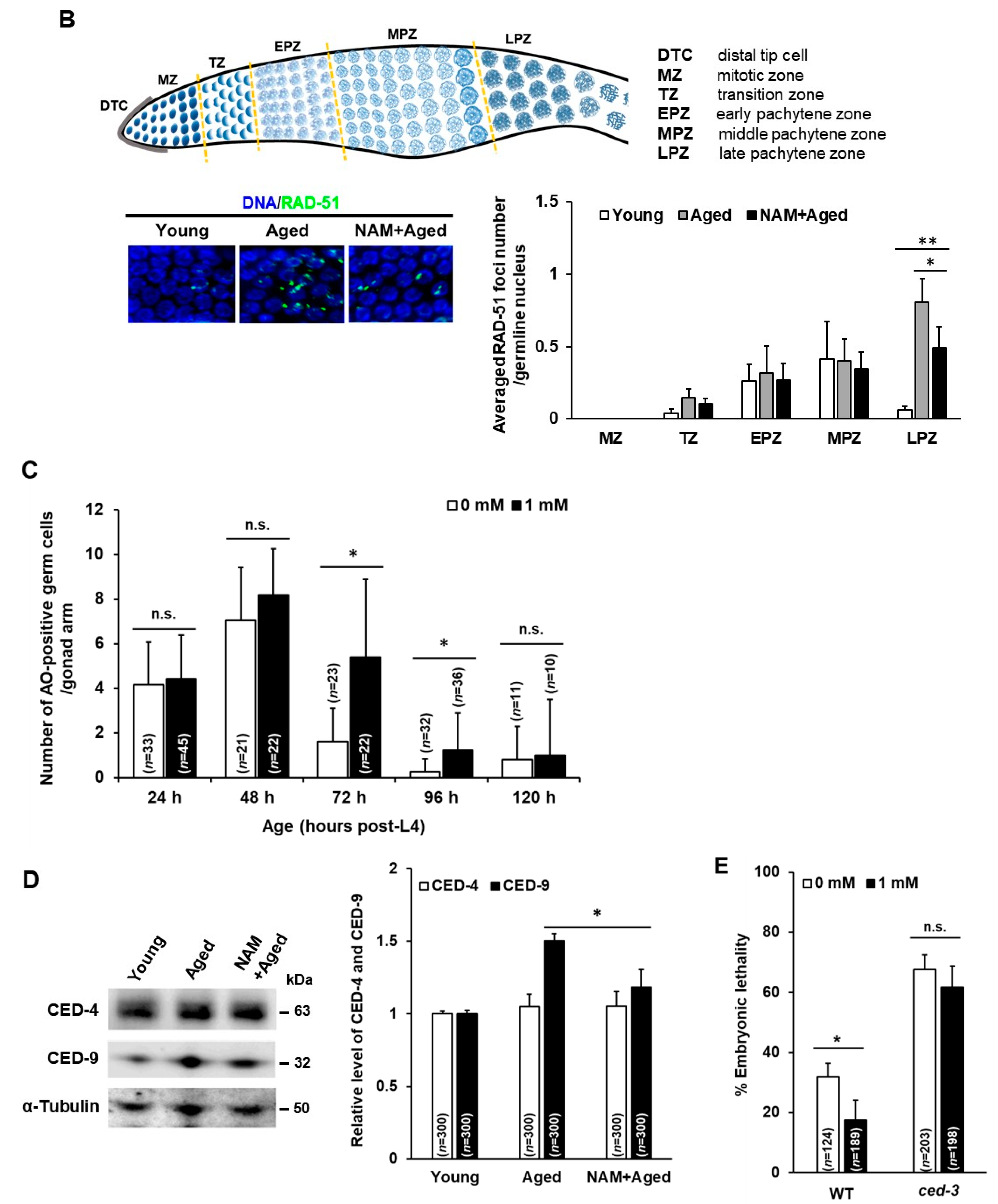
3.2. NAM Supplementation Improves Chromosomal Abnormalities in Aged Oocytes and Decreased Level of Germ Cell Apoptosis in Aged Animals
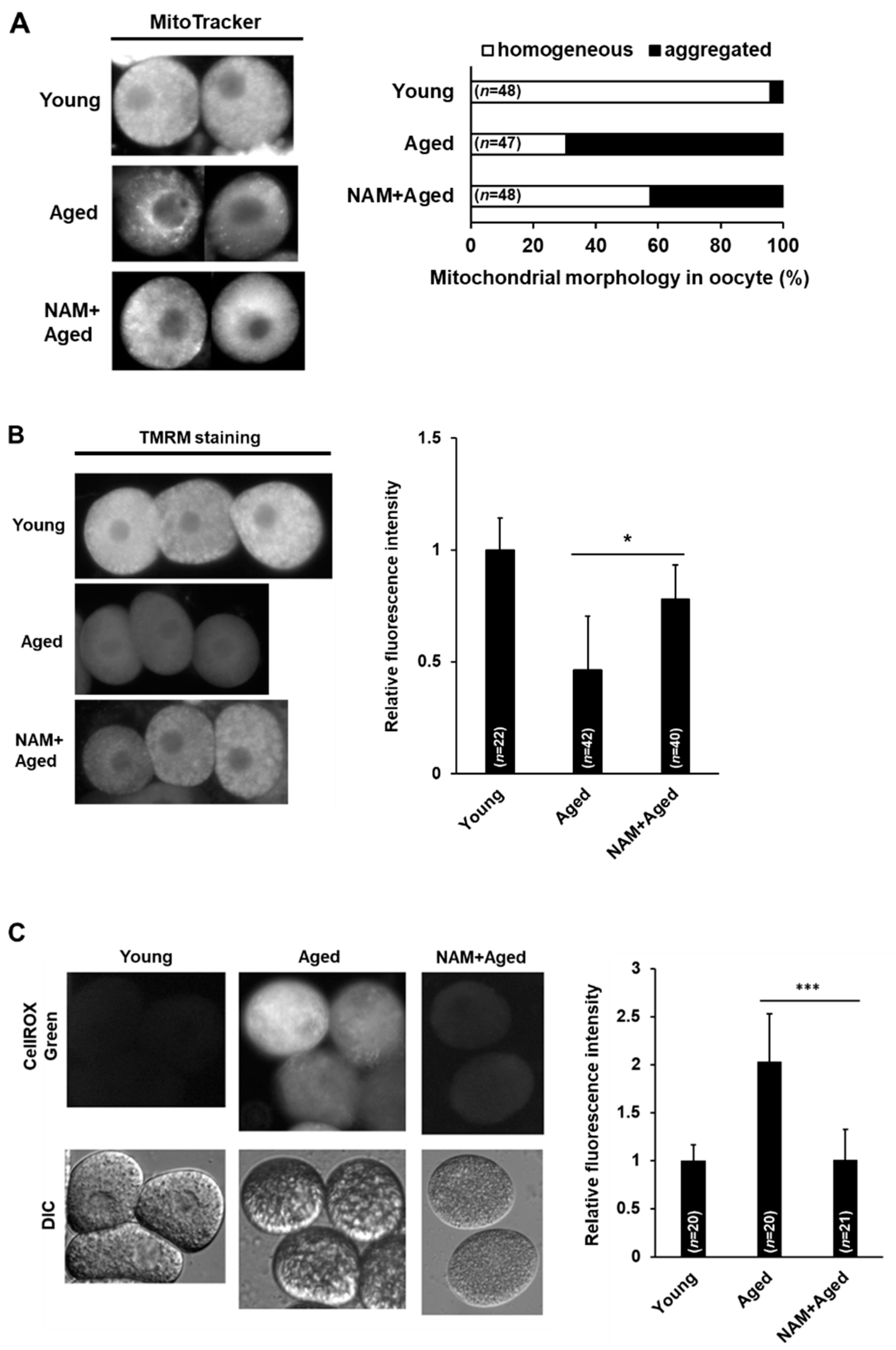
3.3. NAM Supplementation Improves Mitochondrial Dysfunction in Aged Oocytes
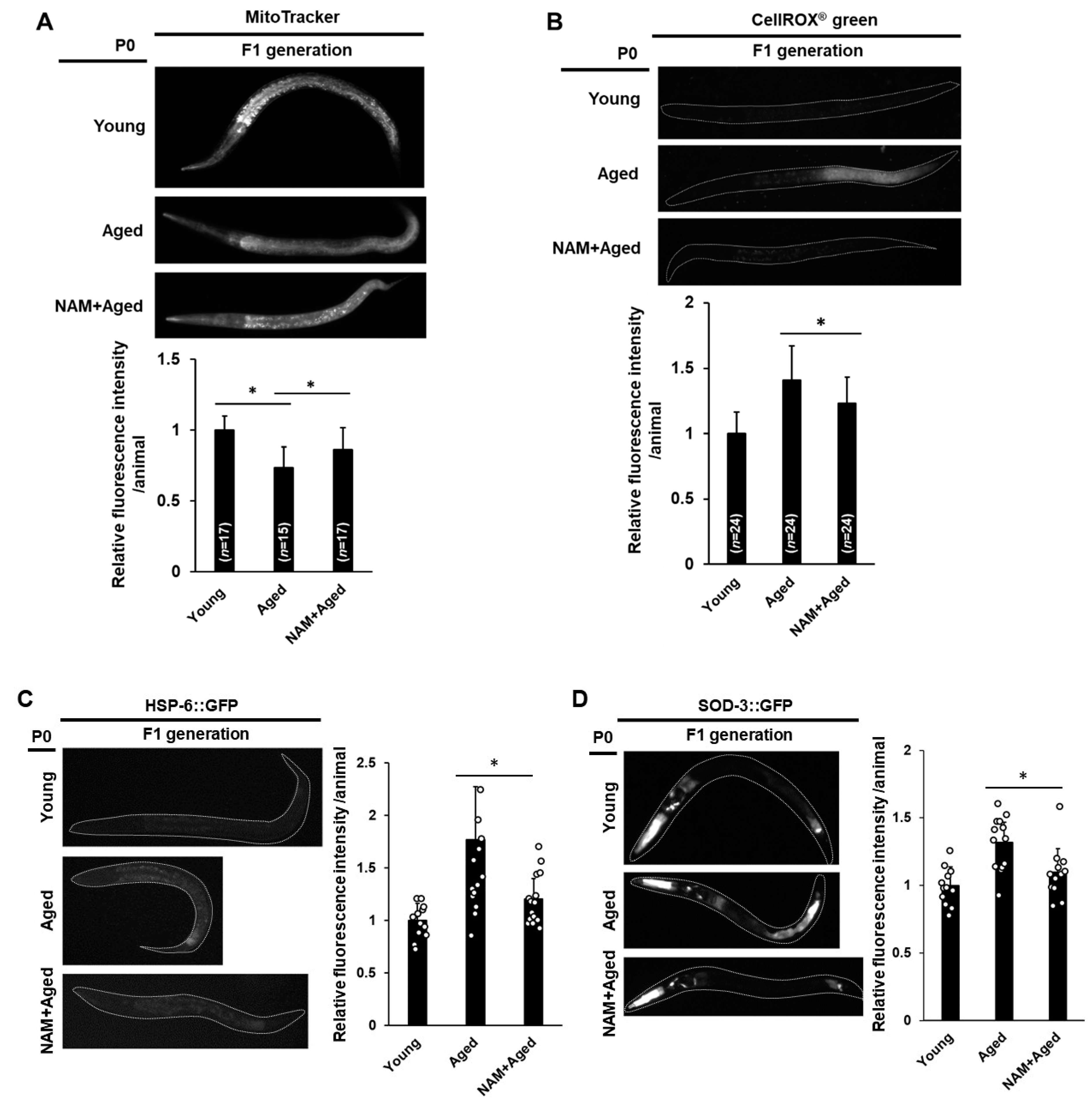
3.4. Maternal NAM Supplementation Improves Mitochondrial Activity and Oxidative Stress Responses in Offspring Produced by Aged Oocytes
3.5. Maternal NAM Supplementation Improves Motility, Oxidative Stress Resistance, and Developmental Growth in Offspring Produced by Aged Oocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, T.Y.; Lau, M.S.K.; Loh, S.F.; Tan, H.H. Female ageing and reproductive outcome in assisted reproduction cycles. Singap. Med. J. 2014, 55, 305–309. [Google Scholar] [CrossRef]
- Crawford, N.M.; Steiner, A.Z. Age-related infertility. Obstet. Gynecol. Clin. N. Am. 2015, 42, 15–25. [Google Scholar] [CrossRef]
- Rodríguez-Varela, C.; Labarta, E. Clinical application of antioxidants to improve human oocyte mitochondrial function: A review. Antioxidants 2020, 9, 1197. [Google Scholar] [CrossRef] [PubMed]
- Fabozzi, G.; Iussig, B.; Cimadomo, D.; Vaiarelli, A.; Maggiulli, R.; Ubaldi, N.; Ubaldi, F.M.; Rienzi, L. The impact of unbalanced maternal nutritional intakes on oocyte mitochondrial activity: Implications for reproductive function. Antioxidants 2021, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Cui, Z.; Gao, Q.; Rui, R.; Xiong, B. Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Rep. 2020, 32, 107987. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, M.J.; Listijono, D.R.; Ho, W.H.J.; Riepsamen, A.H.; Goss, D.M.; Richani, D.; Jin, X.L.; Mahbub, S.; Campbell, J.M.; Habibalahi, A.; et al. NAD+ repletion rescues female fertility during reproductive aging. Cell Rep. 2020, 30, 1670–1681.e7. [Google Scholar] [CrossRef]
- Andux, S.; Ellis, R.E. Apoptosis maintains oocyte quality in aging Caenorhabditis elegans females. PLoS Genet. 2008, 4, e1000295. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Shaw, W.M.; Ashraf, J.; Murphy, C.T. TGF-ß Sma/Mab signaling mutations uncouple reproductive aging from somatic aging. PLoS Genet. 2009, 5, e1000789. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Kleemann, G.A.; Ashraf, J.M.; Shaw, W.M.; Murphy, C.T. TGF-β and insulin signaling regulate reproductive aging via oocyte and germline quality maintenance. Cell 2010, 143, 299–312. [Google Scholar] [CrossRef]
- Luo, S.; Murphy, C.T. Caenorhabditis elegans reproductive aging: Regulation and underlying mechanisms. Genesis 2010, 49, 53–65. [Google Scholar] [CrossRef]
- Greenstein, D. Control of oocyte meiotic maturation and fertilization. WormBook 2005, 1–12. [Google Scholar] [CrossRef]
- Mehlmann, L.M. Stops and starts in mammalian oocytes: Recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction 2005, 130, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sauve, A.A. NAD+ Metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta 2016, 1864, 1787–1800. [Google Scholar] [CrossRef]
- Bonkowski, M.S.; Sinclair, D.A. Slowing ageing by design: The rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679–690. [Google Scholar] [CrossRef]
- Croteau, D.L.; Fang, E.F.; Nilsen, H.; Bohr, V.A. NAD+ in DNA repair and mitochondrial maintenance. Cell Cycle 2017, 16, 491–492. [Google Scholar] [CrossRef]
- Zhang, D.X.; Zhang, J.P.; Hu, J.Y.; Huang, Y.S. The potential regulatory roles of NAD+ and its metabolism in autophagy. Metabolism 2016, 65, 454–462. [Google Scholar] [CrossRef]
- Ewald, C.Y. Redox signaling of NADPH oxidases regulates oxidative stress responses, immunity and aging. Antioxidants 2018, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Navas, L.E.; Carnero, A. NAD+ Metabolism, stemness, the immune response, and cancer. Signal Transduct. Target. Ther. 2021, 6, 2. [Google Scholar] [CrossRef]
- Gomes, A.P.; Price, N.L.; Ling, A.J.Y.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P.; et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef]
- Zhu, X.H.; Lu, M.; Lee, B.Y.; Ugurbil, K.; Chen, W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc. Natl. Acad. Sci. USA 2015, 112, 2876–2881. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Pereira, J.; Tarragó, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Greaney, J.; Zhe, W.; Hayden, H. Regulation of chromosome segregation in oocytes and the cellular basis for female meiotic errors. Hum. Reprod. Update 2018, 24, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Driver, C.; Angela, G. How to re-energise old mitochondria without shooting yourself in the foot. Biogerontology 2002, 3, 103–106. [Google Scholar] [CrossRef]
- Jia, H.; Li, X.; Gao, H.; Feng, Z.; Li, X.; Zhao, L.; Xu, J.; Hongyu, Z.; Liu, J. High doses of nicotinamide prevent oxidative mitochondrial dysfunction in a cellular model and improve motor deficit in a Drosophila model of Parkinson’s disease. J. Neurosci. Res. 2008, 86, 2083–2090. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Altun, Z.F.; Hall, D.H. Introduction; WormAtlas (Cold Spring Harbor Laboratory Press): Long Island, NY, USA, 2009. [Google Scholar] [CrossRef]
- Min, H.; Kim, J.S.; Ahn, J.; Shim, Y.H. Gliadin intake causes disruption of the intestinal barrier and an increase in germ cell apoptosis in a Caenorhabditis elegans model. Nutrients 2019, 11, 2587. [Google Scholar] [CrossRef]
- Min, H.; Youn, E.; Kim, J.; Son, S.Y.; Lee, C.H.; Shim, Y.H. Effects of phosphoethanolamine supplementation on mitochondrial activity and lipogenesis in a caffeine ingestion Caenorhabditis elegans model. Nutrients 2020, 12, 3348. [Google Scholar] [CrossRef]
- Navarro, R.E.; Shim, E.Y.; Kohara, Y.; Singson, A.; Blackwell, T.K. cgh-1, a conserved predicted RNA helicase required for gametogenesis and protection from physiological germline apoptosis in C. elegans. Development 2001, 128, 3221–3232. [Google Scholar] [PubMed]
- Fernández-Cárdenas, L.P.; Villanueva-Chimal, E.; Salinas, L.S.; José-Nuñez, C.; Tuena de Gómez Puyou, M.; Navarro, R.E. Caenorhabditis elegans ATPase inhibitor factor 1 (IF1) MAI-2 preserves the mitochondrial membrane potential (Δψm) and is important to induce germ cell apoptosis. PLoS ONE 2017, 12, e0181984. [Google Scholar] [CrossRef]
- Lim, S.D.; Min, H.; Youn, E.; Kawasaki, I.; Shim, Y.H. Gliadin intake induces oxidative-stress responses in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2018, 503, 2139–2145. [Google Scholar] [CrossRef]
- Lee, H.; Cho, J.S.; Lambacher, N.; Lee, J.; Lee, S.J.; Lee, T.H.; Gartner, A.; Koo, H.S. The Caenorhabditis elegans AMP-activated protein kinase AAK-2 is phosphorylated by LKB1 and is required for resistance to oxidative stress and for normal motility and foraging behavior. J. Biol. Chem. 2008, 283, 14988–14993. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Schatten, H.; Sun, Q.Y. Why is chromosome segregation error in oocytes increased with maternal aging? Physiology 2011, 26, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Magli, M.C.; Gianaroli, L.; Ferraretti, A.P.; Lappi, M.; Ruberti, A.; Farfalli, V. Embryo morphology and development are dependent on the chromosomal complement. Fertil. Steril. 2007, 87, 534–541. [Google Scholar] [CrossRef]
- Watson, E.V.; Elledge, S.J. Aneuploidy police detect chromosomal imbalance triggering immune crackdown! Trends Genet. 2017, 33, 662–664. [Google Scholar] [CrossRef]
- Fang, E.F.; Kassahun, H.; Croteau, D.L.; Scheibye-Knudsen, M.; Marosi, K.; Lu, H.; Shamanna, R.A.; Kalyanasundaram, S.; Bollineni, R.C.; Wilson, M.A.; et al. NAD+ replenishment improves lifespan and healthspan in Ataxia telangiectasia models via mitophagy and DNA repair. Cell Metab. 2016, 24, 566–581. [Google Scholar] [CrossRef]
- Delabaere, L.; Ertl, H.A.; Massey, D.J.; Hofley, C.M.; Sohail, F.; Bienenstock, E.J.; Sebastian, H.; Chiolo, I.; LaRocque, J.R. Aging impairs double-strand break repair by homologous recombination in Drosophila germ cells. Aging Cell 2017, 16, 320–328. [Google Scholar] [CrossRef]
- Dernburg, A.F.; McDonald, K.; Moulder, G.; Barstead, R.; Dresser, M.; Villeneuve, A.M. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 1998, 94, 387–398. [Google Scholar] [CrossRef]
- Yu, Z.; Kim, Y.; Dernburg, A.F. Meiotic recombination and the crossover assurance checkpoint in Caenorhabditis elegans. Semin. Cell. Dev. Biol. 2016, 54, 106–116. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, N.; Rose, A. DNA repair. WormBook 2006, 13, 1–12. [Google Scholar] [CrossRef]
- De la Guardia, Y.; Gilliat, A.F.; Hellberg, J.; Rennert, P.; Cabreiro, F.; Gems, D. Run-on of germline apoptosis promotes gonad senescence in C. elegans. Oncotarget 2016, 7, 39082–39096. [Google Scholar] [CrossRef]
- Gartner, A.; Boag, P.R.; Blackwell, T.K. Germline survival and apoptosis. WormBook 2008, 1–20. [Google Scholar] [CrossRef]
- Schleyer, M.; Schmidt, B.; Neupert, W. Requirement of a membrane potential for the posttranslational transfer of proteins into mitochondria. Eur. J. Biochem. 1982, 125, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Elorza, A.; Molina, A.J.A.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obstet. Gynecol. 2015, 27, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, C.J.; Pollard, A.; Barratt, T.F.; Constantin-Teodosiu, D.; Greenhaff, P.L.; Szewczyk, N.J. Greater Loss of mitochondrial function with ageing is associated with earlier onset of sarcopenia in C. elegans. Aging 2018, 10, 3382–3396. [Google Scholar] [CrossRef]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD+ in aging: Molecular mechanisms and translational implications. Trends Mol. Med. 2017, 23, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Scheibye-Knudsen, M.; Mitchell, S.J.; Fang, E.F.; Iyama, T.; Ward, T.; Wang, J.; Dunn, C.A.; Singh, N.; Veith, S.; Hasan-Olive, M.M.; et al. A High-fat diet and NAD+ activate sirt1 to rescue premature aging in cockayne syndrome. Cell Metab. 2014, 20, 840–855. [Google Scholar] [CrossRef]
- Kaneko, S.; Wang, J.; Kaneko, M.; Yiu, G.; Hurrell, J.M.; Chitnis, T.; Khoury, S.J.; He, Z. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J. Neurosci. 2006, 26, 9794–9804. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H. Is NADH effective in the treatment of Parkinson’s disease? Drugs Aging 1998, 13, 263–268. [Google Scholar] [CrossRef]
- Lee, C.F.; Chavez, J.D.; Garcia-Menendez, L.; Choi, Y.; Roe, N.D.; Chiao, Y.A.; Edgar, J.S.; Goo, Y.A.; Goodlett, D.R.; Bruce, J.E.; et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation 2016, 134, 883–894. [Google Scholar] [CrossRef]
- Balan, V.; Miller, G.S.; Kaplun, L.; Balan, K.; Chong, Z.Z.; Li, F.; Kaplun, A.; VanBerkum, M.F.A.; Arking, R.; Freeman, D.C.; et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J. Biol. Chem. 2008, 283, 27810–27819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; Amico, D.D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Horikawa, M.; Nomura, T.; Sakamoto, K. Nicotinamide adenine dinucleotide extends the lifespan of Caeno-rhabditis elegans mediated by sir-2.1 and daf-16. Biogerontology 2010, 11, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Horvitz, H.R. Worms, life, and death (Nobel lecture). Chembiochem 2003, 4, 697–711. [Google Scholar] [CrossRef]
- Conradt, B. Genetic control of programmed cell death during animal development. Annu. Rev. Genet. 2009, 43, 493–523. [Google Scholar] [CrossRef]
- Lettre, G.; Hengartner, M.O. Developmental apoptosis in C. elegans: A complex CEDnario. Nat. Rev. Mol. Cell Biol. 2006, 7, 97–108. [Google Scholar] [CrossRef]
- Pourkarimi, E.; Greiss, S.; Gartner, A. Evidence that CED-9/Bcl2 and CED-4/Apaf-1 localization is not consistent with the current model for C. elegans apoptosis induction. Cell Death Differ. 2012, 19, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.J.; Husain, M.; Manlandro, C.M.; Koppenol, M.; Fire, A.Z.; Hill, R.B. CED-9 and mitochondrial homeostasis in C. elegans muscle. J. Cell Sci. 2008, 121, 3373–3382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rolland, S.G.; Lu, Y.; David, C.N.; Conradt, B. The BCL-2-like protein CED-9 of C. elegans promotes FZO-1/Mfn1,2- and EAT-3/Opa1-dependent mitochondrial fusion. J. Cell Biol. 2009, 186, 525–540. [Google Scholar] [CrossRef]
- Raiders, S.A.; Eastwood, M.D.; Bacher, M.; Priess, J.R. Binucleate germ cells in Caenorhabditis elegans are removed by physiological apoptosis. PLoS Genet. 2018, 14, e1007417. [Google Scholar] [CrossRef] [PubMed]
- Sutovsky, P.; Moreno, R.D.; Ramalho-Santos, J.; Dominko, T.; Simerly, C.; Schatten, G. Ubiquitin tag for sperm mitochondria. Nature 1999, 402, 371–372. [Google Scholar] [CrossRef] [PubMed]
- St John, J.C. Mitochondria and female germline stem cells-A mitochondrial DNA perspective. Cells 2019, 8, 852. [Google Scholar] [CrossRef]
- Payne, B.A.I.; Wilson, I.J.; Yu-Wai-Man, P.; Coxhead, J.; Deehan, D.; Horvath, R.; Taylor, R.W.; Samuels, D.C.; Santibanez-Koref, M.; Chinnery, P.F. Universal heteroplasmy of human mitochondrial DNA. Hum. Mol. Genet. 2013, 22, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.L.; Boudoures, A.L.; Asghar, Z.; Thompson, A.; Drury, A.; Zhang, W.; Chi, M.; Cusumano, A.; Scheaffer, S.; Moley, K.H. Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Craven, L.; Alston, C.L.; Taylor, R.W.; Turnbull, D.M. Recent advances in mitochondrial disease. Annu. Rev. Genomics Hum. Genet. 2017, 18, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Tuna, S.; Keogh, M.J.; Smith, K.R.; Aitman, T.J.; Beales, P.L.; Bennett, D.L.; Gale, D.P.; Bitner-Glindzicz, M.A.K.; Black, G.C.; et al. Germline selection shapes human mitochondrial DNA diversity. Science 2019, 364, eaau6520. [Google Scholar] [CrossRef]
- Wu, L.L.; Russell, D.L.; Wong, S.L.; Chen, M.; Tsai, T.S.; St John, J.C.; Norman, R.J.; Febbraio, M.A.; Carroll, J.; Robker, R.L. Mitochondrial dysfunction in oocytes of obese mothers: Transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development 2015, 142, 681–691. [Google Scholar] [CrossRef]
- Szymusik, I.; Marianowski, P.; Zygula, A.; Wielgos, M. Poor responders in IVF--is there any evidence-based treatment for them? Neuro. Endocrinol. Lett. 2015, 36, 209–213. [Google Scholar]




| Aligned Condensed | Under- Condensed | Misaligned Condensed | n | |
|---|---|---|---|---|
| Young | 100 | 0 | 0 | 30 |
| Aged | 65.38 | 19.23 | 15.38 | 17 |
| NAM+Aged | 77.76 | 11.13 | 11.11 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, H.; Lee, M.; Cho, K.S.; Lim, H.J.; Shim, Y.-H. Nicotinamide Supplementation Improves Oocyte Quality and Offspring Development by Modulating Mitochondrial Function in an Aged Caenorhabditis elegans Model. Antioxidants 2021, 10, 519. https://doi.org/10.3390/antiox10040519
Min H, Lee M, Cho KS, Lim HJ, Shim Y-H. Nicotinamide Supplementation Improves Oocyte Quality and Offspring Development by Modulating Mitochondrial Function in an Aged Caenorhabditis elegans Model. Antioxidants. 2021; 10(4):519. https://doi.org/10.3390/antiox10040519
Chicago/Turabian StyleMin, Hyemin, Mijin Lee, Kyoung Sang Cho, Hyunjung Jade Lim, and Yhong-Hee Shim. 2021. "Nicotinamide Supplementation Improves Oocyte Quality and Offspring Development by Modulating Mitochondrial Function in an Aged Caenorhabditis elegans Model" Antioxidants 10, no. 4: 519. https://doi.org/10.3390/antiox10040519
APA StyleMin, H., Lee, M., Cho, K. S., Lim, H. J., & Shim, Y.-H. (2021). Nicotinamide Supplementation Improves Oocyte Quality and Offspring Development by Modulating Mitochondrial Function in an Aged Caenorhabditis elegans Model. Antioxidants, 10(4), 519. https://doi.org/10.3390/antiox10040519