NOX4 Mediates Pseudomonas aeruginosa-Induced Nuclear Reactive Oxygen Species Generation and Chromatin Remodeling in Lung Epithelium
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Animal Procedures
2.2. Isolation and Culture of Mouse Lung Alveolar Type II Cells
2.3. Downregulation of PKC δ, SPHK2, NOX2, and NOX4 Proteins with Small Interfering RNA
2.4. Imaging and Measurement of Nuclear ROS Using HyPer Biosensor in Epithelial Cells
2.5. Isolation of Nuclear Fraction from Epithelial Cells
2.6. Measurement of IL-6, TNF-α, IL-4, and IL-12
2.7. HDAC Activity
2.8. Measurement of H2O2
2.9. Immunoblotting and Immunoprecipitation
2.10. RAC1 Activation Assay by Western Blotting
2.11. Detection of Oxidized HDAC2
2.12. Immunohistochemical Staining
2.13. Preparation of P. aeruginosa
2.14. Statistical Analysis
3. Results
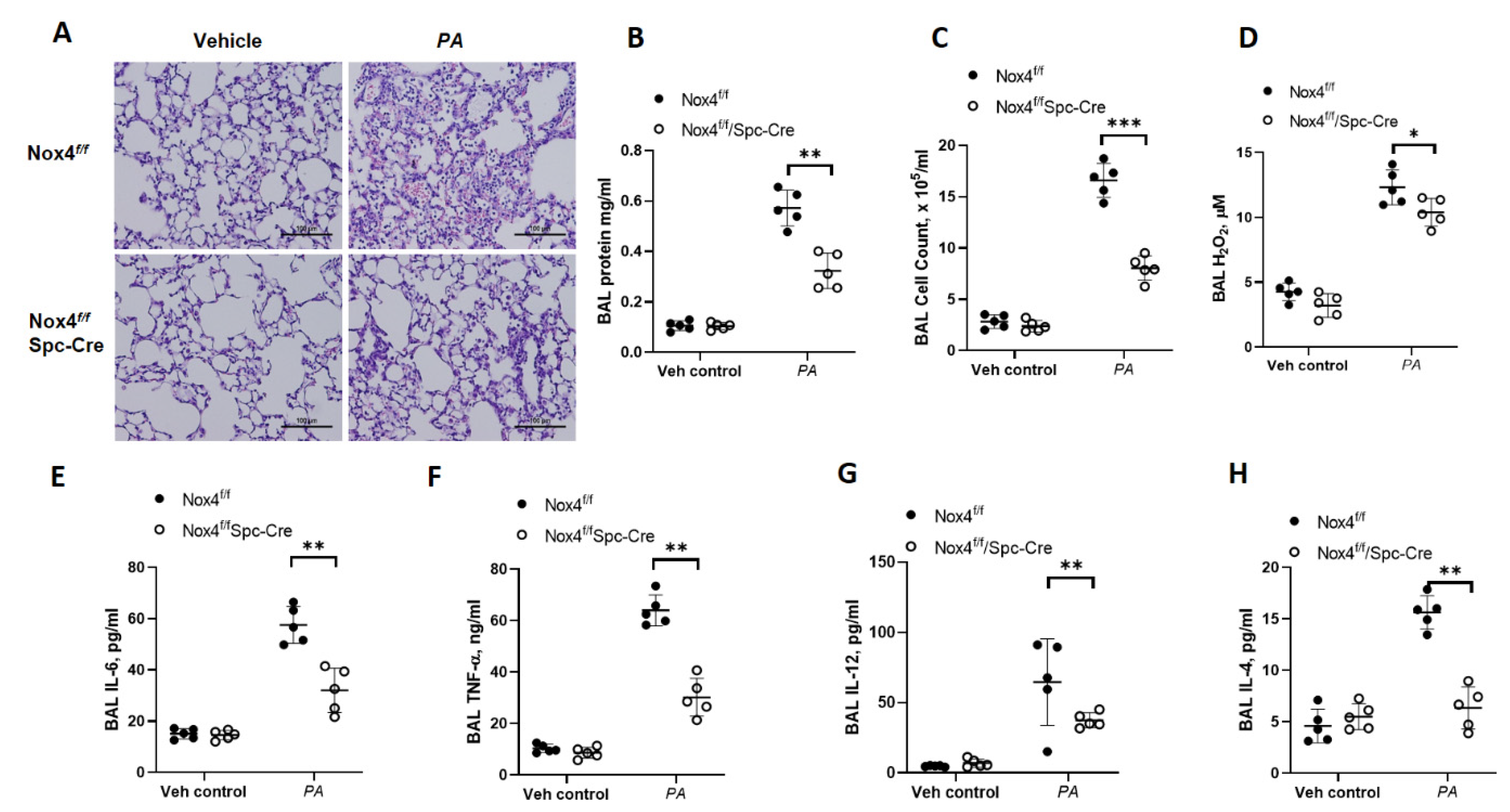
3.1. Genetic Deletion of Nox4 in Alveolar Epithelial Cells Protects Mice from P. aeruginosa-Induced Lung Inflammatory Injury
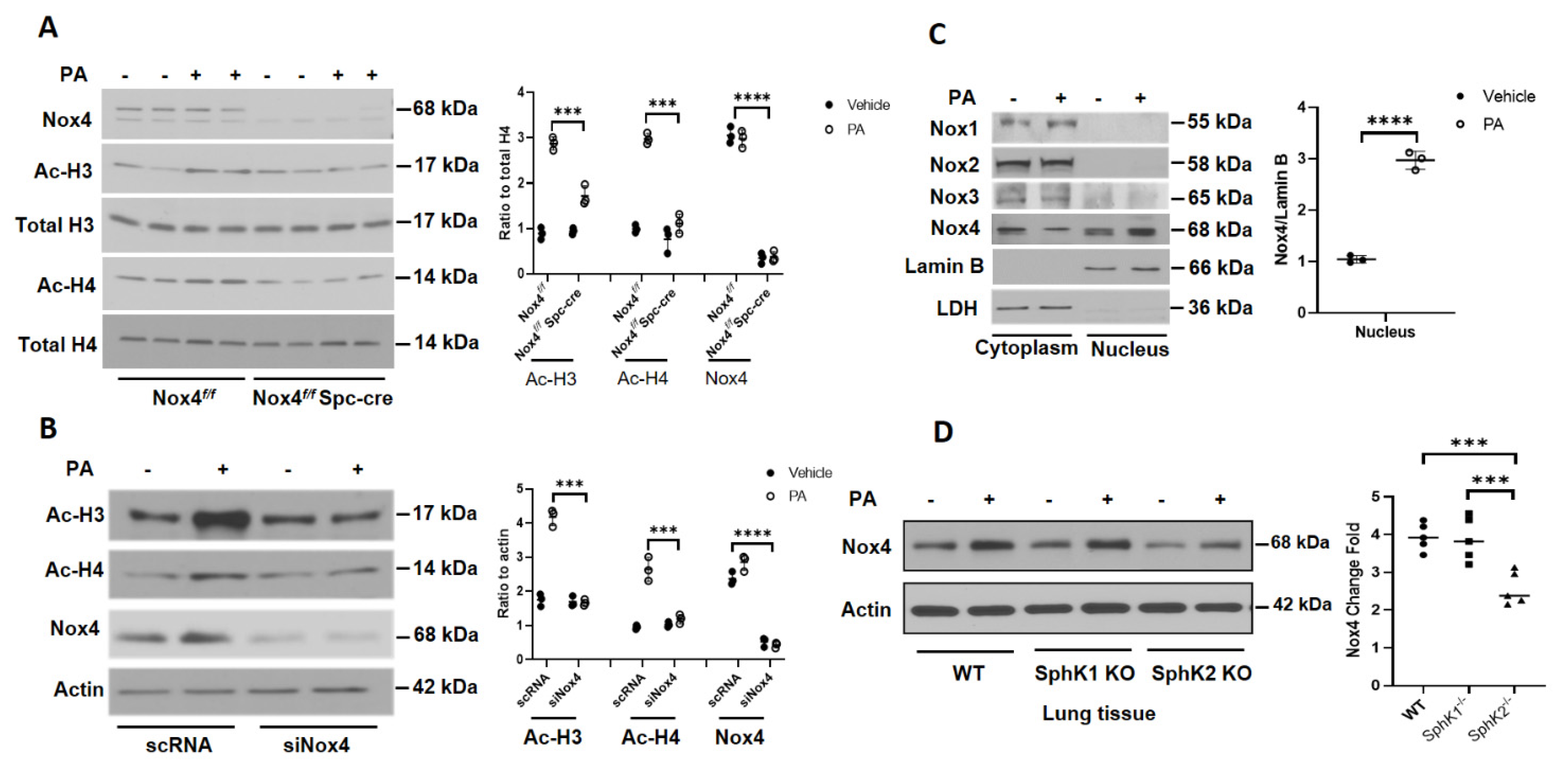
3.2. Deletion of Nox4 Reduces P. aeruginosa-Mediated H3 and H4 Histone Acetylation in Mouse Lung
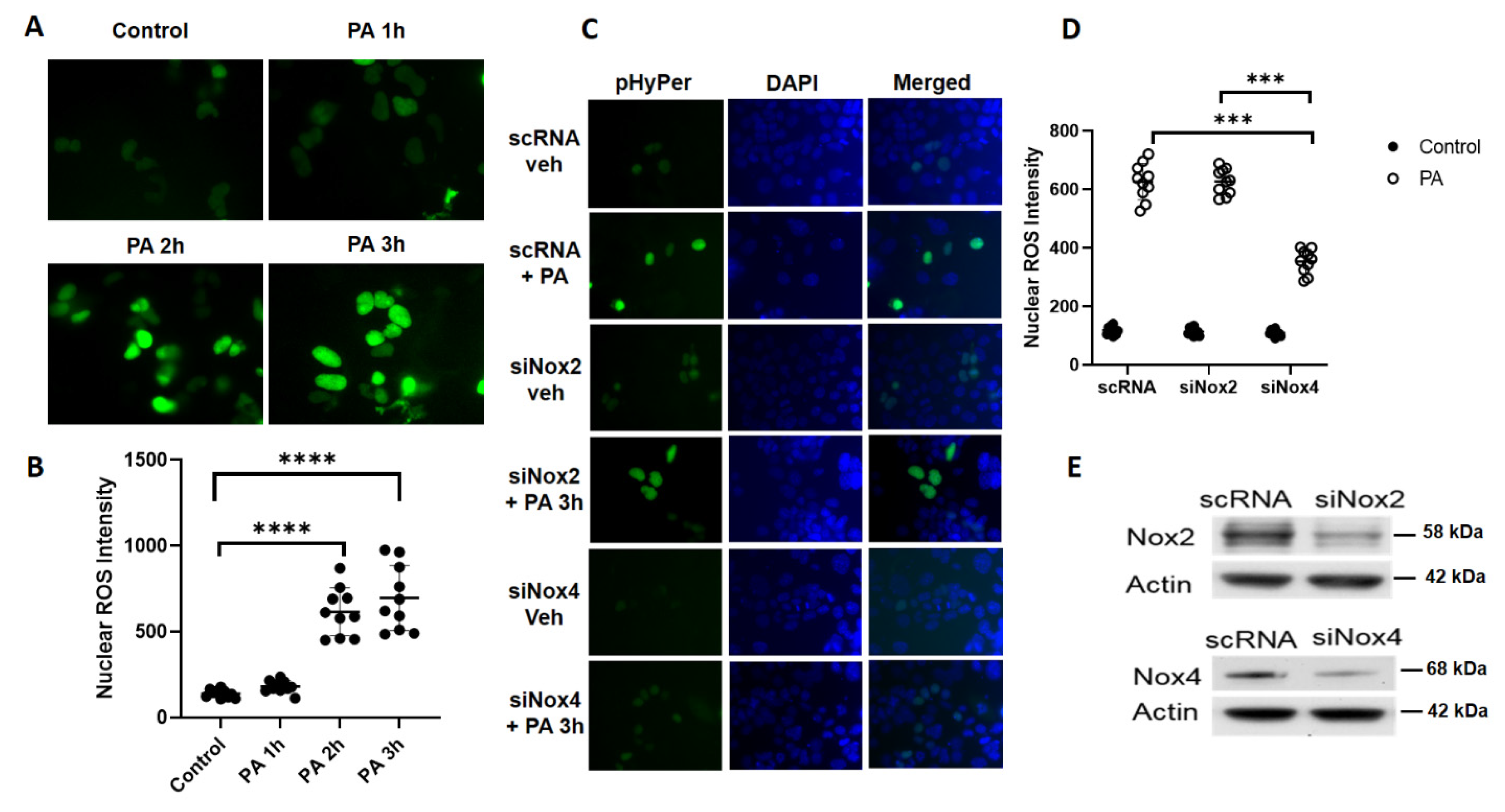
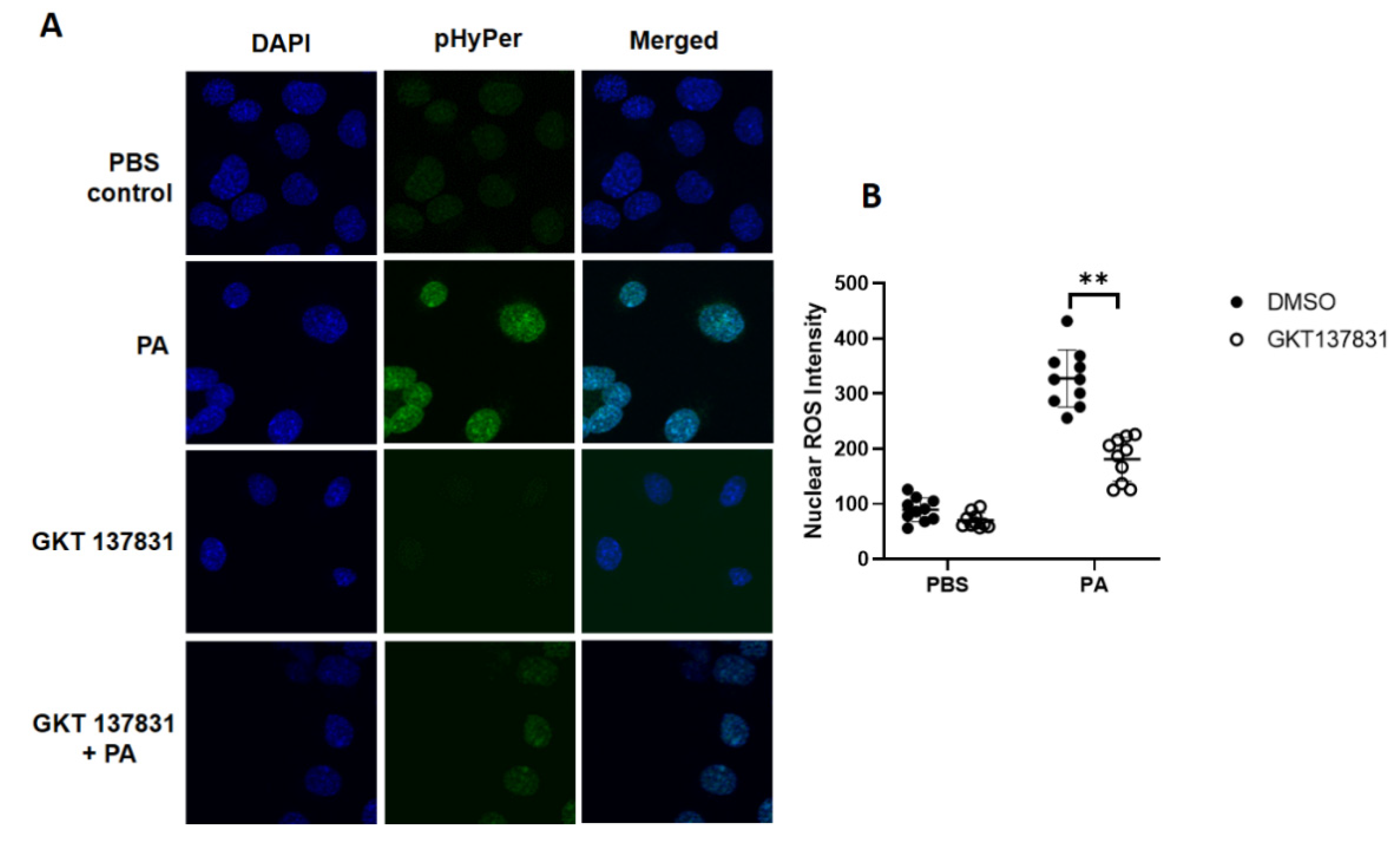
3.3. P. aeruginosa Stimulates Nuclear ROS Generation via Nox4, But Not Nox2, in Lung Epithelial Cells
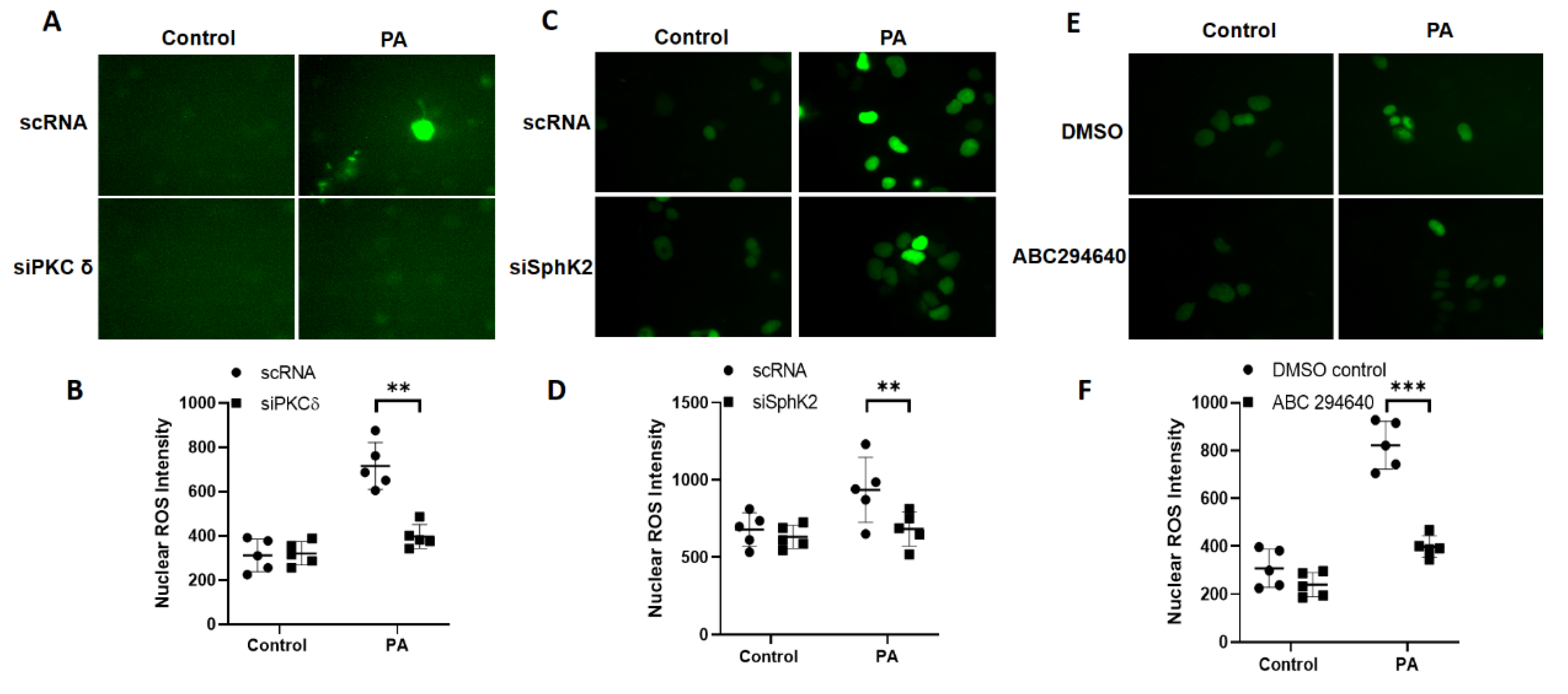
3.4. Downregulation of PKC δ and Sphk2 or Inhibition of SPHK2 Reduces P. aeruginosa-Induced Nuclear ROS in Lung Epithelial Cells
3.5. P. aeruginosa-Induced Oxidation of Nuclear HDAC2 Is Dependent on PKC δ, SPHK2, and NOX4 Signaling in Lung Bronchial Epithelial Cells
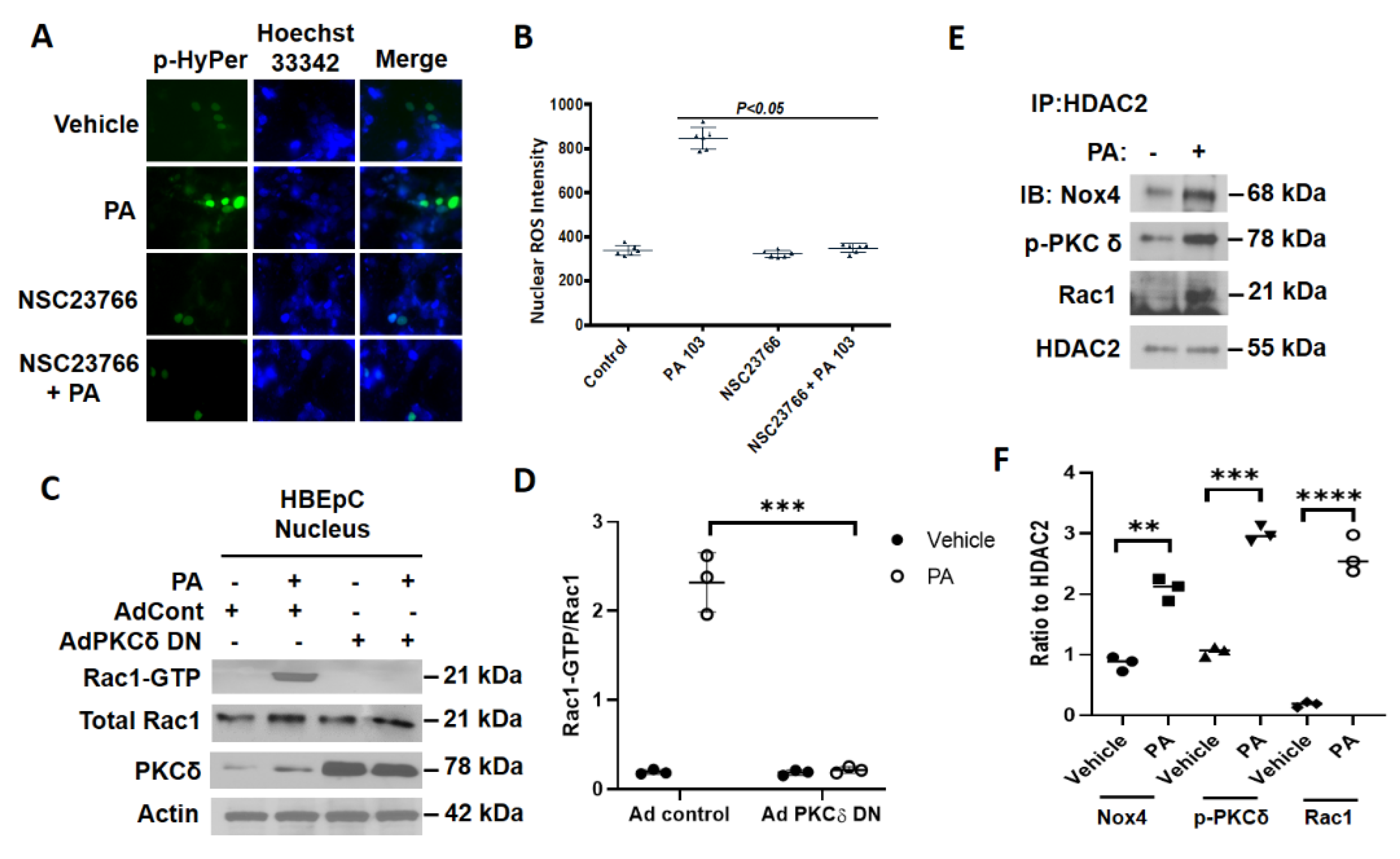
3.6. Inhibition of RAC1 Reduces P. aeruginosa-Mediated Nuclear ROS Production
3.7. P. aeruginosa Enhances Association of NOX4, PKC δ, and RAC1 with HDAC2 in Nucleus of Lung Epithelial Cells
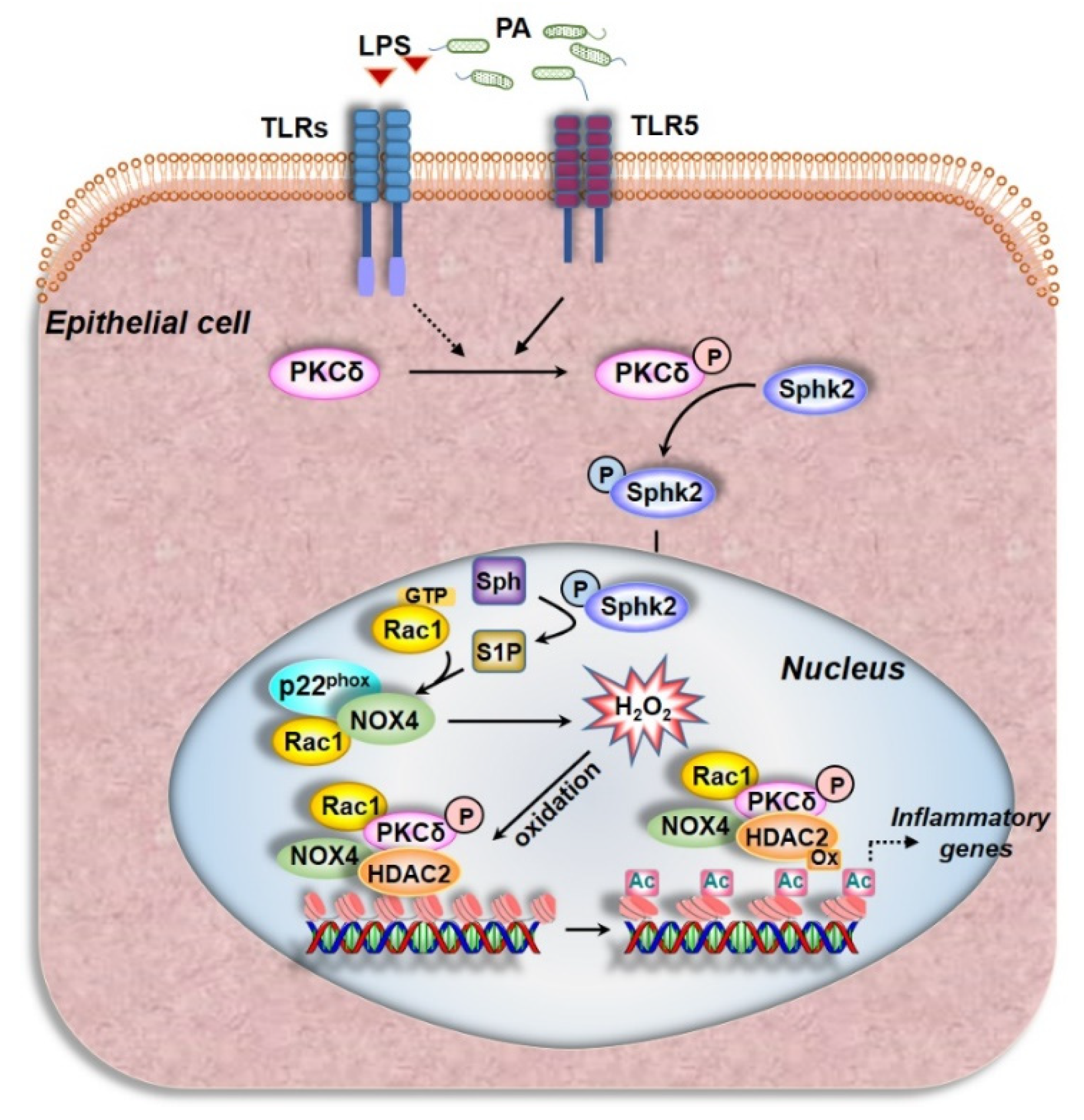
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhagirath, A.Y.; Li, Y.; Somayajula, D.; Dadashi, M.; Badr, S.; Duan, K. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm. Med. 2016, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Chastre, J.; Fagon, J.-Y. Ventilator-associated Pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Hayes, D., Jr.; Wozniak, D.J. Cystic Fibrosis andPseudomonas aeruginosa: The Host-Microbe Interface. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef]
- Murphy, T.F.; Brauer, A.L.; Eschberger, K.; Lobbins, P.; Grove, L.; Cai, X.; Sethi, S. Pseudomonas aeruginosain Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2008, 177, 853–860. [Google Scholar] [CrossRef]
- Park, J.-W.; Shin, I.-S.; Ha, U.-H.; Oh, S.-R.; Kim, J.-H.; Ahn, K.-S. Pathophysiological Changes Induced by Pseudomonas aeruginosa Infection Are Involved in MMP-12 and MMP-13 Upregulation in Human Carcinoma Epithelial Cells and a Pneumonia Mouse Model. Infect. Immun. 2015, 83, 4791–4799. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Estrada, S.R.; Borgatta, B. Pseudomonas aeruginosa ventilator-associated pneumonia management. Infect. Drug Resist. 2016, 9, 7–18. [Google Scholar] [CrossRef]
- Harijith, A.; Natarajan, V.; Fu, P. The Role of Nicotinamide Adenine Dinucleotide Phosphate Oxidases in Lung Architecture Remodeling. Antioxidants 2017, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, D.L.; Fu, P.; Krishnan, Y.; Maienschein-Cline, M.; Hu, H.; Jung, S.; Madduri, R.; Arbieva, Z.; Harijith, A.; Natarajan, V. Genetic deletion of Sphk2 confers protection against Pseudomonas aeruginosa mediated differential expression of genes related to virulent infection and inflammation in mouse lung. BMC Genom. 2019, 20, 984. [Google Scholar] [CrossRef]
- Gomez, J.C.; Dang, H.; Martin, J.R.; Doerschuk, C.M. Nrf2 Modulates Host Defense during Streptococcus pneumoniae Pneumonia in Mice. J. Immunol. 2016, 197, 2864–2879. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, J.K.; Norris, A.; Bangera, M.G.; Geiss, G.K.; Van Wout, A.B.; Bumgarner, R.E.; Lory, S. Interaction of Pseudomonas aeruginosa with epithelial cells: Identification of differentially regulated genes by expression microarray analysis of human cDNAs. Proc. Natl. Acad. Sci. USA 2000, 97, 9659–9664. [Google Scholar] [CrossRef] [PubMed]
- Turkina, M.V.; Vikström, E. Bacteria-Host Crosstalk: Sensing of the Quorum in the Context of Pseudomonas aeruginosa Infections. J. Innate Immun. 2019, 11, 263–279. [Google Scholar] [CrossRef]
- Seitz, A.P.; Grassmé, H.; Edwards, M.J.; Pewzner-Jung, Y.; Gulbins, E. Ceramide and sphingosine in pulmonary infections. Biol. Chem. 2015, 396, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Pewzner-Jung, Y.; Tabazavareh, S.T.; Grassmé, H.; Becker, K.A.; Japtok, L.; Steinmann, J.; Joseph, T.; Lang, S.; Tuemmler, B.; Schuchman, E.H.; et al. Sphingoid long chain bases prevent lung infection by Pseudomonas aeruginosa. EMBO Mol. Med. 2014, 6, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, D.L.; Berdyshev, E.V.; Bronova, I.A.; Liu, Y.; Tiruppathi, C.; Komarova, Y.; Benevolenskaya, E.V.; Suryadevara, V.; Ha, A.W.; Harijith, A.; et al. Pseudomonas aeruginosa stimulates nuclear sphingosine-1-phosphate generation and epigenetic regulation of lung inflammatory injury. Thorax 2019, 74, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Mohan, V.; Mansoor, S.; Tiruppathi, C.; Sadikot, R.T.; Natarajan, V. Role of Nicotinamide Adenine Dinucleotide Phosphate–Reduced Oxidase Proteins in Pseudomonas aeruginosa–Induced Lung Inflammation and Permeability. Am. J. Respir. Cell Mol. Biol. 2013, 48, 477–488. [Google Scholar] [CrossRef]
- Li, H.; Luo, Y.-F.; Wang, Y.-S.; Yang, Q.; Xiao, Y.-L.; Cai, H.-R.; Xie, C.-M. Using ROS as a Second Messenger, NADPH Oxidase 2 Mediates Macrophage Senescence via Interaction with NF-κB during Pseudomonas aeruginosa Infection. Oxidative Med. Cell. Longev. 2018, 2018, 9741838. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Ebenezer, D.L.; Ha, A.W.; Suryadevara, V.; Harijith, A.; Natarajan, V. Nuclear lipid mediators: Role of nuclear sphingolipids and sphingosine-1-phosphate signaling in epigenetic regulation of inflammation and gene expression. J. Cell. Biochem. 2018, 119, 6337–6353. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, S.; Kuroda, J.; Ago, T.; Zhai, P.; Park, J.Y.; Xie, L.-H.; Tian, B.; Sadoshima, J. Increased Oxidative Stress in the Nucleus Caused by Nox4 Mediates Oxidation of HDAC4 and Cardiac Hypertrophy. Circ. Res. 2013, 112, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sadikot, R.T.; Adami, G.R.; Kalinichenko, V.V.; Pendyala, S.; Natarajan, V.; Zhao, Y.-Y.; Malik, A.B. FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J. Exp. Med. 2011, 208, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Ramchandran, R.; Shaaya, M.; Huang, L.; Ebenezer, D.L.; Jiang, Y.; Komarova, Y.; Vogel, S.M.; Malik, A.B.; Minshall, R.D.; et al. Phospholipase D2 restores endothelial barrier function by promoting PTPN14-mediated VE-cadherin dephosphorylation. J. Biol. Chem. 2020, 295, 7669–7685. [Google Scholar] [CrossRef] [PubMed]
- Usatyuk, P.V.; Fu, P.; Mohan, V.; Epshtein, Y.; Jacobson, J.R.; Gomez-Cambronero, J.; Wary, K.K.; Bindokas, V.; Dudek, S.M.; Salgia, R.; et al. Role of c-Met/Phosphatidylinositol 3-Kinase (PI3k)/Akt Signaling in Hepatocyte Growth Factor (HGF)-mediated Lamellipodia Formation, Reactive Oxygen Species (ROS) Generation, and Motility of Lung Endothelial Cells. J. Biol. Chem. 2014, 289, 13476–13491. [Google Scholar] [CrossRef]
- Anilkumar, N.; José, G.S.; Sawyer, I.; Santos, C.X.; Sand, C.; Brewer, A.C.; Warren, D.; Shah, A.M. A 28-kDa Splice Variant of NADPH Oxidase-4 Is Nuclear-Localized and Involved in Redox Signaling in Vascular Cells. Arter. Thromb. Vasc. Biol. 2013, 33, e104–e112. [Google Scholar] [CrossRef]
- Fu, P.; Usatyuk, P.V.; Jacobson, J.; Cress, A.E.; Garcia, J.G.N.; Salgia, R.; Natarajan, V. Role played by paxillin and paxillin tyrosine phosphorylation in hepatocyte growth factor/sphingosine-1-phosphate-mediated reactive oxygen species generation, lamellipodia formation, and endothelial barrier function. Pulm. Circ. 2015, 5, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Pendyala, S.; Gorshkova, I.A.; Usatyuk, P.V.; He, D.; Pennathur, A.; Lambeth, J.D.; Thannickal, V.J.; Natarajan, V. Role of Nox4 and Nox2 in Hyperoxia-Induced Reactive Oxygen Species Generation and Migration of Human Lung Endothelial Cells. Antioxid. Redox Signal. 2009, 11, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.A.; Staroverov, D.B.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 2006, 3, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Malinouski, M.; Zhou, Y.; Belousov, V.V.; Hatfield, L.L.; Gladyshev, V.N. Hydrogen Peroxide Probes Directed to Different Cellular Compartments. PLoS ONE 2011, 6, e14564. [Google Scholar] [CrossRef]
- French, K.J.; Zhuang, Y.; Maines, L.W.; Gao, P.; Wang, W.; Beljanski, V.; Upson, J.J.; Green, C.L.; Keller, S.N.; Smith, C.D. Pharmacology and Antitumor Activity of ABC294640, a Selective Inhibitor of Sphingosine Kinase-2. J. Pharmacol. Exp. Ther. 2010, 333, 129–139. [Google Scholar] [CrossRef]
- Hordijk, P.L.; Danilczyk, U.; Penninger, J.M. Regulation of NADPH Oxidases: The role of Rac proteins. Circ. Res. 2006, 98, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Pendyala, S.; Natarajan, V. Redox regulation of Nox proteins. Respir. Physiol. Neurobiol. 2010, 174, 265–271. [Google Scholar] [CrossRef]
- Pick, E. Role of the Rho GTPase Rac in the activation of the phagocyte NADPH oxidase: Outsourcing a key task. Small GTPases 2014, 5, e27952. [Google Scholar] [CrossRef]
- Miyano, K.; Sumimoto, H. Role of the small GTPase Rac in p22phox-dependent NADPH oxidases. Biochimie 2007, 89, 1133–1144. [Google Scholar] [CrossRef]
- Ekaludercic, N.; Edeshwal, S.; Lisa, F.E. Reactive oxygen species and redox compartmentalization. Front. Physiol. 2014, 5, 285. [Google Scholar] [CrossRef]
- Kietzmann, T. Intracellular Redox Compartments: Mechanisms and Significances. Antioxid. Redox Signal. 2010, 13, 395–398. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W.R.; Lovell, M.A. Damage to Lipids, Proteins, DNA, and RNA in Mild Cognitive Impairment. Arch. Neurol. 2007, 64, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.I.; Dao, V.T.-V.; Daiber, A.; Maghzal, G.J.; Di Lisa, F.; Kaludercic, N.; Leach, S.; Cuadrado, A.; Jaquet, V.; Seredenina, T.; et al. Reactive Oxygen-Related Diseases: Therapeutic Targets and Emerging Clinical Indications. Antioxid. Redox Signal. 2015, 23, 1171–1185. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2017, 175, 1279–1292. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2019, 467, 1–12. [Google Scholar] [CrossRef]
- Sadikot, R.T.; Zeng, H.; Joo, M.; Everhart, M.B.; Sherrill, T.P.; Li, B.; Cheng, D.-S.; Yull, F.E.; Christman, J.W.; Blackwell, T.S. Targeted Immunomodulation of the NF-κB Pathway in Airway Epithelium Impacts Host Defense againstPseudomonas aeruginosa. J. Immunol. 2006, 176, 4923–4930. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Carnesecchi-Acker, S.; Deffert, C.; Donati, Y.R.; Basset, O.; Hinz, B.; Preynat-Seauve, O.; Guichard, C.; Arbiser, J.L.; Banfi, B.; Pache, J.-C.; et al. A Key Role for NOX4 in Epithelial Cell Death During Development of Lung Fibrosis. Antioxid. Redox Signal. 2011, 15, 607–619. [Google Scholar] [CrossRef]
- Block, K.; Eid, A.; Griendling, K.K.; Lee, D.-Y.; Wittrant, Y.; Gorin, Y. Nox4 NAD(P)H Oxidase Mediates Src-dependent Tyrosine Phosphorylation of PDK-1 in Response to Angiotensin II: Role in mesangial cell hypertrophy and fibronectin expression. J. Biol. Chem. 2008, 283, 24061–24076. [Google Scholar] [CrossRef]
- Lyle, A.N.; Deshpande, N.N.; Taniyama, Y.; Seidel-Rogol, B.; Pounkova, L.; Du, P.; Papaharalambus, C.; Lassègue, B.; Griendling, K.K. Poldip2, a Novel Regulator of Nox4 and Cytoskeletal Integrity in Vascular Smooth Muscle Cells. Circ. Res. 2009, 105, 249–259. [Google Scholar] [CrossRef]
- Pendyala, S.; Moitra, J.; Kalari, S.; Kleeberger, S.R.; Zhao, Y.; Reddy, S.P.; Garcia, J.G.; Natarajan, V. Nrf2 regulates hyperoxia-induced Nox4 expression in human lung endothelium: Identification of functional antioxidant response elements on the Nox4 promoter. Free. Radic. Biol. Med. 2011, 50, 1749–1759. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, B.; Dong, W.; Kong, M.; Shao, Y.; Fan, Z.; Yu, L.; Wu, D.; Lu, J.; Guo, J.; et al. The Chromatin Remodeler Brg1 Integrates ROS Production and Endothelial-Mesenchymal Transition to Promote Liver Fibrosis in Mice. Front. Cell Dev. Biol. 2019, 7, 245. [Google Scholar] [CrossRef] [PubMed]
- Sterner, D.E.; Berger, S.L. Acetylation of Histones and Transcription-Related Factors. Microbiol. Mol. Biol. Rev. 2000, 64, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zang, C.; Rosenfeld, J.A.; Schones, D.E.; Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.-Y.; Peng, W.; Zhang, M.Q.; et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 2008, 40, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhang, Y.; Chen, J.; Chen, H.; Lin, C.; Wang, Q.; Ou, Y. Nickel-Induced Histone Hypoacetylation: The Role of Reactive Oxygen Species. Toxicol. Sci. 2003, 74, 279–286. [Google Scholar] [CrossRef]
- Kietzmann, T.; Petry, A.; Shvetsova, A.; Gerhold, J.M.; Görlach, A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1533–1554. [Google Scholar] [CrossRef]
- Zhang, M.; Brewer, A.C.; Schröder, K.; Santos, C.X.C.; Grieve, D.J.; Wang, M.; Anilkumar, N.; Yu, B.; Dong, X.; Walker, S.J.; et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18121–18126. [Google Scholar] [CrossRef]
- Osoata, G.O.; Yamamura, S.; Ito, M.; Vuppusetty, C.; Adcock, I.M.; Barnes, P.J.; Ito, K. Nitration of distinct tyrosine residues causes inactivation of histone deacetylase 2. Biochem. Biophys. Res. Commun. 2009, 384, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Sundar, I.K.; Yao, H.; Rahman, I. Oxidative Stress and Chromatin Remodeling in Chronic Obstructive Pulmonary Disease and Smoking-Related Diseases. Antioxid. Redox Signal. 2013, 18, 1956–1971. [Google Scholar] [CrossRef]
- Schader, T.; Löwe, O.; Reschke, C.; Malacarne, P.; Hahner, F.; Müller, N.; Gajos-Draus, A.; Backs, J.; Schröder, K. Oxidation of HDAC4 by Nox4-derived H2O2 maintains tube formation by endothelial cells. Redox Biol. 2020, 36, 101669. [Google Scholar] [CrossRef] [PubMed]
- Ago, T.; Liu, T.; Zhai, P.; Chen, W.; Li, H.; Molkentin, J.D.; Vatner, S.F.; Sadoshima, J. A Redox-Dependent Pathway for Regulating Class II HDACs and Cardiac Hypertrophy. Cell 2008, 133, 978–993. [Google Scholar] [CrossRef]
- Adams, G.E.; Chandru, A.; Cowley, S.M. Co-repressor, co-activator and general transcription factor: The many faces of the Sin3 histone deacetylase (HDAC) complex. Biochem. J. 2018, 475, 3921–3932. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of Histone Acetylation in the Nucleus by Sphingosine-1-Phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef]
- Ebenezer, D.L.; Fu, P.; Ramchandran, R.; Ha, A.W.; Putherickal, V.; Sudhadevi, T.; Harijith, A.; Schumacher, F.; Kleuser, B.; Natarajan, V. S1P and plasmalogen derived fatty aldehydes in cellular signaling and functions. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158681. [Google Scholar] [CrossRef] [PubMed]
- Gorin, Y.; Ricono, J.M.; Kim, N.-H.; Bhandari, B.; Choudhury, G.G.; Abboud, H.E. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am. J. Physiol. Physiol. 2003, 285, F219–F229. [Google Scholar] [CrossRef] [PubMed]
- Wedgwood, S.; Lakshminrusimha, S.; Czech, L.; Schumacker, P.T.; Steinhorn, R.H. Increased p22phox/Nox4 Expression Is Involved in Remodeling Through Hydrogen Peroxide Signaling in Experimental Persistent Pulmonary Hypertension of the Newborn. Antioxid. Redox Signal. 2013, 18, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Zana, M.; Péterfi, Z.; Kovács, H.A.; Tóth, Z.E.; Enyedi, B.; Morel, F.; Paclet, M.-H.; Donkó, Á.; Morand, S.; Leto, T.L.; et al. Interaction between p22phox and Nox4 in the endoplasmic reticulum suggests a unique mechanism of NADPH oxidase complex formation. Free. Radic. Biol. Med. 2018, 116, 41–49. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, P.; Ramchandran, R.; Sudhadevi, T.; Kumar, P.P.K.; Krishnan, Y.; Liu, Y.; Zhao, Y.; Parinandi, N.L.; Harijith, A.; Sadoshima, J.; et al. NOX4 Mediates Pseudomonas aeruginosa-Induced Nuclear Reactive Oxygen Species Generation and Chromatin Remodeling in Lung Epithelium. Antioxidants 2021, 10, 477. https://doi.org/10.3390/antiox10030477
Fu P, Ramchandran R, Sudhadevi T, Kumar PPK, Krishnan Y, Liu Y, Zhao Y, Parinandi NL, Harijith A, Sadoshima J, et al. NOX4 Mediates Pseudomonas aeruginosa-Induced Nuclear Reactive Oxygen Species Generation and Chromatin Remodeling in Lung Epithelium. Antioxidants. 2021; 10(3):477. https://doi.org/10.3390/antiox10030477
Chicago/Turabian StyleFu, Panfeng, Ramaswamy Ramchandran, Tara Sudhadevi, Prasanth P. K. Kumar, Yashaswin Krishnan, Yuru Liu, Yutong Zhao, Narasimham L. Parinandi, Anantha Harijith, Junichi Sadoshima, and et al. 2021. "NOX4 Mediates Pseudomonas aeruginosa-Induced Nuclear Reactive Oxygen Species Generation and Chromatin Remodeling in Lung Epithelium" Antioxidants 10, no. 3: 477. https://doi.org/10.3390/antiox10030477
APA StyleFu, P., Ramchandran, R., Sudhadevi, T., Kumar, P. P. K., Krishnan, Y., Liu, Y., Zhao, Y., Parinandi, N. L., Harijith, A., Sadoshima, J., & Natarajan, V. (2021). NOX4 Mediates Pseudomonas aeruginosa-Induced Nuclear Reactive Oxygen Species Generation and Chromatin Remodeling in Lung Epithelium. Antioxidants, 10(3), 477. https://doi.org/10.3390/antiox10030477







