IL17A Depletion Affects the Metabolism of Macrophages Treated with Gemcitabine
Abstract
1. Introduction
2. Materials and Methods
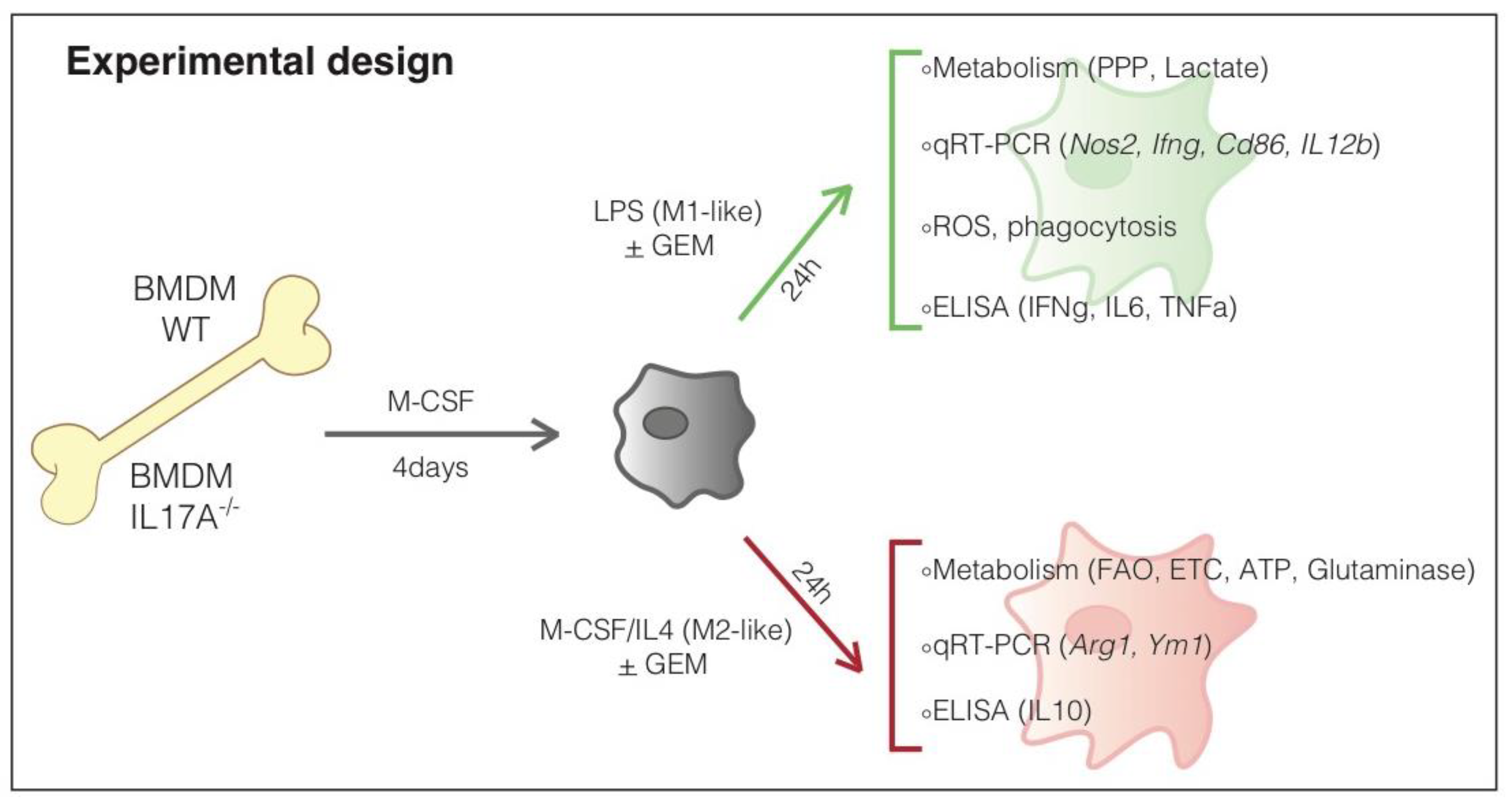
2.1. In Vitro Generation of Murine Bone Marrow-Derived Macrophages (BMDM)
2.2. Quantitative RT-PCR (qPCR)
2.3. ELISA
2.4. MTT Assay
2.5. The Pentose Phosphate Pathway (PPP) and Tricarboxylic Acid (TCA) Cycle
2.6. Lactate
2.7. Fatty Acid β-Oxidation
2.8. Mitochondrial Respiratory Chain Measurement
2.9. ATP Detection
2.10. Glutamine Catabolism
2.11. Formalin-Fixed and Paraffin-Embedded (FFPE) Dissociation and Flow Cytometry Analysis
2.12. ROS Measurement
2.13. Macrophage Uptake Activity
2.14. Immunoblotting
2.15. Statistical Analyses
3. Results
3.1. The Absence of IL17A Induces Unique Features in Both M1- and M2-Like Macrophages
3.2. IL17A Absence Renders Macrophages Differently Responsive to the Gemcitabine-Induced M2-to-M1-Switch
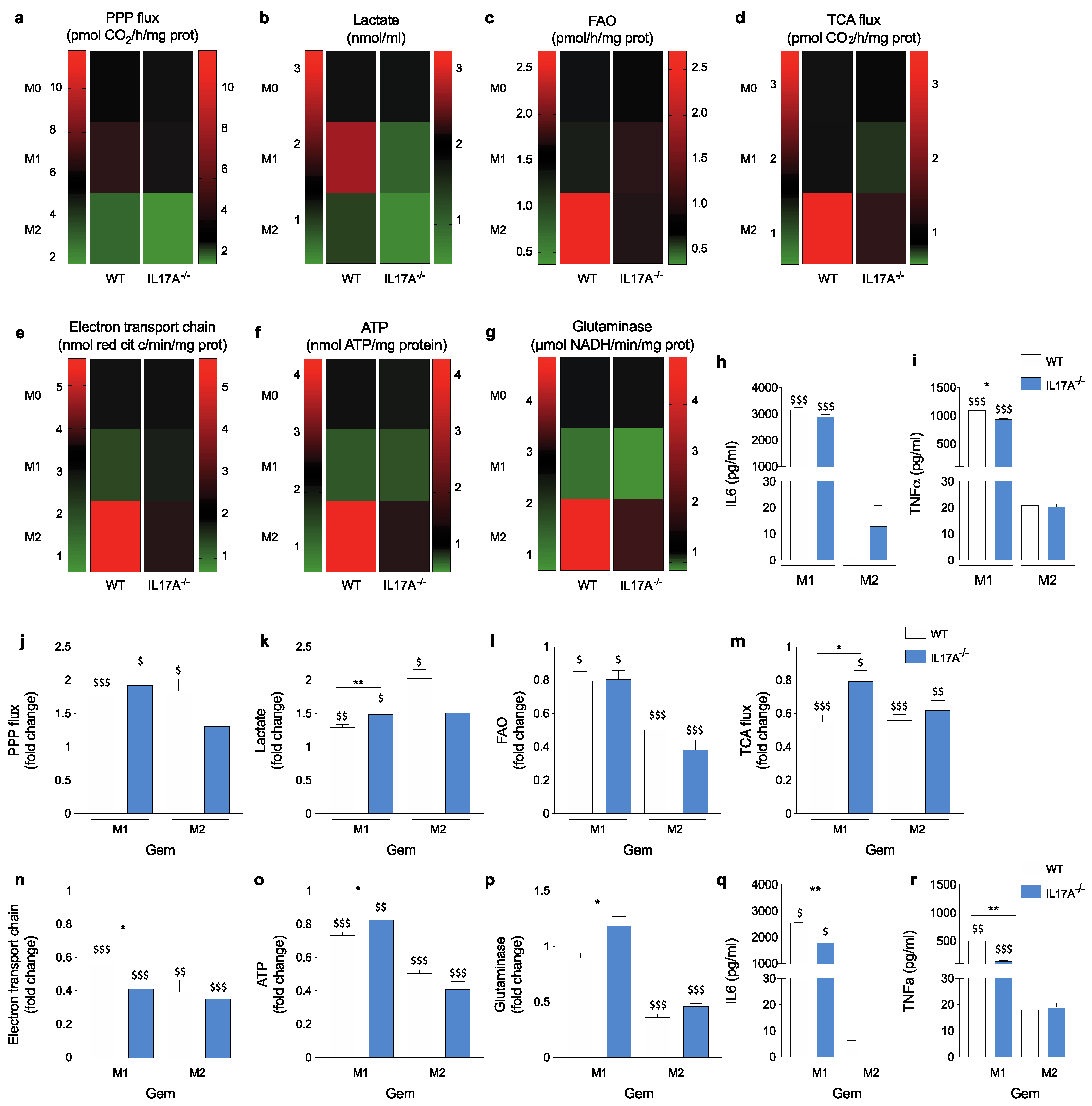
3.3. The Absence of IL17A Attenuates Metabolic Fluxes of M1- and Particularly M2-Like Macrophages
3.4. IL17A Absence Differently Modulates Metabolic Changes Induced by Gemcitabine
3.5. IL17A Neutralization Combined with Gemcitabine Shapes Macrophages towards a “Peculiar” M1-Like Phenotype
3.6. IL17A Depletion Increases the Phagocytosis Rate in Macrophages
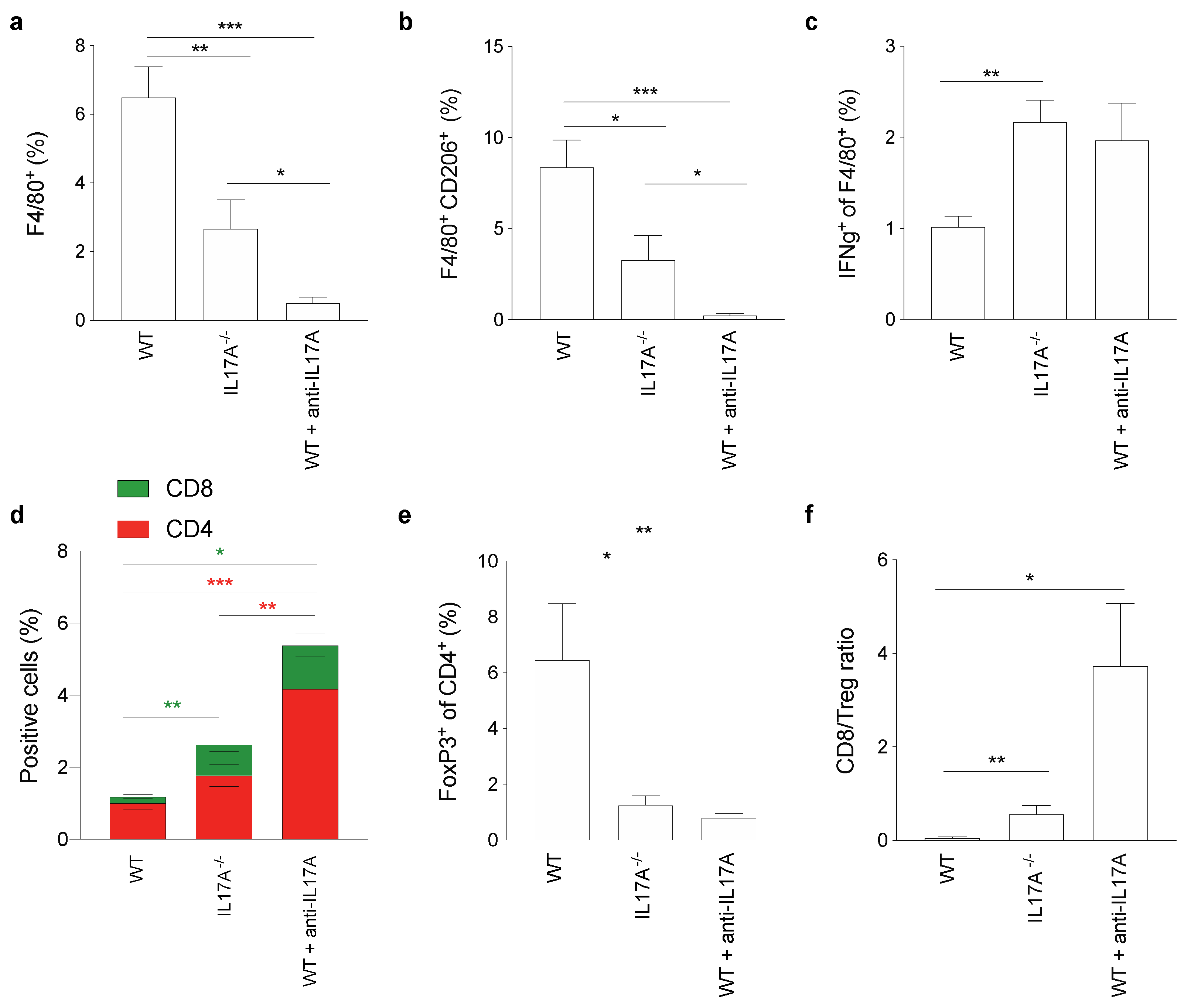
3.7. IL17A Depletion Decreases T Regulatory Cells Infiltrating Pancreatic Cancer
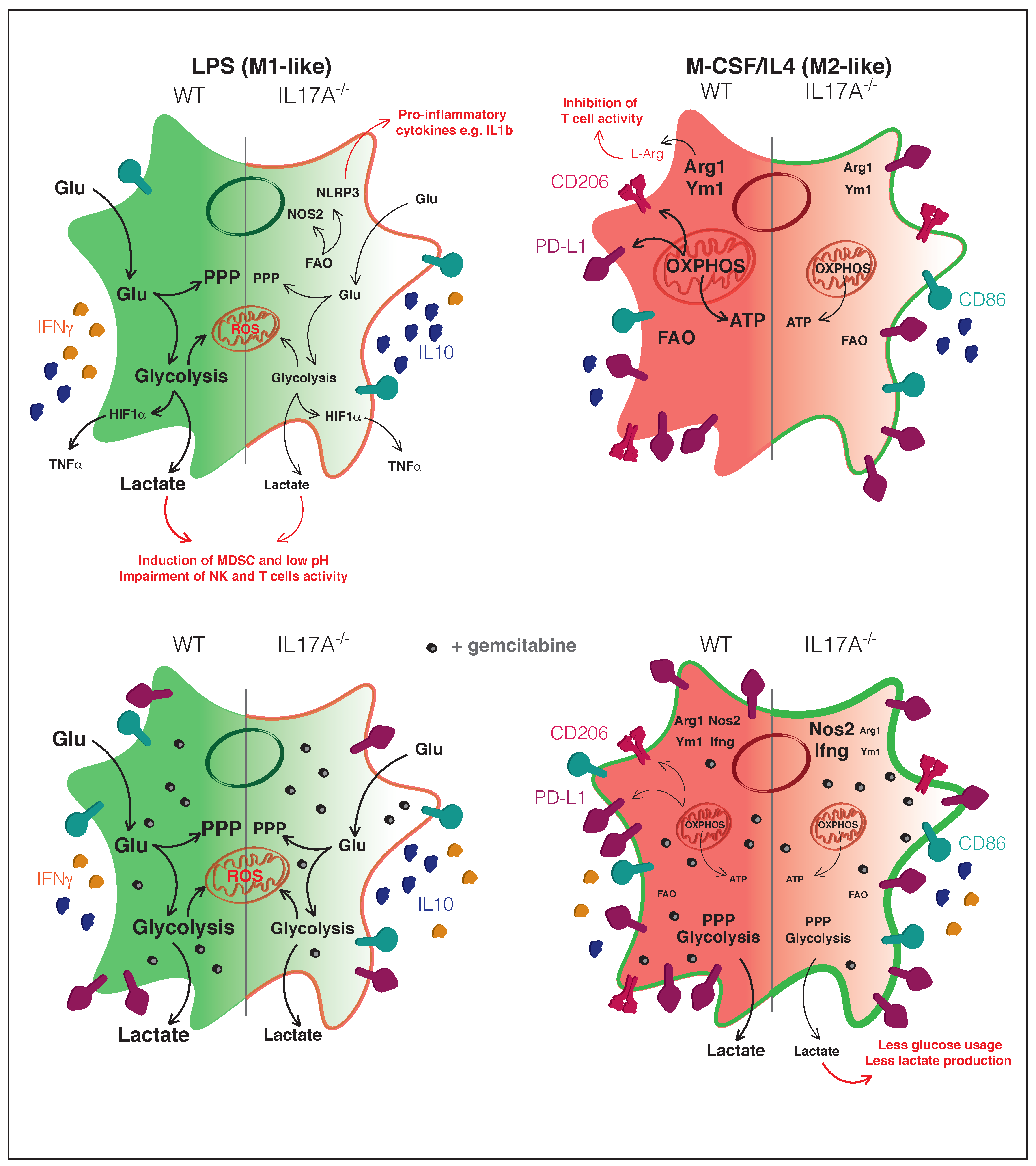
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMDM | Bone Marrow-Derived Macrophages; |
| CD206 | mannose receptor C type 1; |
| CFSE | Carboxyfluorescein Succinimidyl Ester; |
| CPT1 | Carnitine Palmitoyl Transferase 1; |
| ETC | Electron Transport Chain; |
| FAO | Fatty Acid Oxidation; |
| FAS | Fatty Acid Synthesis; |
| FATP1 | Fatty Acid Transporter Protein 1; |
| FFPE | Formalin-Fixed and Paraffin-Embedded; |
| GLS | Glutaminase; |
| IFNγ | Interferon gamma; |
| IL | Interleukin; |
| M-CSF | Macrophage Colony-Stimulating Factor; |
| NOS | Nitric Oxide Synthase; |
| OXPHOS | Oxidative Phosphorylation; |
| PDAC | Pancreatic Ductal Adenocarcinoma; |
| PPP | Pentose Phosphate Pathway; |
| ROS | Reactive Oxygen Species; |
| TAM | Tumor-Associated Macrophages; |
| TCA | Tricarboxylic Acid; |
| TME | Tumor Microenvironment. |
References
- Weaver, C.T. One Road to the T H 17 Pathway: How T H 1 Led to T H 17 (and Vice Versa), and First Came Last. Nat. Immunol. 2020, 21, 819–821. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Harb, H.; Michel, S.; Alhamwe, B.A.; Renz, H.; Tost, J. Epigenetics and Allergy: From Basic Mechanisms to Clinical Applications. Epigenomics 2017, 9, 539–571. [Google Scholar] [CrossRef]
- Ouyang, W.; Kolls, J.K.; Zheng, Y. The Biological Functions of T Helper 17 Cell Effector Cytokines in Inflammation. Immunity 2008, 28, 454–467. [Google Scholar] [CrossRef]
- McAllister, F.; Bailey, J.M.; Alsina, J.; Nirschl, C.J.; Sharma, R.; Fan, H.; Rattigan, Y.; Roeser, J.C.; Lankapalli, R.H.; Zhang, H.; et al. Oncogenic Kras Activates a Hematopoietic-to-Epithelial IL-17 Signaling Axis in Preinvasive Pancreatic Neoplasia. Cancer Cell 2014, 25, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage Polarization: Tumor-Associated Macrophages as a Paradigm for Polarized M2 Mononuclear Phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage Plasticity and Interaction with Lymphocyte Subsets: Cancer as a Paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage Plasticity and Polarization: In Vivo Veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Barin, J.G.; Baldeviano, G.C.; Talor, M.V.; Wu, L.; Ong, S.; Quader, F.; Chen, P.; Zheng, D.; Caturegli, P.; Rose, N.R.; et al. Macrophages Participate in IL17-Mediated Inflammation. Eur. J. Immunol. 2012, 42, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Erbel, C.; Akhavanpoor, M.; Okuyucu, D.; Wangler, S.; Dietz, A.; Zhao, L.; Stellos, K.; Little, K.M.; Lasitschka, F.; Doesch, A.; et al. IL-17A Influences Essential Functions of the Monocyte/Macrophage Lineage and Is Involved in Advanced Murine and Human Atherosclerosis. J. Immunol. Author Choice 2014, 193, 4344–4355. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Seo, N.; Torii, M.; Ma, N.; Muraoka, D.; Tawara, I.; Masuya, M.; Tanaka, K.; Takei, Y.; Shiku, H.; et al. Interleukin-17 Induces an Atypical M2-like Macrophage Subpopulation that Regulates Intestinal Inflammation. PLoS ONE 2014, 9, e108494. [Google Scholar] [CrossRef]
- Roux, C.; Jafari, S.M.; Shinde, R.; Duncan, G.; Cescon, D.W.; Silvester, J.; Chu, M.F.; Hodgson, K.; Berger, T.; Wakeham, A.; et al. Reactive Oxygen Species Modulate Macrophage Immunosuppressive Phenotype through the Up-Regulation of PD-L1. Proc. Natl. Acad. Sci. USA 2019, 116, 4326–4335. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, J.; O’Neill, L.A.; Menon, D. Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol. 2017, 38, 395–406. [Google Scholar] [CrossRef]
- Haschemi, A.; Kosma, P.; Gille, L.; Evans, C.R.; Burant, C.F.; Starkl, P.; Knapp, B.; Haas, R.; Schmid, J.A.; Jandl, C.; et al. The Sedoheptulose Kinase CARKL Directs Macrophage Polarization through Control of Glucose Metabolism. Cell Metab. 2012, 15, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Halbrook, C.J.; Pontious, C.; Kovalenko, I.; Lapienyte, L.; Dreyer, S.; Lee, H.-J.; Thurston, G.; Zhang, Y.; Lazarus, J.; Sajjakulnukit, P.; et al. Macrophage-Released Pyrimidines Inhibit Gemcitabine Therapy in Pancreatic Cancer. Cell Metab. 2019, 29, 1390–1399.e6. [Google Scholar] [CrossRef]
- Di Caro, G.; Cortese, N.; Castino, G.F.; Grizzi, F.; Gavazzi, F.; Ridolfi, C.; Capretti, G.; Mineri, R.; Todoric, J.; Zerbi, A.; et al. Dual Prognostic Significance of Tumour-Associated Macrophages in Human Pancreatic Adenocarcinoma Treated or Untreated with Chemotherapy. Gut 2016, 65, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Tyagi, N.; Khan, M.A.; Srivastava, S.K.; Al-Ghadhban, A.; Dugger, K.; Carter, J.E.; Singh, S.; Singh, A.P. Gemcitabine Treatment Promotes Immunosuppressive Microenvironment in Pancreatic Tumors by Supporting the Infiltration, Growth, and Polarization of Macrophages. Sci. Rep. 2018, 8, 12000. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Aldieri, E.; Bergandi, L.; Fenoglio, I.; Costamagna, C.; Fubini, B.; Bosia, A.; Ghigo, D. Crocidolite Asbestos Inhibits Pentose Phosphate Oxidative Pathway and Glucose 6-Phosphate Dehydrogenase Activity in Human Lung Epithelial Cells. Free Radic. Biol. Med. 2002, 32, 938–949. [Google Scholar] [CrossRef]
- Capello, M.; Ferri-Borgogno, S.; Riganti, C.; Chattaragada, M.S.; Principe, M.; Roux, C.; Zhou, W.; Petricoin, E.F.; Cappello, P.; Novelli, F. Targeting the Warburg Effect in Cancer Cells through ENO1 Knockdown Rescues Oxidative Phosphorylation and Induces Growth Arrest. Oncotarget 2016, 7, 5598. [Google Scholar] [CrossRef]
- Roux, C.; Riganti, C.; Borgogno, S.F.; Curto, R.; Curcio, C.; Catanzaro, V.; Digilio, G.; Padovan, S.; Puccinelli, M.P.; Isabello, M.; et al. Endogenous Glutamine Decrease Is Associated with Pancreatic Cancer Progression. Oncotarget 2017, 8, 95361. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Andrejeva, G.; Rathmell, J.C. Similarities and Distinctions of Cancer and Immune Metabolism in Inflammation and Tumors. Cell Metab. 2017, 26, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Mucciolo, G.; Curcio, C.; Roux, C.; Li, W.Y.; Capello, M.; Curto, R.; Chiarle, R.; Giordano, D.; Satolli, M.A.; Lawlor, R.; et al. IL17A Critically Shapes the Transcriptional Program of Fibroblasts in Pancreatic Cancer and Switches on Their Protumorigenic Functions. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Zhang, Y.; Zoltan, M.; Riquelme, E.; Xu, H.; Sahin, I.; Castro-Pando, S.; Montiel, M.F.; Chang, K.; Jiang, Z.; Ling, J.; et al. Immune Cell Production of Interleukin 17 Induces Stem Cell Features of Pancreatic Intraepithelial Neoplasia Cells. Gastroenterology 2018, 155, 210–223.e3. [Google Scholar] [CrossRef]
- McAllister, F.; Leach, S.D. Targeting IL-17 for Pancreatic Cancer Prevention. Oncotarget 2014, 5, 9530–9531. [Google Scholar] [CrossRef]
- Nakai, K.; He, Y.-Y.; Nishiyama, F.; Naruse, F.; Haba, R.; Kushida, Y.; Katsuki, N.; Moriue, T.; Yoneda, K.; Kubota, Y. IL-17A Induces Heterogeneous Macrophages, and It Does Not Alter the Effects of Lipopolysaccharides on Macrophage Activation in the Skin of Mice. Sci. Rep. 2017, 7, 12473. [Google Scholar] [CrossRef]
- Onishi, R.M.; Gaffen, S.L. Interleukin-17 and Its Target Genes: Mechanisms of Interleukin-17 Function in Disease. Immunology 2010, 129, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Herndon, J.M.; Sojka, D.K.; Kim, K.-W.; Knolhoff, B.L.; Zuo, C.; Cullinan, D.R.; Luo, J.; Bearden, A.R.; Lavine, K.J.; et al. Tissue Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity 2017, 47, 323–338.e6. [Google Scholar] [CrossRef] [PubMed]
- Habtezion, A.; Edderkaoui, M.; Pandol, S.J. Macrophages and Pancreatic Ductal Adenocarcinoma. Cancer Lett. 2016, 381, 211–216. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) Cells in Cancer: Can Treg Cells Be a New Therapeutic Target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Stromnes, I.M.; Brockenbrough, S.; Izeradjene, K.; Carlson, M.A.; Cuevas, C.; Simmons, R.M.; Greenberg, P.D.; Hingorani, S.R. Targeted Depletion of a MDSC Subset Unmasks Pancreatic Ductal Adenocarcinoma to Adaptive Immunity. Gut 2014, 63, 1769–1781. [Google Scholar] [CrossRef]
- Beatty, G.L.; Li, Y.; Long, K.B. Cancer Immunotherapy: Activating Innate and Adaptive Immunity through CD40 Agonists. Expert Rev. Anticancer Ther. 2017, 17, 175–186. [Google Scholar] [CrossRef]
- Kaneda, M.M.; Cappello, P.; Nguyen, A.V.; Ralainirina, N.; Hardamon, C.R.; Foubert, P.; Schmid, M.C.; Sun, P.; Mose, E.; Bouvet, M.; et al. Macrophage PI3Kγ Drives Pancreatic Ductal Adenocarcinoma Progression. Cancer Discov. 2016, 6, 870–885. [Google Scholar] [CrossRef]
- Cerboni, S.; Gehrmann, U.; Preite, S.; Mitra, S. Cytokine-Regulated Th17 Plasticity in Human Health and Diseases. Immunology 2020. [Google Scholar] [CrossRef] [PubMed]
- Borgoni, S.; Iannello, A.; Cutrupi, S.; Allavena, P.; D’Incalci, M.; Novelli, F.; Cappello, P. Depletion of Tumor-Associated Macrophages Switches the Epigenetic Profile of Pancreatic Cancer Infiltrating T Cells and Restores Their Anti-Tumor Phenotype. Oncoimmunology 2018, 7, e1393596. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Huang, S.C.-C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network Integration of Parallel Metabolic and Transcriptional Data Reveals Metabolic Modules that Regulate Macrophage Polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J. A Broken Krebs Cycle in Macrophages. Immunity 2015, 42, 393–394. [Google Scholar] [CrossRef]
- Infantino, V.; Convertini, P.; Cucci, L.; Panaro, M.A.; Di Noia, M.A.; Calvello, R.; Palmieri, F.; Iacobazzi, V. The Mitochondrial Citrate Carrier: A New Player in Inflammation. Biochem. J. 2011, 438, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate Is an Inflammatory Signal that Induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.e13. [Google Scholar] [CrossRef]
- Husain, Z.; Seth, P.; Sukhatme, V.P. Tumor-Derived Lactate and Myeloid-Derived Suppressor Cells: Linking Metabolism to Cancer Immunology. Oncoimmunology 2013, 2, e26383. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.E.N.; Cleveland, J.L. Lactate Wreaks Havoc on Tumor-Infiltrating T and NK Cells. Cell Metab. 2016, 24, 649–650. [Google Scholar] [CrossRef]
- Chang, C.-H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.W.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Penny, H.L.; Sieow, J.L.; Adriani, G.; Yeap, W.H.; See Chi Ee, P.; San Luis, B.; Lee, B.; Lee, T.; Mak, S.Y.; Ho, Y.S.; et al. Warburg Metabolism in Tumor-Conditioned Macrophages Promotes Metastasis in Human Pancreatic Ductal Adenocarcinoma. Oncoimmunology 2016, 5, e1191731. [Google Scholar] [CrossRef]
- Bulle, A.; Dekervel, J.; Deschuttere, L.; Nittner, D.; Libbrecht, L.; Janky, R.; Plaisance, S.; Topal, B.; Coosemans, A.; Lambrechts, D.; et al. Gemcitabine Recruits M2-Type Tumor-Associated Macrophages into the Stroma of Pancreatic Cancer. Transl. Oncol. 2020, 13, 100743. [Google Scholar] [CrossRef]
- Johnson, A.R.; Qin, Y.; Cozzo, A.J.; Freemerman, A.J.; Huang, M.J.; Zhao, L.; Sampey, B.P.; Milner, J.J.; Beck, M.A.; Damania, B.; et al. Metabolic Reprogramming through Fatty Acid Transport Protein 1 (FATP1) Regulates Macrophage Inflammatory Potential and Adipose Inflammation. Mol. Metab. 2016, 5, 506–526. [Google Scholar] [CrossRef]
- Nomura, M.; Liu, J.; Rovira, I.I.; Gonzalez-Hurtado, E.; Lee, J.; Wolfgang, M.J.; Finkel, T. Fatty Acid Oxidation in Macrophage Polarization. Nat. Immunol. 2016, 17, 216–217. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Baardman, J.; Otto, N.A.; van der Velden, S.; Neele, A.E.; van den Berg, S.M.; Luque-Martin, R.; Chen, H.-J.; Boshuizen, M.C.S.; Ahmed, M.; et al. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016, 17, 684–696. [Google Scholar] [CrossRef]



| Gene | Accession Number | Forward | Reverse |
|---|---|---|---|
| Gapdh | 14433 | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
| Nos2 | 18126 | CTTTGCCACGGACGAGAC | TCATTGTACTCTGAGGGCTGAC |
| Ifng | 15978 | ATCTGGAGGAACTGGCAAAA | TTCAAGACTTCAAAGAGTCTGAGGTA |
| Cd86 | 12524 | GAAGCCGAATCAGCCTAGC | CAGCGTTACTATCCCGCTCT |
| Il12b | 16160 | AAGGAACAGTGGGTGTCCAG | GTTAGCTTCTGAGGACACATCTTG |
| Arg1 | 11846 | GAATCTGCATGGGCAACC | GAATCCTGGTACATCTGGGAAC |
| Ym1 | 12655 | AAGAACACTGAGCTAAAAACTCTCCT | GAGACCATGGCACTGAAC G |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roux, C.; Mucciolo, G.; Kopecka, J.; Novelli, F.; Riganti, C.; Cappello, P. IL17A Depletion Affects the Metabolism of Macrophages Treated with Gemcitabine. Antioxidants 2021, 10, 422. https://doi.org/10.3390/antiox10030422
Roux C, Mucciolo G, Kopecka J, Novelli F, Riganti C, Cappello P. IL17A Depletion Affects the Metabolism of Macrophages Treated with Gemcitabine. Antioxidants. 2021; 10(3):422. https://doi.org/10.3390/antiox10030422
Chicago/Turabian StyleRoux, Cecilia, Gianluca Mucciolo, Joanna Kopecka, Francesco Novelli, Chiara Riganti, and Paola Cappello. 2021. "IL17A Depletion Affects the Metabolism of Macrophages Treated with Gemcitabine" Antioxidants 10, no. 3: 422. https://doi.org/10.3390/antiox10030422
APA StyleRoux, C., Mucciolo, G., Kopecka, J., Novelli, F., Riganti, C., & Cappello, P. (2021). IL17A Depletion Affects the Metabolism of Macrophages Treated with Gemcitabine. Antioxidants, 10(3), 422. https://doi.org/10.3390/antiox10030422









