Improving Reproductive Performance and Health of Mammals Using Honeybee Products
Abstract
1. Introduction
2. Honeybee Species and Bioactive Components of Honeybee Products
2.1. Honey
2.2. Royal Jelly
2.3. Propolis
2.4. Bee Venom
2.5. Bee Pollen
2.6. Drone Brood, Beeswax, and Bee Bread
3. Honeybee Products and Reproductive Health
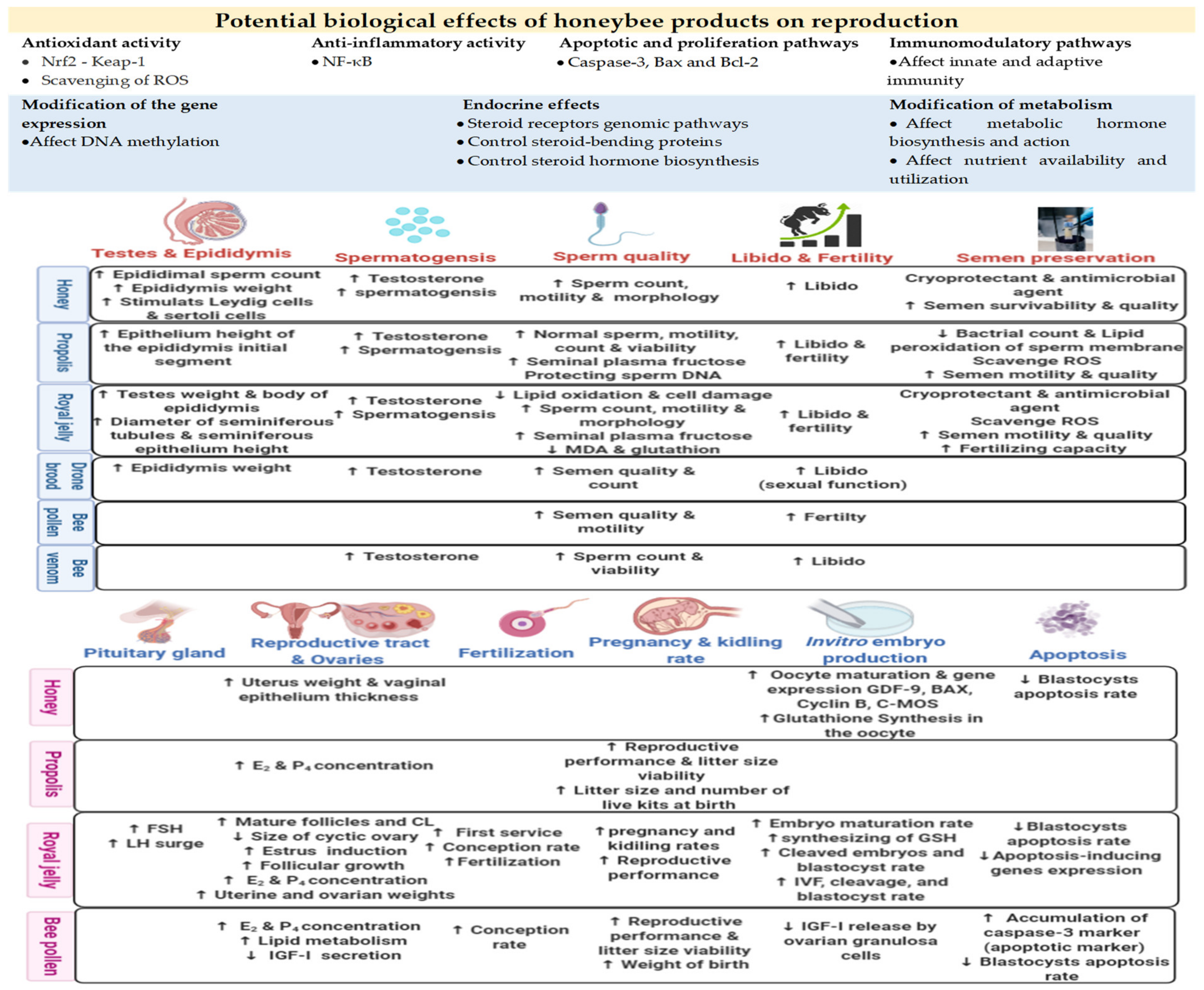
3.1. Biological Effects of Honeybee Products on Reproduction
3.2. Applications for Improving Male Fertility
3.3. Applications for Improving Female Fertility
4. Honeybee Products and Assisted Reproductive Techniques
4.1. Male-Associated Assisted Reproductive Techniques
4.2. Female-Associated Assisted Reproductive Techniques
5. Honeybee Products and Reproductive Disorder Mitigation
5.1. Reproductive Toxicity of Pollutants and Heavy Metals
5.2. Unhealthy Lifestyle and Psychological Stresses
6. Precautions and Hazards
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Zulkhairi Amin, F.A.; Sabri, S.; Mohammad, S.M.; Ismail, M.; Chan, K.W.; Ismail, N.; Norhaizan, M.E.; Zawawi, N. Therapeutic properties of stingless bee honey in comparison with european bee honey. Adv. Pharmacol. Sci. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, A.; Lensky, Y. Bee Products: Properties, Applications, and Apitherapy; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 1475793715. [Google Scholar]
- El-Desoky, N.I.; Hashem, N.M.; Elkomy, A.; Abo-Elezz, Z.R. Physiological response and semen quality of rabbit bucks supplemented with Moringa leaves ethanolic extract during summer season. Animal 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G.; Antunes, M.D.; Faleiro, M.L. Honey as a complementary medicine. Integr. Med. Insights 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Szczêsna, T. Protein content and amino acid composition of bee-collected pollen from selected botanical origins. J. Apic. Sci. 2006, 50, 81–90. [Google Scholar]
- Silici, S. Bal Arısı Ürünleri ve Apiterapi. Turk. J. Agric. Food Sci. Technol. 2019, 7, 1249. [Google Scholar] [CrossRef]
- Hashem, N.M.; Abd El-Hady, A.M.; Hassan, O.A. Inclusion of phytogenic feed additives comparable to vitamin E in diet of growing rabbits: Effects on metabolism and growth. Ann. Agric. Sci. 2017, 62. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Bee venom: Overview of main compounds and bioactivities for therapeutic interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M. Bee Products—Chemical and Biological Properties; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319596891. [Google Scholar]
- Hashem, N.M.; El-Hady, A.A.; Hassan, O. Effect of vitamin E or propolis supplementation on semen quality, oxidative status and hemato-biochemical changes of rabbit bucks during hot season. Livest. Sci. 2013, 157, 520–526. [Google Scholar] [CrossRef]
- Maghsoudlou, A.; Sadeghi Mahoonak, A.; Mohebodini, H.; Toldra, F. Royal jelly: Chemistry, storage and bioactivities. J. Apic. Sci. 2019, 63, 17–40. [Google Scholar] [CrossRef]
- Igbokwe, V.U.; Samuel, O. Pure Honey Potent Fertility Booster: Activities of Honey on Sperm. IOSR J. Dent. Med. Sci. 2013, 9, 43–47. [Google Scholar]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.L.; Ansari, M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017, 24, 975–978. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Hanoun, A.; Bovera, F. Effect of different levels of bee pollen on performance and blood profile of New Zealand White bucks and growth performance of their offspring during summer and winter months. J. Anim. Physiol. Anim. Nutr. 2011, 95, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Handayani, N.; Gofur, A. Does propolis extract alleviate male reproductive performance through gonadotropic hormone levels and sperm quality? IOP Conf. Ser. Earth Environ. Sci. 2019, 276. [Google Scholar] [CrossRef]
- De Moraes, G.V.; Mataveli, M.; de Moura, L.P.P.; Scapinello, C.; Mora, F.; Osmari, M.P. Inclusion of propolis in rabbit diets and semen characteristics. Arq. Ciências Veterinárias Zool. UNIPAR 2015, 17, 227–231. [Google Scholar] [CrossRef]
- Gabr, S. Effect of oral administration of rabbit bucks with egyptian propolis during summer, in Egypt. Egypt. J. Rabbit Sci. 2013, 23, 161–178. [Google Scholar] [CrossRef]
- El-Sherbiny, A. Effect of some bee products on reproductive phenomena of male New Zealand white rabbits. Egypt. J. Rabbit Sci. 2015, 25, 119–136. [Google Scholar] [CrossRef]
- Capucho, C.; Sette, R.; de Souza Predes, F.; de Castro Monteiro, J.; Pigoso, A.A.; Barbieri, R.; Dolder, M.A.H.; Severi-Aguiar, G.D.C. Green Brazilian propolis effects on sperm count and epididymis morphology and oxidative stress. Food Chem. Toxicol. 2012, 50, 3956–3962. [Google Scholar] [CrossRef] [PubMed]
- Rizk, S.M.; Zaki, H.F.; Mina, M.A.M. Propolis attenuates doxorubicin-induced testicular toxicity in rats. Food Chem. Toxicol. 2014, 67, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; Kamel, K.I.; Hassan, M.S.; El-Morsy, A.M.A. Protective role of propolis against reproductive toxicity of triphenyltin in male rabbits. Food Chem. Toxicol. 2010, 48, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ekusa, A.; Iwai, K.; Yonekura, M.; Takahata, Y.; Morimatsu, F. Royal jelly peptides inhibit lipid peroxidation in vitro and in vivo. J. Nutr. Sci. Vitaminol. (Tokyo) 2008, 54, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A. Effect of royal jelly on sexual efficiency in adult male rats. Iraqi J. Vet. Sci. 2009, 23, 155–160. [Google Scholar]
- Shi, Z.; Enayatullah, H.; Lv, Z.; Dai, H.; Wei, Q.; Shen, L.; Karwand, B.; Shi, F. Freeze-dried royal jelly proteins enhanced the testicular development and spermatogenesis in pubescent male mice. Animals 2019, 9. [Google Scholar] [CrossRef]
- El-Hanoun, A.M.; Elkomy, A.E.; Fares, W.A.; Shahien, E.H. Impact of royal jelly to improve reproductive performance of male rabits under hot sumer conditions. World Rabbit Sci. 2014, 22, 241–248. [Google Scholar] [CrossRef]
- Khadr, A.; Abdou, A.; El-Sherbiny, A. Age of puberty and fertility of male new zealand white rabbits orally administered with royal jelly or/ and bee honey. J. Anim. Poult. Prod. 2015, 6, 201–217. [Google Scholar] [CrossRef]
- Abdul-Ghani, A.S.; Dabdoub, N.; Muhammad, R.; Abdul-Ghani, R.; Qazzaz, M. Effect of Palestinian honey on spermatogenesis in rats. J. Med. Food 2008, 11, 799–802. [Google Scholar] [CrossRef]
- Mohamed, M.; Sulaiman, S.A.; Jaafar, H.; Sirajudeen, K.N.S. Effect of different doses of Malaysian honey on reproductive parameters in adult male rats. Andrologia 2012, 44, 182–186. [Google Scholar] [CrossRef]
- Syazana, N.S.; Hashida, N.H.; Majid, A.M.; Durriyyah Sharifah, H.A.; Kamaruddin, M.Y. Effects of Gelam honey on sperm quality and testis of rat. Sains Malaysiana 2011, 40, 1243–1246. [Google Scholar]
- Bolatovna, K.S.; Rustenov, A.; Eleuqalieva, N.; Omirzak, T.; Akhanov, U.K. Improving reproductive qualities of pigs using the drone brood homogenate. Biol. Med. 2015, 7, 2. [Google Scholar]
- Shoinbayeva, K.B.; Omirzak, T.; Bigara, T.; Abubakirova, A.; Dauylbay, A. Biologically active preparation and reproductive function of stud rams. Asian J. Pharm. 2017, 11, 184–191. [Google Scholar]
- El-Hanoun, A.; El-Komy, A.; El-Sabrout, K.; Abdella, M. Effect of bee venom on reproductive performance and immune response of male rabbits. Physiol. Behav. 2020, 223, 112987. [Google Scholar] [CrossRef]
- Yang, A.; Zhou, M.; Zhang, L.; Xie, G.; Chen, H.; Liu, Z.; Ma, W. Influence of royal jelly on the reproductive function of puberty male rats. Food Chem. Toxicol. 2012, 50, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Fikri, A.M.; Sulaeman, A.; Handharyani, E.; Marliyati, S.A.; Fahrudin, M. The effect of propolis administration on fetal development. Heliyon 2019, 5, e02672. [Google Scholar] [CrossRef]
- Uddin, V.; Zuccato, V.; Maza, F.; Schievano, E. Entomological origin of honey discriminated by NMR chloroform extracts in ecuadorian honey. Int. J. Nutr. Food Eng. 2015, 9, 494–497. [Google Scholar]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Braz. J. Pharmacogn. 2016, 26, 657–664. [Google Scholar] [CrossRef]
- Council, E.U. Council Directive 2001/110/EC of 20 December 2001 relating to honey. Off. J. Eur. Communities L 2002, 10, 47–52. [Google Scholar]
- Patricia, V.; Oliverio, V.; Triny, L.; Favián, M. Meliponini biodiversity and medicinal uses of pot-honey from El Oro province in Ecuador. Emirates J. Food Agric. 2015, 27, 502–506. [Google Scholar] [CrossRef]
- Piszcz, P.; Głód, B.K. Antioxidative properties of selected polish honeys. J. Apic. Sci. 2019, 63, 81–91. [Google Scholar] [CrossRef]
- Melliou, E.; Chinou, I. Chemistry and bioactivity of royal jelly from Greece. J. Agric. Food Chem. 2005, 53, 8987–8992. [Google Scholar] [CrossRef]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. More than royal food-Major royal jelly protein genes in sexuals and workers of the honeybee Apis mellifera. Front. Zool. 2013, 10, 1–10. [Google Scholar] [CrossRef]
- Hamid, N.A.; Bakar, A.B.A.; Zain, A.A.M.; Hussain, N.H.N.; Othman, Z.A.; Zakaria, Z.; Mohamed, M. Composition of Royal Jelly (RJ) and its anti-androgenic effect on reproductive parameters in a polycystic ovarian syndrome (PCOS) animal model. Antioxidants 2020, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Balkanska, R.; Marghitas, L.-A.; Pavel, C.I. Antioxidant activity and total polyphenol content of royal jelly from Bulgaria. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 578–585. [Google Scholar] [CrossRef]
- Nozaki, R.; Tamura, S.; Ito, A.; Moriyama, T.; Yamaguchi, K.; Kono, T. A rapid method to isolate soluble royal jelly proteins. Food Chem. 2012, 134, 2332–2337. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Šturm, L.; Ulrih, N.P. Advances in the Propolis chemical composition between 2013 and 2018: A review. eFood 2019, 1, 24. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Hellner, M.; Winter, D.; Von Georgi, R.; Münstedt, K. Apitherapy: Usage and experience in German beekeepers. Evid.-Based Complement. Altern. Med. 2008, 5, 475–479. [Google Scholar] [CrossRef]
- El-Seedi, H.; El-Wahed, A.A.; Yosri, N.; Musharraf, S.G.; Chen, L.; Moustafa, M.; Zou, X.; Al-Mousawi, S.; Guo, Z.; Khatib, A.; et al. Antimicrobial properties of apis mellifera’s bee venom. Toxins 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee Venom: An updating review of its bioactive molecules and its health applications. Nutrients 2020, 12, 3360. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Bae, H. Anti-inflammatory applications of melittin, a major component of bee venom: Detailed mechanism of action and adverse effects. Molecules 2016, 21. [Google Scholar] [CrossRef]
- Sobral, F.; Sampaio, A.; Falcão, S.; Queiroz, M.J.R.P.; Calhelha, R.C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in Northeast Portugal. Food Chem. Toxicol. 2016, 94, 172–177. [Google Scholar] [CrossRef]
- Pucca, M.B.; Cerni, F.A.; Oliveira, I.S.; Jenkins, T.P.; Argemí, L.; Sørensen, C.V.; Ahmadi, S.; Barbosa, J.E.; Laustsen, A.H. Bee Updated: Current Knowledge on Bee Venom and Bee Envenoming Therapy. Front. Immunol. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Dong, J.; Zhang, H. Breaking the cells of rape bee pollen and consecutive extraction of functional oil with supercritical carbon dioxide. Innov. Food Sci. Emerg. Technol. 2009, 10, 42–46. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Alagawany, M.; Farag, M.R.; Elnesr, S.S. Beneficial impacts of bee pollen in animal production, reproduction and health. J. Anim. Physiol. Anim. Nutr. 2019, 103, 477–484. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki Michałand Kabała-Dzik, A.; Kubina, R.; Moździerz, A.; Buszman, E.; Iriti, M. Polyphenols from bee pollen: Structure, absorption, metabolism and biological activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Antioxidant and tyrosinase inhibitory properties of aqueous ethanol extracts from monofloral bee pollen. J. Apic. Sci. 2015, 59, 119–128. [Google Scholar] [CrossRef]
- Sawczuk, R.; Karpinska, J.; Miltyk, W. What do we need to know about drone brood homogenate and what is known. J. Ethnopharmacol. 2019, 245. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. Royal jelly, bee brood: Composition, health, medicine: A review. Lipids 2011, 3, 8–19. [Google Scholar]
- Kacániová, M.; Vuković, N.; Chlebo, R.; Haščík, P.; Rovná, K.; Cubon, J.; Dzugan, M.; Pasternakiewicz, A. The antimicrobial activity of honey, bee pollen loads and beeswax from Slovakia. Arch. Biol. Sci. 2012, 64, 927–934. [Google Scholar] [CrossRef]
- Aguilar, F.; Autrup, H.; Barlow, S.; Castle, L.; Crebelli, R.; Engel, K.; Gontard, N.; Gott, D.; Grilli, S.; Gürtler, R.; et al. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food Adopted on 7 March 2008. EFSA J. 2008, 1–29. [Google Scholar]
- Milojkovic, V. Bee Bread (Perga)—The Source of Health, Vitality and Longevity. In Proceedings of the Apiquality & Apimedica 2018 the XI-th Congress of the XI-th Romanian Society of Apitherapy, Sibiu, Romania, 11–16 October 2018. [Google Scholar]
- Fuenmayor, B.C.; Zuluaga, D.C.; Díaz, M.C.; de Quicazán, C.M.; Cosio, M.; Mannino, S. Evaluation of the physicochemical and functional properties of Colombian bee pollen. Rev. MVZ Córdoba 2014, 4003–4014. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Bakier, S.; Stocki, M. GC-MS investigation of the chemical composition of honeybee drone and queen larva homogenate. J. Apic. Sci. 2016, 60, 111–120. [Google Scholar] [CrossRef]
- Hashem, N.; Gonzalez-Bulnes, A.; Simal-Gandara, J. Polyphenols in Farm Animals: Source of Reproductive Gain or Waste? Antioxidants 2020. [Google Scholar] [CrossRef]
- Hashem, N.M.; El-Azrak, K.M.; Nour El-Din, A.N.M.; Sallam, S.M.; Taha, T.A.; Salem, M.H. Effects of Trifolium alexandrinum phytoestrogens on oestrous behaviour, ovarian activity and reproductive performance of ewes during the non-breeding season. Anim. Reprod. Sci. 2018. [Google Scholar] [CrossRef]
- Hashem, N.M.; El-Azrak, K.M.; Sallam, S.M.A. Hormonal concentrations and reproductive performance of Holstein heifers fed Trifolium alexandrinum as a phytoestrogenic roughage. Anim. Reprod. Sci. 2016, 170. [Google Scholar] [CrossRef]
- Sİlİcİ, S. Chemical Content and Bioactive Properties of Drone Larvae (Apilarnil). Mellifera 2019, 19, 14–22. [Google Scholar]
- Hosny, N.S.; Hashem, N.M.; Morsy, A.S.; Abo-Elezz, Z.R. Effects of organic selenium on the physiological response, blood metabolites, redox status, semen quality, and fertility of rabbit bucks kept under natural heat stress conditions. Front. Vet. Sci. 2020, 7, 290. [Google Scholar] [CrossRef]
- Banihani, S.A. Mechanisms of honey on testosterone levels. Heliyon 2019, 5, e02029. [Google Scholar] [CrossRef]
- Zaid, S.S.M.; Sulaiman, S.A.; Sirajudeen, K.N.M.; Othman, N.H. The effects of tualang honey on female reproductive organs, tibia bone and hormonal profile in ovariectomised rats—Animal model for menopause. BMC Complement. Altern. Med. 2010, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Husein, M.Q.; Haddad, S.G. A new approach to enhance reproductive performance in sheep using royal jelly in comparison with equine chorionic gonadotropin. Anim. Reprod. Sci. 2006, 93, 24–33. [Google Scholar] [CrossRef]
- Gimenez-Diaz, C. Improved reproductive response of sheep in intrauterine insemination program with the use of royal jelly. Afr. J. Biotechnol. 2012, 11, 12518–12521. [Google Scholar] [CrossRef]
- Husein, M.Q.; Kridli, R.T. Reproductive responses following royal jelly treatment administered orally or intramuscularly into progesterone-treated Awassi ewes. Anim. Reprod. Sci. 2002, 74, 45–53. [Google Scholar] [CrossRef]
- Kridli, R.T.; Husein, M.Q.; Humphrey, W.D. Effect of royal jelly and GnRH on the estrus synchronization and pregnancy rate in ewes using intravaginal sponges. Small Rumin. Res. 2003, 49, 25–30. [Google Scholar] [CrossRef]
- Ghanbari, E.; Khazaei, M.R.; Khazaei, M.; Nejati, V. Royal jelly promotes ovarian follicles growth and increases steroid hormones in immature rats. Int. J. Fertil. Steril. 2018, 11, 263–269. [Google Scholar] [CrossRef]
- Attia, Y.A.; Bovera, F.; Abd Elhamid, A.E.H.; Nagadi, S.A.; Mandour, M.A.; Hassan, S.S. Bee pollen and propolis as dietary supplements for rabbit: Effect on reproductive performance of does and on immunological response of does and their offspring. J. Anim. Physiol. Anim. Nutr. 2019, 103, 959–968. [Google Scholar] [CrossRef]
- Attia, Y.; Bovera, F.; El-Tahawy, W.; El-Hanoun, A.; Al-Harthi, M.; Habiba, H.I. Productive and reproductive performance of rabbits does as affected by bee pollen and/or propolis, inulin and/or mannan-oligosaccharides. World Rabbit Sci. 2015, 23, 273–282. [Google Scholar] [CrossRef]
- Kolesarova, A.; Bakova, Z.; Capcarova, M.; Galik, B.; Juracek, M.; Simko, M.; Toman, R.; Sirotkin, A.V. Consumption of bee pollen affects rat ovarian functions. J. Anim. Physiol. Anim. Nutr. 2013, 97, 1059–1065. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Hanoun, A.; Tag El-Din, A.E.; Bovera, F.; Shewika, Y.E. Effect of bee pollen levels on productive, reproductive and blood traits of NZW rabbits. J. Anim. Physiol. Anim. Nutr. 2011, 95, 294–303. [Google Scholar] [CrossRef]
- Kridli, R.T.; Al-Khetib, S.S. Reproductive responses in ewes treated with eCG or increasing doses of royal jelly. Anim. Reprod. Sci. 2006, 92, 75–85. [Google Scholar] [CrossRef]
- Adriana, K.; Capcarova, M.; Bakova, Z.; Branislav, G.; Miroslav, J.; Milan, S.; Sirotkin, A.V. The effect of bee pollen on secretion activity, markers of proliferation and apoptosis of porcine ovarian granulosa cells in vitro. J. Environ. Sci. Heal. Part B Pestic. Food Contam. Agric. Wastes 2011, 46, 207–212. [Google Scholar] [CrossRef]
- Abdelhafiz, A.T.; Muhamad, J.A. Midcycle pericoital intravaginal bee honey and royal jelly for male factor infertility. Int. J. Gynecol. Obstet. 2008, 101, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Makarevich, A.V.; Spalekova, E.; Olexikova, L.; Lukac, N.; Kubovicova, E.; Hegedusova, Z. Functional characteristics of ram cooling-stored spermatozoa under the influence of epidermal growth factor. Gen. Physiol. Biophys. 2011, 30, S36–S43. [Google Scholar] [CrossRef]
- Hashem, N.; Gonzalez-Bulnes, A. State-of-the-Art and Prospective of Nanotechnologies for Smart Reproductive Management of Farm Animals. Animals 2020. [Google Scholar] [CrossRef]
- Fakhrildin, M.-B.M.-R.; Alsaadi, R.A.-R. Honey Supplementation to Semen-Freezing Medium ImprovesHuman Sperm Parameters Post-Thawing. J. Fam. Reprod. Heal. 2014, 8, 27–31. [Google Scholar]
- Chung, E.L.T.; Nayan, N.; Nasir, N.S.M.; Hing, P.S.A.; Ramli, S.; Rahman, M.H.A.; Kamalludin, M.H. Effect of honey as an additive for cryopreservation on bull semen quality from different cattle breeds under tropical condition. J. Anim. Heal. Prod 2019, 7, 171–178. [Google Scholar] [CrossRef]
- Yimer, N.; Sarsaifi, K.; Haron, A.W. Effect of honey supplementation into Tris Extender on Cryopreservation of Bull Spermatozoa Application of assisted reproductive biotechnology in Rusa deer View project Enhancement of the quality of semen cryopreservation View project. Malays. J. Anim. Sci. 2016, 18, 47–54. [Google Scholar]
- El-Nattat, W.S.; El-Sheshtawy, R.I.; El-Batawy, K.A.; Shahba, M.I.; El-Seadawy, I.E. Research article Preservability of buffalo bull semen in tris-citrate extender enriched with bee’s honey. J. Innov. Pharm. Biol. Sci. 2016, 3, 180–185. [Google Scholar]
- Mu, A. Effect of bee honey and royal jelly addition to extender on rabbit semen fertilizing capacity at room temperaturE. J. Chem. Inf. Model. 2019, 53, 1689–1699. [Google Scholar] [CrossRef]
- El-Speiy, M.; El-Sawy, M.; Badri, F.; Sadaka, T. Effect of Honey Bees Supplementation for Semen Extender on Cryopreservation, Bacterial Activity and Fertility Traits of Rabbits. Egypt. J. Rabbit Sci. 2017, 27, 1–22. [Google Scholar] [CrossRef]
- Machebe, N.S.; Ugwu, S.O.; Akandi, A. Survivability of boar sperm stored under room temperature in extenders containing some natural products. Open Access Anim. Physiol. 2015, 57. [Google Scholar] [CrossRef]
- Chua, L.S.; Rahaman, N.L.A.; Adnan, N.A.; Eddie Tan, T.T. Antioxidant activity of three honey samples in relation with their biochemical components. J. Anal. Methods Chem. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Gautam, S.; Sharma, A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010, 118, 391–397. [Google Scholar] [CrossRef]
- Khan, F.R.; Abadin, Z.U.; Rauf, N. Honey: Nutritional and medicinal value. Int. J. Clin. Pract. 2007, 61, 1705–1707. [Google Scholar] [CrossRef]
- Mullai, V.; Menon, T. Bactericidal activity of different types of honey against clinical and environmental isolates of Pseudomonas aeruginosa. J. Altern. Complement. Med. 2007, 13, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Masoumi, R.; Rostami, B.; Shahir, M.H.; Taghilou, P.; Arslan, H.O. Effects of supplementation of Tris-egg yolk extender with royal jelly on chilled and frozen-thawed ram semen characteristics. Cryobiology 2019, 88, 75–80. [Google Scholar] [CrossRef]
- Moradi, A.R.; Malekinejad, H.; Farrokhi-Ardabili, F.; Bernousi, I. Royal Jelly improves the sperm parameters of ram semen during liquid storage and serves as an antioxidant source. Small Rumin. Res. 2013, 113, 346–352. [Google Scholar] [CrossRef]
- Alcay, S.; Toker, M.B.; Onder, N.T.; Gokce, E. Royal jelly supplemented soybean lecithin-based extenders improve post-thaw quality and incubation resilience of goat spermatozoa. Cryobiology 2017, 74, 81–85. [Google Scholar] [CrossRef]
- Shahzad, Q.; Mehmood, M.U.; Khan, H.; ul Husna, A.; Qadeer, S.; Azam, A.; Naseer, Z.; Ahmad, E.; Safdar, M.; Ahmad, M. Royal jelly supplementation in semen extender enhances post-thaw quality and fertility of Nili-Ravi buffalo bull sperm. Anim. Reprod. Sci. 2016, 167, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, S.M. Effect of royal jelly on the fertilizing ability of buffalo spermatozoa in vitro. J. Buffalo Sci. 2012, 1, 1–4. [Google Scholar] [CrossRef]
- Kodai, T.; Umebayashi, K.; Nakatani, T.; Ishiyama, K.; Noda, N. Compositions of royal jelly II. Organic acid glycosides and sterols of the royal jelly of honeybees (Apis mellifera). Chem. Pharm. Bull. 2007, 55, 1528–1531. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; Al-Gabri, N.A.; Hashem, N.M.; Gonzalez-Bulnes, A. Supplementation with Proline Improves Haemato-Biochemical and Reproductive Indicators in Male Rabbits Affected by Environmental Heat-Stress. Animals 2021, 11. [Google Scholar] [CrossRef]
- Rahnama, G.; Deldar, H.; Ansari Pirsaraei, Z.; Kazemifard, M. Oral administration of royal jelly may improve the preservation of rooster spermatozoa. J. Anim. Physiol. Anim. Nutr. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Toker, M.B.; Alcay, S.; Gokce, E.; Ustuner, B. Cryopreservation of ram semen with antioxidant supplemented soybean lecithin-based extenders and impacts on incubation resilience. Cryobiology 2016, 72, 205–209. [Google Scholar] [CrossRef]
- El-Seadawy, I.E.S.; El-Nattat, W.S.; El-Tohamy, M.M.; Aziza, S.A.H.; El-Senosy, Y.A.; Hussein, A.S. Preservability of rabbit semen after chilled storage in tris based extender enriched with different concentrations of Propolis ethanolic extract (PEE). Asian Pac. J. Reprod. 2017, 6, 68–76. [Google Scholar] [CrossRef]
- Virgili, F.; Marino, M. Regulation of cellular signals from nutritional molecules: A specific role for phytochemicals, beyond antioxidant activity. Free Radic. Biol. Med. 2008, 45, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M. Antimicrobial Effects of Propolis on Preservation of Ram’s Semen Extender and Its Fertility Rate. J. Anim. Poult. Prod. 2017, 8, 203–213. [Google Scholar] [CrossRef]
- Khalifa, E.I.; Mohamed, M.Y. Possibility of using propolis as natural antibiotic instead of synthetic antibiotics in ram semen extenders. Egypt. J. Sheep Goat Sci. 2016, 11, 1–14. [Google Scholar] [CrossRef]
- Barakat, I.A.H.; Alajmi, R.A.; Zoheir, K.M.A.; Salem, L.M.; Al-Hemidiy, A.R. Gene expression and maturation evaluation of sheep oocytes cultured in medium supplemented with natural antioxidant source. S. Afr. J. Anim. Sci. 2018, 48, 261–270. [Google Scholar] [CrossRef]
- Zhu, J.; Moawad, A.R.; Wang, C.Y.; Li, H.F.; Ren, J.Y.; Dai, Y.F. Advances in in vitro production of sheep embryos. Int. J. Vet. Sci. Med. 2018, 6, S15–S26. [Google Scholar] [CrossRef] [PubMed]
- Veshkini, A.; Mohammadi-Sangcheshmeh, A.; Ghanem, N.; Abazari-kia, A.H.; Mottaghi, E.; Kamaledini, R.; Deldar, H.; Ozturk, I.; Gastal, E.L. Oocyte maturation with royal jelly increases embryo development and reduces apoptosis in goats. Anim. Reprod. 2018, 15, 124–134. [Google Scholar] [CrossRef]
- Eshtiyaghi, M.; Deldar, H.; Pirsaraei, Z.A.; Shohreh, B. Royal jelly may improve the metabolism of glucose and redox state of ovine oocytes matured in vitro and embryonic development following in vitro fertilization. Theriogenology 2016, 86, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Kaabi, A.M.; Barakat, I.A.H.; Alajmi, R.A.; Abdel-Daim, M.M. Use of black seed (Nigella sativa) honey bee to improve sheep oocyte maturation medium. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Hassan, A.A.; Hammodi, A.S.; Kasem, Y.Y. Effect of royal jelly on reproductive performance in cadmium-treated male rats. Iraqi J. Vet. Sci. 2012, 26, 225–231. [Google Scholar]
- Seven, I.; Tatli Seven, P.; Gul Baykalir, B.; Parlak Ak, T.; Ozer Kaya, S.; Yaman, M. Bee glue (propolis) improves reproductive organs, sperm quality and histological changes and antioxidant parameters of testis tissues in rats exposed to excess copper. Andrologia 2020, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mahran, A.; AlRashidy, A.; ElMawla, A. Role of propolis in improving male rat fertility affected with aluminum chloride cytotoxicity. Hematology 2011, 1, 189. [Google Scholar] [CrossRef]
- Al-Sanafi, A.; Mohssin, S.; Abdulla, S. Effect of Royal Jelly on male Infertility. Iraqi J. Vet. Sci. 2012, 1, 1–12. [Google Scholar]
- ElMazoudy, R.H.; Attia, A.A.; El-Shenawy, N.S. Protective role of propolis against reproductive toxicity of chlorpyrifos in male rats. Pestic. Biochem. Physiol. 2011, 101, 175–181. [Google Scholar] [CrossRef]
- Khatab, A.E.; Hashem, N.M.; El-Kodary, L.M.; Lotfy, F.M.; Hassan, G.A. Evaluation of the Effects of Cypermethrin on Female Reproductive Function by Using Rabbit Model and of the Protective Role of Chinese Propolis. Biomed. Environ. Sci. 2016, 29. [Google Scholar] [CrossRef]
- El-Nekeety, A.A.; El-Kholy, W.; Abbas, N.F.; Ebaid, A.; Amra, H.A.; Abdel-Wahhab, M.A. Efficacy of royal jelly against the oxidative stress of fumonisin in rats. Toxicon 2007, 50, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Biedenharn, K.R.; Fedor, J.M.; Agarwal, A. Lifestyle factors and reproductive health: Taking control of your fertility. Reprod. Biol. Endocrinol. 2013, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Azad, F.; Nejati, V.; Shalizar-Jalali, A.; Najafi, G.; Rahmani, F. Royal jelly protects male mice against nicotine-induced reproductive failure. Vet. Res. Forum 2018, 9, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Troncoso, N.; Sanchez, F.; Garbarino, J.A.; Vanella, A. Propolis protects human spermatozoa from DNA damage caused by benzo [a] pyrene and exogenous reactive oxygen species. Life Sci. 2006, 78, 1401–1406. [Google Scholar] [CrossRef]
- Mohamed, M. Honey and male reproductive health. Honey Curr. Res. Clin. Appl. 2012, 131–142. [Google Scholar]
- Mohamed, M.; Sulaiman, S.A.; Sirajudeen, K.N.S. Protective effect of honey against cigarette smoke induced-impaired sexual behavior and fertility of male rats. Toxicol. Ind. Health 2013, 29, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Eweka, A.O.; OmIniabohs, F.A.E. Histological studies of the effects of monosodium glutamate on the kidney of adult Wistar rats. Internet J. Heal. 2007, 6, 2. [Google Scholar]
- ElMetwally, A. Immunohistopathologic Study On The Ameliorative Effect Of Propolis Against Fluoride Cytotoxicity On Rabbit Buck Fertility. Alexandria J. Vet. Sci. 2017, 53, 1. [Google Scholar] [CrossRef]
- Khaled, F.A.; Yousef, M.I.; Kamel, K.I. The protective role of propolis against the reproductive toxicity of mono-sodium glutamine in male rabbits. IJCS 2016, 4, 4–9. [Google Scholar]
- Haron, M.N.; Rahman, W.F.W.A.; Sulaiman, S.A.; Mohamed, M. Tualang honey ameliorates restraint stress-induced impaired pregnancy outcomes in rats. Eur. J. Integr. Med. 2014, 6, 657–663. [Google Scholar] [CrossRef]
- Haron, M.N.; Mohamed, M. Effect of honey on the reproductive system of male rat offspring exposed to prenatal restraint stress. Andrologia 2016, 48, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Rajabzadeh, A.; Sagha, M.; Gholami, M.R.; Hemmati, R. Honey and vitamin E restore the plasma level of gonadal hormones and improve the fertilization capacity in noise-stressed rats. Crescent J. Med. Biol. Sci. 2015, 2, 64–68. [Google Scholar]
- Hashem, N.M.; Abo-elsoud, M.A.; Nour El-Din, A.N.M.; Kamel, K.I.; Hassan, G.A. Prolonged exposure of dietary phytoestrogens on semen characteristics and reproductive performance of rabbit bucks. Domest. Anim. Endocrinol. 2018, 64. [Google Scholar] [CrossRef]
- Abo-elsoud, M.A.; Hashem, N.M.; Nour El-Din, A.N.M.; Kamel, K.I.; Hassan, G.A. Soybean isoflavone affects in rabbits: Effects on metabolism, antioxidant capacity, hormonal balance and reproductive performance. Anim. Reprod. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
| Honeybee Species (Scientific Name) | Common Name/Domestication | Region |
|---|---|---|
| A. mellifera | Western honeybee/domesticated | Old World Europe, Eastern Mediterranean, and Africa |
| A. cerana | Asiatic honeybee/domesticated | Southern, Southeastern, and Eastern Asia |
| A. dorsata | Giant honeybee/wild | Southern and Southeastern Asia |
| A.florea | Red dwarf honeybee/wild | Southern and Southeastern Asia |
| A. andreniformis | Black dwarf honeybee/wild | Southeastern Asia |
| A. laboriosa | Himalayan giant honey bee/wild | Asia (Himalayas, mountainous regions of Bhutan and China, India, and Vietnam) |
| A. koschevnikovi | Koschevnikov’s honeybee/wild | Asia (Malaysian and Indonesian Borneo) |
| A. nigrocincta | Philippine honeybeelwild | Asia (The Philippine island of Mindanao and Indonesian islands of Sangihe and Sulawesi) |
| Honeybee Product | Main Component | Individual Components |
|---|---|---|
| Honey [8,43,44] | Sugars | Disaccharides (maltose and sucrose), monosaccharides (glucose and fructose), and oligosaccharides (maltotriose and panose) |
| Amino acids | Arginine, glutamic, histidine, lysine, phenylalanine, proline, tyrosine, and valine | |
| Organic acids | Gluconic, acetic, butyric, citric, formic, lactic, malic, pyroglutamic, and succinic acids | |
| Vitamins | B1, B2, B3, B5, B6, B8, B9, B12, and C | |
| Minerals | Ca, Cu, Fe, K, Mg, Mn, Na, P, S, and Zn | |
| Enzymes | Amylase, glucose oxidase, and sucrase (α-glucosidase) | |
| Phenolic compounds | Acacetin, apigenin, benzoic acids, caffeic, chlorogenic, chrysin, ellagic, ferulic, fisetin, galangin, gallic, genistein, 3-hydroxybenzoic, 4-hydroxybenzoic, hesperetin, kaempferol, luteolin, myricetin, naringenin, p-coumaric, pinobanksin, pinocembrin, quercetin, rosmarinic, syringic, and vanillic | |
| Royal jelly [15,45,46,47,48,49] | Sugars | Glucose and fructose |
| Proteins/peptides | Apisimin, major royal jelly proteins (MRJPs 1–9), jelleines, and royalisin | |
| Fatty acids (carboxylic acids) | 10-hydroxy-2-decenoic acid, 10-hydroxydecanoic acid, 4-hydroxyperoxy-2-decenoic acid ethyl ester, and decanoic acid (sebacic acid) | |
| Vitamins | B1, B2,B3, B5, B6, B9, and β-carotene | |
| Minerals | Cu, Fe, K, Mg, and Zn | |
| Hormones | Estradiol, progesterone, prolactin, and testosterone | |
| Phenolic compounds | Apigenin, caffeic acid, gallic acid, 4-hydroxy-3-methoxyphenylethanol, 4-hydroxybenzoic acid-methyl ester, 4-hydroxybenzoic acid, 4-hydroxyhydrocinnamic acid, hydroquinone, isorhamnetin, kaempferol, luteolin, methyl salicylate, 2-methoxy-p-cresol, 2-methoxyphenol, naringenin, p-coumaric, pinobanksin, pyrocatechol, quercetin, and rutin | |
| Propolis [50,51,58] | Sugars | Fructose, glucose, and sucrose |
| Fatty acids | Arachidonic acid, cis-13,16-docosadienoic acid, cis-11,14,17-eicosatrienoic acid, cis-5,8,11,14,17-eicosapentaenoic acid, eicosadienoic acid, elaidic acid, heneicosylic acid, linoleic acid, oleic acid, palmitic acid, palmitoleic acid, and α- and γ- linoleic acids | |
| Terpenoids | Clerodane diterpenoids, farnesol, isocupressic acid, labdane, and 13-symphyoreticulic acid | |
| Phenolic compounds | 2,2,dimethyl-8-prenylchromene, apigenin, benzofuran, caffeic acid and its derivatives, chrysin, cinnamic acid and its derivatives, ferulic acid, galangin, kaempferol and its derivatives, naringenin, p-coumaric acid, pinobanksin, pinocembrin, pinostrobin, quercetin, and tectochrysin | |
| Bee venom [14,15,16,17,18,19,20,21,22] | Sugars | Fructose and glucose |
| Proteins/peptides/amines | Adolapin, apamin, dopamine, histamine, mast cell degranulating peptide, melittin, noradrenaline, procamine, protease inhibitors, secapin, and tertiapin | |
| Minerals | Ca, Mg, and P | |
| Enzymes | Glucosidase, hyaluronidase acid, lysophospholipase, phospholipase A2, phospholipase B, and phosphomonoesterase | |
| Bee pollen [58,59,60,68] | Sugars | Fructose, glucose, and sucrose |
| Fatty acids | Linoleic acid and linolenic acid | |
| Phenolic compounds | Apigenin, caffeic acid, catechin, delphinidin, ferulic acid, galangin, gallic acid, isorhamnetin, luteolin, naringenin, p-coumaric acid, protocatechuic acid, quercetin, rutin, and syringic acid | |
| Beeswax [66] | Fatty acids | 15,hydroxypalmitic acid, oleic acid, and palmitic acid |
| Vitamins | A, B1, B6, choline, and rutin | |
| Minerals | Ca, Cu, Fe, K, Mn, Na, P, and Zn | |
| Hydrocarbons | Hentriacontane, heptacosane, nonacosane, pentacosane, and tricosane | |
| complex wax esters | 15-hydroxypalmitic acid and diols | |
| Bee bread [67] | Sugars | Monosaccharides (glucose and fructose) and disaccharides (erlose, maltose, turanose, and trehalose) |
| Fatty acids | Arachidic acid, arachidonic acid, docosahexaenoic acid, eicosapentaenoic acid, linoleic acid, myristic acid, oleic acid, palmitic acid, and α-linolenic acid | |
| Vitamins | B1, B2, B3,B5, B6, B9, C, E (α, β, and γ-tocopherol), and K | |
| Minerals | Ca, Cu, Fe, K, Mg, P, Se, and Zn | |
| Enzymes | Amylase, phosphatases, and saccharase | |
| Phenolic compounds | Apigenin, chrysin, kaempferol, naringin, p-coumaric acid, quercetin, and rutin | |
| Sugars | Lactulose, melizitoze, neo, trehalose, raffinose, sucrose, trehalose, (α- and β-) isomaltose, and (α- and β-) maltose | |
| Drone brood [63,69] | Amino acids | Alanine, asparagine, aspartic acid, glutamine, glycine, histidine homoserine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, pyroglutamic acid, sarcosine, serine, threonine, tyrosine, valine, and γ-aminobutyric acid |
| Fatty acids | 3-ethylglutaconic acid, 2-hydroxyglutaric acid, 3-hydroxybutyric acid, adipic acid, fumaric acid, glyceric acid, lactic acid, malic acid, octadecanoic acid, oleic acid, palmitic acid, and succinic acid | |
| N, containing compounds | Adenosine- tetra-TMS, uracil, urea, uric acid, and uridine | |
| P, containing compounds | Glucopyranosyl phosphate, phosphoric acid, and (α- and β-) glycerylphosphate | |
| Sterols | Avenasterol, campesterol, and β-sitosterol |
| Animal Species | Treatment/Honeybee Product | Result | Suggested Mode of Action |
|---|---|---|---|
| Rats [16] | 1 mL/100 g BW of honey for 65 days |
|
|
| Rats [31] | Drinking 5% solution of Palestinian honey for 20 days |
| |
| Rats [32] | 0, 0.2, 1.2, and 2.4 g/kg BW/day of Malaysian honey for 4 weeks |
| |
| Rats [33] | 1.0 mL/100 g BW/day of Gelam honey for 60 days |
| |
| Rats [19] | 2.5%, 5%, 7.5%, 10%, and 12.5% of propolis extract for 18 days |
|
|
| Rabbits [20] | 0, 0.25, 0.50, 0.75, 1.0, and 1.25 g/kg diet of propolis for 94 days |
|
|
| Rabbits [21] | 0.5 and 1 g/animal/day of Egyptian propolis for 6 weeks during summer months |
|
|
| Rabbits [14] | 150 mg/kg diet of vitamin E or propolis for 10 consecutive weeks during summer months |
|
|
| Pre-pubertal rabbits [22] | 15 mg/kg BW of propolis with/without 200 mg royal jelly + 0.25 mL bee honey |
|
|
| Rats [23] | 3, 6, and 10 mg/kg BW/day of Brazilian green propolis extract for 56 days |
|
|
| Rats [27] | 1 g/kg BW of royal jelly with or without hydrogen peroxide (0.5%) in drinking water for one month |
|
|
| Pups [28] | 125, 250, and 500 mg/day/kg diet of royal jelly proteins |
|
|
| Rabbits [29] | 0, 50, 100, and 150 mg/kg BW of Chinese royal jelly |
|
|
| Pre-pubertal rabbits [30] | 0.25 mL honey, 200 mg royal jelly, and 200 mg royal jelly + 0.25 mL honey |
|
|
| Sheep [35] | 10, 15, and 20 mg/kg diet of apistimul preparation (drone brood) for 95 days |
|
|
| Pigs (junior boars) [34] | Parenteral injection with alcohol extracts of the drone brood |
| |
| Rabbits [36] | Injection of 0.1, 0.2, and 0.3 mg/rabbit of bee venom twice weekly for 20 weeks |
|
|
| Rabbits [18] | 0, 100, 200, and 300 mg of bee pollen/kg BW |
|
|
| Animal Species | Treatment/Honeybee Product | Result | Suggested Mode of Action |
|---|---|---|---|
| Ovariectomized rats a model for menopausal symptoms in women [76] | 0.2, 1.0, and 2.0 g/kg/day of Tualang honey for 2 weeks |
|
|
| Rabbits and offspring [82] | 150 and 300 mg/kg diet/day of bee pollen and/or propolis (Bp + Pro) three times a week along eight parities |
|
|
| Rabbits [83] | 0.2 g/kg BW of bee pollen and/or propolis compared with 35 mg/kg BW prebiotic (inulin and/or MOS) |
|
|
| Rats [84] | 3 and 5 g/kg feed mixture of rape seed bee pollen |
|
|
| Rabbits and offspring [85] | 100, 200, and 300 mg/kg BW of bee pollen extract for 1 week before and after mating |
|
|
| Polycystic ovarian syndrome animal model using female rats [46] | 200 and 400 mg/kg/day of royal jelly for 4 weeks |
|
|
| Sheep [80] | 250 mg/ewe of royal jelly during 12 days of estrous synchronization |
|
|
| Sheep [77] | 400 mg/ewe of royal jelly during the period of CIDR-treatment |
|
|
| Rats [81] | Intraperitoneal treatment with 100, 200, and 400 mg/kg BW/day royal jelly for 14 days |
|
|
| Animal Species | Treatment/Honeybee Product | Result | Suggested Mode of Action |
|---|---|---|---|
| Humans [90] | 0%, 5%, or 10% of honey as cryoprotectant |
|
|
| Buffalos [94] | 1%, 2%, 3%, 4%, and 5% of honey to cooled and frozen tris-based semen extenders |
| |
| Boars [99] | 1.0%, 1.5%, and 2.0% of honey to liquid storage semen extender |
| |
| Bulls [92] | 1%, 2.5%, 5%, 10%, and 15% of honey to frozen semen extender |
| |
| Bulls [93] | 2.5%, 5%, and 10% of honey to frozen semen extender |
| |
| Rabbits [96] | 0%, 1%, 3%, and 5% of honey to cooled semen extender (cooling at 4 °C for 72 h) |
| |
| Sheep [103] | 0%, 0.5%, 1%, 1.5%, and 2% of royal jelly to liquid storage semen extender |
|
|
| Buffaloes [105] | 0%, 0.05%, 0.1%, 0.2%, 0.3%, and 0.4% of royal jelly to semen freezing extender |
| |
| Rabbits [111] | 0.8, 1.2, 1.6, and 2.0 mg of propolis ethanolic extract/5 mL of frozen semen extender |
|
|
| Sheep [113] | 400 and 600 µl of propolis powder/propolis glue compared with synthetic antibiotic |
|
| Animal Species. | Treatment/Honeybee Product | Result | Suggested Mode of Action |
|---|---|---|---|
| Sheep [119] | Grade A and B oocytes cultured for 24 h in IVM supplemented with 0, 5, 10, and 20% honey |
|
|
| Goats [117] | 2.5, 5, and 10 mg/mL of royal jelly to oocyte IVM media |
|
|
| Sheep [118] | 0, 2.5, 5, and 10 mg/mL of royal jelly to oocyte IVM media |
|
| Animal Species/Chemical Stress | Treatment/Honeybee Product | Result | Suggested Mode of Action |
|---|---|---|---|
| Rats/Copper [121] | 100 mg/kg BW/day of propolis ethanolic extract |
|
|
| Rats/Cadmium [120] | 0.5 mg/L water of cadmium chloride and 400mg/kg BW of royal jelly |
|
|
| Rats/Aluminum chloride [122] | mg/kg BW, 1/25 L of aluminum chloride and 50 mg/kg BW of propolis for 70 days |
|
|
| Rats/Chlorpyrifos [124] | 9 mg/kg BW chlorpyrifos and 50 mg/kg BW of propolis for 70 days |
|
|
| Rabbits/Cypermethrin [125] | 50 mg/kg BW of cypermethrin and 50 mg/kg BW of propolis |
|
|
| Rats/Cadmium [120] | 0.5 ppm/L water cadmium and 400 mg/kg BW/day of royal jelly for 60 days |
|
|
| Rats/Fumonisin [126] | 200 mg/kg diet Fumonisin B-contaminated diet and 100 or 150 mg/kg BW of royal jelly |
|
|
| Lifestyle and Psychological Stress/Animal Species | Treatment/Honeybee Product | Result | Suggested Mode of Action |
|---|---|---|---|
| Cigarette (CS) smoke/Rats [131] | CS for 8 min three times/day and 1.2 g/kg BW/day of honey |
|
|
| Nicotine/Mice [128] | 0.5 and 1 mg/kg/day of nicotine and 100 mg/kg BW/day royal jelly |
|
|
| Fluoride/Rabbits [133] | 10 mg/kg BW/day of sodium fluoride and 25 mg/kg BW/day of propolis for 70-day |
|
|
| Mono-sodium glutamine/Rabbits [134] | 8 mg/kg BW of mono-sodium glutamine and 50 mg/kg BW of propolis |
|
|
| Noise/Rats [137] | Exposure to noise as a natural teratogenic factor and 5% honey of solution and 75 mg/mL vitamin E |
|
|
| Prenatal stress/Rats [136] | Exposure to a restraint stress and 1.2 g /kg BW of honey |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashem, N.M.; Hassanein, E.M.; Simal-Gandara, J. Improving Reproductive Performance and Health of Mammals Using Honeybee Products. Antioxidants 2021, 10, 336. https://doi.org/10.3390/antiox10030336
Hashem NM, Hassanein EM, Simal-Gandara J. Improving Reproductive Performance and Health of Mammals Using Honeybee Products. Antioxidants. 2021; 10(3):336. https://doi.org/10.3390/antiox10030336
Chicago/Turabian StyleHashem, Nesrein M., Eman M. Hassanein, and Jesus Simal-Gandara. 2021. "Improving Reproductive Performance and Health of Mammals Using Honeybee Products" Antioxidants 10, no. 3: 336. https://doi.org/10.3390/antiox10030336
APA StyleHashem, N. M., Hassanein, E. M., & Simal-Gandara, J. (2021). Improving Reproductive Performance and Health of Mammals Using Honeybee Products. Antioxidants, 10(3), 336. https://doi.org/10.3390/antiox10030336








