Ursodeoxycholic Acid May Inhibit Environmental Aging-Associated Hyperpigmentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Measurement of Melanin Content
2.3. Intracellular Tyrosinase Activity Assay
2.4. Exposure to UV Radiation, Particulate Matter (PM), or Growth Factors
2.5. 2′,7′-Dichlorofluorescein Diacetate (DCF-DA) Microplate Assay
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Western Blotting
2.8. MTT Assay
2.9. Statistical Analysis
3. Results
3.1. Antioxidant Property of UDCA
3.1.1. UDCA Decreases ROS Levels Induced by UVA and UVB in HDFs
3.1.2. UDCA Attenuates the Increased ROS Level Following Exposure to PM in NHKs
3.2. Anti-Inflammatory Property of UDCA
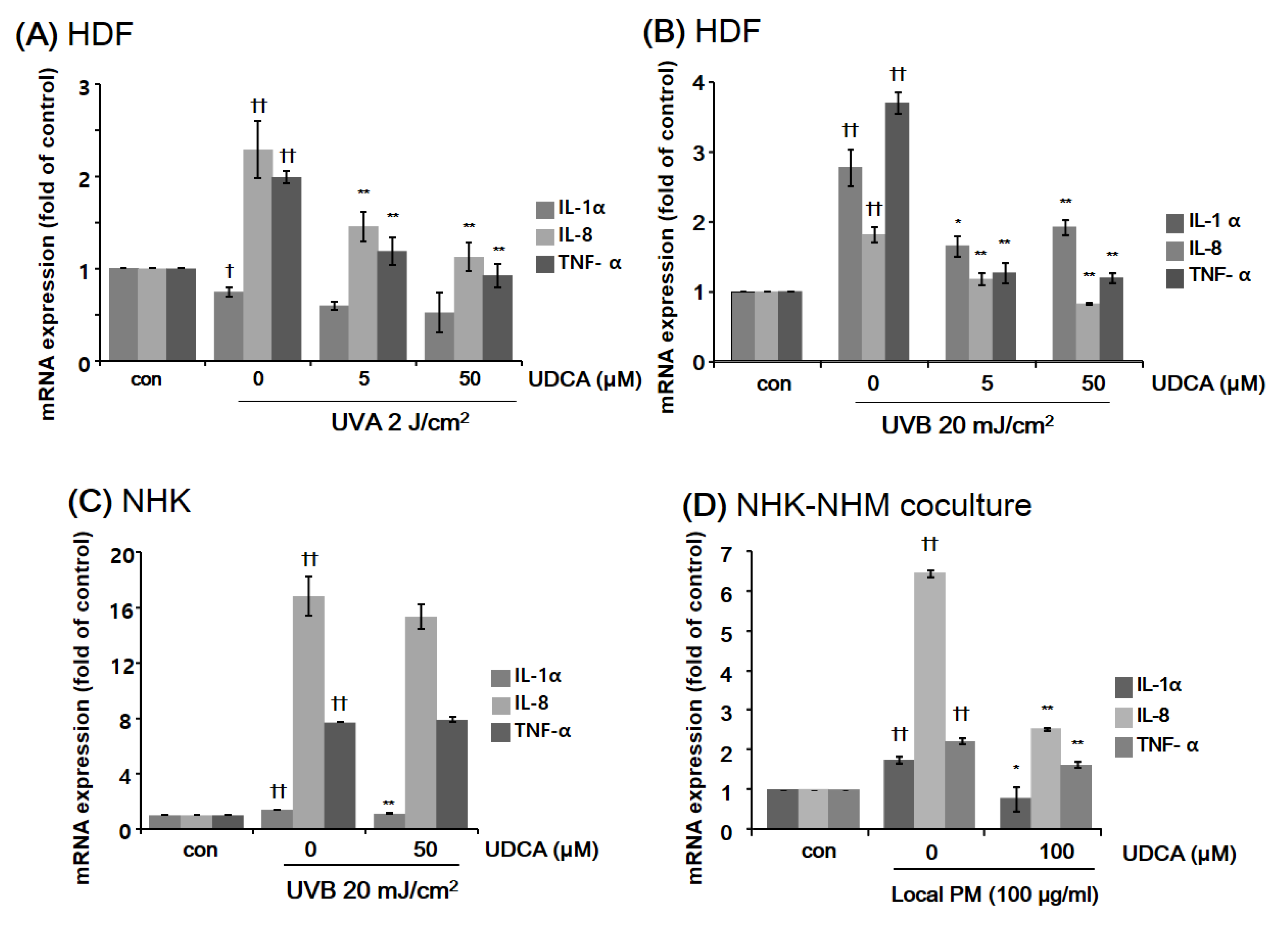
3.2.1. UDCA Treatment Had an Anti-Inflammatory Effect against the Inflammatory Cellular Microenvironment Resulting from Exposure to UV or PM
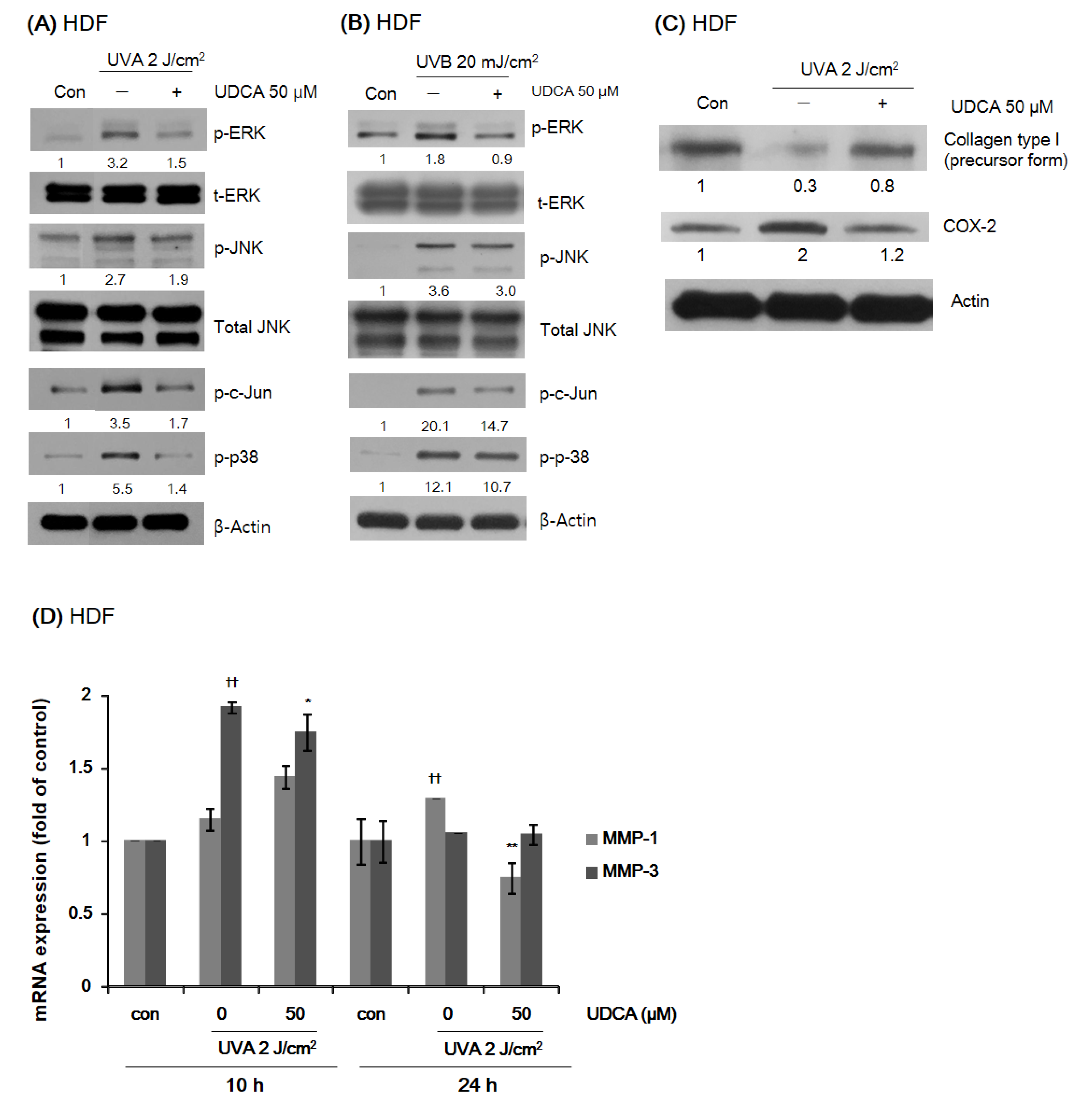
3.2.2. UDCA Reduces the Expression of Proteins Associated with Environmental Aging and Inflammation in HDFs While Restoring Type I Collagen Expression
3.3. Anti-Melanogenic Property of UDCA
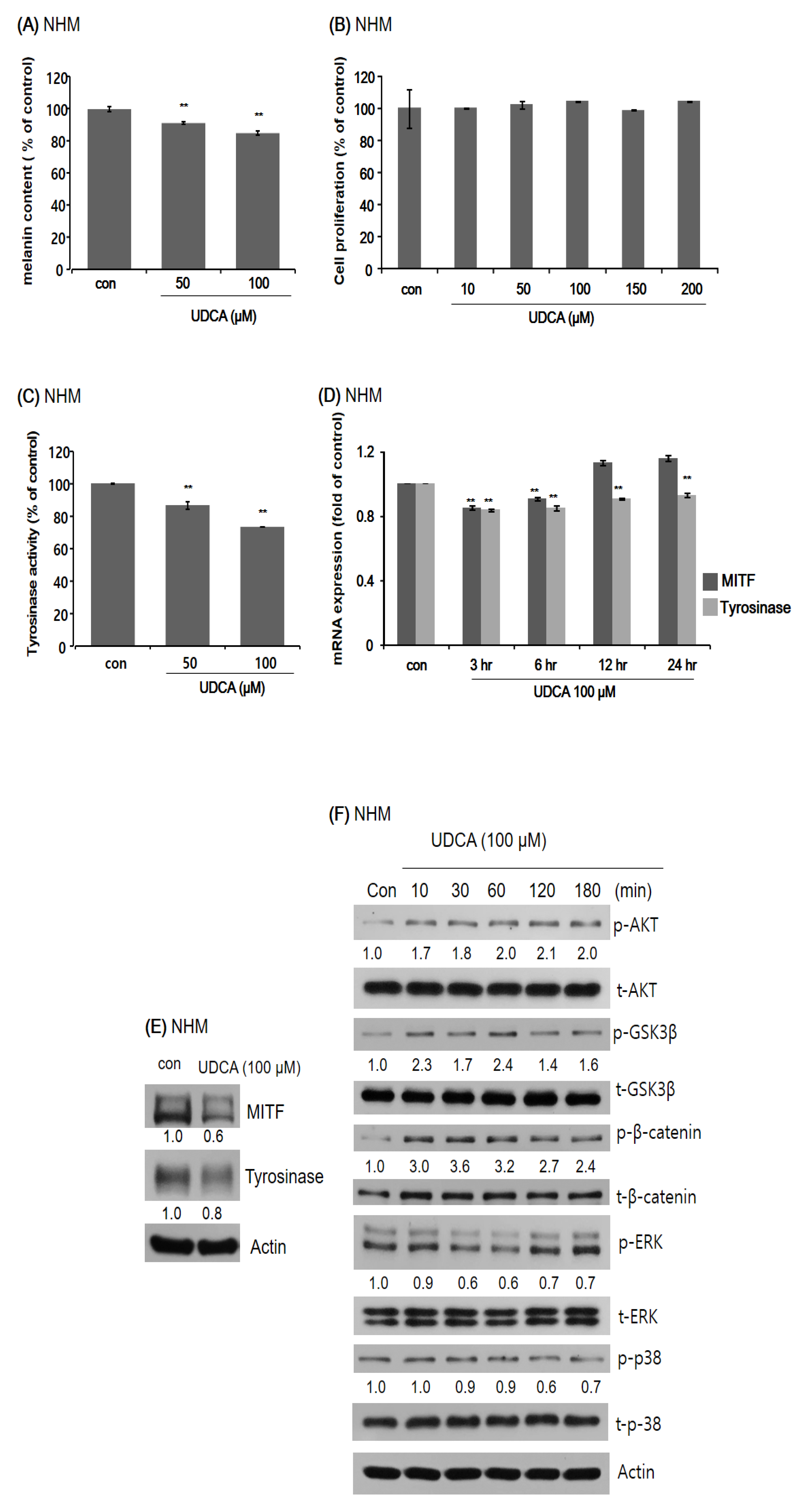
3.3.1. UDCA Decreases Melanogenesis in Human Melanocytes
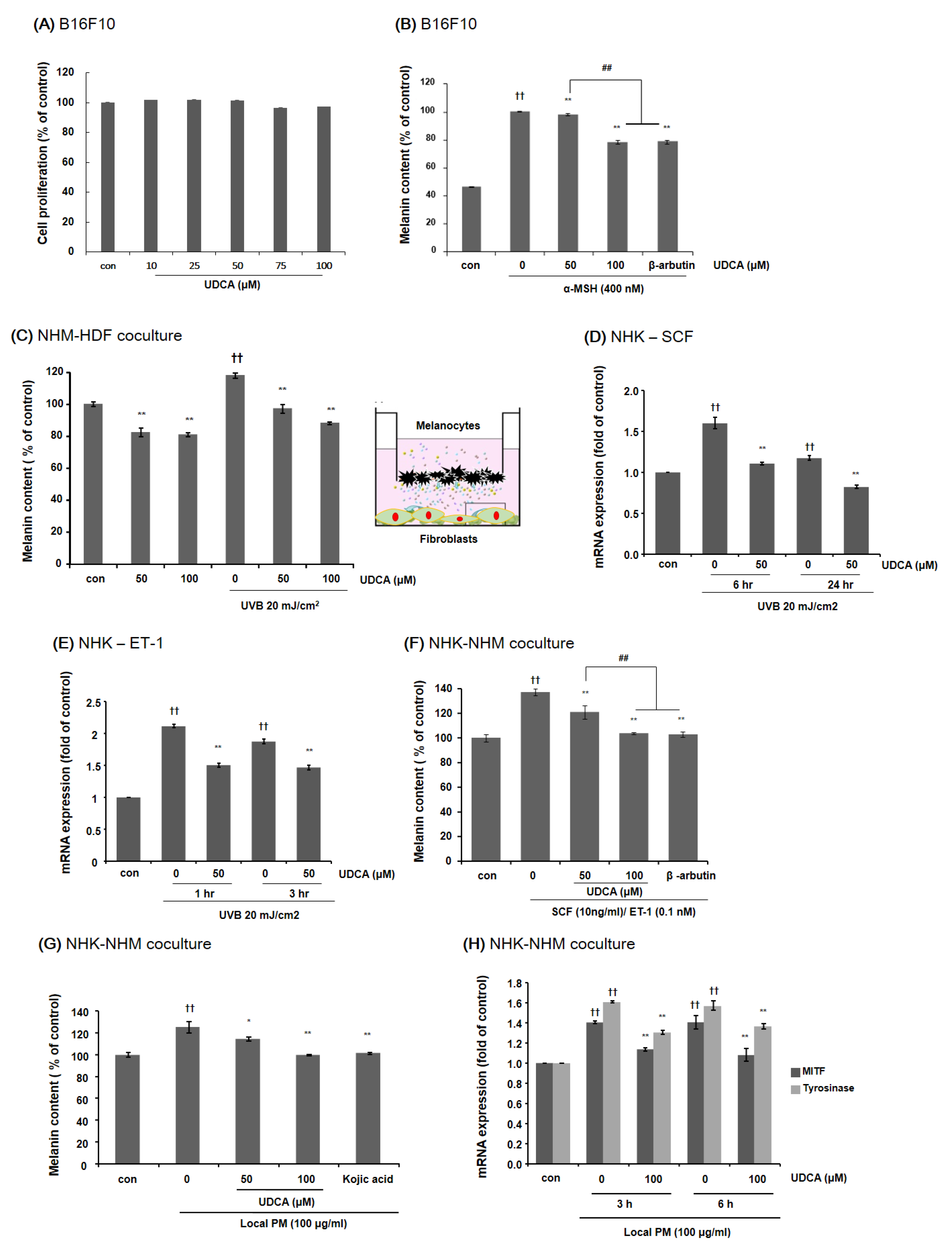
3.3.2. UDCA Decreases the Melanin Content in B16F10 Cells and HDF-NHM Coculture under Stimulated Conditions
3.3.3. UDCA Attenuates the Increase in Melanin Content Induced by Treatment with PM in NHK-NHM Coculture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burke, K.E. Photoaging: The role of oxidative stress. G. Ital. Derm. Venereol 2010, 145, 445–459. [Google Scholar]
- Gilchrest, B.A. Skin aging and photoaging: An overview. J. Am. Acad. Derm. 1989, 21, 610–613. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Derm. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Derm. 2006, 55, 1–19. [Google Scholar] [CrossRef]
- Chung, J.H. Photoaging in Asians. Photodermatol. Photoimmunol. Photomed. 2003, 19, 109–121. [Google Scholar] [CrossRef]
- Won, K.H.; Lee, S.H.; Lee, M.H.; Rhee, D.Y.; Yeo, U.C.; Chang, S.E. A prospective, split-face, double-blinded, randomized study of the efficacy and safety of a fractional 1064-nm Q-switched Nd:YAG laser for photoaging-associated mottled pigmentation in Asian skin. J. Cosmet. Laser Ther. 2016, 18, 381–386. [Google Scholar] [CrossRef]
- Hüls, A.; Sugiri, D.; Fuks, K.; Krutmann, J.; Schikowski, T. Lentigine Formation in Caucasian Women-Interaction between Particulate Matter and Solar UVR. J. Investig. Derm. 2019, 139, 974–976. [Google Scholar] [CrossRef]
- Dijkhoff, I.M.; Drasler, B.; Karakocak, B.B.; Petri-Fink, A.; Valacchi, G.; Eeman, M.; Rothen-Rutishauser, B. Impact of airborne particulate matter on skin: A systematic review from epidemiology to in vitro studies. Part. Fibre Toxicol 2020, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Bossis, G.; Hyun, Y.M.; Park, C.O.; Hyun, J.W. Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Ho, S.G.; Chan, H.H. The Asian dermatologic patient: Review of common pigmentary disorders and cutaneous diseases. Am. J. Clin. Derm. 2009, 10, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Duval, C.; Cohen, C.; Chagnoleau, C.; Flouret, V.; Bourreau, E.; Bernerd, F. Key regulatory role of dermal fibroblasts in pigmentation as demonstrated using a reconstructed skin model: Impact of photo-aging. PLoS ONE 2014, 9, e114182. [Google Scholar] [CrossRef]
- Wang, Y.; Viennet, C.; Robin, S.; Berthon, J.Y.; He, L.; Humbert, P. Precise role of dermal fibroblasts on melanocyte pigmentation. J. Derm. Sci. 2017, 88, 159–166. [Google Scholar] [CrossRef]
- Lapenna, D.; Ciofani, G.; Festi, D.; Neri, M.; Pierdomenico, S.D.; Giamberardino, M.A.; Cuccurullo, F. Antioxidant properties of ursodeoxycholic acid. Biochem Pharm. 2002, 64, 1661–1667. [Google Scholar] [CrossRef]
- Rodrigues, C.M.; Steer, C.J. The therapeutic effects of ursodeoxycholic acid as an anti-apoptotic agent. Expert Opin. Investig. Drugs 2001, 10, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Kono, M.; Akimoto, T. Psoriasis treated with ursodeoxycholic acid: Three case reports. Clin. Exp. Derm. 2007, 32, 398–400. [Google Scholar] [CrossRef]
- Oh, A.R.; Bae, J.S.; Lee, J.; Shin, E.; Oh, B.C.; Park, S.C.; Cha, J.Y. Ursodeoxycholic acid decreases age-related adiposity and inflammation in mice. BMB Rep. 2016, 49, 105–110. [Google Scholar] [CrossRef]
- Roma, M.G.; Toledo, F.D.; Boaglio, A.C.; Basiglio, C.L.; Crocenzi, F.A.; Sanchez Pozzi, E.J. Ursodeoxycholic acid in cholestasis: Linking action mechanisms to therapeutic applications. Clin. Sci. 2011, 121, 523–544. [Google Scholar] [CrossRef]
- Moon, H.R.; Jung, J.M.; Kim, S.Y.; Song, Y.; Chang, S.E. TGF-β3 suppresses melanogenesis in human melanocytes cocultured with UV-irradiated neighboring cells and human skin. J. Derm. Sci. 2020, 99, 100–108. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Borlon, C.; Pascal, T.; Royer, V.; Eliaers, F.; Ninane, N.; Carrard, G.; Friguet, B.; de Longueville, F.; Boffe, S.; et al. Repeated exposure of human skin fibroblasts to UVB at subcytotoxic level triggers premature senescence through the TGF-beta1 signaling pathway. J. Cell Sci. 2005, 118, 743–758. [Google Scholar] [CrossRef]
- Dobrzynska, I.; Szachowicz-Petelska, B.; Skrzydlewska, E.; Figaszewski, Z.A. Effects of UVB Radiation on the Physicochemical Properties of Fibroblasts and Keratinocytes. J. Membr. Biol. 2016, 249, 319–325. [Google Scholar] [CrossRef]
- Lei, L.; Zeng, Q.; Lu, J.; Ding, S.; Xia, F.; Kang, J.; Tan, L.; Gao, L.; Kang, L.; Cao, K.; et al. MALAT1 participates in ultraviolet B-induced photo-aging via regulation of the ERK/MAPK signaling pathway. Mol. Med. Rep. 2017, 15, 3977–3982. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S. Dandelion Extracts Protect Human Skin Fibroblasts from UVB Damage and Cellular Senescence. Oxid. Med. Cell Longev. 2015, 2015, 619560. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.P.; Li, Z.; Choi, E.K.; Lee, S.; Kim, Y.K.; Seo, E.Y.; Chung, J.H.; Cho, S. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J. Derm. Sci. 2018, 91, 175–183. [Google Scholar] [CrossRef]
- McDaniel, D.; Farris, P.; Valacchi, G. Atmospheric skin aging-Contributors and inhibitors. J. Cosmet. Derm. 2018, 17, 124–137. [Google Scholar] [CrossRef]
- Magnani, N.D.; Muresan, X.M.; Belmonte, G.; Cervellati, F.; Sticozzi, C.; Pecorelli, A.; Miracco, C.; Marchini, T.; Evelson, P.; Valacchi, G. Skin Damage Mechanisms Related to Airborne Particulate Matter Exposure. Toxicol. Sci. 2015, 149, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Morita, A.; Seité, S.; Haarmann-Stemmann, T.; Grether-Beck, S.; Krutmann, J. Environment-induced lentigines: Formation of solar lentigines beyond ultraviolet radiation. Exp. Derm. 2015, 24, 407–411. [Google Scholar] [CrossRef]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Dürr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Nobile, V.; Michelotti, A.; Cestone, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Pérez-Sánchez, A.; Micol, V. Skin photoprotective and antiageing effects of a combination of rosemary (Rosmarinus officinalis) and grapefruit (Citrus paradisi) polyphenols. Food Nutr. Res. 2016, 60, 31871. [Google Scholar] [CrossRef] [PubMed]
- Karapetsas, A.; Voulgaridou, G.P.; Iliadi, D.; Tsochantaridis, I.; Michail, P.; Kynigopoulos, S.; Lambropoulou, M.; Stavropoulou, M.I.; Stathopoulou, K.; Karabournioti, S.; et al. Honey Extracts Exhibit Cytoprotective Properties against UVB-Induced Photodamage in Human Experimental Skin Models. Antioxidants 2020, 9, 566. [Google Scholar] [CrossRef]
- Jo, K.; Bae, G.Y.; Cho, K.; Park, S.S.; Suh, H.J.; Hong, K.B. An Anthocyanin-Enriched Extract from Vaccinium uliginosum Improves Signs of Skin Aging in UVB-Induced Photodamage. Antioxidants 2020, 9, 844. [Google Scholar] [CrossRef]
- Chen, Y.S.; Liu, H.M.; Lee, T.Y. Ursodeoxycholic Acid Regulates Hepatic Energy Homeostasis and White Adipose Tissue Macrophages Polarization in Leptin-Deficiency Obese Mice. Cells 2019, 8, 253. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef]
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.M. Skin ageing. J. Eur. Acad. Derm. Venereol. 2011, 25, 873–884. [Google Scholar] [CrossRef]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Toutfaire, M.; Bauwens, E.; Debacq-Chainiaux, F. The impact of cellular senescence in skin ageing: A notion of mosaic and therapeutic strategies. Biochem. Pharm. 2017, 142, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.W.; Park, I.S.; Choi, G.S.; Shin, J. Role of fibroblast-derived factors in the pathogenesis of melasma. Clin. Exp. Derm. 2016, 41, 601–609. [Google Scholar] [CrossRef]
- Hirobe, T.; Hasegawa, K.; Furuya, R.; Fujiwara, R.; Sato, K. Effects of fibroblast-derived factors on the proliferation and differentiation of human melanocytes in culture. J. Derm. Sci. 2013, 71, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Cardinali, G.; Aspite, N.; Cota, C.; Luzi, F.; Bellei, B.; Briganti, S.; Amantea, A.; Torrisi, M.R.; Picardo, M. Role of fibroblast-derived growth factors in regulating hyperpigmentation of solar lentigo. Br. J. Derm. 2010, 163, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Salducci, M.; Andre, N.; Guere, C.; Martin, M.; Fitoussi, R.; Vie, K.; Cario-Andre, M. Factors secreted by irradiated aged fibroblasts induce solar lentigo in pigmented reconstructed epidermis. Pigment Cell Melanoma Res. 2014, 27, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Itami, S.; Watabe, H.; Yasumoto, K.; Abdel-Malek, Z.A.; Kubo, T.; Rouzaud, F.; Tanemura, A.; Yoshikawa, K.; Hearing, V.J. Mesenchymal-epithelial interactions in the skin: Increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J. Cell Biol. 2004, 165, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.E.; Kim, Y.; Kwon, S.; Kim, M.; Kim, Y.H.; Kim, J.H.; Park, T.J.; Kang, H.Y. Senescent fibroblasts drive ageing pigmentation: A potential therapeutic target for senile lentigo. Theranostics 2018, 8, 4620–4632. [Google Scholar] [CrossRef] [PubMed]

| Name | Accession Number | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|---|
| IL-1α | NM_000575.5 | AGGGCGTCATTCAGGATGAA | CGCCAATGACTCAGAGGAAGA |
| IL-8 | NM_000584.4 | AACCCTCTGCACCCAGTTTTC | ACTGAGAGTGATTGAGAGTGGAC |
| TNF-α | NM_000594.4 | AGCTGCCCCTCAGCTTGAG | CCCAGGGACCTCTCTCTAATCA |
| RPLP0 | NM_001002.4 | GGCGACCTGGAAGTCCAACT | CCATCAGCACCACAGCCTTC |
| MITF | NM_000248.4 | TCTACCGTCTCTCACTGGATTGG | GCTTTACCTGCTGCCGTTGG |
| Tyrosinase | NM_000372.5 | GGCCTCAATTTCCCTTCACA | CAGAGCACTGGCAGGTCCTAT |
| MMP-1 | NM_001145938.2 | CTCTGGAGTAATGTCACACCTCT | TGTTGGTCCACCTTTCATCTTC |
| MMP-3 | NM_002422.5 | CGGTTCCGCCTGTCTCAAG | CGCCAAAAGTGCCTGTCTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, I.J.; Yoo, H.; Paik, S.H.; Kim, H.T.; Kim, S.Y.; Song, Y.; Chang, S.E. Ursodeoxycholic Acid May Inhibit Environmental Aging-Associated Hyperpigmentation. Antioxidants 2021, 10, 267. https://doi.org/10.3390/antiox10020267
Moon IJ, Yoo H, Paik SH, Kim HT, Kim SY, Song Y, Chang SE. Ursodeoxycholic Acid May Inhibit Environmental Aging-Associated Hyperpigmentation. Antioxidants. 2021; 10(2):267. https://doi.org/10.3390/antiox10020267
Chicago/Turabian StyleMoon, Ik Jun, Hanju Yoo, Seung Hwan Paik, Hak Tae Kim, Su Yeon Kim, Youngsup Song, and Sung Eun Chang. 2021. "Ursodeoxycholic Acid May Inhibit Environmental Aging-Associated Hyperpigmentation" Antioxidants 10, no. 2: 267. https://doi.org/10.3390/antiox10020267
APA StyleMoon, I. J., Yoo, H., Paik, S. H., Kim, H. T., Kim, S. Y., Song, Y., & Chang, S. E. (2021). Ursodeoxycholic Acid May Inhibit Environmental Aging-Associated Hyperpigmentation. Antioxidants, 10(2), 267. https://doi.org/10.3390/antiox10020267





