Rebaudioside A Enhances Resistance to Oxidative Stress and Extends Lifespan and Healthspan in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Culture Conditions
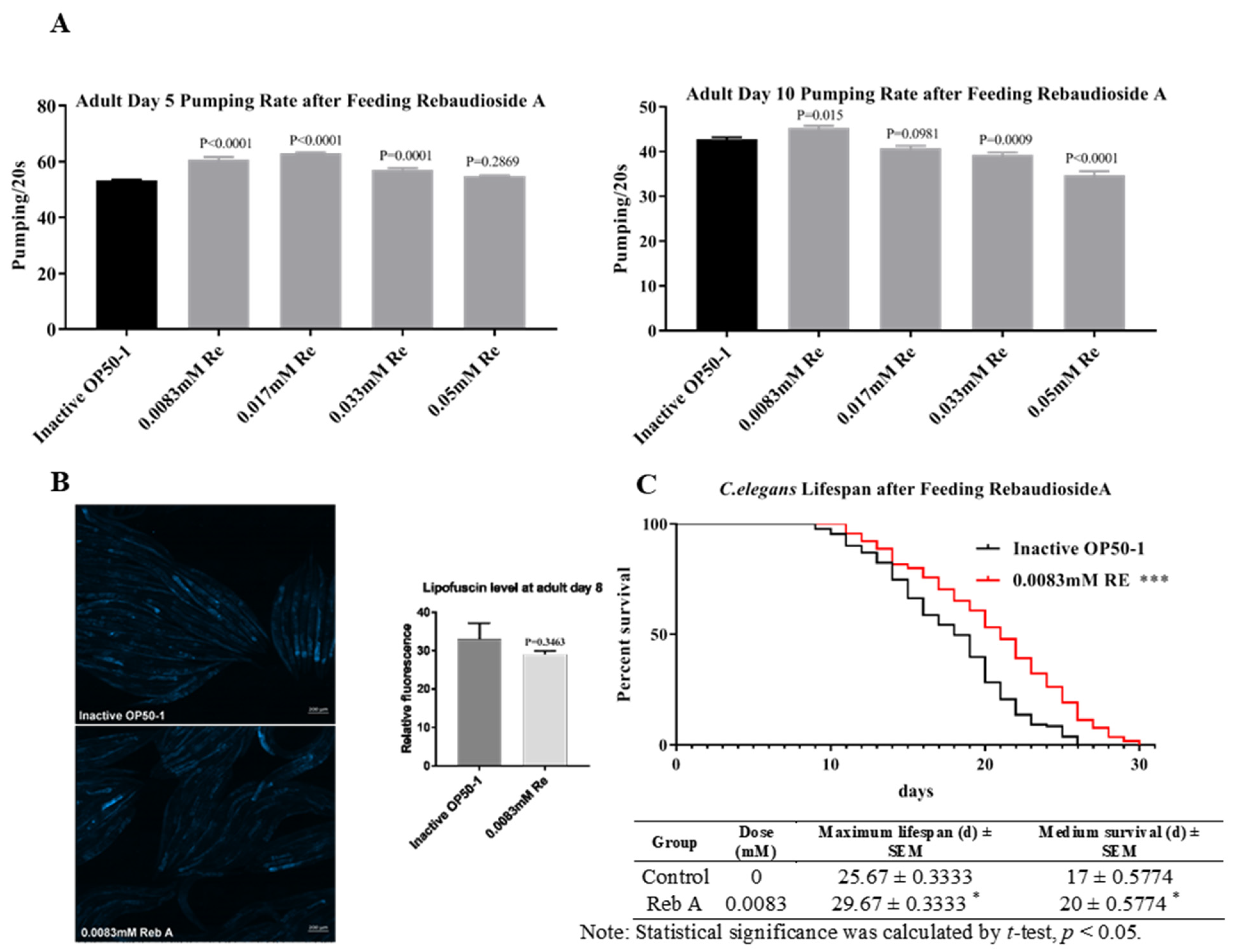
2.2. Pharyngeal Pumping Rate Assay
2.3. Lipofuscin Assay
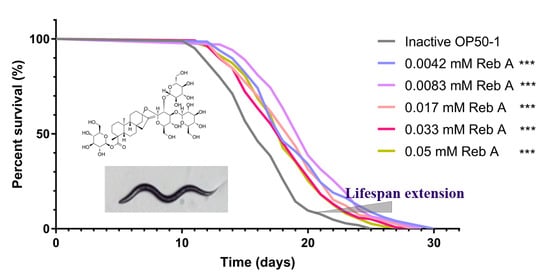
2.4. Lifespan Assay
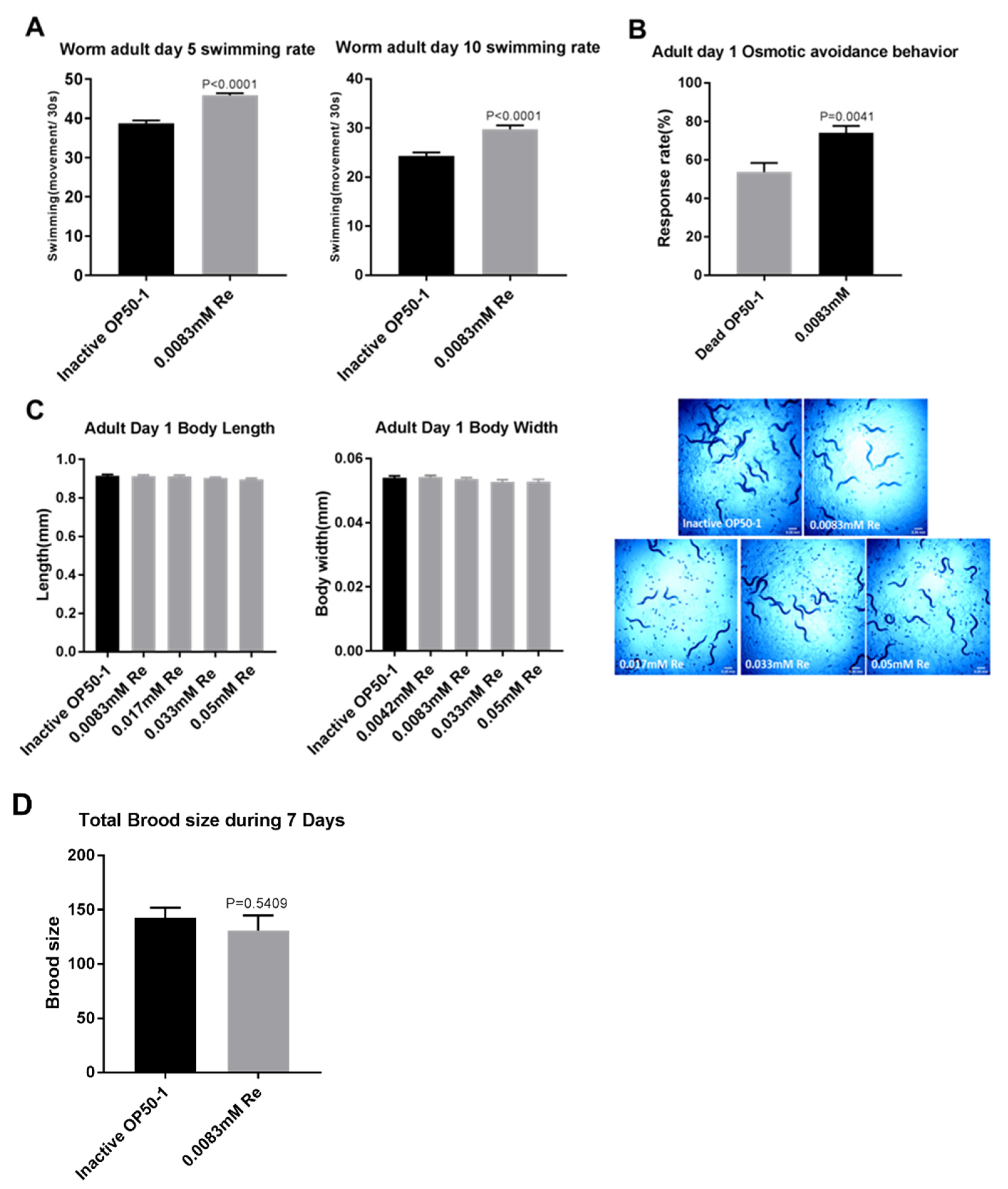
2.5. Swimming Assay
2.6. Osmotic Avoidance Assay
2.7. Brood Size Assay
2.8. Body Length and Body Width
2.9. Heat Stress Assay
2.10. Acute Oxidative Stress Assay
2.11. Accumulation of Cellular Reactive Oxygen Species (ROS)
2.12. Food Intake Assay
2.13. RNA Extraction and Transcriptome Analysis
2.14. Postfix Nile Red (NR) Staining
2.15. Oxygen Consumption Rate (OCR) Measurement
2.16. Malondialdehyde (MDA) Content Assay
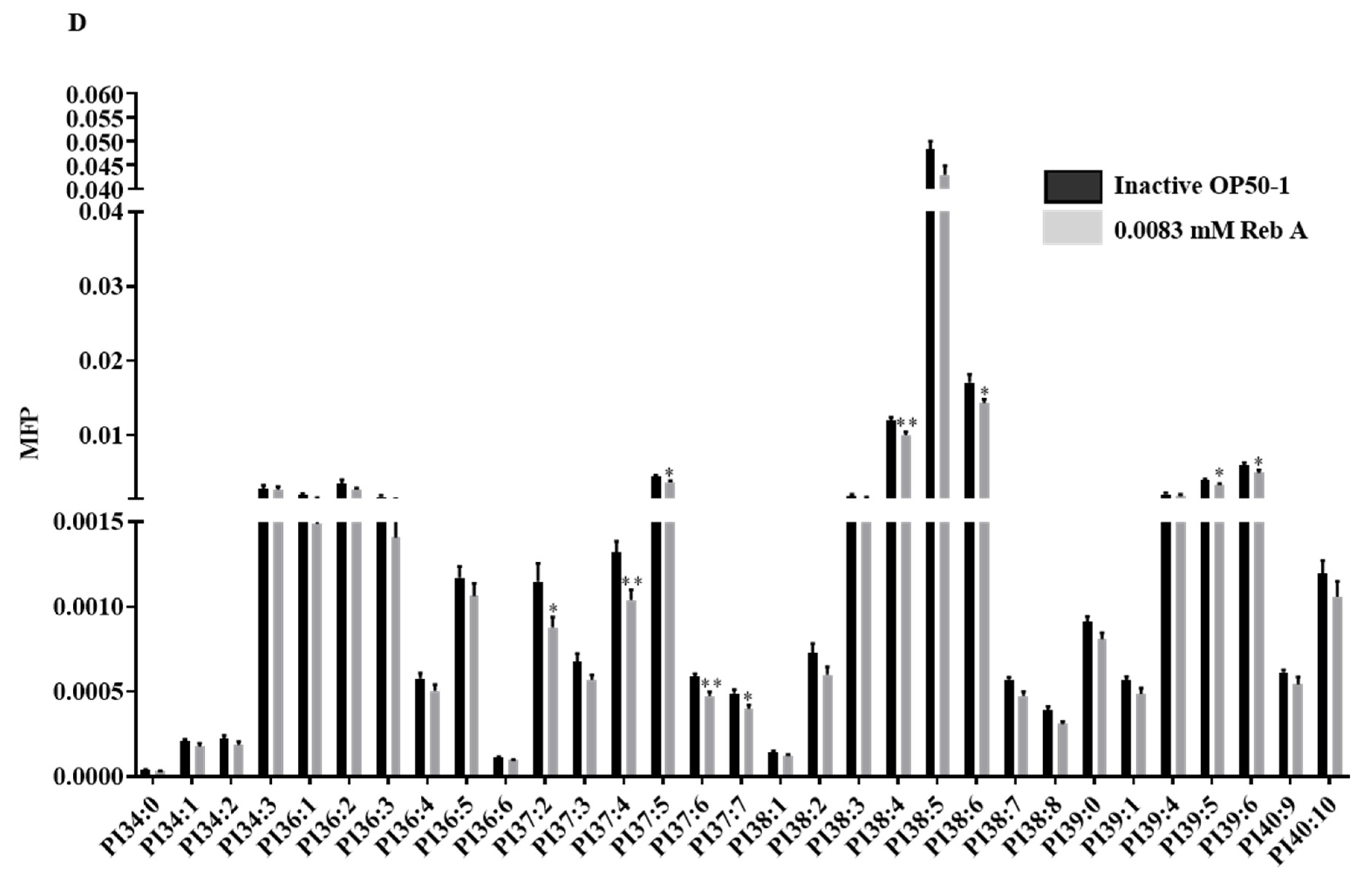
2.17. Lipid Extraction and MS Analysis
2.18. Statistical Analysis
3. Results
3.1. Reb A Extends Lifespan in C. elegans
3.2. Reb A Extends Healthspan in C. elegans
3.3. Reb A Enhances Resistance to Oxidative Stress in C. elegans
3.4. Reb A Inhibited the CeTOR Signaling Pathway
3.5. Reb A Supplementation Lowers Lipid Storage in C. elegans
3.6. Reb A Alters Lipid Metabolism in C. elegans
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pradhan, S.; Shah, U.H.; Mathur, A.; Sharma, S. Experimental evaluation of antipyretic and analgesic activities of aspartame reply. Indian J. Pharmacol. 2016, 43, 486–487. [Google Scholar]
- Walbolt, J.; Koh, Y. Non-nutritive sweeteners and their associations with obesity and Type 2 diabetes. J. Obes. Metab. Syndr. 2020, 29, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.Y.; Friel, J.K.; Mackay, D.S. Effect of sucralose and aspartame on glucose metabolism and gut hormones. Nutr. Rev. 2020, 78, 725–746. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A. Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk. Adv. Nutr. 2014, 5, 797–808. [Google Scholar] [CrossRef]
- Uebanso, T.; Ohnishi, A.; Kitayama, R.; Yoshimoto, A.; Nakahashi, M.; Shimohata, T.; Takahashi, A. Effects of low-dose non-caloric sweetener consumption on gut microbiota in mice. Nutrients 2017, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, A.; Gürtler, R.; Schäfer, B. Heating of food containing sucralose might result in the generation of potentially toxic chlorinated compounds. Food Chem. 2020, 321, 126700. [Google Scholar] [CrossRef]
- Alcántar-Fernández, J.; Navarro, R.E.; Salazar-Martínez, A.M.; Pérez-Andrade, M.E.; Miranda-Ríos, J. Caenorhabditis elegans respond to high-glucose diets through a network of stress-responsive transcription factors. PLoS ONE 2018, 13, e0199888. [Google Scholar] [CrossRef]
- Kundu, N.; Domingues, C.C.; Patel, J.; Aljishi, M.; Ahmadi, N.; Fakhri, M.; Sylvetsky, A.C.; Sen, S. Sucralose promotes accumulation of reactive oxygen species (ROS) and adipogenesis in mesenchymal stromal cells. Stem Cell Res. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Praveena, S.M.; Cheema, M.S.; Guo, H.-R. Non-nutritive artificial sweeteners as an emerging contaminant in environment: A global review and risks perspectives. Ecotoxicol. Environ. Saf. 2019, 170, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Prakash, I.; Chaturvedula, V.S.P. Steviol Glycosides: Natural Non-Caloric Sweeteners (Phytochemistry); Springer: Cham, Switzerland, 2016. [Google Scholar]
- Fitch, C.; Keim, K.S. Position of the academy of nutrition and dietetics: Use of nutritive and nonnutritive sweeteners. J. Acad. Nutr. Diet. 2012, 112, 739–758. [Google Scholar] [CrossRef]
- Ohta, M.; Sasa, S.; Inoue, A.; Tamai, T.; Fujita, I.; Morita, K.; Matsuura, F. Characterization of novel steviol glycosides from leaves of Stevia rebaudiana morita. J. Appl. Glycosci. 2010, 57, 199–209. [Google Scholar] [CrossRef]
- Chatsudthipong, V.; Muanprasat, C. Stevioside and related compounds: Therapeutic benefits beyond sweetness. Pharmacol. Ther. 2009, 121, 41–54. [Google Scholar] [CrossRef]
- Schiano, C.; Grimaldi, V.; Franzese, M.; Fiorito, C.; De Nigris, F.; Donatelli, F.; Soricelli, A.; Salvatore, M.; Napoli, C. Non-nutritional sweeteners effects on endothelial vascular function. Toxicol. In Vitro 2020, 62, 104694. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Cho, U.M.; Hwang, H.S. Anti-inflammation effect of Rebaudioside A by inhibition of the MAPK and NF-κB signal pathway in RAW264.7 macrophage. J. Appl. Biol. Chem. 2018, 61, 205–211. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, Y.; Zhu, X.; Jiang, M.; Song, E.; Song, Y. New application of the commercial sweetener rebaudioside a as a hepatoprotective candidate: Induction of the Nrf2 signaling pathway. Eur. J. Pharmacol. 2018, 822, 128–137. [Google Scholar] [CrossRef]
- Nettleton, J.E.; Klancic, T.; Schick, A.; Choo, A.C.; Shearer, J.; Borgland, S.L.; Chleilat, F.; Mayengbam, S.; Reimer, R.A. Low-dose Stevia (Rebaudioside A) consumption perturbs gut microbiota and the mesolimbic dopamine reward system. Nutrients 2019, 11, 1248. [Google Scholar] [CrossRef] [PubMed]
- Casas-Grajales, S.; Ramos-Tovar, E.; Chávez-Estrada, E.; Alvarez-Suarez, D.; Hernández-Aquino, E.; Reyes-Gordillo, K.; Cerda-García-Rojas, C.M.; Camacho, J.; Tsutsumi, V.; Lakshman, M.R.; et al. Antioxidant and immunomodulatory activity induced by stevioside in liver damage: In Vivo, In Vitro and in silico assays. Life Sci. 2019, 224, 187–196. [Google Scholar] [CrossRef]
- Xi, D.; Bhattacharjee, J.; Salazar-Gonzalez, R.-M.; Park, S.; Jang, A.; Warren, M.; Merritt, R.; Michail, S.; Bouret, S.; Kohli, R. Rebaudioside affords hepatoprotection ameliorating sugar sweetened beverage- induced nonalcoholic steatohepatitis. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–399. [Google Scholar] [CrossRef]
- O’Reilly, L.P.; Luke, C.J.; Perlmutter, D.H.; Silverman, G.A.; Pak, S.C. C. elegans in high-throughput drug discovery. Adv. Drug Deliv. Rev. 2014, 70, 247–253. [Google Scholar]
- Pincus, Z.; Mazer, T.C.; Slack, F.J. Autofluorescence as a measure of senescence in C. elegans: Look to red, not blue or green. Aging 2016, 8, 889–898. [Google Scholar] [CrossRef]
- Golegaonkar, S.; Tabrez, S.S.; Pandit, A.; Sethurathinam, S.; Jagadeeshaprasad, M.G.; Bansode, S.; Sampathkumar, S.G.; Mukhopadhyay, A. Rifampicin reduces advanced glycation end products and activates DAF-16 to increase lifespan in Caenorhabditis elegans. Aging Cell 2015, 14, 463–473. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Moribe, H.; Konakawa, R.; Koga, D.; Ushiki, T.; Nakamura, K.; Mekada, E. Tetraspanin is required for generation of reactive oxygen species by the dual oxidase system in Caenorhabditis elegans. PLoS Genet. 2012, 8, e1002957. [Google Scholar] [CrossRef]
- Ewald, C.Y. Redox Signaling of NADPH oxidases regulates oxidative stress responses, immunity and aging. Antioxidants 2018, 7, 130. [Google Scholar] [CrossRef]
- De Diego, I.; Peleg, S.; Fuchs, B.; Tamayo, I.D.D. The role of lipids in aging-related metabolic changes. Chem. Phys. Lipids 2019, 222, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, B.; Lam, S.-M.; Shui, G. Integration of lipidomics and metabolomics for in-depth understanding of cellular mechanism and disease progression. J. Genet. Genom. 2020, 47, 69–83. [Google Scholar] [CrossRef]
- Menon, D.; Salloum, D.; Bernfeld, E.; Gorodetsky, E.; Akselrod, A.; Frias, M.A.; Sudderth, J.; Chen, P.-H.; De Berardinis, R.; Foster, D.A.; et al. Lipid sensing by mTOR complexes via de novo synthesis of phosphatidic acid. J. Biol. Chem. 2017, 292, 6303–6311. [Google Scholar] [CrossRef] [PubMed]
- Shamalnasab, M.; Gravel, S.-P.; St-Pierre, J.; Breton, L.; Jäger, S.; Aguilaniu, H. A salicylic acid derivative extends the lifespan of Caenorhabditis elegans by activating autophagy and the mitochondrial unfolded protein response. Aging Cell 2018, 17, e12830. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-Q.; Ding, A.-J.; Li, G.-P.; Wu, G.-S.; Luo, H.-R. Drug absorption efficiency in Caenorhbditis elegans delivered by different methods. PLoS ONE 2013, 8, e56877. [Google Scholar] [CrossRef]
- Huang, C.; Xiong, C.; Kornfeld, K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2004, 101, 8084–8089. [Google Scholar] [CrossRef] [PubMed]
- Liao, V.H.-C.; Yu, C.-W.; Chu, Y.-J.; Li, W.-H.; Hsieh, Y.-C.; Wang, T.-T. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech. Ageing Dev. 2011, 132, 480–487. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, X.; Su, Z.; Xiao, J.; Lv, M.; Cao, Y.; Chen, Y. Carnosol improved lifespan and healthspan by promoting antioxidant capacity in Caenorhabditis elegans. Oxidative Med. Cell. Longev. 2019, 2019, 5958043. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.S.; Taylor, R.C.; Dillin, A. Chapter 12-analysis of aging in Caenorhabditis elegans. Methods Cell Biol. 2012, 107, 353–381. [Google Scholar] [PubMed]
- Fang, E.F.; Waltz, T.B.; Kassahun, H.; Lu, Q.; Kerr, J.S.; Morevati, M.; Fivenson, E.M.; Wollman, B.N.; Marosi, K.; Wilson, M.A.; et al. Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf2 pathway. Sci. Rep. 2017, 7, 46208. [Google Scholar] [CrossRef]
- Li, G.; Gong, J.; Lei, H.; Liu, J.; Xu, X.Z.S. Promotion of behavior and neuronal function by reactive oxygen species in C. elegans. Nat. Commun. 2016, 7, 13234. [Google Scholar] [CrossRef] [PubMed]
- Rangsinth, P.; Prasansuklab, A.; Duangjan, C.; Gu, X.; Meemon, K.; Wink, M.; Tencomnao, T. Leaf extract of Caesalpinia mimosoides enhances oxidative stress resistance and prolongs lifespan in Caenorhabditis elegans. BMC Complement. Altern. Med. 2019, 19, 164. [Google Scholar] [CrossRef] [PubMed]
- Farias-Pereira, R.; Park, Y. Cafestol increases fat oxidation and energy expenditure in Caenorhabditis elegans via DAF-12-dependent pathway. Food Chem. 2020, 3. [Google Scholar] [CrossRef]
- Rathor, L.; Pant, A.; Awasthi, H.; Mani, D.; Pandey, R. An antidiabetic polyherbal phytomedicine confers stress resistance and extends lifespan in Caenorhabditis elegans. Biogerontology 2017, 18, 131–147. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.J.; Zhang, A.H.; Zhang, N.; Sun, W.J. Liuwei Dihuang Wan, a traditional Chinese medicinal formula, protects against osteoporosis. Anal. Chim. Acta 2013, 4, 1–6. [Google Scholar]
- Sarasija, S.; Norman, K.R. Measurement of ROS in Caenorhabditis elegans using a reduced form of fluorescein. Bio-Protocol 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Amaro, R.L.; Valentine, E.R.; Carretero, M.; Leboeuf, S.E.; Rangaraju, S.; Broaddus, C.D.; Solis, G.M.; Williamson, J.R.; Petrascheck, M. Measuring food intake and nutrient absorption in Caenorhabditis elegans. Genetics 2015, 200, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2007, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- O’Rourke, E.J.; Soukas, A.A.; Carr, C.E.; Ruvkun, G. C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab. 2009, 10, 430–435. [Google Scholar]
- Koopman, M.; Michels, H.; Dancy, B.M.; Kamble, R.; Mouchiroud, L.; Auwerx, J.; Houtkooper, R.H. A screening-based platform for the assessment of cellular respiration in Caenorhabditis elegans. Nat. Protoc. 2016, 11, 1798–1816. [Google Scholar] [CrossRef]
- Lam, S.M.; Wang, Z.; Li, J.; Huang, X.; Shui, G. Sequestration of polyunsaturated fatty acids in membrane phospholipids of Caenorhabditis elegans dauer larva attenuates eicosanoid biosynthesis for prolonged survival. Redox Biol. 2017, 12, 967–977. [Google Scholar] [CrossRef]
- Lam, S.M.; Tong, L.; Reux, B.; Duan, X.; Petznick, A.; Yong, S.S.; Khee, C.B.S.; Lear, M.J.; Wenk, M.R.; Shui, G. Lipidomic analysis of human tear fluid reveals structure-specific lipid alterations in dry eye syndrome. J. Lipid Res. 2014, 55, 299–306. [Google Scholar] [CrossRef]
- Song, J.W.; Lam, S.M.; Fan, X.; Cao, W.J.; Wang, S.Y.; Tian, H.; Li, B.; Jiang, T.J.; Wang, R.; Shui, G.; et al. Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis. Cell Metab. 2020, 32, 188–202. [Google Scholar] [CrossRef]
- Diomede, L.; Rognoni, P.; Lavatelli, F.; Romeo, M.; Di Fonzo, A.; Foray, C.; Fiordaliso, F.; Palladini, G.; Valentini, V.; Perfetti, V.; et al. Investigating heart-specific toxicity of amyloidogenic immunoglobulin light chains: A lesson from C. elegans. Worm 2014, 3, e965590. [Google Scholar] [CrossRef][Green Version]
- Bansal, A.; Zhu, L.J.; Yen, K.; Tissenbaum, H.A. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc. Natl. Acad. Sci. USA 2015, 112, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Kim, H.; Di Tano, J.; Manion, D.; King, B.L.; Strange, K. Abnormal osmotic avoidance behavior in C. elegans is associated with increased hypertonic stress resistance and improved proteostasis. PLoS ONE 2016, 11, e0154156. [Google Scholar] [CrossRef]
- Lu, M.; Tan, L.; Zhou, X.G.; Yang, Z.L.; Zhu, Q.; Chen, J.N.; Wu, G.S. Secoisolariciresinol diglucoside delays the progression of aging-related diseases and extends the lifespan of Caenorhabditis elegans via DAF-16 and HSF-Oxid. Med. Cell Longev. 2020, 2020, 1293935. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, R.; Li, H.; Xiang, Y.; Xiao, L.; Hu, M.; Ma, F.; Ma, C.W.; Huang, Z. Antioxidant and neuroprotective effects of Dictyophora indusiata polysaccharide in Caenorhabditis elegans. J. Ethnopharmacol. 2016, 192, 413–422. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Yue, Y.; Zheng, J.; Park, Y. Caenorhabditis elegans: A convenient In Vivo model for assessing the impact of food bioactive compounds on obesity, aging, and Alzheimer’s disease. Annu. Rev. Food Sci. Technol. 2018, 9, 1–22. [Google Scholar] [CrossRef]
- Blackwell, T.K.; Sewell, A.K.; Wu, Z.; Han, M. TOR signaling in Caenorhabditis elegans development, metabolism, and aging. Genetics 2019, 213, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- Lapierre, L.R.; Hansen, M. Lessons from C. elegans: Signaling pathways for longevity. Trends Endocrinol. Metab. 2012, 23, 637–644. [Google Scholar] [CrossRef]
- Wang, X.; Lam, S.M.; Cao, M.; Wang, T.; Wang, Z.; Yu, M.; Li, B.; Yhang, H.; Ping, F.; Shui, G.; et al. Localized increases in CEPT1 and ATGL elevate plasmalogen phosphatidylcholines in HDLs contributing to atheroprotective lipid profiles in hyperglycemic GCK-MODY. Redox Biol. 2021, 40, 101855. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Wang, Z.; Li, B.; Shui, G. High-coverage lipidomics for functional lipid and pathway analyses. Anal. Chim. Acta 2021, 1147, 199–2101. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Zhou, T.; Li, J.; Zhang, S.; Chua, G.H.; Li, B.; Shui, G. A robust, integrated platform for comprehensive analyses of acyl-coenzyme As and acyl-carnitines revealed chain length-dependent disparity in fatty acyl metabolic fates across Drosophila development. Sci. Bull. 2020, 65, 1840–1848. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Wang, Z.; Lam, S.M.; Shui, G. Rebaudioside A Enhances Resistance to Oxidative Stress and Extends Lifespan and Healthspan in Caenorhabditis elegans. Antioxidants 2021, 10, 262. https://doi.org/10.3390/antiox10020262
Li P, Wang Z, Lam SM, Shui G. Rebaudioside A Enhances Resistance to Oxidative Stress and Extends Lifespan and Healthspan in Caenorhabditis elegans. Antioxidants. 2021; 10(2):262. https://doi.org/10.3390/antiox10020262
Chicago/Turabian StyleLi, Pan, Zehua Wang, Sin Man Lam, and Guanghou Shui. 2021. "Rebaudioside A Enhances Resistance to Oxidative Stress and Extends Lifespan and Healthspan in Caenorhabditis elegans" Antioxidants 10, no. 2: 262. https://doi.org/10.3390/antiox10020262
APA StyleLi, P., Wang, Z., Lam, S. M., & Shui, G. (2021). Rebaudioside A Enhances Resistance to Oxidative Stress and Extends Lifespan and Healthspan in Caenorhabditis elegans. Antioxidants, 10(2), 262. https://doi.org/10.3390/antiox10020262