Oxidative Stress in the Pathogenesis of Crohn’s Disease and the Interconnection with Immunological Response, Microbiota, External Environmental Factors, and Epigenetics
Abstract
1. Introduction
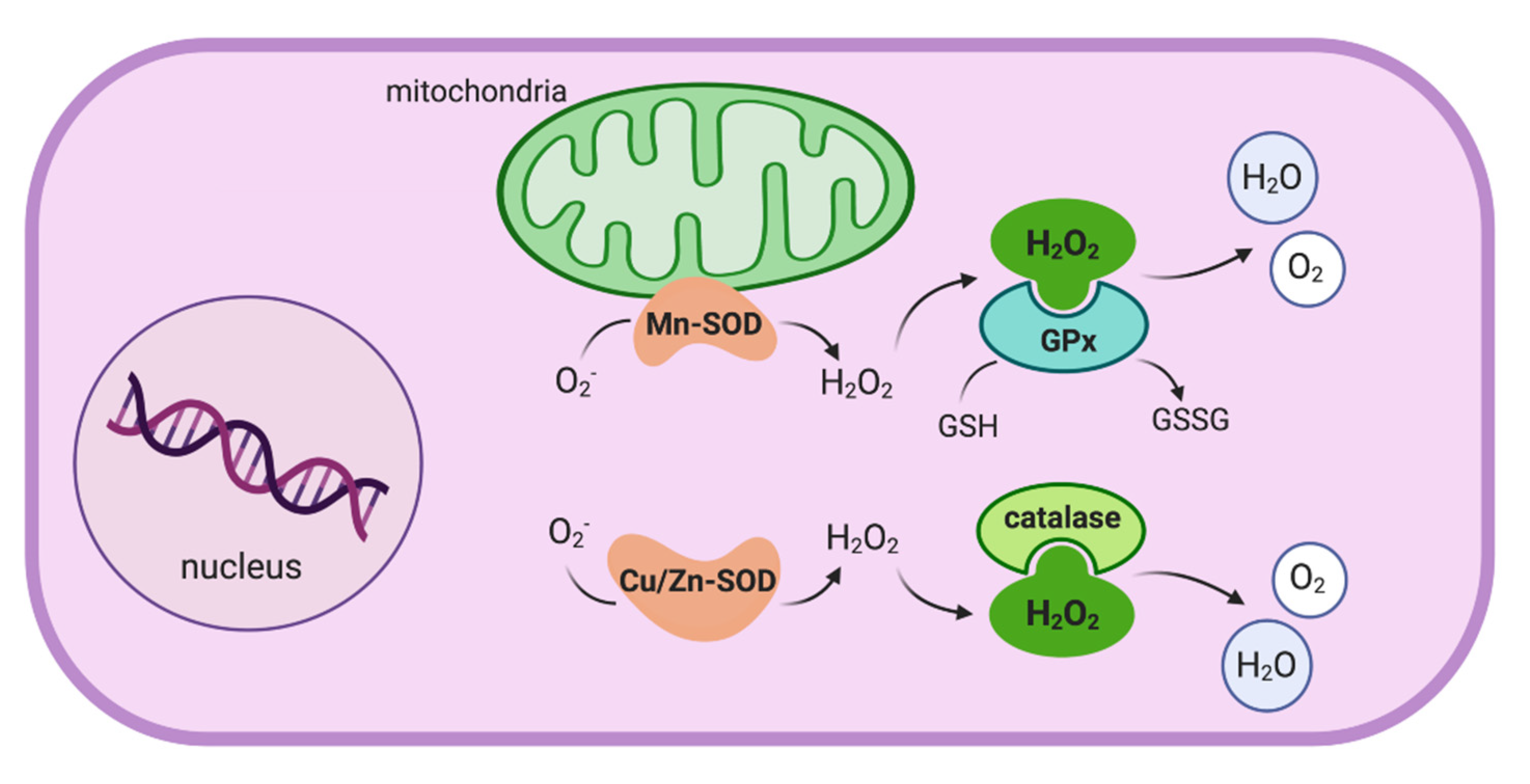
2. Oxidative Stress in Crohn’s Disease
2.1. Oxidative Stress and the Impaired Immunological Response
2.2. Oxidative Stress as a Key Effector Mechanism in CD Pathogenesis
3. The Role of the Environment in Crohn’s Disease
3.1. The “In-Vironment”: The Microbiota
3.2. External and Environmental Factors
3.2.1. Western Diet Versus the Mediterranean Diet and Their Impact on Crohn’s Disease
3.2.2. Other Lifestyle Factors and Health Conditions Relevant to the Pathogenesis
3.3. Epigenetics as a Transducer of Environmental Factors in Crohn’s Disease
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bernstein, C.N. Review article: Changes in the epidemiology of inflammatory bowel disease-clues for aetiology. Aliment. Pharmacol. Ther. 2017, 46, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Stappenbeck, T.S. Autophagy and intestinal homeostasis. Annu. Rev. Physiol. 2013, 75, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Moret, I.; Cerrillo, E.; Navarro-Puche, A.; Iborra, M.; Rausell, F.; Tortosa, L.; Beltrán, B. Oxidative stress in Crohn’s disease. Gastroenterol. Hepatol. 2014, 37, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Iborra, M.; Moret, I.; Rausell, F.; Bastida, G.; Aguas, M.; Cerrillo, E.; Nos, P.; Beltrán, B. Role of oxidative stress and antioxidant enzymes in Crohn’s disease. Biochem. Soc. Trans. 2011, 39, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Moret-Tatay, I.; Iborra, M.; Cerrillo, E.; Tortosa, L.; Nos, P.; Beltrán, B. Possible Biomarkers in Blood for Crohn’s Disease: Oxidative Stress and MicroRNAs-Current Evidences and Further Aspects to Unravel. Oxid. Med. Cell. Longev. 2016, 2016, 2325162. [Google Scholar] [CrossRef]
- Beltrán, B.; Nos, P.; Dasí, F.; Iborra, M.; Bastida, G.; Martínez, M.; OʼConnor, J.-E.; Sáez, G.; Moret, I.; Ponce, J. Mitochondrial dysfunction, persistent oxidative damage, and catalase inhibition in immune cells of naïve and treated Crohn’s disease. Inflamm. Bowel Dis. 2010, 16, 76–86. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Pereira, C.; Grácio, D.; Teixeira, J.P.; Magro, F. Oxidative Stress and DNA Damage: Implications in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y.R. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: Updated experimental and clinical evidence. Exp. Biol. Med. 2012, 237, 474–480. [Google Scholar] [CrossRef]
- Alzoghaibi, M.A. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 2013, 19, 6540. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Redox biology of the intestine. Free Radic. Res. 2011, 45, 1245–1266. [Google Scholar] [CrossRef] [PubMed]
- Kitahora, T.; Suzuki, K.; Asakura, H.; Yoshida, T.; Suematsu, M.; Watanabe, M.; Aiso, S.; Tsuchiya, M. Active oxygen species generated by monocytes and polymorphonuclear cells in Crohn’s disease. Dig. Dis. Sci. 1988, 33, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Raddatz, D.; Bockemühl, M.; Ramadori, G. Quantitative measurement of cytokine mRNA in inflammatory bowel disease: Relation to clinical and endoscopic activity and outcome. Eur. J. Gastroenterol. Hepatol. 2005, 17, 547–557. [Google Scholar] [CrossRef]
- Balmus, I.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3. [Google Scholar] [CrossRef]
- Kruidenier, L.; Kuiper, I.; van Duijn, W.; Mieremet-Ooms, M.A.; van Hogezand, R.A.; Lamers, C.B.; Verspaget, H.W. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J. Pathol. 2003, 201, 17–27. [Google Scholar] [CrossRef]
- D’Odorico, A.; Bortolan, S.; Cardin, R.; D’Inca’, R.; Martines, D.; Ferronato, A.; Sturniolo, G.C. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand. J. Gastroenterol. 2001, 36, 1289–1294. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Fichna, J. Review article: The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative stress and pathogenesis of inflammatory bowel disease: An epiphenomenon or the cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef]
- Yuksel, M.; Ates, I.; Kaplan, M.; Arikan, M.F.; Ozin, Y.O.; Kilic, Z.M.Y.; Topcuoglu, C.; Kayacetin, E. Is Oxidative Stress Associated with Activation and Pathogenesis of Inflammatory Bowel Disease? J. Med. Biochem. 2017, 36, 341–348. [Google Scholar] [CrossRef]
- Sido, B.; Hack, V.; Hochlehnert, A.; Lipps, H.; Herfarth, C.; Dröge, W. Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut 1998, 42, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Iantomasi, T.; Marraccini, P.; Favilli, F.; Vincenzini, M.T.; Ferretti, P.; Tonelli, F. Glutathione Metabolism in Crohn′s Disease. Biochem. Med. Metab. Biol. 1994, 53, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kruidenier, L.; Kuiper, I.; Lamers, C.B.H.W.; Verspaget, H.W. Intestinal oxidative damage in inflammatory bowel disease: Semi-quantification, localization, and association with mucosal antioxidants. J. Pathol. 2003, 201, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.A.S.; Lopes, M.S.-M.S.; Bastos, S.T.; Reigada, C.L.; Dantas, R.F.; Neto, J.C.; Luna, A.S.; Madi, K.; Nunes, T.; Zaltman, C. Does active Crohn’s disease have decreased intestinal antioxidant capacity? J. Crohn’s Colitis 2013, 7, e358–e366. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; von Martels, J.Z.H.; Bulthuis, M.L.C.; van Londen, M.; Faber, K.N.; Dijkstra, G.; van Goor, H. Crohn’s Disease in Clinical Remission Is Marked by Systemic Oxidative Stress. Front. Physiol. 2019, 10, 499. [Google Scholar] [CrossRef]
- Cosnes, J.; Gower–Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and Natural History of Inflammatory Bowel Diseases. Gastroenterology 2011, 140, 1785–1794.e4. [Google Scholar] [CrossRef]
- Chen, J.J.; Bertrand, H.; Yu, B.P. Inhibition of adenine nucleotide translocator by lipid peroxidation products. Free Radic. Biol. Med. 1995, 19, 583–590. [Google Scholar] [CrossRef]
- Catalá, A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids 2009, 157, 1–11. [Google Scholar] [CrossRef]
- Pelli, M.A.; Trovarelli, G.; Capodicasa, E.; De Medio, G.E.; Bassotti, G. Breath alkanes determination in ulcerative colitis and Crohnʼs disease. Dis. Colon Rectum 1999, 42, 71–76. [Google Scholar] [CrossRef]
- Sampietro, G.M.; Cristaldi, M.; Cervato, G.; Maconi, G.; Danelli, P.; Cervellione, R.; Rovati, M.; Bianchi Porro, G.; Cestaro, B.; Taschieri, A.M. Oxidative stress, vitamin A and vitamin E behaviour in patients submitted to conservative surgery for complicated Crohn’s disease. Dig. Liver Dis. 2002, 34, 696–701. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Yang, H.; Shao, D.; Zhao, X.; Zhang, G. Intraperitoneal injection of 4-hydroxynonenal (4-HNE), a lipid peroxidation product, exacerbates colonic inflammation through activation of Toll-like receptor 4 signaling. Free Radic. Biol. Med. 2019, 131, 237–242. [Google Scholar] [CrossRef]
- Szczeklik, K.; Krzyściak, W.; Cibor, D.; Domagała-Rodacka, R.; Pytko-Polończyk, J.; Mach, T.; Owczarek, D. Markers of lipid peroxidation and antioxidant status in the serum and saliva of patients with active Crohn disease. Pol. Arch. Intern. Med. 2018, 128, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Sedelnikova, O.A.; Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Georgakilas, A.G.; Bonner, W.M. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat. Res. 2010, 704, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313 Pt 1, 17–29. [Google Scholar] [CrossRef]
- Dean, R.T.; Fu, S.; Stocker, R.; Davies, M.J. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 1997, 324 Pt 1, 1–18. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Neubauer, K.; Berdowska, I.; Boehm, D.; Zielinski, B.; Petryszyn, P.; Terlecki, G.; Paradowski, L.; Gamian, A. Enhanced formation of advanced oxidation protein products in IBD. Inflamm. Bowel Dis. 2008, 14, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.A.; Neubauer, K. Oxidative Stress Markers in Inflammatory Bowel Diseases: Systematic Review. Diagnostics 2020, 10, 601. [Google Scholar] [CrossRef]
- Biddlestone, J.; Bandarra, D.; Rocha, S. The role of hypoxia in inflammatory disease (review). Int. J. Mol. Med. 2015, 35, 859–869. [Google Scholar] [CrossRef]
- Cummins, E.P.; Crean, D. Hypoxia and inflammatory bowel disease. Microbes Infect. 2017, 19, 210–221. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 1–18. [Google Scholar] [CrossRef]
- Dryden, G.W.; Deaciuc, I.; Arteel, G.; McClain, C.J. Clinical implications of oxidative stress and antioxidant therapy. Curr. Gastroenterol. Rep. 2005, 7, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Luceri, C.; Bigagli, E.; Agostiniani, S.; Giudici, F.; Zambonin, D.; Scaringi, S.; Ficari, F.; Lodovici, M.; Malentacchi, C. Analysis of Oxidative Stress-Related Markers in Crohn’s Disease Patients at Surgery and Correlations with Clinical Findings. Antioxidants 2019, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Herszenyi, L.; Miheller, P.; Tulassay, Z. Carcinogenesis in inflammatory bowel disease. Dig. Dis. 2007, 25, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Fiocchi, C. Genes and “in-vironment”: How will our concepts on the pathophysiology of inflammatory bowel disease develop in the future? Dig. Dis. 2012, 30 (Suppl. 3), 2–11. [Google Scholar] [CrossRef]
- Biasi, F.; Leonarduzzi, G.; Oteiza, P.I.; Poli, G. Inflammatory Bowel Disease: Mechanisms, Redox Considerations, and Therapeutic Targets. Antioxid. Redox Signal. 2013, 19, 1711–1747. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, G.-Z.; Zhou, J.-Q.; Xia, B.-Q.; Wang, X.-Y.; Jiang, B. Serum antibodies to microbial antigens for Crohn’s disease progression: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2014, 26, 733–742. [Google Scholar] [CrossRef]
- Sheehan, D.; Moran, C.; Shanahan, F. The microbiota in inflammatory bowel disease. J. Gastroenterol. 2015, 50, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Roy, B.C.; Khan, S.A.; Septer, S.; Umar, S. Microbiome, Metabolome and Inflammatory Bowel Disease. Microorganisms 2016, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Mazzola, M.; Leone, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Gerges Geagea, A.; Jurjus, A.; et al. Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed. Pap. 2016, 160, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Yu, B. The Bidirectional Interactions between Resveratrol and Gut Microbiota: An Insight into Oxidative Stress and Inflammatory Bowel Disease Therapy. BioMed Res. Int. 2019, 2019, 5403761. [Google Scholar] [CrossRef]
- Knights, D.; Lassen, K.G.; Xavier, R.J. Advances in inflammatory bowel disease pathogenesis: Linking host genetics and the microbiome. Gut 2013, 62, 1505–1510. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Rapozo, D.C.M.; Bernardazzi, C.; de Souza, H.S.P. Diet and microbiota in inflammatory bowel disease: The gut in disharmony. World J. Gastroenterol. 2017, 23, 2124. [Google Scholar] [CrossRef]
- Khanna, S.; Raffals, L.E. The Microbiome in Crohn’s Disease. Gastroenterol. Clin. N. Am. 2017, 46, 481–492. [Google Scholar] [CrossRef]
- The International IBD Genetics Consortium (IIBDGC); Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Philip Schumm, L.; Sharma, Y.; et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef]
- Becker, C.; Neurath, M.F.; Wirtz, S. The Intestinal Microbiota in Inflammatory Bowel Disease. ILAR J. 2015, 56, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Hampe, J.; Franke, A.; Rosenstiel, P.; Till, A.; Teuber, M.; Huse, K.; Albrecht, M.; Mayr, G.; De La Vega, F.M.; Briggs, J.; et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 2007, 39, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Parkes, M.; Barrett, J.C.; Prescott, N.J.; Tremelling, M.; Anderson, C.A.; Fisher, S.A.; Roberts, R.G.; Nimmo, E.R.; Cummings, F.R.; Soars, D.; et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat. Genet. 2007, 39, 830–832. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Bonen, D.K.; Inohara, N.; Nicolae, D.L.; Chen, F.F.; Ramos, R.; Britton, H.; Moran, T.; Karaliuskas, R.; Duerr, R.H.; et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001, 411, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Hugot, J.P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cézard, J.P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Harder, J.; Weichenthal, M.; Schwab, M.; Schäffeler, E.; Schlee, M.; Herrlinger, K.R.; Stallmach, A.; Noack, F.; Fritz, P.; et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut 2004, 53, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; Robertson, C.E.; Hamm, C.M.; Kpadeh, Z.; Zhang, T.; Chen, H.; Zhu, W.; Sartor, R.B.; Boedeker, E.C.; Harpaz, N.; et al. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 179–184. [Google Scholar] [CrossRef]
- Li, E.; Hamm, C.M.; Gulati, A.S.; Sartor, R.B.; Chen, H.; Wu, X.; Zhang, T.; Rohlf, F.J.; Zhu, W.; Gu, C.; et al. Inflammatory bowel diseases phenotype, C. difficile and NOD2 genotype are associated with shifts in human ileum associated microbial composition. PLoS ONE 2012, 7, e26284. [Google Scholar] [CrossRef]
- Cooney, R.; Baker, J.; Brain, O.; Danis, B.; Pichulik, T.; Allan, P.; Ferguson, D.J.P.; Campbell, B.J.; Jewell, D.; Simmons, A. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat. Med. 2010, 16, 90–97. [Google Scholar] [CrossRef]
- Shaw, M.H.; Kamada, N.; Warner, N.; Kim, Y.-G.; Nuñez, G. The ever-expanding function of NOD2: Autophagy, viral recognition, and T cell activation. Trends Immunol. 2011, 32, 73–79. [Google Scholar] [CrossRef]
- Chu, H.; Khosravi, A.; Kusumawardhani, I.P.; Kwon, A.H.K.; Vasconcelos, A.C.; Cunha, L.D.; Mayer, A.E.; Shen, Y.; Wu, W.-L.; Kambal, A.; et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science 2016, 352, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Hedin, C.R.; McCarthy, N.E.; Louis, P.; Farquharson, F.M.; McCartney, S.; Taylor, K.; Prescott, N.J.; Murrells, T.; Stagg, A.J.; Whelan, K.; et al. Altered intestinal microbiota and blood T cell phenotype are shared by patients with Crohn’s disease and their unaffected siblings. Gut 2014, 63, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Willing, B.P.; Dicksved, J.; Halfvarson, J.; Andersson, A.F.; Lucio, M.; Zheng, Z.; Järnerot, G.; Tysk, C.; Jansson, J.K.; Engstrand, L. A Pyrosequencing Study in Twins Shows That Gastrointestinal Microbial Profiles Vary with Inflammatory Bowel Disease Phenotypes. Gastroenterology 2010, 139, 1844–1854.e1. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wu, L.; Xu, W.; Zhou, X.; Ye, K.; Xiong, H.; Song, C.; Xie, Y. Correlation between antibiotic use in childhood and subsequent inflammatory bowel disease: A systematic review and meta-analysis. Scand. J. Gastroenterol. 2020, 55, 301–311. [Google Scholar] [CrossRef]
- Dutta, A.K. Influence of environmental factors on the onset and course of inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 1088. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Ng, S.C. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology 2017, 152, 313–321.e2. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Shen, J. Role of environmental factors in the pathogenesis of Crohn’s disease: A critical review. Int. J. Colorectal Dis. 2019, 34, 2023–2034. [Google Scholar] [CrossRef]
- Rogler, G.; Vavricka, S. Exposome in IBD: Recent Insights in Environmental Factors that Influence the Onset and Course of IBD. Inflamm. Bowel Dis. 2015, 21, 400–408. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary Intake and Risk of Developing Inflammatory Bowel Disease: A Systematic Review of the Literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Ruemmele, F.M. Role of Diet in Inflammatory Bowel Disease. Ann. Nutr. Metab. 2016, 68, 32–41. [Google Scholar] [CrossRef]
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E. coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Sáez-González, E.; Mateos, B.; López-Muñoz, P.; Iborra, M.; Moret, I.; Nos, P.; Beltrán, B. Bases for the Adequate Development of Nutritional Recommendations for Patients with Inflammatory Bowel Disease. Nutrients 2019, 11, 1062. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef]
- Saeedi, B.J.; Kao, D.J.; Kitzenberg, D.A.; Dobrinskikh, E.; Schwisow, K.D.; Masterson, J.C.; Kendrick, A.A.; Kelly, C.J.; Bayless, A.J.; Kominsky, D.J.; et al. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol. Biol. Cell 2015, 26, 2252–2262. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; van der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; van Seuningen, I.; Renes, I.B. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef]
- Nielsen, D.S.G.; Jensen, B.B.; Theil, P.K.; Nielsen, T.S.; Knudsen, K.E.B.; Purup, S. Effect of butyrate and fermentation products on epithelial integrity in a mucus-secreting human colon cell line. J. Funct. Foods 2018, 40, 9–17. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.R.; Cantarel, B.L.; Lamendella, R.; Darzi, Y.; Mongodin, E.F.; Pan, C.; Shah, M.; Halfvarson, J.; Tysk, C.; Henrissat, B.; et al. Integrated Metagenomics/Metaproteomics Reveals Human Host-Microbiota Signatures of Crohn’s Disease. PLoS ONE 2012, 7, e49138. [Google Scholar] [CrossRef] [PubMed]
- Geirnaert, A.; Calatayud, M.; Grootaert, C.; Laukens, D.; Devriese, S.; Smagghe, G.; De Vos, M.; Boon, N.; Van de Wiele, T. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci. Rep. 2017, 7, 11450. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Polyunsaturated fatty acids and inflammation: PUFA and Inflammation. IUBMB Life 2015, 67, 659–667. [Google Scholar] [CrossRef]
- Shoda, R.; Matsueda, K.; Yamato, S.; Umeda, N. Epidemiologic analysis of Crohn disease in Japan: Increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am. J. Clin. Nutr. 1996, 63, 741–745. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Mittmann, U.; Bar-Meir, S.; D’Haens, G.; Bradette, M.; Cohen, A.; Dallaire, C.; Ponich, T.P.; McDonald, J.W.D.; et al. Omega-3 Free Fatty Acids for the Maintenance of Remission in Crohn Disease: The EPIC Randomized Controlled Trials. JAMA 2008, 299, 1690. [Google Scholar] [CrossRef]
- Lev-Tzion, R.; Griffiths, A.M.; Ledder, O.; Turner, D. Omega 3 fatty acids (fish oil) for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2014, CD006320. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Wang, Y.; Huang, C.; Fu, Y.; Li, J.; Wang, H.; Jia, X.; Ba, Q. Effect of Long-Term Intake of Dietaryint Titanium Dioxide Nanoparticles on Intestine Inflammation in Mice. J. Agric. Food Chem. 2019, 67, 9382–9389. [Google Scholar] [CrossRef] [PubMed]
- Reddavide, R.; Rotolo, O.; Caruso, M.G.; Stasi, E.; Notarnicola, M.; Miraglia, C.; Nouvenne, A.; Meschi, T.; De’ Angelis, G.L.; Di Mario, F.; et al. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Bio-Med. Atenei Parm. 2018, 89, 60–75. [Google Scholar] [CrossRef]
- D’Souza, S.; Levy, E.; Mack, D.; Israel, D.; Lambrette, P.; Ghadirian, P.; Deslandres, C.; Morgan, K.; Seidman, E.G.; Amre, D.K. Dietary patterns and risk for Crohnʼs disease in children. Inflamm. Bowel Dis. 2008, 14, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genomics 2013, 7, 24. [Google Scholar] [CrossRef]
- Nagpal, R.; Shively, C.A.; Appt, S.A.; Register, T.C.; Michalson, K.T.; Vitolins, M.Z.; Yadav, H. Gut Microbiome Composition in Non-human Primates Consuming a Western or Mediterranean Diet. Front. Nutr. 2018, 5, 28. [Google Scholar] [CrossRef]
- Oliveras-López, M.-J.; Berná, G.; Jurado-Ruiz, E.; López-García de la Serrana, H.; Martín, F. Consumption of extra-virgin olive oil rich in phenolic compounds has beneficial antioxidant effects in healthy human adults. J. Funct. Foods 2014, 10, 475–484. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology 2019, 157, 647–659.e4. [Google Scholar] [CrossRef]
- To, N.; Gracie, D.J.; Ford, A.C. Systematic review with meta-analysis: The adverse effects of tobacco smoking on the natural history of Crohn’s disease. Aliment. Pharmacol. Ther. 2016, 43, 549–561. [Google Scholar] [CrossRef]
- Benjamin, J.L.; Hedin, C.R.H.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Prescott, N.J.; Pessoa-Lopes, P.; Mathew, C.G.; Sanderson, J.; Hart, A.L.; et al. Smokers with active Crohnʼs disease have a clinically relevant dysbiosis of the gastrointestinal microbiota *. Inflamm. Bowel Dis. 2012, 18, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, V.; Grondin, V.; Rajca, S.; Maubert, M.-A.; Pigneur, B.; Thomas, G.; Trugnan, G.; Beaugerie, L.; Cosnes, J.; Masliah, J.; et al. Current smoking differentially affects blood mononuclear cells from patients with crohnʼs disease and ulcerative colitis: Relevance to its adverse role in the disease. Inflamm. Bowel Dis. 2012, 18, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, L.; Schultz, B.M.; Salazar, G.A.; Pardo-Roa, C.; Sebastián, V.P.; Álvarez-Lobos, M.M.; Bueno, S.M. Impact of Cigarette Smoking on the Gastrointestinal Tract Inflammation: Opposing Effects in Crohn’s Disease and Ulcerative Colitis. Front. Immunol. 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Yanbaeva, D.G.; Dentener, M.A.; Creutzberg, E.C.; Wesseling, G.; Wouters, E.F.M. Systemic Effects of Smoking. Chest 2007, 131, 1557–1566. [Google Scholar] [CrossRef]
- Mateos, B.; Palanca-Ballester, C.; Saez-Gonzalez, E.; Moret, I.; Lopez, A.; Sandoval, J. Epigenetics of Inflammatory Bowel Disease: Unraveling Pathogenic Events. Crohns Colitis 360 2019, 1, otz017. [Google Scholar] [CrossRef]
- Dawson, M.A.; Kouzarides, T. Cancer Epigenetics: From Mechanism to Therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef]
- Scarpa, M.; Stylianou, E. Epigenetics: Concepts and relevance to IBD pathogenesis. Inflamm. Bowel Dis. 2012, 18, 1982–1996. [Google Scholar] [CrossRef]
- Ventham, N.T.; Kennedy, N.A.; Nimmo, E.R.; Satsangi, J. Beyond Gene Discovery in Inflammatory Bowel Disease: The Emerging Role of Epigenetics. Gastroenterology 2013, 145, 293–308. [Google Scholar] [CrossRef]
- Wawrzyniak, M.; Scharl, M. Genetics and epigenetics of inflammatory bowel disease. Swiss Med. Wkly. 2018, 148, w14671. [Google Scholar] [CrossRef]
- Moret-Tatay, I.; Cerrillo, E.; Sáez-González, E.; Hervás, D.; Iborra, M.; Sandoval, J.; Busó, E.; Tortosa, L.; Nos, P.; Beltrán, B. Identification of Epigenetic Methylation Signatures With Clinical Value in Crohnʼs Disease. Clin. Transl. Gastroenterol. 2019, 10, e00083. [Google Scholar] [CrossRef]
- Nimmo, E.R.; Prendergast, J.G.; Aldhous, M.C.; Kennedy, N.A.; Henderson, P.; Drummond, H.E.; Ramsahoye, B.H.; Wilson, D.C.; Semple, C.A.; Satsangi, J. Genome-wide methylation profiling in Crohn’s disease identifies altered epigenetic regulation of key host defense mechanisms including the Th17 pathway. Inflamm. Bowel Dis. 2012, 18, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Somineni, H.K.; Venkateswaran, S.; Kilaru, V.; Marigorta, U.M.; Mo, A.; Okou, D.T.; Kellermayer, R.; Mondal, K.; Cobb, D.; Walters, T.D.; et al. Blood-Derived DNA Methylation Signatures of Crohn’s Disease and Severity of Intestinal Inflammation. Gastroenterology 2019, 156, 2254–2265.e3. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Loddo, I.; Romano, C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front. Immunol. 2015, 6, 551. [Google Scholar] [CrossRef] [PubMed]
- Kalla, R.; Ventham, N.T.; Kennedy, N.A.; Quintana, J.F.; Nimmo, E.R.; Buck, A.H.; Satsangi, J. MicroRNAs: New players in IBD. Gut 2015, 64, 504–517. [Google Scholar] [CrossRef]
- Silva, J.P.B.; Navegantes-Lima, K.C.; de Oliveira, A.L.B.; Rodrigues, D.V.S.; Gaspar, S.L.F.; Monteiro, V.V.S.; Moura, D.P.; Monteiro, M.C. Protective Mechanisms of Butyrate on Inflammatory Bowel Disease. Curr. Pharm. Des. 2019, 24, 4154–4166. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol. Cell 2016, 64, 982–992. [Google Scholar] [CrossRef]
- Kreuz, S.; Fischle, W. Oxidative stress signaling to chromatin in health and disease. Epigenomics 2016, 8, 843–862. [Google Scholar] [CrossRef]
- Niu, Y.; DesMarais, T.L.; Tong, Z.; Yao, Y.; Costa, M. Oxidative stress alters global histone modification and DNA methylation. Free Radic. Biol. Med. 2015, 82, 22–28. [Google Scholar] [CrossRef]
- Kelly, D.; Kotliar, M.; Woo, V.; Jagannathan, S.; Whitt, J.; Moncivaiz, J.; Aronow, B.J.; Dubinsky, M.C.; Hyams, J.S.; Markowitz, J.F.; et al. Microbiota-sensitive epigenetic signature predicts inflammation in Crohn’s disease. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Rogler, G.; Biedermann, L.; Scharl, M. New insights into the pathophysiology of inflammatory bowel disease: Microbiota, epigenetics and common signalling pathways. Swiss Med. Wkly. 2018, 148, w14599. [Google Scholar] [CrossRef] [PubMed]

| Study | Type of Study | Methodology | Main Findings |
|---|---|---|---|
| Agus et al. (2016) [81] | In vivo study | WT and CEABAC10 mice 1 were fed with a high-fat/high-sugar diet (HF/HS) (N = 6) vs. a conventional diet (N = 5) over a period of 18 weeks. Germ-Free (GF) mice were transplanted with fecal pellets of HF/HS donor mice (N = 5) or conventional donor mice (N = 5), followed by an infection with an adherent-invasive E. coli (AEIC) LF82 strain isolated from a CD patient | Western diet causes an inflammatory environment in the digestive tract associated with microbiome perturbations; favors the emergence of E. coli associated with the ileal, cecal, and colonic mucosa; and decreases the level of SCFA produced by intestinal microbiota modulating immune response. Transplantation of feces from HF/HS treated mice to GF mice increases susceptibility to AIEC infection |
| Martinez-Medina, M. et al. (2014) [82] | In vivo study | WT and CEABAC10 mice 1 were fed with a HF/HS diet vs. conventional diet for 12 weeks 2, and orally infected with AIEC strain LF82 | Western diet induces changes in gut microbiota composition with an increase in the mucin-degrading bacterium Ruminococcus torques and the group Bacteroides/Prevotella; alters intestinal permeability, decreases barrier function, and affects the host homeostasis promoting AEIC gut colonization in genetically susceptible mice |
| Geirnaert et al. (2017) [94] | Clinical research—In vitro study | Butyrate-producing bacteria supplemented to the fecal microbial communities of CD patients (N = 10) in an in vitro system simulating the mucus- and lumen-associated microbiota, and an in vitro study of the resulting microbiota influence on epithelial barrier integrity with a Caco-2 model | In vitro supplementation of microbiota of CD patients with butyrate-producing bacteria results in a higher butyrate production, with and enhanced epithelial barrier integrity in a Caco-2 model. Supports the preclinical development of a probiotic product containing butyrate-producing species |
| Desai et al. (2016) [95] | In vivo study | Assembled synthetic gut microbiota from fully sequenced human gut bacteria in gnotobiotic mice were fed with fiber-rich vs. fiber-free diets 2 | In the chronic or intermittent absence of dietary fiber mucolytic bacteria become the predominant species within the gut microbiota with the consequent degradation of the colonic mucus layer and increased pathogen susceptibility |
| Chassaing et al. (2015) [101] | In vivo study | WT mice and two engineered strains of mice, namely IL10−/− and TLR5−/− (prone to develop shifts in microbiota composition and inflammation) exposed to emulsifiers in the drinking water or to water alone (control group) for 12 weeks 2 | Relatively low concentrations of commonly used dietary emulsifiers (carboxymethylcellulose and polysorbate-80) can disturb the host-microbiota relationship, induce low-grade inflammation and obesity/metabolic syndrome in WT hosts and promote robust colitis in mice predisposed to this disorder |
| Mu et al. (2019) [102] | In vivo study | WT mice and DSS-induced colitis mice treated with titanium dioxide nanoparticles vs. standard (control) diet for 3 months from weaning 2 | First demonstration that long-term dietary intake of titanium dioxide nanoparticles (which are used as food additives) results in lower body weigh along with colorectal inflammation in mice; and it aggravates DSS-induced chronic colitis and immune response in vivo, reduces the population of CD4+ T cells, regulatory T cells, and macrophages in mesenteric lymph node |
| Marlow, G. et al. (2013) [105] | Clinical research | 6-week intervention with a Mediterranean-inspired diet in CD patients (N = 8). Obtention of blood and fecal samples at the beginning and the end of the diet | A Mediterranean-inspired diet appears to benefit the health of CD patients: shows a trend for reducing inflammation and normalizing the microbiota |
| To, N. et al. (2016) [110] | Systematic review with metanalysis of the effects of smoking on disease course in CD | Search of MEDLINE, EMBASE and EMBASE classic carried out up to July 2015 (with the resulting 33 eligible studies) | Smokers, compared with non-smokers, have 55–85% higher rates of flares of disease activity, clinical recurrence rates after surgery that are two-fold higher, between 54% and 68% higher rates of need for first surgery, and are twice as likely to need a second operation. Quitting smoking appears to have a beneficial effect on CD course, especially for flare of disease activity or need for a second operation |
| Benjamin, J.L. et al. (2012) [111] | Clinical research | Fecal samples from patients with active CD (N = 101; 29 of whom current smokers) and healthy controls (N = 66; 8 of whom current smokers) were analyzed by fluorescent in situ hybridization (using probes targeting 16S rRNA of bacteria previously shown to be altered in active CD) | Smokers with active CD have a clinically relevant dysbiosis of the gastrointestinal microbiota; with strong and significant associations between smoking and higher bacteroides (this novel finding is also present in healthy controls) |
| Bergeron, V. et al. (2012) [112] | Clinical research—In vitro study | Study of mononuclear cells extracted from blood samples of CD patients (smokers N = 19, and non-smokers N = 26), UC patients (smokers N = 7, and non-smokers N = 18), and healthy controls (smokers N = 13, and non-smokers N = 18); following either in vivo or in vitro exposure to cigarette smoke | Mononuclear cells from CD patients who smoke are functionally impaired, present a defective sensitivity to anti-inflammatory or antioxidant protection, and particularly synthesize lower levels of cytoprotective Hsp70. Findings suggest that the effects of cigarette smoke are largely dependent on the oxidative stress generated rather than on the nicotine component |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alemany-Cosme, E.; Sáez-González, E.; Moret, I.; Mateos, B.; Iborra, M.; Nos, P.; Sandoval, J.; Beltrán, B. Oxidative Stress in the Pathogenesis of Crohn’s Disease and the Interconnection with Immunological Response, Microbiota, External Environmental Factors, and Epigenetics. Antioxidants 2021, 10, 64. https://doi.org/10.3390/antiox10010064
Alemany-Cosme E, Sáez-González E, Moret I, Mateos B, Iborra M, Nos P, Sandoval J, Beltrán B. Oxidative Stress in the Pathogenesis of Crohn’s Disease and the Interconnection with Immunological Response, Microbiota, External Environmental Factors, and Epigenetics. Antioxidants. 2021; 10(1):64. https://doi.org/10.3390/antiox10010064
Chicago/Turabian StyleAlemany-Cosme, Ester, Esteban Sáez-González, Inés Moret, Beatriz Mateos, Marisa Iborra, Pilar Nos, Juan Sandoval, and Belén Beltrán. 2021. "Oxidative Stress in the Pathogenesis of Crohn’s Disease and the Interconnection with Immunological Response, Microbiota, External Environmental Factors, and Epigenetics" Antioxidants 10, no. 1: 64. https://doi.org/10.3390/antiox10010064
APA StyleAlemany-Cosme, E., Sáez-González, E., Moret, I., Mateos, B., Iborra, M., Nos, P., Sandoval, J., & Beltrán, B. (2021). Oxidative Stress in the Pathogenesis of Crohn’s Disease and the Interconnection with Immunological Response, Microbiota, External Environmental Factors, and Epigenetics. Antioxidants, 10(1), 64. https://doi.org/10.3390/antiox10010064





