Abstract
The global burden of chronic kidney disease (CKD) intertwined with cardiovascular disease has become a major health problem. Oxidative stress (OS) plays an important role in the pathophysiology of CKD. The nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant responsive element (ARE) antioxidant system plays a critical role in kidney protection by regulating antioxidants during OS. Heme oxygenase-1 (HO-1), one of the targets of Nrf2-ARE, plays an important role in regulating OS and is protective in a variety of human and animal models of kidney disease. Thus, activation of Nrf2-HO-1 signaling may offer a potential approach to the design of novel therapeutic agents for kidney diseases. In this review, we have discussed the association between OS and the pathogenesis of CKD. We propose Nrf2-HO-1 signaling-mediated cell survival systems be explored as pharmacological targets for the treatment of CKD and have reviewed the literature on the beneficial effects of small molecule natural products that may provide protection against CKD.
1. Introduction
The incidence and prevalence of chronic kidney disease (CKD) patients is increasing worldwide. The prevalence of CKD between male and female patients is not constant between countries, however, kidney functions decline faster in males than females [1]. Importantly, CKD is not only a risk factor for increasing global mortality but it is also a critical factor involved in cardiovascular disease (CVD) [2]. The close link between CKD and CVD has been known for a long time [3,4,5]. Not only traditional risk factors such as hypertension, dyslipidemia, and diabetes, but also non-traditional risk factors such as disturbed minerals and vitamins in CKD may play important roles in the progression of CVD. The current treatment options for CKD are controlling blood pressure, serum glucose, and serum lipid profile [6], as well as a modification of lifestyle [7,8]. Since the efficacy of the current therapeutic strategy is still limited [9], there is a need to develop a more effective therapeutic option for treating CKD. Although the exact mechanism involved in the development of CKD is elusive, many lines of evidence strongly suggest that oxidative stress (OS) plays a critical role in the progression of CKD [10,11,12,13].
OS is an imbalance between cellular reactive oxygen species (ROS) levels and antioxidant enzymes, leading to a pathological condition. ROS regulates various signaling pathways, including the growth and differentiation of cells, mitogenesis, production, and breakdown of the extracellular matrix (ECM), inflammation, and apoptosis [14]. OS-mediated damaging effects of cells are controlled by activating the antioxidant defense system. OS has also been noticed to be affected by sex hormones in ischemic kidney injury [15]. Unfortunately, there is an impairment of antioxidative defense and a reduced activity of antioxidant enzymes in CKD [16]. Hence, promoting the endogenous antioxidants defense system may become an important strategy in inhibiting OS-mediated cellular damage in CKD.
Phytochemicals and other natural products are cytoprotective against OS by scavenging oxygen-free radicals and enhancing the level of antioxidants [17]. The literature on protective effects of antioxidant natural products against CKD has been reported [18,19,20]. Nuclear factor erythroid 2-related factor 2 (Nrf2) is the master regulator of the cellular antioxidant defense system [17]. Studies review that augmentation of Nrf2 activity prevents the progression of acute kidney injury (AKI) to CKD transition [21,22]. Natural bioactive compounds and their sources have been demonstrated to have kidney protective potential by activating Nrf2 in experimental CKD models [23,24]. In a recent review on clinical studies, bardoxolone methyl (CDDO-me), a semi-synthetic triterpenoid activating the Nrf2 pathway, has been reported as an effective therapeutic for diabetic kidney disease (DKD), although it has limitations in that it increases the risk of heart failure [25]. Heme oxygenase-1 (HO-1), one of the target molecules of Nrf2, attenuates the overall production of ROS through its ability to degrade heme and to produce carbon monoxide (CO), biliverdin/bilirubin, and the release of free iron. Induction of HO-1 mediates many beneficial effects in the cardiovascular system and kidney [26]. Also, the modulatory role of HO-1 has been reported in various kidney injury models including CKD [27,28,29,30,31,32,33,34]. Several natural HO-1 inducers and their therapeutic applications in various diseases, including CKD, have been reported [35].
In this review, we have explored the causes responsible for the development of OS and its involvement in the pathophysiology of CKD. We then introduced Nrf2-HO-1 signaling as pharmacological targets for the treatment of CKD. Finally, we have discussed the recent literature on its protective effects on the kidney and the underlying pharmacological mechanisms of bioactive phytochemicals that activate Nrf2-HO-1-mediated kidney protective actions.
2. Oxidative Stress in Chronic Kidney Disease
OS plays a critical role in the progression of CKD [36,37], including diabetic kidney disease (DKD), glomerulosclerosis, glomerulonephritis, lupus nephritis (LN), systemic lupus erythematosus (SLE), tubulointerstitial fibrosis, and chronic renal allograft dysfunction (CRAD). The mechanistic link between OS and CKD has been widely investigated [10,11,12]. Briefly, mitochondria and NADPH oxidases are the important sources of intracellular ROS which activate signal transduction cascade and transcription factors, leading to upregulation of genes and proteins involved in remodeling of ECM in the diabetic kidney [10]. In addition, ROS disturb the excretory function of the nephron, leading to homeostasis imbalance and accumulation of metabolic products. ROS also disturb regulatory mechanisms of kidney, such as tubular glomerular feedback, myogenic reflex in the arteriole, and the renin–angiotensin–aldosterone system. As a result, the kidney fails to compensate for water–electrolyte and acid–base imbalances, leading to an additional increase in OS. Ultimately, the progression of CKD is occurred with a variety of complications [11]. This section reviews the mechanisms involved in the different models of CKD, as below and in Figure 1.

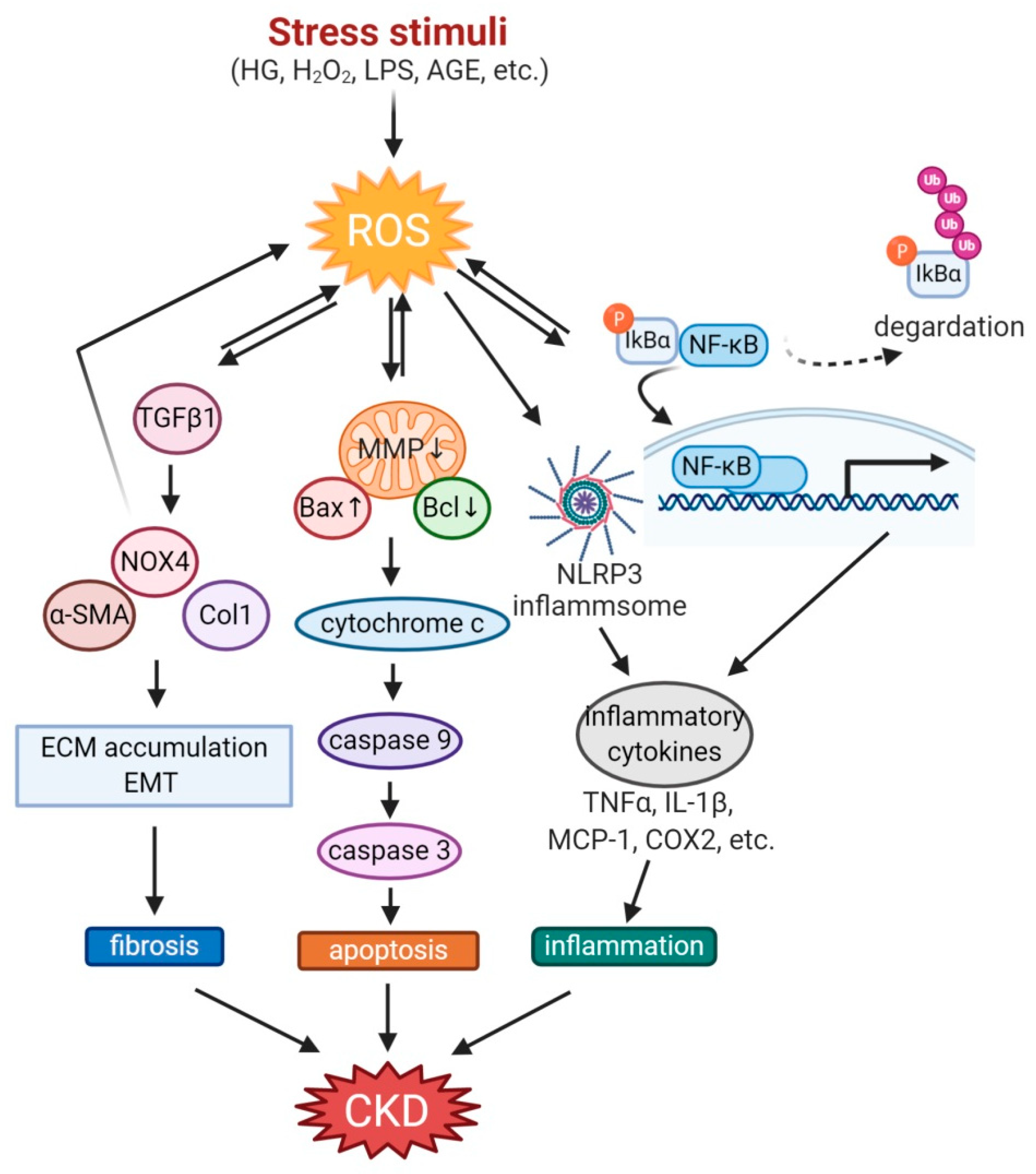
Figure 1.
Mechanisms involved in the pathogenesis of OS in CKD. Stress stimuli such as high glucose, H2O2, and AGE generate ROS in the kidney. The higher ROS increases TGFβ activation, causing kidney fibrosis through the accumulation of ECM and EMT. ROS also decreases MMP and regulates Bax/Bcl2 in mitochondria to activate caspase3, resulting in apoptosis. Not only nuclear localization of NF-kB through degradation of phosphorylated IkBα but also NLRP3 inflammasome activation induces inflammation with inflammatory cytokines secretion. These ROS-induced pathophysiologic conditions exacerbate CKD. AGE, advanced glycation end products; CKD, chronic kidney disease; COX2, cyclooxygenase 2; ECM, extracellular matrix; EMT, epithelial-to-mesenchymal transition; HG, high glucose; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; MMP, mitochondrial membrane permeability; NLRP3, NLR family pyrin domain containing 3; ROS, reactive oxygen species; TNFα, tumor necrosis factor α; TGFβ, transforming growth factor β.
2.1. Diabetic Kidney Disease (DKD)
DKD is a key microvascular complication of diabetes. NADPH oxidases (Nox)-derived ROS play an important role in inflammation and in the accumulation of ECM in DKD. Both hyperglycemia and dyslipidemia play roles in OS and mitochondrial dysfunction [38,39]. In mice, streptozotocin (STZ) increases the ROS level and the expression of monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor α (TNFα), macrophage marker (F4/80), transforming growth factor β (TGFβ), and fibronectin (FN). Also, STZ treatment induces mitochondrial and peroxisomal dysfunction. These effects are decreased by APX-115, a pan-Nox inhibitor [40], indicating the involvement of OS in mitochondrial and peroxisomal dysfunction. Increased ROS levels and oxidative DNA damage were found to be associated with increased TGFβ1 and ECM accumulation in STZ-induced diabetic mice [41]. In STZ-induced diabetic rats, methylglyoxal increased the levels of ROS, and Bax, cytochrome C, caspase-9, and caspase-3 expression, while it decreased Bcl2 expression in the kidneys [42]. In high-fat diet (HFD)-induced type 2 diabetic mice, accumulation of kidney OS markers such as 8-isoprostane and 4-hydroxynonenal (HNE) were associated with 12/15-lipoxygenase upregulation, resulting in prediabetic nephropathic phenomenon [43]. HFD-induced OS is contributed by inducible nitric oxide synthase (iNOS) and Nox4, as well as mitochondrial oxidants, induced kidney fibrosis, and glomerular hypertrophy in mice [44]. The Zucker diabetic fatty rat, a model of type 2 diabetes, shows increased ROS, nitric oxide, and lipid and protein peroxidation levels in the kidney associated with alterations in glutathione (GSH)-dependent metabolism and mitochondrial function, leading to the development of kidney injury [45]. In db/db type 2 diabetic mice, treatment with an inhibitor of OS, biliverdin, has also been identified as a potential therapeutic option for translation into clinical application [46]. Under high glucose (HG), peripheral blood mononuclear cells showed decreased catalase (CAT), CuZn superoxide dismutase (SOD), and glutathione peroxidase (GPx) mRNA expression in patients with type 1 diabetic nephropathy [47]. Besides, OS as indicated by the plasma carbonyl group [48], nitrotyrosine [49], and malondialdehyde (MDA) [50] were increased in type 2 diabetic patients.
2.2. Glomerulosclerosis and Glomerulonephritis
Glomerulosclerosis is a common final pathological feature of CKD. In Charles Dawley rats, doxorubicin-induced glomerulosclerosis (glomerular podocyte damage) increased proteinuria and reduced their body weights. Doxorubicin increased OS, as indicated by neutrophil cytosolic factor 1 (p47phox) and Nox2 (p91phox) mRNA. Increased oxidative enzyme expression was accompanied by increased lipid peroxidation, as demonstrated by HNE and increased protein nitrosylation demonstrated by nitrotyrosine in doxorubicin nephropathy in Charles Dawley rats. Besides, fibrosis markers such as TGFβ1, CTGF, and Col1α1 mRNA were also upregulated [51].
Acute glomerulonephritis (AGN), an inflammatory and proliferative glomerular disease, is characterized by marked proliferation of mesangial and endothelial cells in the glomerulus, together with infiltration of neutrophils. During the disease process, ROS levels might be increased by neutrophils, monocytes, and mesangial cells. Thus, OS may play an important role in the pathogenesis of AGN [52]. The involvement of OS with experimental AGN is further supported by an increase in lipid peroxidation products and alterations in antioxidants [53]. Membranous glomerulonephritis (MGN) is a nephrotic syndrome that may lead to CKD. In cationic bovine serum albumin (BSA)-induced MGN in the rat, kidney dysfunction and histopathological changes were observed, while oxidants such as MDA were enhanced with a decline in the level of antioxidants, including SOD, CAT, and GPx [54]. Altogether, the oxidant–antioxidant imbalance may cause the development of pathogenic alterations in these glomerulonephritis models.
2.3. Lupus Nephritis (LN) and Systemic Lupus Erythematosus (SLE)
LN is a severe and frequent complication of SLE. The important role of OS in LN has been previously described [55]. The New Zealand black/white F1 lupus-prone mice showed impaired kidney function with severe kidney lesions and increased ROS-mediated OS. Besides, they showed enhanced expression of the NLRP3 inflammasome as well as NF-kB activation [56]. The pristine-induced lupus nephritic mice resulted in LN with glomerular oxidative damage associated with an increase in ROS, TGFβ1, FN, and iNOS [57]. A recent review has also explained the role of OS in SLE. Here, mitochondrial dysfunction increased OS, leading to lupus pathogenesis [58]. In lupus nephritic patients, immunohistochemistry analysis of the kidney showed an increased accumulation of 8-oxo-dG [57].
2.4. Tubulointerstitial Fibrosis
Tubulointerstitial fibrosis is the most important feature associated with progressive CKD. The remnant kidney of 5/6 nephrectomy-induced CKD in Sprague-Dawley rats showed increased profibrotic cytokines, lipid peroxidation, GSH depletion, and Nox, suggesting the involvement of OS with tubulointerstitial fibrosis [48]. The involvement of OS in kidney tubulointerstitial inflammation and fibrosis in a unilateral ureteral obstruction (UUO) mouse model has been established for a long time. DNA oxidant markers such as 8-oxo-dG and lipid peroxidation markers such as MDA are increased in UUO kidneys. Also, the increase in ROS levels and a reduction in antioxidants, including SOD and CAT, were found to be involved with TGFβ-mediated inflammation and fibrosis [59]. Adenine administration significantly increased the plasma and urinary OS biomarkers and caused morphological and histological damage in the kidney tubules in rats. Adenine also increased the inflammatory biomarkers and reduced the levels of antioxidant enzymes [60,61].
2.5. Chronic Renal Allograft Dysfunction (CRAD)
CRAD is defined as a progressive kidney dysfunction with features on biopsy of chronic allograft nephropathy. F344 rat kidneys were orthotopically transplanted into Lewis rats. At 12 weeks following surgery, the kidney expressed a higher level of MDA and lower levels of SOD activity, with increased levels of pathological damaging biomarkers such as serum creatinine levels and the infiltration of tubulointerstitial mononuclear cells, indicating the role of OS in CRAD [62].
2.6. The Role of Oxidative Stress in Kidney Cells
Various kidney cells are used to dissect the molecular mechanisms involved in CKD. In mouse proximal tubular epithelial (mProx) cells, Ang II induced hypertrophy. Ang II induced superoxide anion (O2●−) in cultured tubular epithelial cells. This induction of O2●− might be due to p22phox-mediated activation of Nox [63]. In human proximal tubular epithelial (HK-2) cells, lipopolysaccharide (LPS) enhanced the expression of Nox4 and iNOS, leading to an increase in the levels of nitric oxide and O2●−. Subsequently, these ROS reduced cytochrome C oxidase activity and caused mitochondrial dysfunction by interrupting mitochondrial oxidative phosphorylation [64]. HG increased ROS-induced phosphoinositide 3-kinase (PI3K)/ protein kinase B (Akt)/ glycogen synthase kinase 3β (GSK3β) activity and accelerated epithelial-to-mesenchymal transition (EMT) in HK-2 cells [65]. Treatment with 4-hydroxy-2-hexenal increased ROS levels and increased ERK and JNK expression, triggering NF-kB activation and IkBα degradation in HK-2 cells. Eventually, activation of NF-kB promoted apoptosis by inhibiting Bcl2 and increasing Bax expression [66]. H2O2 increased levels of ROS in the cytosol and mitochondria, leading to apoptosis in HK-2 cells [67]. Treatment with TGFβ1 increased the levels of ROS and decreased the levels of GSH in HK-2 cells. TGFβ1 stimulated the expression of EMT genes, FN, and collagen1 [68]. When Madin-Darby canine kidney epithelial cells were exposed to oxalate, a constituent of many kidney stones, phospholipase A2 was activated and the ROS level was increased with depolarization of the mitochondrial membrane potential, implying mitochondrial dysfunction through OS is involved in oxalate toxicity [69]. In rat kidney epithelial (NRK-52E) cells, methylglyoxal increased the ROS levels, along with inducing increased expression of Bax, cytochrome C, caspase-9, and caspase-3, while it decreased the mitochondrial membrane permeability and Bcl2 expression [42].
HG induces intracellular ROS in mProx cells and mesangial cells. Also, ROS are induced in glomerular mesangial by advanced glycation end products (AGE) and cytokines. This study suggests that Nox may play a role in ROS generation, leading to DKD [10]. In rat kidney mesangial cells, the role of OS was examined via alleviation of AGE-induced activation of NF-kB, protein kinase C (PKC) activity, and TGFβ1 transcription with antioxidants such as vitamin E and nitecapone [70].
HG induces micro-vesicles generation (which may cause the pathogenesis of many diseases, such as CVD and diabetes) through the ROS/Nox4 pathway in mouse podocyte clone 5 cells. Also, HG-induced micro-vesicles were significantly decreased after pretreatment with N-Acetyl-l-cysteine (NAC, an antioxidant) [71]. In mouse podocyte cell lines, exposure to aldosterone elevated ROS levels. Treatment with NAC prevented OS and attenuated podocyte injury by increasing nephrin expression and inhibiting apoptosis [72].
3. Role of Nrf2 and HO-1 against Oxidative Stress
Cellular antioxidant defense systems against OS include SOD, CAT, sulfiredoxin, thioredoxin, γ-glutamine cysteine ligase and synthase, NADPH quinone oxidoreductase (Nqo1), Nrf2, and HO-1. The cellular antioxidant defense system is mainly controlled by the Keap1-Nrf2 pathway, which is activated by stimuli such as electrophilic compounds, ROS, and ER stress. The transcription factor Nrf2 is a master regulator of this system. Under normal conditions, the function of Nrf2 is negatively regulated by Keap1 [73], which promotes its degradation via the ubiquitin-proteasome system [74]. Under stress conditions, after a conformational change in Keap1, Nrf2 is released from the proteasome pathway and it translocates into the nucleus [75]. In the nucleus, Nrf2 binds to the gene regulator antioxidant responsive element (ARE) region and mediates the transcription of antioxidant genes [76]. The association of Nrf2 with kidney diseases has been described in various reports. In brief, Nrf2 plays an important role in improving STZ-induced DKD in mice [41]. In this study, Nrf2−/− mice showed increased ROS levels and higher oxidative DNA damage. Besides, increased TGFβ1 and ECM accumulation were found in diabetic mice. These all were decreased by Nrf2 activation [41]. A study reviewed that genetic or pharmacological augmentation of Nrf2 activity reduces OS in the kidney tubules and significantly prevents the progression of AKI to CKD transition [21]. This review explained that a deficiency of Nrf2 accelerates kidney injury in various models, such as LN, and STZ-induced DKD. On the other hand, a deficiency of Keap1 reduced tubular injury in ischemia reperfusion injury (IRI) and diminished kidney fibrosis in UUO [21]. A recent study suggested a role of GSK3β overexpression-mediated Keap1-independent regulation of the Nrf2 antioxidant response against the folic acid-induced AKI to CKD transition in mice [22]. In LN patients, immunohistochemistry analysis of the kidney showed an increased accumulation of 8-oxo-dG, while the expression of Nrf2 and Nqo1 was decreased [57]. The pristine-induced LN mice had glomerular oxidative damage, while the Nrf2-deficient mice had accelerated kidney damage with an increase in ROS, TGFβ1, FN, and iNOS, suggesting a role of Nrf2 in the regulation of ROS levels [57]. In cationic BSA-induced MGN in the rat, kidney dysfunction and histopathological changes were observed. Also, Nrf2 expression and its downstream antioxidants were responsible for the protective response against OS in MGN rats, while negative regulators of Nrf2, such as Keap1 and oxidants such as MDA, were enhanced, with a decline in the level of antioxidants, including SOD, CAT, and GPx [54]. HG increased ROS-induced PI3K/Akt/GSK3β activity and accelerated EMT in HK-2 cells. Further, the accumulation of EMT was reduced by treatment with an Nrf2 activator, sulforaphane, emphasizing the therapeutic potential of targeting Nrf2-HO-1 signaling [65]. The TGFβ1-increased ROS levels, EMT genes, FN, and collagen 1, were further increased by Nrf2 knockdown and suppressed by Keap1 knockdown in HK-2 cells [68], suggesting a critical role of Nrf2 in kidney fibrosis.
HO-1 is one of the targets of Nrf2 and it degrades heme into CO, iron (induction of ferritin), and biliverdin-IXα [77], all of which have antioxidative, anti-inflammatory, and cytoprotective effects against various diseases, including kidney diseases [78,79,80,81,82,83,84]. Cobalt protoporphyrin (CoPP) and curcumin, inducers of HO-1, induce NQO1 expression in HepG2 cells. In addition, endogenous CO, a by-product of HO-1, induces NQO1 expression [85]. Treatment of CoPP increases the SOD expression and catalase activity in STZ-treated rats [86]. These observations suggest a major and functional role of HO-1 in antioxidant defense. HO-1 has been found to be effective against several injurious stimuli and many clinically relevant diseases such as sepsis, hypertension, atherosclerosis, and acute lung and kidney injury [87,88]. Hyperglycemia is the major reason for increased ROS levels, while it is also an important cause of CKD [89]. High levels of glucose inhibit HO-1 activity [90], which leads to increased OS in the vascular system [91]. Decreased HO-1 expression and activity are observed in type 1 diabetes [92], and upregulation of HO-1 reduces diabetic vascular dysfunction [93]. All of these observations suggest that decreased HO-1 expression and activity in the vascular system may lead to the progression of CKD, since vascular abnormalities are strongly linked with kidney dysfunction [3,4].
Induction of HO-1 improves hyperhomocysteinemia-induced AKI to CKD progression in mice. In this study, administration of the HO-1 inducer, cobalt protoporphyrin-IX, significantly hampered ROS and kidney fibrotic lesions [27]. Hemin, another HO-1 inducer, also improved the kidney function and decreased the expression of markers of OS (as indicated by the levels of MDA) in IRI [28]. Ginkgo biloba extract attenuated the production of ROS in HG-stimulated podocytes, and HO-1 inhibitor treatment abolished these effects [29]. There is a strong link between HO-1 and mitochondrial function [30]. Mitochondria-targeted HO-1 attenuates ROS in kidney epithelial cells [31]. HO-1 induction is cytoprotective against ROS-mediated OS in the kidney through an increase in the levels of mitochondrial transporters and cytochrome c oxidase activity [30]. HO-1 overexpression decreased ROS levels, suggesting a decrease in levels of Nox, a heme-dependent protein [32]. Therefore, the induction of HO-1 results in a superior cellular environment due to its good antioxidant capabilities.
4. Functional Link between Nrf2 and HO-1
Since under electrophiles and ROS stress Keap1 is modified at its cysteine residues, Nrf2 is released and translocated to the nucleus and binds to the ARE regions [94]. As a result, Nrf2 induces transcription of ARE regulatory genes, including HO-1 in various tissues and cells under OS conditions [95,96,97,98]. Considering the important cytoprotective role of HO-1 in the kidney and other tissues [33,34], in this review, we have focused on the Nrf2-HO-1 pathways (Figure 2).

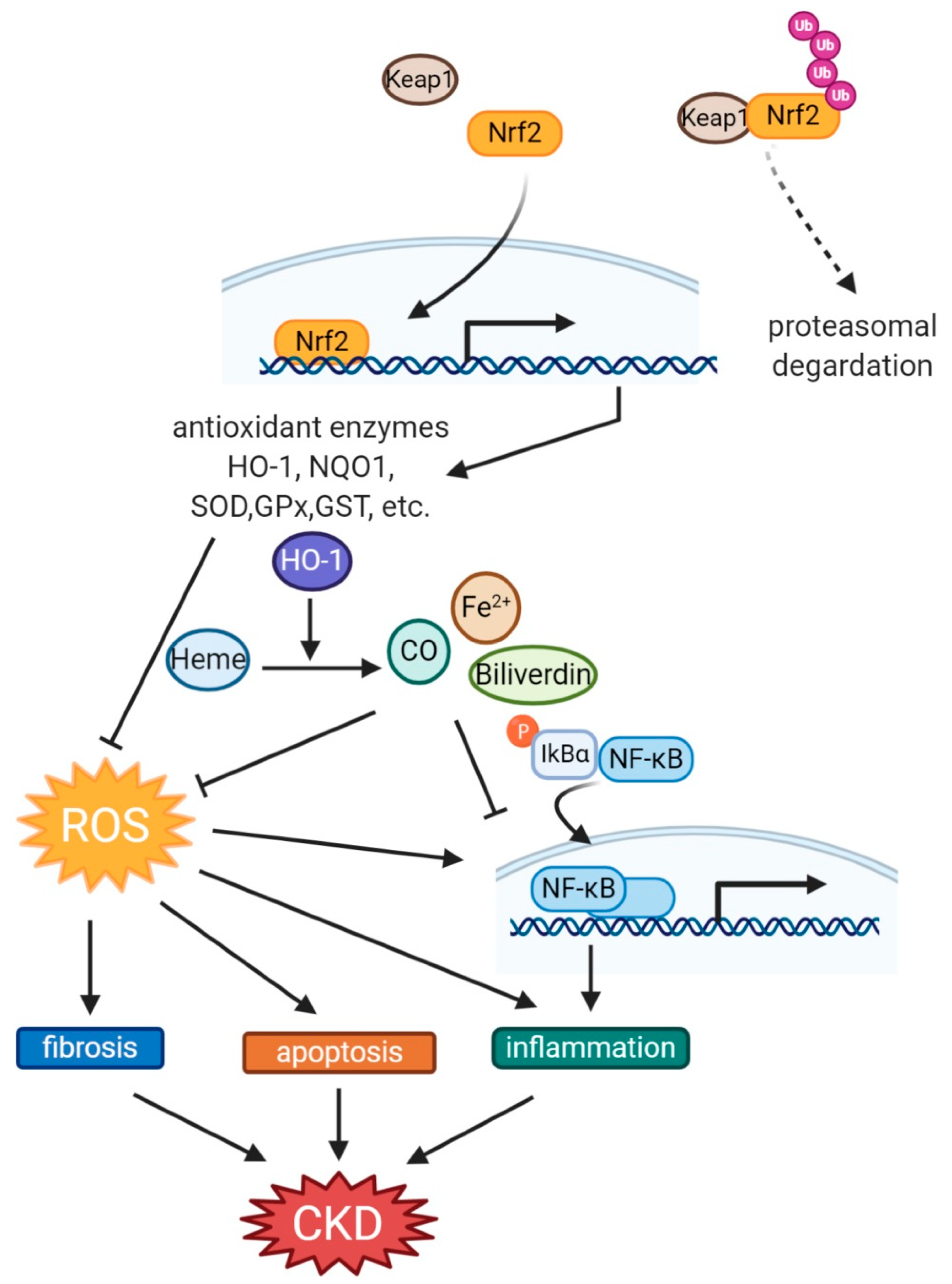
Figure 2.
The protective mechanism via the Nrf2-HO-1 pathway on CKD. When Keap1, which targets Nrf2 for ubiquitination and proteasomal degradation, is decreased or inactivated, phosphorylated Nrf2 is translocated into the nucleus, and transcription of downstream antioxidant enzymes is activated. In particular, by HO-1, fibrosis, apoptosis, and inflammation are attenuated either directly or indirectly through inhibition of OS, thus improving kidney damage. CKD, chronic kidney disease; CO, carbon monoxide; GPx, glutathione peroxidase; GST, Glutathione-S-transferase; HO-1, Heme oxygenase-1; Nqo1, NADPH quinone oxidoreductase; Nrf2, nuclear factor erythroid 2-related factor 2; SOD, superoxide dismutase; ROS, reactive oxygen species.
Nrf2-induced HO-1 may protect the kidney from remote organ injury in mice and rats [99]. HO-1 induction could be a potential therapeutic approach to prevent CKD complications by activating antioxidative and antiapoptotic signaling. Yoh and colleagues speculate that the decrease in HO-1 expression might be associated with the pathogenesis of LN in Nrf2−/− female mice [100]. Also, adenine-induced OS and inflammation were associated with kidney tubulointerstitial fibrosis with impairment of Nrf2 activation and downregulation of its target gene products, including HO-1 [60]. Small molecule activator of Nrf2, CDDO-Im (1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl] imidazole), protects against LPS-induced dysregulation of the innate immune response in mice. Specifically, CDDO-Im treatment reduced LPS-induced ROS levels and inflammatory cytokines such as TNFα and IL-6, as well as increased antioxidative genes such as HO-1, GCLC, GCLM, and Nqo1 in Nrf2+/+ neutrophils but not Nrf2−/− neutrophils in mice [101]. Also, CDDO-Me treatment attenuates retinal vascular degeneration following IRI and increases the expression of HO-1 in wild-type, but not Nrf2−/−, retinas in mice [102], suggesting that activation of Nrf2-dependent compensatory antioxidative pathways by CDDO compounds may protect tissues or cells from OS-induced injury.
The protective effect of insulin-mediated HO-1 was through the PI3K/Akt pathway and the Nrf2 transcription factor in mProx cells [103]. In HK-2 cells, the Nrf2 activator sulforaphane increased HO-1 protein expression. HG increased the expression of vimentin and FN and decreased E-cadherin expression, which were attenuated by the treatment with sulforaphane [65], indicating an inhibitory effect of Nrf2-HO-1 on HG-induced EMT. In mouse mesangial cells, Nrf2 overexpression upregulated Nrf2 and its downstream HO-1 expression under HG, while Nrf2 siRNA-treatment reduced the expression of Nrf2 and HO-1, leading to an increase in ROS and TGFβ1 [61,104]. In another study, Nrf2 deficiency upregulated the NF-kB and TGFβ1 signaling pathway and decreased the expression of downstream antioxidants of HO-1 and Nqo1 in mouse mesangial cells [57], indicating that Nrf2-dependent HO-1 expression limits the activation of NF-kB and inhibits pro-inflammatory cytokines production. Besides, in human umbilical vein endothelial cells (HUVECs), 3-hydroxyanthranilic acid (HA) treatment increases ARE-driven luciferase activity. TNFα-induced NF-kB/DNA-binding activity was suppressed by HA-induced Nrf2 transcription and HO-1 activity, and the NF-kB/DNA-binding activity was restored by treatment with tin protoporphyrin IX dichloride (SnPP, an HO-1 inhibitor) [105]. In HUVECs, constitutively active PKCε enhanced HO-1 mRNA and protein levels, while aortas or cardiac endothelial cells from PKCε-deficient mice showed decreased levels of HO-1. Also, Ang II stimulated PKCε and produced HO-1 in a PKCε-dependent manner. Nrf2 siRNA blocked PKCε-mediated HO-1 induction [106]. In rat pheochromocytoma PC12 cells, dominant-negative Nrf2 significantly inhibited PI3K-induced HO-1 promoter activity. Thus, PI3K is necessary to initiate activation of the HO-1 promoter through the AREs in an Nrf2-dependent manner [107].
However, Nrf2-independent HO-1 expression has also been reported [108]. Bach1 is regarded as a critical physiological repressor of HO-1. Higher levels of HO-1 mRNA were observed in the thymus, heart, lung, and liver in the bach1−/− mice [108]. Since HO-1 is activated by Nrf2 [95,96,97,98], higher levels of HO-1 expression in the bach1−/− mice seem to be due to Nrf2 activation. Surprisingly, the enhanced HO-1 levels in the Bach1-deficient thymus were independent of Nrf2, since the expression of HO-1 was not affected by Nrf2 deficiency [108]. In addition, CO/HO-1 induce NQO1 expression via Nrf2 activation [85], suggesting a crosstalk between HO-1 and Nrf2.
5. Small Molecule Natural Products Activating Nrf2-HO-1 Signaling
A substantial quantity of natural products has been reported to confer renoprotection and improve disease outcomes of the various types of CKD, primarily through activating the Nrf2/HO-1 antioxidant defense systems and attenuating the proinflammatory signaling pathways. Here, we reviewed the existing literature over the past decade to compile comprehensive information on the kidney protective potential of naturally occurring compounds. Experimental and disease models, the pathobiology involved, the research outcomes, and the molecular markers altered by these compounds are summarized in Table 1 and Table 2 and Figure 3. To facilitate the discussion, we have categorized the kidney protective effects of these natural compounds into two distinct chemical groups: phenolic and non-phenolics. This categorization also highlights common bioactive compounds, belonging to phenolic group which represents the largest chemical class showing enormous bioactivity with the potential to be future drug candidates.

Table 1.
Kidney protective effects provided by phenolic compounds of phytochemicals targeting the Nrf2-HO-1 signaling pathway.

Table 2.
Kidney protective effects provided by non-phenolic compounds of phytochemicals targeting the Nrf2-HO-1 signaling pathway.

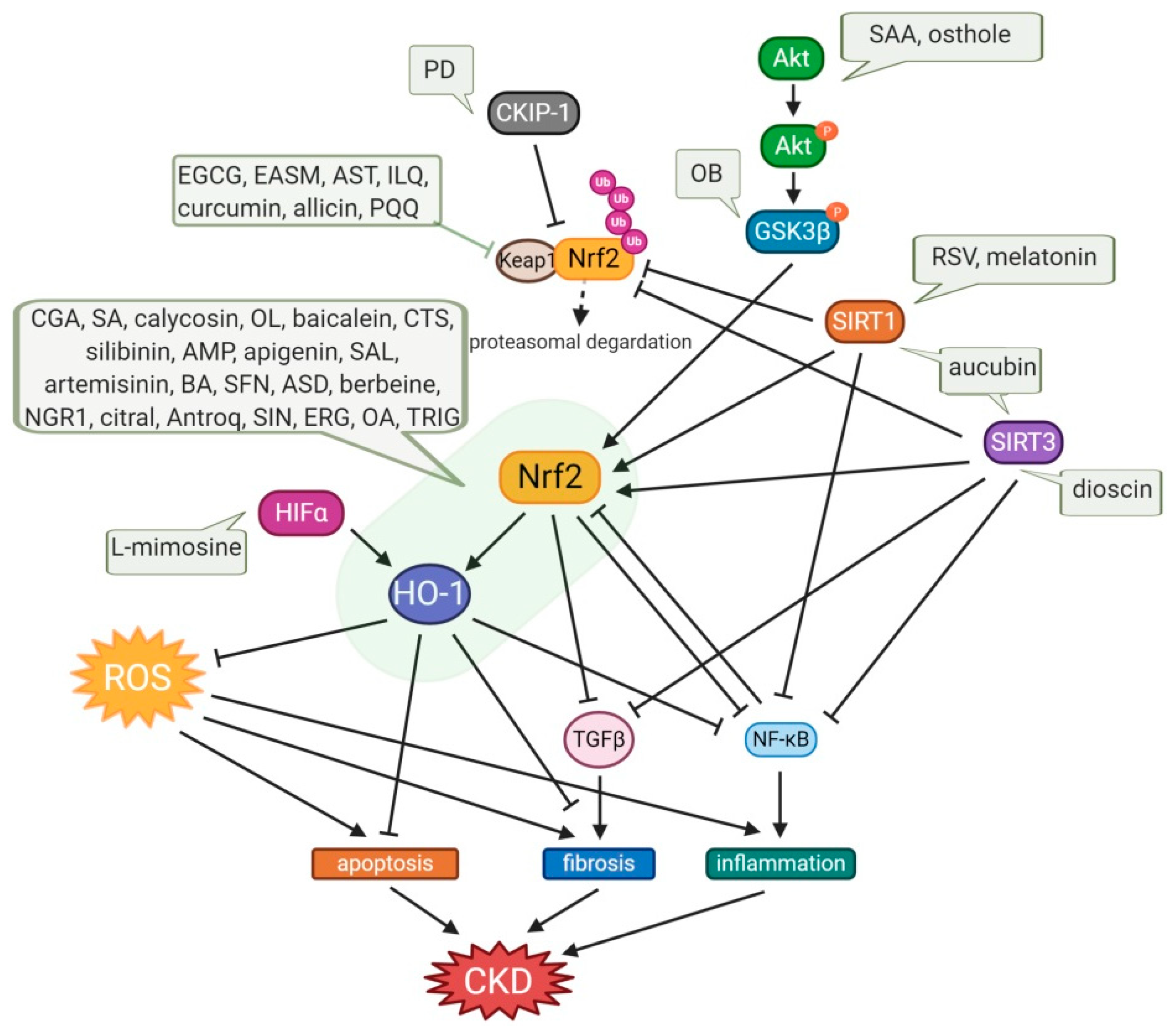
Figure 3.
Protective effects of small-molecule natural products on OS in CKD. Osthole and SAA enhance the activation of the Akt/Nrf2/HO-1 signaling pathway with suppression of NF-kB and TGFβ1, consequently attenuating OS, inflammation, and fibrosis. OB induces the phosphorylation of GSK3β, which inhibits Fyn-mediated Nrf2 nuclear export, and activates the transcription of Nrf2-driven antioxidant genes. Expression of SIRT1, which inhibits NF-kB activity, and the activation of Nrf2 are enhanced by aucubin, melatonin, and RSV, which also upregulates SIRT3, resulting in amelioration of kidney injury. Dioscin upregulates SIRT3 level, promotes Nrf2, and suppresses Keap1 expression, resulting in inhibition of inflammation, lipid metabolism, OS, and kidney fibrosis. PD increases the CKIP-1 expression level and promotes the interaction of CKIP-1 with Nrf2, consequently activating the Nrf2-ARE antioxidative pathway. Allicin, AST, curcumin, EASM, EGCG, ILQ, and PQQ attenuate OS via the Nrf2/HO-1 signaling pathway with inhibition of Keap1, and they also reduce TGFβ-mediated fibrosis and NF-kB-induced inflammation. In the cases of an anti-fibrotic effect of apigenin, ASD, baicalein, BA, CGA, CTS, ERG, OL, and SFN, AMP, antroq, artemisinin, berbeine, calycosin, SA, SIN, and TRIG, they are mediated not only by upregulation of the Nrf2/HO-1 antioxidant signaling pathway and downregulation of NF-kB-induced inflammation, but also via TGFβ suppression. Treatments with citral, NGR1, OA, SAL, and silibinin have potency for anti-apoptotic effects with regulation of Bcl2/Bax and caspase3. The decrease in the NLRP3 inflammasome was also observed in treatments with baicalein, EGCG, and OL. L-mimosine activates HIF1α, which upregulates renoprotective HIF target genes, such as VEGF, HO-1, and GLUT1, and decreases fibrosis markers. AMP, ampelopsin; Antroq, antroquinonol; ASD, akebia saponin D; AST, astaxanthin; BA, betulinic acid; CGA, chlorogenic acid; CTS, cryptotanshinone; EASM, ethyl acetate extract of Salvia miltiorrhiza; EGCG, Epigallocatechin gallate; ERG, ergone; GSK3β, glycogen synthase kinase 3β; HIFα, hypoxia-inducible factor α; ILQ, isoliquiritin; NGR1, notoginsenoside R1; OA, oleanolic acid; OB, obacunone; OL, oleuropein; PD, polydatin; PQQ, pyrroloquinoline quinone; RSV, resveratrol; SA, sinapic acid; SAA, salvianolic acid A; SAL, salidroside; SFN, sulforaphane; SIN, sinomenine; TRIG, trigonelline.
5.1. Phenolic Compounds
A significant number of phenolic compounds have shown protection against DKD (Table 1). Administration of resveratrol, a versatile bioactive phenolic found in many plant sources, including red grapes, peanuts, and berries, ameliorated diabetes-induced changes in the kidney tissues of STZ-induced rats by attenuating inflammatory signaling pathways through a mechanism that involved regulation of the NF-κB and Nrf2 signaling pathways [125]. Alone or in combination with metformin, salvianolic acid A attenuated diabetes-induced macrovascular and kidney injury in STZ-injected mice by activating the Nrf2/ARE pathways [129]. In a similar experimental setup, Gong and colleagues reported nephroprotective effects of a stilbenoid glucoside polydatin, which relieved HG-induced kidney damage through activating the CKIP-1-Nrf2-ARE pathway [153]. In STZ-injected mice, epigallocatechin gallate (EGCG) prevented diabetes-induced kidney damage by upregulating Nrf2 expression, which was mediated by disrupting the Nrf2-Keap1 complex [120]. Chlorogenic acid attenuated diabetes-induced kidney damage in STZ-injected and HFD-fed Sprague-Dawley rats by mitigating OS and inflammation through a mechanism that involved modulation of the Nrf2/HO-1 and NF-ĸB signaling pathways [115]. Astaxanthin attenuated HG-induced OS and FN accumulation in glomerular mesangial cells and improved the metabolic status and kidney morphology in STZ-induced diabetic rats [111]. These renoprotective activities of AST were attributed to its activation of Nrf2/ARE signaling [111]. Sinapic acid prevented STZ-induced DKD in rats by attenuating inflammation and OS through upregulating Nrf2/HO-1 signaling pathways [132]. Calycosin ameliorated kidney injury and dysfunction in HFD-fed/STZ-induced diabetic rats by inhibiting inflammation, and OS through modulating the IL33/ST2, NF-κB, and Nrf2 signaling pathways [114].
Wang et al. demonstrated restoration of kidney function by EGCG relieving oxidative and inflammatory damage in UUO, which is attributed to the regulatory roles of this common tea polyphenol on the NF-κB and Nrf2/HO-1 signaling pathways [121]. In a subsequent study, Wang et al. reported that the administration of cryptotanshinone at 50 and 100 mg/kg/day prevented OS and inflammation by suppressing NF-κB signaling and activating Nrf2 signaling in a mouse model of UUO [116]. Rotenone, a mitochondrial complex I inhibitor, ameliorated chronic obstructive kidney injury through attenuating mitochondrial OS, inflammation, and fibrosis [127].
Silibinin (75 mg/kg day) significantly reversed arsenic (As)-induced biochemical changes in the kidney, reduced lipid peroxidation, and improved the antioxidant defense system [131]. These nephroprotective effects of silibinin against As-induced CKD were attributed to its antioxidant, anti-inflammatory, and metal chelating properties [131]. Co-administration of osthole (40 mg/kg, intravenously) along with 2% adenine suspension attenuated inflammatory damage in a rat model of CKD through a mechanism that involved downregulation of NF-κB and TGFβ1 and activation of PI3K/Akt/Nrf2 signaling [124].
EGCG protected against LN in mice by activating the Nrf2 antioxidant signaling pathway and inhibiting the NLRP3 inflammasome [56]. In a similar study, Li et al. showed that baicalein improved pristane-induced LN symptoms in mice through preventing inflammation and OS by a mechanism that involved activation of the Nrf2/HO-1 signaling pathway and upregulation of NLRP3 expression [113]. Mice fed oleuropein- and peracetylated oleuropein-supplemented diets experienced a lower intensity of pristane-induced kidney damage. These protective effects of oleuropein and peracetylated oleuropein against LN were attributed to its activating role of HO-1/Nrf2 signaling and its suppressive effect on the JAK/STAT, NF-κB, MAPK, and NLRP3 inflammasome signaling pathways [123].
Curcumin protected against the changes in kidney functions in 5/6 nephrectomy, an experimental CKD model, through activating the Nrf2-Keap1 and kidney dopamine pathways, an effect that was comparable to the standard agent mycophenolate mofetil [117]. Curcumin ameliorated adenine-induced alteration of kidney functions, including hypertension and albuminuria, in a rat model of CKD by attenuating inflammation and OS through activating Nrf2 signaling [118]. Liu et al. demonstrated kidney protective effects of isoliquiritin against cationic BSA-induced MGN in an experimental rat model, which was attributed to its antioxidative (activation of Nrf2 signaling) and anti-inflammatory properties (inhibition of NF-κB signaling) [54].
Moreover, resveratrol, curcumin, ampelopsin, and apigenin were shown to have attenuating effects against oxidative damage in various cellular models of kidney injury [109,110,119,126]. Besides, salidroside has anti-apoptotic effects in HG-treated mouse podocytes, showing therapeutic promise in the management of kidney disease [128].
5.2. Non-Phenolic Compounds
Like phenolics, several non-phenolic compounds have been shown to protect against DKD (Table 1). Most notable is sulforaphane, which improved kidney morphological and functional alterations in STZ-injected and meglumine diatrizoate-injected diabetic rats through activation of the Nrf2/HO-1 pathway [150]. Sulforaphane treatment also resulted in functional and morphological improvements of CRAD by attenuating OS through inducing the Nrf2-HO-1/Nqo1 signaling pathway [151]. Artemisinin prevented OS-induced kidney damage in STZ-injected DN. These renoprotective effects of artemisinin were due to its inhibitory role on the TGFβ1 regulator and its activating role in the Nrf2 signaling pathway [136]. Betulinic acid ameliorated DKD in STZ-induced rats, which was mediated by activating the AMPK/NF-κB/Nrf2 signaling pathway [139]. Akebia Saponin D protected against diabetes-induced kidney damage and improved kidney function by antioxidant and anti-inflammatory functions, which was attributed to its activation of the Nrf2/HO-1 pathway and its inhibition of the NF-κB pathway [133]. In STZ-induced diabetic mice, berberine can ameliorate tubulointerstitial fibrosis via activating the Nrf2 pathway and inhibiting the TGFβ/Smad/EMT signaling pathway [138]. Aucubin, a natural iridoid glucoside, improved symptoms of DKD through inhibiting NF-κB activation and inducing the SIRT1/SIRT3-FOXO3a signaling pathway in HFD/STZ-induced diabetic mice [137]. Notoginsenoside R1 protected against OS and ameliorated DKD in db/db mice through upregulation of Nrf2-mediated HO-1 expression [145].
Sinomenine ameliorated kidney fibrosis in UUO-operated ICR mice by preventing OS through Nrf2 activation and interfering with pro-fibrogenic signaling of TGFβ/Smad and Wnt/β-catenin [149]. Allicin can protect against 5/6 nephrectomy-induced hypertension and kidney dysfunction (uremia, high serum creatinine, and albuminuria) through activating the Nrf2/Keap1 antioxidant defense system, an effect that was similar to, or even better, than that of losartan [134]. Administration of L-mimosine at a later stage of kidney ablation (from week 5 to week 12) caused transient activation of hypoxia-inducible factors (HIF-1α and HIF-2α proteins), increased expression of VEGF, HO-1, and GLUT-1, and a reduction in fibrosis markers [143].
Dioscin prevented high fructose-induced kidney damage via attenuating SIRT3-mediated OS and inflammation and adjusting lipid metabolism and TGFβ1/Smad signaling to inhibit kidney fibrosis [141]. When treated with ergone, alisol B 23-acetate, and pachymic acid B, these compounds prevented ECM accumulation in HK-2 cells and attenuated podocyte injury by inhibiting Ang II-induced RAS/Wnt/β-catenin axis activation and thereby ameliorating tubulointerstitial nephropathy [142]. Oleanolic acid treatment of cyclosporine-treated ICR mice ameliorated tubulointerstitial fibrosis in chronic nephropathy by activating the Nrf2/HO-1 signaling pathway [147]. Trigonelline can prevent the effects of oxalate-induced EMT in kidney tubular epithelial cells, offering a promising anti-fibrotic agent in the management of CKD [152].
Melatonin can protect against pristane-induced LN in mice, and this effect was attributed to its enhancing role on the Nrf2 signaling pathway and its ability to inhibit kidney NLRP3 inflammasome activation [144]. Also, obacunone and pyrroloquinoline quinone have shown promise in CKD for their antioxidant and anti-inflammatory potential [146,148].
6. Conclusions and Future Perspectives
OS has been involved in the pathobiology of CKD and thus, developing a treatment strategy targeting OS might be a potential option against CKD. Cells have their own antioxidant defense system to tackle the effects of OS, and Nrf2-HO-1 signaling is an important antioxidant defense system against various diseases, including CKD. Several recent clinical trials are investigating the protective potential of phytochemicals, such as resveratrol, curcumin, and sulforaphane, in CKD patients. In those studies, the expression of Nrf2 and HO-1 were set as outcome measures (Table 3), although Nrf2 activates HO-1 under OS in many preclinical settings of CKD. Targeting Nrf2-HO-1 may provide a means of controlling OS. Pharmacological modulators that can activate Nrf2-HO-1 antioxidant systems offer promise for the treatment of diseases associated with OS-associated kidney injury. In this perspective, several phytochemicals have been described to protect against kidney injury by activating Nrf2-HO-1 systems, suggesting that they could be used to design novel therapeutic agents for treating CKD.

Table 3.
Antioxidant natural compounds used for treating CKD patients.
Although the kidney protective actions of the mentioned phytochemicals are promising, their protective effects have only been studied in preclinical settings. Although few clinical trials are ongoing, they may fail in clinical studies. For instance, resveratrol has been proven to be a potent kidney protective agent that activates the Nrf2-HO-1 pathway in various cells, but it has shown poor bioavailability. Thus, it needs advanced drug delivery systems, such as nanoparticle-mediated drug delivery, in order to achieve proper doses of the drug. Likewise, investigating the detailed molecular mechanism of the kidney protective effects of these phytochemicals is important to discover which cellular defense system between the Nrf2 and HO-1 pathways is involved. Also, it would be useful to study the pharmacokinetics as well as pharmacodynamics of these phytochemicals on the gender differences in CKD.
Author Contributions
Conceptualization, M.J.U. and H.H.; Writing—Original Draft Preparation, M.J.U., E.H.K. and M.A.H.; Writing—Review and Editing, M.J.U., M.A.H. and H.H.; Supervision, H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Research Foundation (No. 2020R1I1A1A01072879 and 2019R1A2C2002720), and Brain Pool program funded by the Ministry of Science and ICT through the National Research Foundation (No. 2020H1D3A2A02110924), Republic of Korea.
Conflicts of Interest
No conflict of interest with the authors is present regarding the publication of this manuscript.
References
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Carney, E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.C.; Lu, L.; Cao, Y.; Sun, R.R.; Chen, S.; Zhang, P.Y. Cardiovascular disease and its relationship with chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2918–2926. [Google Scholar]
- Clausen, P.; Jensen, J.S.; Jensen, G.; Borch-Johnsen, K.; Feldt-Rasmussen, B. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation 2001, 103, 1869–1874. [Google Scholar] [CrossRef]
- Chan, D.T.; Irish, A.B.; Dogra, G.K.; Watts, G.F. Dyslipidaemia and cardiorenal disease: Mechanisms, therapeutic opportunities and clinical trials. Atherosclerosis 2008, 196, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, F.A.; De Zeeuw, D.; Thomas, M.C.; Cooper, M.E.; De Graeff, P.A.; Hillege, H.J.L.; Parving, H.H.; Brenner, B.M.; Shahinfar, S.; Heerspink, H.J.L. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011, 80, 282–287. [Google Scholar] [CrossRef]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; Von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Gregg, L.P.; Hedayati, S.S. Management of traditional cardiovascular risk factors in CKD: What are the data? Am. J. Kidney Dis. 2018, 72, 728–744. [Google Scholar] [CrossRef]
- Cruz, M.C.; Andrade, C.; Urrutia, M.; Draibe, S.; Nogueira-Martins, L.A.; Sesso, R.C.C. Quality of life in patients with chronic kidney disease. Clinics 2011, 66, 991–995. [Google Scholar] [CrossRef]
- Lee, H.B.; Yu, M.R.; Yang, Y.; Jiang, Z.; Ha, H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14, S241–S245. [Google Scholar] [CrossRef]
- Podkowińska, A.; Formanowicz, D. Chronic kidney disease as oxidative stress-and inflammatory-mediated cardiovascular disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef]
- Noh, H.; Ha, H. Reactive oxygen species and oxidative stress. In Contributions to Nephrology; Karger: Basel, Switzerland, 2011; Volume 170, pp. 102–112. [Google Scholar]
- Ha, H.; Hwang, I.A.; Park, J.H.; Lee, H.B. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res. Clin. Pract. 2008, 82, S42–S45. [Google Scholar] [CrossRef]
- Harman, D. The aging process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124. [Google Scholar] [CrossRef]
- Kim, J.; Kil, I.S.; Seok, Y.M.; Yang, E.S.; Kim, D.K.; Lim, D.G.; Park, J.W.; Bonventre, J.V.; Park, K.M. Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice. A role for manganese superoxide dismutase. J. Biol. Chem. 2006, 281, 20349–20356. [Google Scholar] [CrossRef] [PubMed]
- Stępniewska, J.; Gołembiewska, E.; Dołęgowska, B.; Domański, M.; Ciechanowski, K. Oxidative stress and antioxidative enzyme activities in chronic kidney disease and different types of renal replacement therapy. Curr. Protein Pept. Sci. 2015, 16, 243–248. [Google Scholar] [CrossRef]
- Surh, Y.J.; Kundu, J.K.; Na, H.K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar] [CrossRef]
- Lv, W.; Booz, G.W.; Fan, F.; Wang, Y.; Roman, R.J. Oxidative stress and renal fibrosis: Recent insights for the development of novel therapeutic strategies. Front. Physiol. 2018, 9, 105. [Google Scholar] [CrossRef]
- Chen, D.Q.; Hu, H.H.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Zhao, Y.Y. Natural products for the prevention and treatment of kidney disease. Phytomedicine 2018, 50, 50–60. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and oxidative stress in chronic kidney disease-potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Nezu, M.; Suzuki, N.; Yamamoto, M. Targeting the KEAP1-NRF2 system to prevent kidney disease progression. Am. J. Nephrol. 2017, 45, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wang, P.; Qiao, Y.; Jiang, C.; Ge, Y.; Flickinger, B.; Malhotra, D.K.; Dworkin, L.D.; Liu, Z.; Gong, R. GSK3β-mediated Keap1-independent regulation of Nrf2 antioxidant response: A molecular rheostat of acute kidney injury to chronic kidney disease transition. Redox Biol. 2019, 26, 101275. [Google Scholar] [CrossRef]
- Yamawaki, K.; Kanda, H.; Shimazaki, R. Nrf2 activator for the treatment of kidney diseases. Toxicol. Appl. Pharmacol. 2018, 360, 30–37. [Google Scholar] [CrossRef]
- Choi, B.H.; Kang, K.S.; Kwak, M.K. Effect of redox modulating NRF2 activators on chronic kidney disease. Molecules 2014, 19, 12727–12759. [Google Scholar] [CrossRef]
- Kanda, H.; Yamawaki, K. Bardoxolone methyl: Drug development for diabetic kidney disease. Clin. Exp. Nephrol. 2020, 24, 857–864. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Heme oxygenase and the cardiovascular–renal system. Free Radic. Biol. Med. 2005, 39, 1–25. [Google Scholar] [CrossRef]
- Li, S.; Qiu, B.; Lu, H.; Lai, Y.; Liu, J.; Luo, J.; Zhu, F.; Hu, Z.; Zhou, M.; Tian, J.; et al. Hyperhomocysteinemia accelerates acute kidney injury to chronic kidney disease progression by downregulating Heme Oxygenase-1 expression. Antioxid. Redox Signal. 2019, 30, 1635–1650. [Google Scholar] [CrossRef]
- Demirogullari, B.; Ekingen, G.; Guz, G.; Bukan, N.; Erdem, O.; Ozen, I.O.; Memis, L.; Sert, S. A comparative study of the effects of hemin and bilirubin on bilateral renal ischemia reperfusion injury. Nephron Exp. Nephrol. 2006, 103, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T.; Chen, Y.A.; Li, S.Y.; Chen, J.W. Nrf-2 mediated heme oxygenase-1 activation contributes to the anti-inflammatory and renal protective effects of Ginkgo biloba extract in diabetic nephropathy. J. Ethnopharmacol. 2020, 266, 113474. [Google Scholar] [CrossRef]
- Di Noia, M.A.; Van Driesche, S.; Palmieri, F.; Yang, L.M.; Quan, S.; Goodman, A.I.; Abraham, N.G. Heme oxygenase-1 enhances renal mitochondrial transport carriers and cytochrome C oxidase activity in experimental diabetes. J. Biol. Chem. 2006, 281, 15687–15693. [Google Scholar] [CrossRef]
- Bolisetty, S.; Traylor, A.; Zarjou, A.; Johnson, M.S.; Benavides, G.A.; Ricart, K.; Boddu, R.; Moore, R.D.; Landar, A.; Barnes, S.; et al. Mitochondria-targeted heme oxygenase-1 decreases oxidative stress in renal epithelial cells. Am. J. Physiol. Ren. Physiol. 2013, 305, F255–F264. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Takeshige, K.; Cheung, B.S.; Minakami, S. Bilirubin inhibits the activation of superoxide-producing NADPH oxidase in a neutrophil cell-free system. Biochim. Biophys. Acta 1991, 1076, 369–373. [Google Scholar] [CrossRef]
- Nath, K.A. Heme oxygenase-1: A provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006, 70, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Lever, J.M.; Boddu, R.; George, J.F.; Agarwal, A. Heme oxygenase-1 in kidney health and disease. Antioxid. Redox Signal. 2016, 25, 165–183. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Fernández-Fierro, A.; Covián, C.; Bueno, S.M.; Riedel, C.A.; Mackern-Oberti, J.P.; Kalergis, A.M. Naturally derived Heme-Oxygenase 1 inducers and their therapeutic application to immune-mediated diseases. Front. Immunol. 2020, 11, 1467. [Google Scholar] [CrossRef] [PubMed]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Manning, R.D., Jr.; Tian, N.; Meng, S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am. J. Nephrol. 2005, 25, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, M.T.; Sharma, K. Challenging the dogma of mitochondrial reactive oxygen species overproduction in diabetic kidney disease. Kidney Int. 2016, 90, 272–279. [Google Scholar] [CrossRef]
- Hallan, S.; Sharma, K. The role of mitochondria in diabetic kidney disease. Curr. Diabetes Rep. 2016, 16, 61. [Google Scholar] [CrossRef]
- Kwon, G.; Uddin, M.J.; Lee, G.; Jiang, S.; Cho, A.; Lee, J.H.; Lee, S.R.; Bae, Y.S.; Moon, S.H.; Lee, S.J.; et al. A novel pan-Nox inhibitor, APX-115, protects kidney injury in streptozotocin-induced diabetic mice: Possible role of peroxisomal and mitochondrial biogenesis. Oncotarget 2017, 8, 74217–74232. [Google Scholar] [CrossRef]
- Jiang, T.; Huang, Z.; Lin, Y.; Zhang, Z.; Fang, D.; Zhang, D.D. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 2010, 59, 850–860. [Google Scholar] [CrossRef]
- Shopit, A.; Niu, M.; Wang, H.; Tang, Z.; Li, X.; Tesfaldet, T.; Ai, J.; Ahmad, N.; Al-Azab, M.; Tang, Z. Protection of diabetes-induced kidney injury by phosphocreatine via the regulation of ERK/Nrf2/HO-1 signaling pathway. Life Sci. 2020, 242, 117248. [Google Scholar] [CrossRef]
- Shevalye, H.; Lupachyk, S.; Watcho, P.; Stavniichuk, R.; Khazim, K.; Abboud, H.E.; Obrosova, I.G. Prediabetic nephropathy as an early consequence of the high-calorie/high- fat diet: Relation to oxidative stress. Endocrinology 2012, 153, 1152–1161. [Google Scholar] [CrossRef]
- Ruggiero, C.; Ehrenshaft, M.; Cleland, E.; Stadler, K. High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E1047–E1058. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; John, A.; Howarth, F.C. Increased metabolic stress in Zucker diabetic fatty rat kidney and pancreas. Cell. Physiol. Biochem. 2013, 32, 1610–1620. [Google Scholar] [CrossRef]
- Tesch, G.H.; Lim, A.K.H. Recent insights into diabetic renal injury from the db/db mouse model of type 2 diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2011, 300, F301–F310. [Google Scholar] [CrossRef]
- Hodgkinson, A.D.; Bartlett, T.; Oates, P.J.; Millward, B.A.; Demaine, A.G. The response of antioxidant genes to hyperglycemia is abnormal in patients with type 1 diabetes and diabetic nephropathy. Diabetes 2003, 52, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Vaziri, N.D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am. J. Physiol. Ren. Physiol. 2010, 298, F662–F671. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Quagliaro, L.; Catone, B.; Pascon, R.; Piazzola, M.; Bais, B.; Marra, G.; Tonutti, L.; Taboga, C.; Motz, E. Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care 2002, 25, 1439–1443. [Google Scholar] [CrossRef]
- Ceriello, A.; Bortolotti, N.; Motz, E.; Crescentini, A.; Lizzio, T.S.; Russo, A.; Tonutti, L.; Taboga, C. Meal-generated oxidative stress in type 2 diabetic patients. Diabetes Care 1998, 21, 1529–1533. [Google Scholar] [CrossRef] [PubMed]
- Szalay, C.I.; Erdélyi, K.; Kökény, G.; Lajtár, E.; Godó, M.; Révész, C.; Kaucsár, T.; Kiss, N.; Sárközy, M.; Csont, T.; et al. Oxidative/nitrative stress and inflammation drive progression of doxorubicin-induced renal fibrosis in rats as revealed by comparing a normal and a fibrosis-resistant rat strain. PLoS ONE 2015, 10, e0127090. [Google Scholar] [CrossRef] [PubMed]
- Bülbül, M.; Öner, A.; Demircin, G.; Erdoğan, Ö. Oxidative stress in children with acute glomerulonephritis. Ren. Fail. 2008, 30, 209–214. [Google Scholar] [CrossRef]
- Wójcicka, G.; Marciniak, A.; Bełtowski, J.; Górny, D.; Chibowski, D.; Korolczuk, A.; Czabak-Garbacz, R. Oxidative stress in experimental acute glomerulonephritis. Prz. Lek. 2004, 61, 135–140. [Google Scholar]
- Liu, Y.; Xu, X.; Xu, R.; Zhang, S. Renoprotective effects of isoliquiritin against cationic bovine serum albumin-induced membranous glomerulonephritis in experimental rat model through its anti-oxidative and anti- inflammatory properties. Drug Des. Dev. Ther. 2019, 13, 3735–3751. [Google Scholar] [CrossRef] [PubMed]
- Bona, N.; Pezzarini, E.; Balbi, B.; Daniele, S.M.; Rossi, M.F.; Monje, A.L.; Basiglio, C.L.; Pelusa, H.F.; Arriaga, S.M.M. Oxidative stress, inflammation and disease activity biomarkers in lupus nephropathy. Lupus 2020, 29, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.Y.; Ka, S.M.; Chang, J.M.; Chen, H.C.; Shui, H.A.; Li, C.Y.; Hua, K.F.; Chang, W.L.; Huang, J.J.; Yang, S.S.; et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic. Biol. Med. 2011, 51, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Tian, F.; Zheng, H.; Whitman, S.A.; Lin, Y.; Zhang, Z.; Zhang, N.; Zhang, D.D. Nrf2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-κB-mediated inflammatory response. Kidney Int. 2014, 85, 333–343. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Hsieh, S.-C.; Lu, C.-S.; Wu, T.-H.; Liao, H.-T.; Wu, C.-H.; Li, K.-J.; Kuo, Y.-M.; Lee, H.-T.; Shen, C.-Y.; et al. Cross-talk between mitochondrial dysfunction-provoked oxidative stress and aberrant noncoding RNA expression in the pathogenesis and pathophysiology of SLE. Int. J. Mol. Sci. 2019, 20, 5183. [Google Scholar] [CrossRef]
- Dendooven, A.; Ishola, D.A., Jr.; Nguyen, T.Q.; Van der Giezen, D.M.; Kok, R.J.; Goldschmeding, R.; Joles, J.A. Oxidative stress in obstructive nephropathy. Int. J. Exp. Pathol. 2011, 92, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh, M.A.; Nicholas, S.B.; Norris, K.C.; Vaziri, N.D. Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol. Dial. Transplant. 2013, 28, 2038–2045. [Google Scholar] [CrossRef]
- Al Za’abi, M.; Al Busaidi, M.; Yasin, J.; Schupp, N.; Nemmar, A.; Ali, B.H. Development of a new model for the induction of chronic kidney disease via intraperitoneal adenine administration, and the effect of treatment with gum acacia thereon. Am. J. Transl. Res. 2015, 7, 28–38. [Google Scholar] [PubMed]
- Zhou, Q.; Lv, D.; Xia, Y.; Zhao, Z.; Zou, H. Decreased expression of sirtuin 3 protein correlates with early stage chronic renal allograft dysfunction in a rat kidney model. Exp. Ther. Med. 2018, 15, 3725–3732. [Google Scholar] [CrossRef] [PubMed]
- Hannken, T.; Schroeder, R.; Stahl, R.A.K.; Wolf, G. Angiotensin II-mediated expression of p27(Kip1) and induction of cellular hypertrophy in renal tubular cells depend on the generation of oxygen radicals. Kidney Int. 1998, 54, 1923–1933. [Google Scholar] [CrossRef]
- Quoilin, C.; Mouithys-Mickalad, A.; Lécart, S.; Fontaine-Aupart, M.P.; Hoebeke, M. Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 1790–1800. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Kim, K.M.; Jeong, J.U.; Shin, J.M.; Kang, J.H.; Bang, K.; Kim, J.H. Nrf2-Heme Oxygenase-1 attenuates high-glucose-induced epithelial-to-mesenchymal transition of renal tubule cells by inhibiting ROS-mediated PI3K/Akt/GSK-3β signaling. J. Diabetes Res. 2019, 2019, 2510105. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.H.; Cho, S.; Joo, S.Y.; Ma, S.K.; Kim, S.H.; Lee, J.; Kim, S.W. 4-Hydroxy-2-hexenal-induced apoptosis in human renal proximal tubular epithelial cells. Nephrol. Dial. Transplant. 2011, 26, 3866–3873. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.I.; Kim, H.J.; Park, J.S.; Kim, I.J.; Bae, E.H.; Ma, S.K.; Kim, S.W. PGC-1α attenuates hydrogen peroxide-induced apoptotic cell death by upregulating Nrf-2 via GSK3β inactivation mediated by activated p38 in HK-2 Cells. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Ryoo, I.G.; Ha, H.; Kwak, M.K. Inhibitory role of the KEAP1-NRF2 pathway in TGFbβ1-stimulated renal epithelial transition to fibroblastic cells: A modulatory effect on SMAD signaling. PLoS ONE 2014, 9, e93265. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.C.; Honeyman, T.W.; Cooney, R.; Kennington, L.; Scheid, C.R.; Jonassen, J.A. Mitochondrial dysfunction is a primary event in renal cell oxalate toxicity. Kidney Int. 2004, 66, 1890–1900. [Google Scholar] [CrossRef]
- Lal, M.A.; Brismar, H.; Eklöf, A.C.; Aperia, A. Role of oxidative stress in advanced glycation end product-induced mesangial cell activation. Kidney Int. 2002, 61, 2006–2014. [Google Scholar] [CrossRef]
- Li, M.; Zhang, T.; Wu, X.; Chen, Y.; Sun, L. High glucose provokes microvesicles generation from glomerular podocytes via NOX4/ROS pathway. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Zhao, C.; Zhao, M.; Wang, H.; Zhang, B.; Wang, N.; Mao, H.; Zhang, A.; Xing, C. The roles of oxidative stress, endoplasmic reticulum stress, and autophagy in aldosterone/mineralocorticoid receptor-induced podocyte injury. Lab. Investig. 2015, 95, 1374–1386. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003, 278, 21592–21600. [Google Scholar] [CrossRef]
- Ruiz, S.; Pergola, P.E.; Zager, R.A.; Vaziri, N.D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013, 83, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Maines, M.D. The heme oxygenase system: A regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef]
- Uddin, M.J.; Li, C.-S.; Joe, Y.; Chen, Y.; Zhang, Q.; Ryter, S.W.; Chung, H.T. Carbon monoxide inhibits tenascin-C mediated inflammation via IL-10 expression in a septic mouse model. Mediat. Inflamm. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Jeong, S.-O.; Zheng, M.; Chen, Y.; Cho, G.J.; Chung, H.T.; Joe, Y. Carbon monoxide attenuates dextran sulfate sodium-induced colitis via inhibition of GSK-3beta Signaling. Oxidative Med. Cell. Longev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.; Pak, E.; Ha, H. Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress. Korean J. Physiol. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Pei, G.; Wang, P.; Yang, J.; Zhu, F.; Guo, Y.; Wang, M.; Yao, Y.; Zeng, R.; Liao, W.; et al. Biliverdin Reductase A (BVRA) mediates macrophage expression of Interleukin-10 in injured kidney. Int. J. Mol. Sci. 2015, 16, 22621–22635. [Google Scholar] [CrossRef]
- Llesuy, S.F.; Tomaro, M.L. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. BBA Mol. Cell Res. 1994, 1223, 9–14. [Google Scholar] [CrossRef]
- Balla, G.; Jacob, H.S.; Balla, J.; Rosenberg, M.; Nath, K.; Apple, F.; Eaton, J.W.; Vercellotti, G.M. Ferritin: A cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 1992, 267, 18148–18153. [Google Scholar] [CrossRef]
- Kim, H.J.; Zheng, M.; Kim, S.K.; Cho, J.J.; Shin, C.H.; Joe, Y.; Chung, H.T. CO/HO-1 Induces NQO-1 Expression via Nrf2 Activation. Immune Netw. 2011, 11, 376–382. [Google Scholar] [CrossRef]
- Turkseven, S.; Kruger, A.; Mingone, C.J.; Kaminski, P.; Inaba, M.; Rodella, L.F.; Ikehara, S.; Wolin, M.S.; Abraham, N.G. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H701–H707. [Google Scholar] [CrossRef]
- Jarmi, T.; Agarwal, A. Heme oxygenase and renal disease. Curr. Hypertens. Rep. 2009, 11, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Hill-Kapturczak, N.; Chang, S.H.; Agarwal, A. Heme oxygenase and the kidney. DNA Cell Biol. 2002, 21, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Díaz, A.G.; Pazarín-Villaseñor, L.; Yanowsky-Escatell, F.G.; Andrade-Sierra, J. Oxidative stress in diabetic nephropathy with early chronic kidney disease. J. Diabetes Res. 2016, 2016, 7047238. [Google Scholar] [CrossRef]
- Chang, S.H.; Barbosa-Tessmann, I.; Chen, C.; Kilberg, M.S.; Agarwal, A. Glucose deprivation induces heme oxygenase-1 gene expression by a pathway independent of the unfolded protein response. J. Biol. Chem. 2002, 277, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, C.; Marfella, R.; Cuzzocrea, S.; Piegari, E.; Petronella, P.; Giugliano, D.; Rossi, F.; D’Amico, M. Hyperglycemia in streptozotocin-induced diabetic rat increases infarct size associated with low levels of myocardial HO-1 during ischemia/reperfusion. Diabetes 2005, 54, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Kruger, A.L.; Peterson, S.; Turkseven, S.; Kaminski, P.M.; Zhang, F.F.; Quan, S.; Wolin, M.S.; Abraham, N.G. D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation 2005, 111, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Stewart, D.; Touchard, C.; Boinapally, S.; Choi, A.M.; Cook, J.L. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999, 274, 26071–26078. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Chen, X.; Han, K.; Zhang, T.; Qi, G.; Jiang, Z.; Hu, C. Grass carp (Ctenopharyngodon idella) NRF2 alleviates the oxidative stress and enhances cell viability through upregulating the expression of HO-1. Fish Physiol. Biochem. 2020, 46, 417–428. [Google Scholar] [CrossRef]
- Tanaka, Y.; Maher, J.M.; Chen, C.; Klaassen, C.D. Hepatic ischemia-reperfusion induces renal heme oxygenase-1 via NF-E2-related factor 2 in rats and mice. Mol. Pharmacol. 2007, 71, 817–825. [Google Scholar] [CrossRef]
- Yoh, K.; Itoh, K.; Enomoto, A.; Hirayama, A.; Yamaguchi, N.; Kobayashi, M.; Morito, N.; Koyama, A.; Yamamoto, M.; Takahashi, S. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001, 60, 1343–1353. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Scollick, C.; Traore, K.; Yates, M.; Trush, M.A.; Liby, K.T.; Sporn, M.B.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem. Biophys. Res. Commun. 2006, 351, 883–889. [Google Scholar] [CrossRef]
- Wei, Y.; Gong, J.; Yoshida, T.; Eberhart, C.G.; Xu, Z.; Kombairaju, P.; Sporn, M.B.; Handa, J.T.; Duh, E.J. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free Radic. Biol. Med. 2011, 51, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.M.; McNally, S.J.; Devey, L.; Garden, O.J.; Ross, J.A.; Wigmore, S.J. Insulin induces heme oxygenase-1 through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in renal cells. FEBS J. 2006, 273, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, F.; Zhang, L.; Cao, Y.; Liu, W.; Hao, J.; Liu, Q.; Duan, H. Modulation of Nrf2 expression alters high glucose-induced oxidative stress and antioxidant gene expression in mouse mesangial cells. Cell. Signal. 2011, 23, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Oh, G.S.; Lee, B.S.; Rim, J.S.; Kim, Y.M.; Chung, H.T. 3-Hydroxyanthranilic acid, one of l-tryptophan metabolites, inhibits monocyte chemoattractant protein-1 secretion and vascular cell adhesion molecule-1 expression via heme oxygenase-1 induction in human umbilical vein endothelial cells. Atherosclerosis 2006, 187, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Mylroie, H.; Dumont, O.; Bauer, A.; Thornton, C.C.; Mackey, J.; Calay, D.; Hamdulay, S.S.; Choo, J.R.; Boyle, J.J.; Samarel, A.M.; et al. PKCε-CREB-Nrf2 signalling induces HO-1 in the vascular endothelium and enhances resistance to inflammation and apoptosis. Cardiovasc. Res. 2015, 106, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Rojo, A.I.; Salinas, M.; Diaz, R.; Gallardo, G.; Alam, J.; De Galarreta, C.M.; Cuadrado, A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004, 279, 8919–8929. [Google Scholar] [CrossRef]
- Sun, J.; Hoshino, H.; Takaku, K.; Nakajima, O.; Muto, A.; Suzuki, H.; Tashiro, S.; Takahashi, S.; Shibahara, S.; Alam, J.; et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002, 21, 5216–5224. [Google Scholar] [CrossRef]
- Dong, C.; Wu, G.; Li, H.; Qiao, Y.; Gao, S. Ampelopsin inhibits high glucose-induced extracellular matrix accumulation and oxidative stress in mesangial cells through activating the Nrf2/HO-1 pathway. Phytother. Res. 2020, 34, 2044–2052. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Zhu, H.; Wang, J.; Ma, J.; Gu, M. Apigenin protects against renal tubular epithelial cell injury and oxidative stress by high glucose via regulation of NF-E2-related factor 2 (Nrf2) pathway. Med. Sci. Monit. 2019, 25, 5280–5288. [Google Scholar] [CrossRef]
- Xie, X.; Chen, Q.; Tao, J. Astaxanthin promotes Nrf2/ARE signaling to inhibit hg-induced renal fibrosis in GMCs. Mar. Drugs 2018, 16, 117. [Google Scholar] [CrossRef]
- He, L.; Liu, G.; Shi, Y.; Peng, X.; Liu, H.; Peng, Y. Astaxanthin attenuates adriamycin-induced focal segmental glomerulosclerosis. Pharmacology 2015, 95, 193–200. [Google Scholar] [CrossRef]
- Li, D.; Shi, G.; Wang, J.; Zhang, D.; Pan, Y.; Dou, H.; Hou, Y. Baicalein ameliorates pristane-induced lupus nephritis via activating Nrf2/HO-1 in myeloid-derived suppressor cells. Arthritis Res. Ther. 2019, 21. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Said, E.; Atef, H.; Zaitone, S.A. Renoprotective effect of calycosin in high fat diet-fed/STZ injected rats: Effect on IL-33/ST2 signaling, oxidative stress and fibrosis suppression. Chem. Biol. Interact. 2020, 315. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Zhang, X.S.; Liang, C.Z. Cryptotanshinone attenuates oxidative stress and inflammation through the regulation of Nrf-2 and NF-κB in mice with unilateral ureteral obstruction. Basic Clin. Pharmacol. Toxicol. 2018, 123, 714–720. [Google Scholar] [CrossRef]
- Tapia, E.; García-Arroyo, F.; Silverio, O.; Rodríguez-Alcocer, A.N.; Jiménez-Flores, A.B.; Cristobal, M.; Arellano, A.S.; Soto, V.; Osorio-Alonso, H.; Molina-Jijón, E.; et al. Mycophenolate mofetil and curcumin provide comparable therapeutic benefit in experimental chronic kidney disease: Role of Nrf2-Keap1 and renal dopamine pathways. Free Radic. Res. 2016, 50, 781–792. [Google Scholar] [CrossRef]
- Ali, B.H.; Al-Salam, S.; Al Suleimani, Y.; Al Kalbani, J.; Al Bahlani, S.; Ashique, M.; Manoj, P.; Al Dhahli, B.; Al Abri, N.; Naser, H.T.; et al. Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic Clin. Pharmacol. Toxicol. 2018, 122, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, D.; Guo, L.; Liang, W.; Jiang, Y.; Li, H.; Zhao, Y.; Lu, S.; Chi, Z.H. Curcumin protects renal tubular epithelial cells from high glucose-induced epithelial-to-mesenchymal transition through Nrf2-mediated upregulation of heme oxygenase-1. Mol. Med. Rep. 2015, 12, 1347–1355. [Google Scholar] [CrossRef]
- Sun, W.; Liu, X.; Zhang, H.; Song, Y.; Li, T.; Liu, X.; Liu, Y.; Guo, L.; Wang, F.; Yang, T.; et al. Epigallocatechin gallate upregulates NRF2 to prevent diabetic nephropathy via disabling KEAP1. Free Radic. Biol. Med. 2017, 108, 840–857. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Du, F.; Su, X.; Sun, G.; Zhou, G.; Bian, X.; Liu, N. Epigallocatechin-3-Gallate attenuates oxidative stress and inflammation in obstructive nephropathy via NF-κB and Nrf2/HO-1 signalling pathway regulation. Basic Clin. Pharmacol. Toxicol. 2015, 117, 164–172. [Google Scholar] [CrossRef]
- An, L.; Zhou, M.; Marikar, F.M.M.T.; Hu, X.W.; Miao, Q.Y.; Li, P.; Chen, J. Salvia miltiorrhiza lipophilic fraction attenuates oxidative stress in diabetic nephropathy through activation of nuclear factor erythroid 2-related factor 2. Am. J. Chin. Med. 2017, 45, 1441–1457. [Google Scholar] [CrossRef]
- Castejon, M.L.; Sánchez-Hidalgo, M.; Aparicio-Soto, M.; Montoya, T.; Martín-LaCave, I.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Dietary oleuropein and its new acyl-derivate attenuate murine lupus nephritis through HO-1/Nrf2 activation and suppressing JAK/STAT, NF-κB, MAPK and NLRP3 inflammasome signaling pathways. J. Nutr. Biochem. 2019, 74. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Dong, Z. Osthole protects against inflammation in a rat model of chronic kidney failure via suppression of nuclear factor-κB, transforming growth factor-β1 and activation of phosphoinositide 3-kinase/protein kinase B/nuclear factor (erythroid-derived 2)-like 2 signaling. Mol. Med. Rep. 2017, 16, 4915–4921. [Google Scholar] [CrossRef] [PubMed]
- Koca, H.B.; Pektas, M.B.; Koca, S.; Pektas, G.; Sadi, G. Diabetes-induced renal failure is associated with tissue inflammation and neutrophil gelatinase-associated lipocalin: Effects of resveratrol. Arch. Biol. Sci. 2016, 68, 747–752. [Google Scholar] [CrossRef]
- Bae, E.H.; Joo, S.Y.; Ma, S.K.; Lee, J.U.; Kim, S.W. Resveratrol attenuates 4-hydroxy-2-hexenal-induced oxidative stress in mouse cortical collecting duct cells. Korean J. Physiol. Pharmacol. 2016, 20, 229–236. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Zhao, D.; Ding, G.; Huang, S.; Zhang, A.; Jia, Z. Rotenone remarkably attenuates oxidative stress, inflammation, and fibrosis in chronic obstructive uropathy. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef]
- Lu, H.; Li, Y.; Zhang, T.; Liu, M.; Chi, Y.; Liu, S.; Shi, Y. Salidroside reduces high-glucose-induced podocyte apoptosis and oxidative stress via upregulating heme oxygenase-1 (HO-1) expression. Med. Sci. Monit. 2017, 23, 4067–4076. [Google Scholar] [CrossRef]
- Wu, P.; Yan, Y.; Ma, L.L.; Hou, B.Y.; He, Y.Y.; Zhang, L.; Niu, Z.R.; Song, J.K.; Pang, X.C.; Yang, X.Y.; et al. Effects of the Nrf2 protein modulator salvianolic acid a alone or combined with metformin on diabetes-associated macrovascular and renal injury. J. Biol. Chem. 2016, 291, 22288–22301. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zhang, H.F.; Wang, J.H.; Wang, Y.L.; Gao, C.; Gu, Y.T.; Huang, J.; Zhang, Z. Salvianolic acid A protects the kidney against oxidative stress by activating the Akt/GSK-3 β /Nrf2 signaling pathway and inhibiting the NF- B signaling pathway in 5/6 nephrectomized rats. Oxidative Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Prabu, S.M.; Muthumani, M. Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol. Biol. Rep. 2012, 39, 11201–11216. [Google Scholar] [CrossRef]
- Alaofi, A.L. Sinapic acid ameliorates the progression of streptozotocin (STZ)-induced diabetic nephropathy in rats via NRF2/HO-1 mediated pathways. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Lu, C.; Fan, G.; Wang, D. Akebia Saponin D ameliorated kidney injury and exerted anti-inflammatory and anti-apoptotic effects in diabetic nephropathy by activation of NRF2/HO-1 and inhibition of NF-KB pathway. Int. Immunopharmacol. 2020, 84. [Google Scholar] [CrossRef] [PubMed]
- García Trejo, E.M.Á.; Buendía, A.S.A.; Reyes, O.S.; Arroyo, F.E.G.; García, R.A.; Mendoza, M.L.L.; Tapia, E.; Lozada, L.G.S.; Alonso, H.O. The beneficial effects of Allicin in chronic kidney disease are comparable to Losartan. Int. J. Mol. Sci. 2017, 18, 1980. [Google Scholar] [CrossRef]
- Tsai, P.Y.; Ka, S.M.; Chao, T.K.; Chang, J.M.; Lin, S.H.; Li, C.Y.; Kuo, M.T.; Chen, P.; Chen, A. Antroquinonol reduces oxidative stress by enhancing the Nrf2 signaling pathway and inhibits inflammation and sclerosis in focal segmental glomerulosclerosis mice. Free Radic. Biol. Med. 2011, 50, 1503–1516. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, S.; Song, Y.; Ling, C. Artemisinin attenuates early renal damage on diabetic nephropathy rats through suppressing TGF-β1 regulator and activating the Nrf2 signaling pathway. Life Sci. 2020, 256. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhu, Z.; Zhang, J.; Ren, C.; Zhang, Q. Aucubin alleviates diabetic nephropathy by inhibiting NF-κB activation and inducing SIRT1/SIRT3-FOXO3a signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. J. Funct. Foods 2020, 64. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Liang, D.; Jiang, Y.; Liang, W.; Chi, Z.H.; Ma, J. Protective effects of berberine on renal injury in streptozotocin (STZ)-Induced diabetic mice. Int. J. Mol. Sci. 2016, 17, 1327. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Zhang, H.; Wang, X.Z.; Yang, X.Z.; Wu, S.N.; Wang, H.G.; Shen, P.; Ma, T.H. The protective effect of betulinic acid (BA) diabetic nephropathy on streptozotocin (STZ)-induced diabetic rats. Food Funct. 2017, 8, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Hua, K.F.; Lin, Y.C.; Chen, A.; Chang, J.M.; Kuoping Chao, L.; Ho, C.L.; Ka, S.M. Citral is renoprotective for focal segmental glomerulosclerosis by inhibiting oxidative stress and apoptosis and activating Nrf2 pathway in mice. PLoS ONE 2013, 8, e74871. [Google Scholar] [CrossRef]
- Qiao, Y.; Xu, L.; Tao, X.; Yin, L.; Qi, Y.; Xu, Y.; Han, X.; Tang, Z.; Ma, X.; Liu, K.; et al. Protective effects of dioscin against fructose-induced renal damage via adjusting Sirt3-mediated oxidative stress, fibrosis, lipid metabolism and inflammation. Toxicol. Lett. 2018, 284, 37–45. [Google Scholar] [CrossRef]
- Chen, L.; Chen, D.Q.; Wang, M.; Liu, D.; Chen, H.; Dou, F.; Vaziri, N.D.; Zhao, Y.Y. Role of RAS/Wnt/β-catenin axis activation in the pathogenesis of podocyte injury and tubulo-interstitial nephropathy. Chem. Biol. Interact. 2017, 273, 56–72. [Google Scholar] [CrossRef]
- Yu, X.; Fang, Y.; Ding, X.; Liu, H.; Zhu, J.; Zou, J.; Xu, X.; Zhong, Y. Transient hypoxia-inducible factor activation in rat renal ablation and reduced fibrosis with L-mimosine. Nephrology 2012, 17, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Dos Santos, M.; Veronese, F.V.; Rezzani, R. NLRP3 inflammasome modulation by melatonin supplementation in chronic pristane-induced lupus nephritis. Int. J. Mol. Sci. 2019, 20, 3466. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.; Zhang, C.; Shen, Q.; Sun, G.; Sun, X. Notoginsenoside R1 protects db/db mice against diabetic nephropathy via upregulation of Nrf2-mediated HO-1 expression. Molecules 2019, 24, 247. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, T.; Wang, H.; Jiang, Y.; Peng, S. Obacunone attenuates high glucose-induced oxidative damage in NRK-52E cells by inhibiting the activity of GSK-3β. Biochem. Biophys. Res. Commun. 2019, 513, 226–233. [Google Scholar] [CrossRef]
- Hong, Y.A.; Lim, J.H.; Kim, M.Y.; Kim, E.N.; Koh, E.S.; Shin, S.J.; Choi, B.S.; Park, C.W.; Chang, Y.S.; Chung, S. Delayed treatment with oleanolic acid attenuates tubulointerstitial fibrosis in chronic cyclosporine nephropathy through Nrf2/HO-1 signaling. J. Transl. Med. 2014, 12. [Google Scholar] [CrossRef]
- Wang, Z.; Han, N.; Zhao, K.; Li, Y.; Chi, Y.; Wang, B. Protective effects of pyrroloquinoline quinine against oxidative stress-induced cellular senescence and inflammation in human renal tubular epithelial cells via Keap1/Nrf2 signaling pathway. Int. Immunopharmacol. 2019, 72, 445–453. [Google Scholar] [CrossRef]
- Qin, T.; Yin, S.; Yang, J.; Zhang, Q.; Liu, Y.; Huang, F.; Cao, W. Sinomenine attenuates renal fibrosis through Nrf2-mediated inhibition of oxidative stress and TGFβ signaling. Toxicol. Appl. Pharmacol. 2016, 304, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, S.A.; Raslan, N.A.; Alzokaky, A.A.; Ewees, M.G.; Ashour, A.A.; Abdel-Hamied, H.E.; Abd-Allah, A.R. Contrast media (meglumine diatrizoate) aggravates renal inflammation, oxidative DNA damage and apoptosis in diabetic rats which is restored by sulforaphane through Nrf2/HO-1 reactivation. Chem. Biol. Interact. 2019, 309. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Zhou, Q.; Xia, Y.; You, X.; Zhao, Z.; Li, Y.; Zou, H. The association between oxidative stress alleviation via sulforaphane-induced Nrf2-HO-1/NQO-1 signaling pathway activation and chronic renal allograft dysfunction improvement. Kidney Blood Press. Res. 2018, 43, 191–205. [Google Scholar] [CrossRef]
- Peerapen, P.; Thongboonkerd, V. Protective roles of trigonelline against oxalate-induced epithelial-to-mesenchymal transition in renal tubular epithelial cells: An in vitro study. Food Chem. Toxicol. 2020, 135. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Li, J.; Chen, Z.; Huang, J.; Chen, Q.; Cai, W.; Liu, P.; Huang, H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating CKIP-1 to resist HG-induced up-regulation of FN and ICAM-1 in GMCs and diabetic mice kidneys. Free Radic. Biol. Med. 2017, 106, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Osorio, A.S.; García-Niño, W.R.; González-Reyes, S.; Álvarez-Mejía, A.E.; Guerra-León, S.; Salazar-Segovia, J.; Falcón, I.; Montes de Oca-Solano, H.; Madero, M.; Pedraza-Chaverri, J. The effect of dietary supplementation with curcumin on redox status and Nrf2 activation in patients with nondiabetic or diabetic proteinuric chronic kidney disease: A pilot study. J. Ren. Nutr. 2016, 26, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, L.; Salarolli, R.; Cardozo, L.F.M.F.; Santos, R.S.; de Brito, J.S.; Kemp, J.A.; Reis, D.; de Paiva, B.R.; Stenvinkel, P.; Lindholm, B.; et al. Impact of curcumin supplementation on expression of inflammatory transcription factors in hemodialysis patients: A pilot randomized, double-blind, controlled study. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).