Therapeutic Effects of Specialized Pro-Resolving Lipids Mediators on Cardiac Fibrosis via NRF2 Activation
Abstract
1. Introduction
2. Cardiac Fibrosis and Its Mediators
2.1. Cardiac Fibrosis
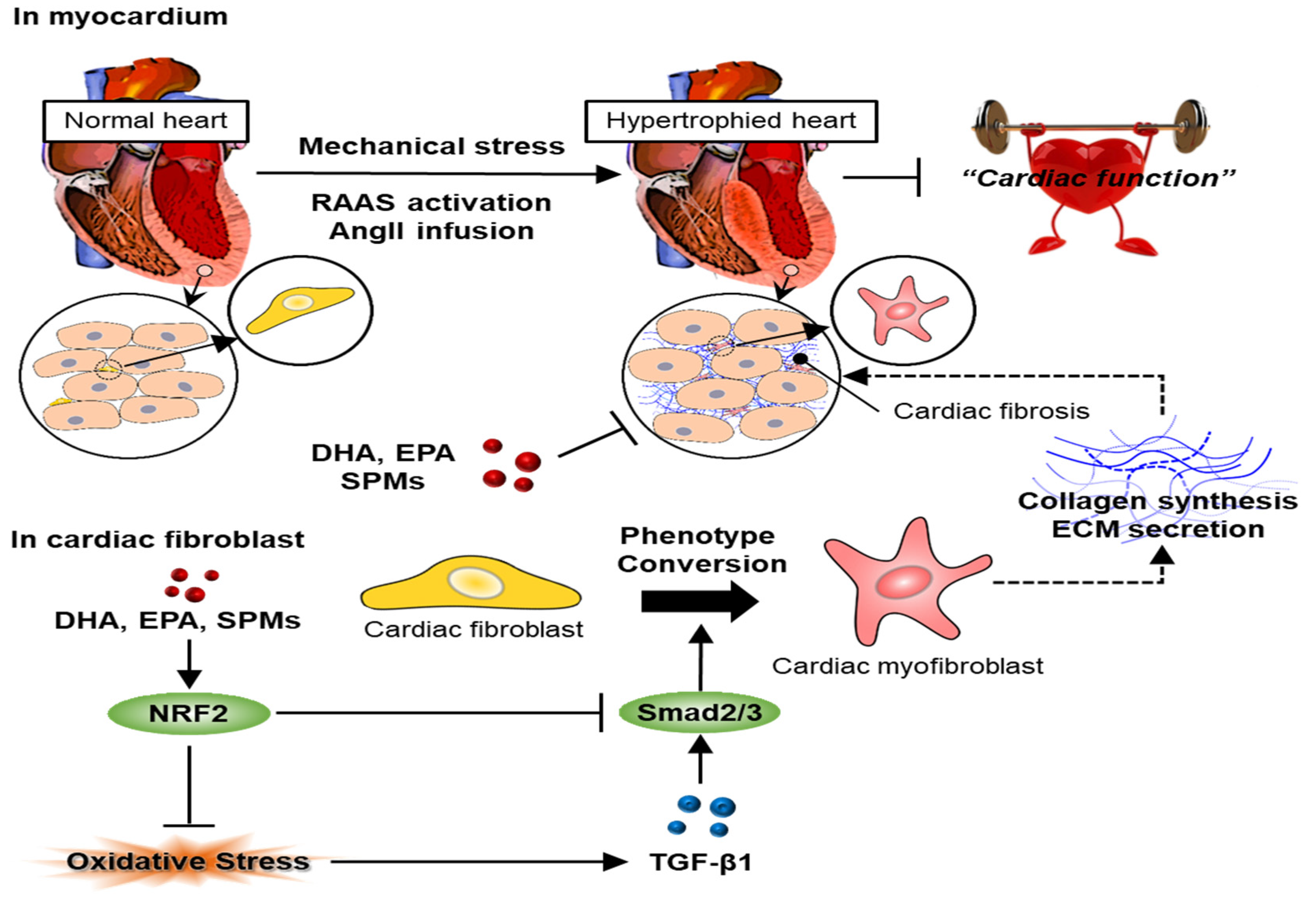
2.2. Mediators of Cardiac Fibrosis
2.3. Cellular Interaction in Cardiac Fibrosis
2.3.1. Cardiac Fibroblasts and Myofibroblasts
2.3.2. Inflammatory Cells
3. Role of NRF2 in Cardiac Fibrosis
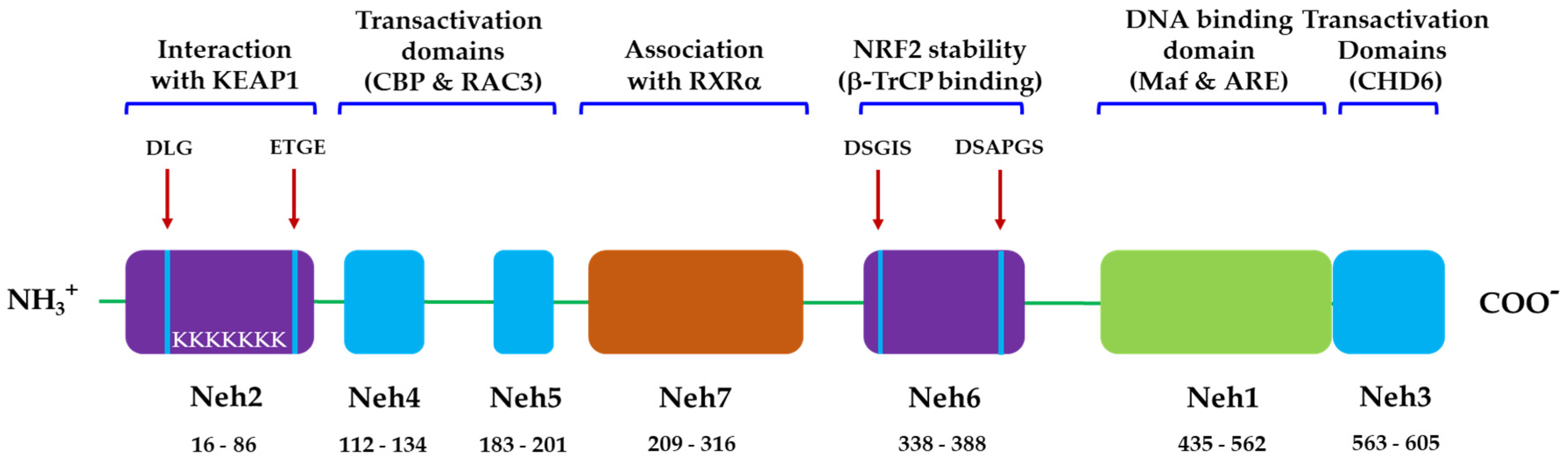
3.1. NRF2
3.2. Role of NRF2 in Cardiac Fibrosis
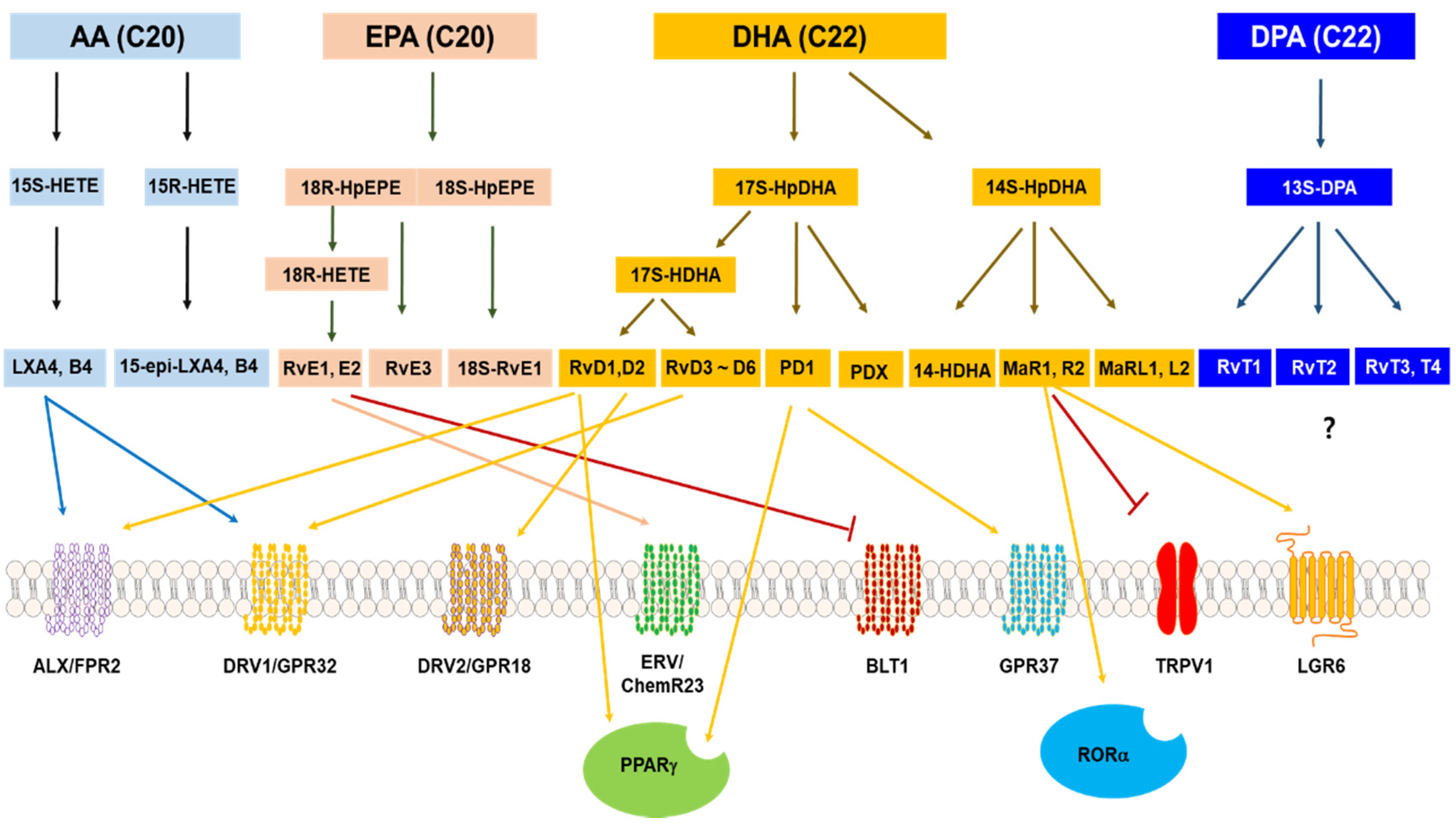
4. Specialized Pro-Resolving Lipid Mediators and Their Receptors
4.1. Types of Specialized Pro-Resolving Lipids
4.1.1. Lipoxins (LXs)
4.1.2. Resolvins (Rvs)
4.1.3. Maresins (MaRs) and Neuroprotectins (NPDs)
4.2. SPM Receptors
4.2.1. Chemerin Receptor 1
4.2.2. N-Formyl Peptide Receptor 2/LXA4 Receptor (FPR2/ALX)
4.2.3. GPR18
4.2.4. GPR32
4.2.5. GPR37
4.2.6. Leukotriene B4 Receptor 1 (BLT1)
4.2.7. Miscellaneous SPMs Receptors
5. Specialized Pro-Resolving Lipid Mediators and Cardiac Fibrosis
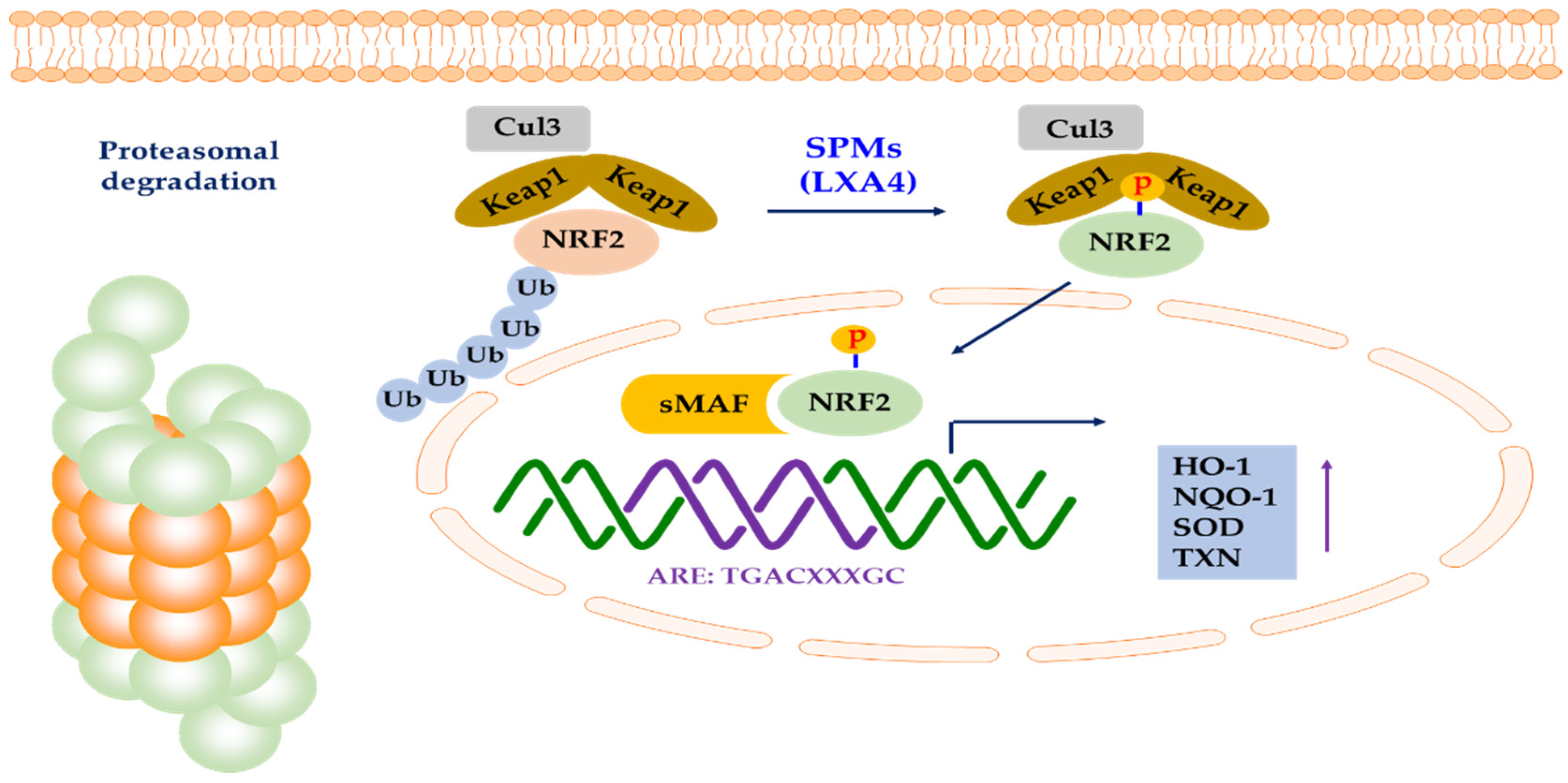
5.1. Connection between NRF2 and SPMs
5.1.1. Connection between NRF2 and DHA/EPA
5.1.2. Connection between NRF2 and LXs
5.1.3. Connection between NRF2 and Rvs
5.1.4. Connection between NRF2 and MaRs
5.2. Therapeutic Effects of DHA and EPA, and SPMs on Cardiac Fibrosis
5.2.1. Therapeutic Effects of DHPA and EPA on Cardiac Fibrosis
5.2.2. Therapeutic Effects of SPMs on Cardiac Fibrosis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Tao, H.; Shi, K.H.; Yang, J.J.; Huang, C.; Zhan, H.Y.; Li, J. Histone deacetylases in cardiac fibrosis: Current perspectives for therapy. Cell Signal. 2014, 26, 521–527. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef]
- Leask, A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ. Res. 2010, 106, 1675–1680. [Google Scholar] [CrossRef]
- Piek, A.; de Boer, R.A.; Sillje, H.H. The fibrosis-cell death axis in heart failure. Heart Fail. Rev. 2016, 21, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.R.; Fan, X.H.; Chen, G.; Zeng, G.W.; Xue, Y.G.; Liu, X.T.; Wang, C.Y. Irisin attenuates angiotensin II-induced cardiac fibrosis via Nrf2 mediated inhibition of ROS/TGFbeta1/Smad2/3 signaling axis. Chem. Biol. Interact. 2019, 302, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.H.; Mouton, A.J.; DeLeon-Pennell, K.Y.; Genovese, F.; Karsdal, M.; Lindsey, M.L. Understanding cardiac extracellular matrix remodeling to develop biomarkers of myocardial infarction outcomes. Matrix Biol. 2019, 75–76, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2011, 57, 891–903. [Google Scholar] [CrossRef]
- Brower, G.L.; Gardner, J.D.; Forman, M.F.; Murray, D.B.; Voloshenyuk, T.; Levick, S.P.; Janicki, J.S. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur. J. Cardiothorac. Surg. 2006, 30, 604–610. [Google Scholar] [CrossRef]
- Dai, Z.; Aoki, T.; Fukumoto, Y.; Shimokawa, H. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J. Cardiol. 2012, 60, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S. How does fibrosis promote atrial fibrillation persistence: In silico findings, clinical observations, and experimental data. Cardiovasc. Res. 2016, 110, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Baci, D.; Bosi, A.; Parisi, L.; Buono, G.; Mortara, L.; Ambrosio, G.; Bruno, A. Innate Immunity Effector Cells as Inflammatory Drivers of Cardiac Fibrosis. Int. J. Mol. Sci. 2020, 21, 7165. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Sun, Y.; Bhattacharya, S.K.; Ahokas, R.A.; Gerling, I.C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 2013, 10, 15–26. [Google Scholar] [CrossRef]
- Crabos, M.; Roth, M.; Hahn, A.W.; Erne, P. Characterization of angiotensin II receptors in cultured adult rat cardiac fibroblasts. Coupling to signaling systems and gene expression. J. Clin. Investig. 1994, 93, 2372–2378. [Google Scholar] [CrossRef]
- Kurisu, S.; Ozono, R.; Oshima, T.; Kambe, M.; Ishida, T.; Sugino, H.; Matsuura, H.; Chayama, K.; Teranishi, Y.; Iba, O.; et al. Cardiac angiotensin II type 2 receptor activates the kinin/NO system and inhibits fibrosis. Hypertension 2003, 41, 99–107. [Google Scholar] [CrossRef]
- Lijnen, P.; Petrov, V. Induction of cardiac fibrosis by aldosterone. J. Mol. Cell. Cardiol. 2000, 32, 865–879. [Google Scholar] [CrossRef]
- Messaoudi, S.; Gravez, B.; Tarjus, A.; Pelloux, V.; Ouvrard-Pascaud, A.; Delcayre, C.; Samuel, J.; Launay, J.M.; Sierra-Ramos, C.; Alvarez de la Rosa, D.; et al. Aldosterone-specific activation of cardiomyocyte mineralocorticoid receptor in vivo. Hypertension 2013, 61, 361–367. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-beta signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef]
- Villarreal, F.J.; Dillmann, W.H. Cardiac hypertrophy-induced changes in mRNA levels for TGF-beta 1, fibronectin, and collagen. Am. J. Physiol. 1992, 262, H1861–H1866. [Google Scholar] [CrossRef]
- Lyons, R.M.; Keski-Oja, J.; Moses, H.L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J. Cell Biol. 1988, 106, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc. Res. 2004, 63, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Desmouliere, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Schiller, M.; Javelaud, D.; Mauviel, A. TGF-beta-induced SMAD signaling and gene regulation: Consequences for extracellular matrix remodeling and wound healing. J. Dermatol. Sci. 2004, 35, 83–92. [Google Scholar] [CrossRef]
- Shi-Wen, X.; Rodriguez-Pascual, F.; Lamas, S.; Holmes, A.; Howat, S.; Pearson, J.D.; Dashwood, M.R.; du Bois, R.M.; Denton, C.P.; Black, C.M.; et al. Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: Evidence of an autocrine endothelin loop operating through the endothelin A and B receptors. Mol. Cell. Biol. 2006, 26, 5518–5527. [Google Scholar] [CrossRef]
- Leask, A. Targeting the TGFbeta, endothelin-1 and CCN2 axis to combat fibrosis in scleroderma. Cell Signal. 2008, 20, 1409–1414. [Google Scholar] [CrossRef]
- Shi-wen, X.; Kennedy, L.; Renzoni, E.A.; Bou-Gharios, G.; du Bois, R.M.; Black, C.M.; Denton, C.P.; Abraham, D.J.; Leask, A. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor beta in human lung fibroblasts. Arthritis Rheum. 2007, 56, 4189–4194. [Google Scholar] [CrossRef]
- Gevaert, A.B.; Shakeri, H.; Leloup, A.J.; Van Hove, C.E.; De Meyer, G.R.Y.; Vrints, C.J.; Lemmens, K.; Van Craenenbroeck, E.M. Endothelial Senescence Contributes to Heart Failure With Preserved Ejection Fraction in an Aging Mouse Model. Circ. Heart Fail. 2017, 10. [Google Scholar] [CrossRef]
- Testa, M.; Yeh, M.; Lee, P.; Fanelli, R.; Loperfido, F.; Berman, J.W.; LeJemtel, T.H. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J. Am. Coll. Cardiol. 1996, 28, 964–971. [Google Scholar] [CrossRef]
- Hofmann, U.; Knorr, S.; Vogel, B.; Weirather, J.; Frey, A.; Ertl, G.; Frantz, S. Interleukin-13 deficiency aggravates healing and remodeling in male mice after experimental myocardial infarction. Circ. Heart Fail. 2014, 7, 822–830. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Zhang, C.; Li, P.; Cui, W.; Hao, J.; Ma, X.; Yin, Z.; Du, J. gammadeltaT Cell-derived interleukin-17A via an interleukin-1beta-dependent mechanism mediates cardiac injury and fibrosis in hypertension. Hypertension 2014, 64, 305–314. [Google Scholar] [CrossRef]
- Kanisicak, O.; Khalil, H.; Ivey, M.J.; Karch, J.; Maliken, B.D.; Correll, R.N.; Brody, M.J.; Suh-Chin, J.L.; Aronow, B.J.; Tallquist, M.D.; et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 2016, 7, 12260. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.C.; van den Berg, A.; Nunes-Silva, V.; Weirather, J.; Peters, L.; Burkard, M.; Friedrich, M.; Pinnecker, J.; Abesser, M.; Heinze, K.G.; et al. Myocardial aging as a T-cell-mediated phenomenon. Proc. Natl. Acad. Sci. USA 2017, 114, E2420–E2429. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; King, K.R.; Hanley, A.; Hucker, W.J.; Wulfers, E.M.; Seemann, G.; Courties, G.; et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017, 169, 510–522.e20. [Google Scholar] [CrossRef] [PubMed]
- Dick, S.A.; Macklin, J.A.; Nejat, S.; Momen, A.; Clemente-Casares, X.; Althagafi, M.G.; Chen, J.; Kantores, C.; Hosseinzadeh, S.; Aronoff, L.; et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 2019, 20, 29–39. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Song, E.; Ouyang, N.; Horbelt, M.; Antus, B.; Wang, M.; Exton, M.S. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell. Immunol. 2000, 204, 19–28. [Google Scholar] [CrossRef]
- Simoes, F.C.; Cahill, T.J.; Kenyon, A.; Gavriouchkina, D.; Vieira, J.M.; Sun, X.; Pezzolla, D.; Ravaud, C.; Masmanian, E.; Weinberger, M.; et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat. Commun. 2020, 11, 600. [Google Scholar] [CrossRef]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Weinberg, S.; DeBerge, M.; Gainullina, A.; Schipma, M.; Kinchen, J.M.; Ben-Sahra, I.; Gius, D.R.; Yvan-Charvet, L.; Chandel, N.S.; et al. Efferocytosis Fuels Requirements of Fatty Acid Oxidation and the Electron Transport Chain to Polarize Macrophages for Tissue Repair. Cell Metab. 2019, 29, 443–456.e5. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Nahrendorf, M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Gentek, R.; Hoeffel, G. The Innate Immune Response in Myocardial Infarction, Repair, and Regeneration. Adv. Exp. Med. Biol. 2017, 1003, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The immune system and cardiac repair. Pharmacol. Res. 2008, 58, 88–111. [Google Scholar] [CrossRef]
- Nuamnaichati, N.; Sato, V.H.; Moongkarndi, P.; Parichatikanond, W.; Mangmool, S. Sustained beta-AR stimulation induces synthesis and secretion of growth factors in cardiac myocytes that affect on cardiac fibroblast activation. Life Sci. 2018, 193, 257–269. [Google Scholar] [CrossRef]
- Zhao, D.; Li, C.; Yan, H.; Li, T.; Qian, M.; Zheng, N.; Jiang, H.; Liu, L.; Xu, B.; Wu, Q.; et al. Cardiomyocyte Derived miR-328 Promotes Cardiac Fibrosis by Paracrinely Regulating Adjacent Fibroblasts. Cell. Physiol. Biochem. 2018, 46, 1555–1565. [Google Scholar] [CrossRef]
- Ge, Z.D.; Lian, Q.; Mao, X.; Xia, Z. Current Status and Challenges of NRF2 as a Potential Therapeutic Target for Diabetic Cardiomyopathy. Int. Heart J. 2019, 60, 512–520. [Google Scholar] [CrossRef]
- Wu, J.; Xia, S.; Kalionis, B.; Wan, W.; Sun, T. The role of oxidative stress and inflammation in cardiovascular aging. BioMed Res. Int. 2014, 2014, 615312. [Google Scholar] [CrossRef]
- Parim, B.; Sathibabu Uddandrao, V.V.; Saravanan, G. Diabetic cardiomyopathy: Molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail. Rev. 2019, 24, 279–299. [Google Scholar] [CrossRef]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Dodson, M.; de la Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in Disease: Timing Is Everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; Katsuoka, F.; Engel, J.D.; Yamamoto, M. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 6379–6384. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
- Nioi, P.; Nguyen, T.; Sherratt, P.J.; Pickett, C.B. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol. Cell. Biol. 2005, 25, 10895–10906. [Google Scholar] [CrossRef]
- Ahn, J.H.; Hwang, S.H.; Cho, H.S.; Lee, M. Differential Gene Expression Common to Acquired and Intrinsic Resistance to BRAF Inhibitor Revealed by RNA-Seq Analysis. Biomol. Ther. 2019, 27, 302–310. [Google Scholar] [CrossRef]
- Ge, C.; Hu, L.; Lou, D.; Li, Q.; Feng, J.; Wu, Y.; Tan, J.; Xu, M. Nrf2 deficiency aggravates PM2.5-induced cardiomyopathy by enhancing oxidative stress, fibrosis and inflammation via RIPK3-regulated mitochondrial disorder. Aging 2020, 12, 4836–4865. [Google Scholar] [CrossRef]
- Li, J.; Ichikawa, T.; Villacorta, L.; Janicki, J.S.; Brower, G.L.; Yamamoto, M.; Cui, T. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arter. Thromb. Vasc. Biol. 2009, 29, 1843–1850. [Google Scholar] [CrossRef]
- Tian, C.; Gao, L.; Zimmerman, M.C.; Zucker, I.H. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H928–H939. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duan, J.Z.; He, Q.; Wang, C.Q. miR155 modulates high glucoseinduced cardiac fibrosis via the Nrf2/HO1 signaling pathway. Mol. Med. Rep. 2020. [Google Scholar] [CrossRef]
- Cai, S.A.; Hou, N.; Zhao, G.J.; Liu, X.W.; He, Y.Y.; Liu, H.L.; Hua, Y.Q.; Li, L.R.; Huang, Y.; Ou, C.W.; et al. Nrf2 Is a Key Regulator on Puerarin Preventing Cardiac Fibrosis and Upregulating Metabolic Enzymes UGT1A1 in Rats. Front. Pharmacol. 2018, 9, 540. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Fu, M.; Cheng, B.; Kang, Y.; Xie, D. Galanthamine improves myocardial ischemia-reperfusion-induced cardiac dysfunction, endoplasmic reticulum stress-related apoptosis, and myocardial fibrosis by suppressing AMPK/Nrf2 pathway in rats. Ann. Transl. Med. 2019, 7, 634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, Y.; Dai, S.; Deng, W.; Wang, H.; Qin, W.; Yang, H.; Liu, H.; Yue, J.; Wu, D.; et al. Isorhynchophylline enhances Nrf2 and inhibits MAPK pathway in cardiac hypertrophy. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 203–212. [Google Scholar] [CrossRef]
- Song, J.; Meng, Y.; Wang, M.; Li, L.; Liu, Z.; Zheng, K.; Wu, L.; Liu, B.; Hou, F.; Li, A. Mangiferin activates Nrf2 to attenuate cardiac fibrosis via redistributing glutaminolysis-derived glutamate. Pharmacol. Res. 2020, 157, 104845. [Google Scholar] [CrossRef]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-kappaB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef]
- Pirault, J.; Back, M. Lipoxin and Resolvin Receptors Transducing the Resolution of Inflammation in Cardiovascular Disease. Front. Pharmacol. 2018, 9, 1273. [Google Scholar] [CrossRef]
- Romano, M.; Cianci, E.; Simiele, F.; Recchiuti, A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur. J. Pharmacol. 2015, 760, 49–63. [Google Scholar] [CrossRef]
- Markworth, J.F.; Maddipati, K.R.; Cameron-Smith, D. Emerging roles of pro-resolving lipid mediators in immunological and adaptive responses to exercise-induced muscle injury. Exerc. Immunol. Rev. 2016, 22, 110–134. [Google Scholar]
- Chandrasekharan, J.A.; Sharma-Walia, N. Lipoxins: Nature’s way to resolve inflammation. J. Inflamm. Res. 2015, 8, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014, 7, a016311. [Google Scholar] [CrossRef] [PubMed]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wang, C.W.; Arnardottir, H.H.; Li, Y.; Cheng, C.Y.; Dalli, J.; Serhan, C.N. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS ONE 2014, 9, e102362. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Gronert, K.; Devchand, P.R.; Moussignac, R.L.; Serhan, C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 2003, 278, 14677–14687. [Google Scholar] [CrossRef]
- Bazan, N.G. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 136–141. [Google Scholar] [CrossRef]
- Shinohara, M.; Mirakaj, V.; Serhan, C.N. Functional Metabolomics Reveals Novel Active Products in the DHA Metabolome. Front. Immunol. 2012, 3, 81. [Google Scholar] [CrossRef]
- Balas, L.; Durand, T. Dihydroxylated E,E,Z-docosatrienes. An overview of their synthesis and biological significance. Prog. Lipid Res. 2016, 61, 1–18. [Google Scholar] [CrossRef]
- Arita, M.; Bianchini, F.; Aliberti, J.; Sher, A.; Chiang, N.; Hong, S.; Yang, R.; Petasis, N.A.; Serhan, C.N. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005, 201, 713–722. [Google Scholar] [CrossRef]
- Arita, M.; Ohira, T.; Sun, Y.P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007, 178, 3912–3917. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Langmead, C.J.; Riddy, D.M. New Advances in Targeting the Resolution of Inflammation: Implications for Specialized Pro-Resolving Mediator GPCR Drug Discovery. ACS Pharmacol. Transl. Sci. 2020, 3, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Im, D.S. Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Prog. Lipid Res. 2012, 51, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Gantz, I.; Konda, Y.; Yang, Y.K.; Miller, D.E.; Dierick, H.A.; Yamada, T. Molecular cloning of a novel receptor (CMKLR1) with homology to the chemotactic factor receptors. Cytogenet. Cell Genet. 1996, 74, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.; Edinger, A.L.; Stordeur, P.; Rucker, J.; Verhasselt, V.; Sharron, M.; Govaerts, C.; Mollereau, C.; Vassart, G.; Doms, R.W.; et al. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur. J. Immunol. 1998, 28, 1689–1700. [Google Scholar] [CrossRef]
- Meder, W.; Wendland, M.; Busmann, A.; Kutzleb, C.; Spodsberg, N.; John, H.; Richter, R.; Schleuder, D.; Meyer, M.; Forssmann, W.G. Characterization of human circulating TIG2 as a ligand for the orphan receptor ChemR23. FEBS Lett. 2003, 555, 495–499. [Google Scholar] [CrossRef]
- Wittamer, V.; Gregoire, F.; Robberecht, P.; Vassart, G.; Communi, D.; Parmentier, M. The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J. Biol. Chem. 2004, 279, 9956–9962. [Google Scholar] [CrossRef]
- Isobe, Y.; Arita, M.; Matsueda, S.; Iwamoto, R.; Fujihara, T.; Nakanishi, H.; Taguchi, R.; Masuda, K.; Sasaki, K.; Urabe, D.; et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 2012, 287, 10525–10534. [Google Scholar] [CrossRef]
- Luangsay, S.; Wittamer, V.; Bondue, B.; De Henau, O.; Rouger, L.; Brait, M.; Franssen, J.D.; de Nadai, P.; Huaux, F.; Parmentier, M. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J. Immunol. 2009, 183, 6489–6499. [Google Scholar] [CrossRef]
- Freire, M.O.; Dalli, J.; Serhan, C.N.; Van Dyke, T.E. Neutrophil Resolvin E1 Receptor Expression and Function in Type 2 Diabetes. J. Immunol. 2017, 198, 718–728. [Google Scholar] [CrossRef]
- Mocker, A.; Hilgers, K.F.; Cordasic, N.; Wachtveitl, R.; Menendez-Castro, C.; Woelfle, J.; Hartner, A.; Fahlbusch, F.B. Renal Chemerin Expression is Induced in Models of Hypertensive Nephropathy and Glomerulonephritis and Correlates with Markers of Inflammation and Fibrosis. Int. J. Mol. Sci. 2019, 20, 6240. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Kurt, R.; Gurdal, A.; Alahdab, Y.O.; Yonal, O.; Senates, E.; Polat, N.; Eren, F.; Imeryuz, N.; Oflaz, H. Circulating vaspin levels and epicardial adipose tissue thickness are associated with impaired coronary flow reserve in patients with nonalcoholic fatty liver disease. Atherosclerosis 2011, 217, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.D.; Cavanagh, S.L.; Quehenberger, O.; Prossnitz, E.R.; Cochrane, C.G. Isolation of a cDNA that encodes a novel granulocyte N-formyl peptide receptor. Biochem. Biophys. Res. Commun. 1992, 184, 582–589. [Google Scholar] [CrossRef]
- Fiore, S.; Maddox, J.F.; Perez, H.D.; Serhan, C.N. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J. Exp. Med. 1994, 180, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Brink, C.; Dahlen, S.E.; Drazen, J.; Evans, J.F.; Hay, D.W.; Nicosia, S.; Serhan, C.N.; Shimizu, T.; Yokomizo, T. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol. Rev. 2003, 55, 195–227. [Google Scholar] [CrossRef] [PubMed]
- Maderna, P.; Cottell, D.C.; Toivonen, T.; Dufton, N.; Dalli, J.; Perretti, M.; Godson, C. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J. 2010, 24, 4240–4249. [Google Scholar] [CrossRef]
- Maddox, J.F.; Serhan, C.N. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: Selective inactivation by dehydrogenation and reduction. J. Exp. Med. 1996, 183, 137–146. [Google Scholar] [CrossRef]
- Simiele, F.; Recchiuti, A.; Mattoscio, D.; De Luca, A.; Cianci, E.; Franchi, S.; Gatta, V.; Parolari, A.; Werba, J.P.; Camera, M.; et al. Transcriptional regulation of the human FPR2/ALX gene: Evidence of a heritable genetic variant that impairs promoter activity. FASEB J. 2012, 26, 1323–1333. [Google Scholar] [CrossRef]
- Waechter, V.; Schmid, M.; Herova, M.; Weber, A.; Gunther, V.; Marti-Jaun, J.; Wust, S.; Rosinger, M.; Gemperle, C.; Hersberger, M. Characterization of the promoter and the transcriptional regulation of the lipoxin A4 receptor (FPR2/ALX) gene in human monocytes and macrophages. J. Immunol. 2012, 188, 1856–1867. [Google Scholar] [CrossRef]
- Chen, K.; Bao, Z.; Gong, W.; Tang, P.; Yoshimura, T.; Wang, J.M. Regulation of inflammation by members of the formyl-peptide receptor family. J. Autoimmun. 2017, 85, 64–77. [Google Scholar] [CrossRef]
- Takano, T.; Fiore, S.; Maddox, J.F.; Brady, H.R.; Petasis, N.A.; Serhan, C.N. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: Evidence for anti-inflammatory receptors. J. Exp. Med. 1997, 185, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Cooray, S.N.; Gobbetti, T.; Montero-Melendez, T.; McArthur, S.; Thompson, D.; Clark, A.J.; Flower, R.J.; Perretti, M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc. Natl. Acad. Sci. USA 2013, 110, 18232–18237. [Google Scholar] [CrossRef] [PubMed]
- Bozinovski, S.; Uddin, M.; Vlahos, R.; Thompson, M.; McQualter, J.L.; Merritt, A.S.; Wark, P.A.; Hutchinson, A.; Irving, L.B.; Levy, B.D.; et al. Serum amyloid A opposes lipoxin A(4) to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 2012, 109, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Godson, C.; Guiry, P.J.; Agerberth, B.; Haeggstrom, J.Z. Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counterregulated by lipoxin A4 and resolvin E1. FASEB J. 2011, 25, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, S.H.; Han, S.Y.; Lee, Y.S.; Cho, J.; Kim, J.M. LXA4-FPR2 signaling regulates radiation-induced pulmonary fibrosis via crosstalk with TGF-beta/Smad signaling. Cell Death Dis. 2020, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Park, G.T.; Kwon, Y.W.; Lee, T.W.; Kwon, S.G.; Ko, H.C.; Kim, M.B.; Kim, J.H. Formyl Peptide Receptor 2 Activation Ameliorates Dermal Fibrosis and Inflammation in Bleomycin-Induced Scleroderma. Front. Immunol. 2019, 10, 2095. [Google Scholar] [CrossRef]
- Higgins, G.; Fustero Torre, C.; Tyrrell, J.; McNally, P.; Harvey, B.J.; Urbach, V. Lipoxin A4 prevents tight junction disruption and delays the colonization of cystic fibrosis bronchial epithelial cells by Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L1053–L1061. [Google Scholar] [CrossRef]
- Kain, V.; Ingle, K.A.; Colas, R.A.; Dalli, J.; Prabhu, S.D.; Serhan, C.N.; Joshi, M.; Halade, G.V. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J. Mol. Cell. Cardiol. 2015, 84, 24–35. [Google Scholar] [CrossRef]
- Chiang, N.; Dalli, J.; Colas, R.A.; Serhan, C.N. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 2015, 212, 1203–1217. [Google Scholar] [CrossRef]
- Chiang, N.; de la Rosa, X.; Libreros, S.; Serhan, C.N. Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J. Immunol. 2017, 198, 842–851. [Google Scholar] [CrossRef]
- Chiang, N.; Barnaeva, E.; Hu, X.; Marugan, J.; Southall, N.; Ferrer, M.; Serhan, C.N. Identification of Chemotype Agonists for Human Resolvin D1 Receptor DRV1 with Pro-Resolving Functions. Cell Chem. Biol. 2019, 26, 244–254.e4. [Google Scholar] [CrossRef] [PubMed]
- Offertaler, L.; Mo, F.M.; Batkai, S.; Liu, J.; Begg, M.; Razdan, R.K.; Martin, B.R.; Bukoski, R.D.; Kunos, G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol. Pharmacol. 2003, 63, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Hasegawa, H.; Inoue, A.; Muraoka, M.; Miyazaki, T.; Oka, K.; Yasukawa, M. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem. Biophys. Res. Commun. 2006, 347, 827–832. [Google Scholar] [CrossRef]
- Wang, X.; Sumida, H.; Cyster, J.G. GPR18 is required for a normal CD8alphaalpha intestinal intraepithelial lymphocyte compartment. J. Exp. Med. 2014, 211, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Jones, C.N.; Yu, Y.M.; Fischman, A.J.; Watada, S.; Tompkins, R.G.; Fagan, S.P.; Irimia, D. Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB J. 2013, 27, 2270–2281. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, C.; Yang, L.; Zhang, Z.; Zhang, Q.; Wang, B.; Wang, X. GPR18 expression on PMNs as biomarker for outcome in patient with sepsis. Life Sci. 2019, 217, 49–56. [Google Scholar] [CrossRef]
- Sansbury, B.E.; Spite, M. Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology. Circ. Res. 2016, 119, 113–130. [Google Scholar] [CrossRef]
- Norling, L.V.; Dalli, J.; Flower, R.J.; Serhan, C.N.; Perretti, M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: Receptor-dependent actions. Arter. Thromb. Vasc. Biol. 2012, 32, 1970–1978. [Google Scholar] [CrossRef]
- Schmid, M.; Gemperle, C.; Rimann, N.; Hersberger, M. Resolvin D1 Polarizes Primary Human Macrophages toward a Proresolution Phenotype through GPR32. J. Immunol. 2016, 196, 3429–3437. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, M.K.; Lee, E.J.; Lee, C.H. Resolvin D1 inhibits TGF-beta1-induced epithelial mesenchymal transition of A549 lung cancer cells via lipoxin A4 receptor/formyl peptide receptor 2 and GPR32. Int. J. Biochem. Cell Biol. 2013, 45, 2801–2807. [Google Scholar] [CrossRef]
- Dalli, J.; Winkler, J.W.; Colas, R.A.; Arnardottir, H.; Cheng, C.Y.; Chiang, N.; Petasis, N.A.; Serhan, C.N. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem. Biol. 2013, 20, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Fredman, G.; Backhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, Q.; D’Souza, V.; Bartis, D.; Dancer, R.; Parekh, D.; Gao, F.; Lian, Q.; Jin, S.; Thickett, D.R. ResolvinD1 stimulates epithelial wound repair and inhibits TGF-beta-induced EMT whilst reducing fibroproliferation and collagen production. Lab. Investig. 2018, 98, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Marazziti, D.; Golini, E.; Gallo, A.; Lombardi, M.S.; Matteoni, R.; Tocchini-Valentini, G.P. Cloning of GPR37, a gene located on chromosome 7 encoding a putative G-protein-coupled peptide receptor, from a human frontal brain EST library. Genomics 1997, 45, 68–77. [Google Scholar] [CrossRef]
- Lopes, J.P.; Morato, X.; Souza, C.; Pinhal, C.; Machado, N.J.; Canas, P.M.; Silva, H.B.; Stagljar, I.; Gandia, J.; Fernandez-Duenas, V.; et al. The role of parkinson’s disease-associated receptor GPR37 in the hippocampus: Functional interplay with the adenosinergic system. J. Neurochem. 2015, 134, 135–146. [Google Scholar] [CrossRef]
- Fujita-Jimbo, E.; Yu, Z.L.; Li, H.; Yamagata, T.; Mori, M.; Momoi, T.; Momoi, M.Y. Mutation in Parkinson disease-associated, G-protein-coupled receptor 37 (GPR37/PaelR) is related to autism spectrum disorder. PLoS ONE 2012, 7, e51155. [Google Scholar] [CrossRef]
- Marazziti, D.; Mandillo, S.; Di Pietro, C.; Golini, E.; Matteoni, R.; Tocchini-Valentini, G.P. GPR37 associates with the dopamine transporter to modulate dopamine uptake and behavioral responses to dopaminergic drugs. Proc. Natl. Acad. Sci. USA 2007, 104, 9846–9851. [Google Scholar] [CrossRef]
- Yang, H.J.; Vainshtein, A.; Maik-Rachline, G.; Peles, E. G protein-coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination. Nat. Commun. 2016, 7, 10884. [Google Scholar] [CrossRef]
- Bang, S.; Xie, Y.K.; Zhang, Z.J.; Wang, Z.; Xu, Z.Z.; Ji, R.R. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J. Clin. Investig. 2018, 128, 3568–3582. [Google Scholar] [CrossRef]
- McCrary, M.R.; Jiang, M.Q.; Giddens, M.M.; Zhang, J.Y.; Owino, S.; Wei, Z.Z.; Zhong, W.; Gu, X.; Xin, H.; Hall, R.A.; et al. Protective effects of GPR37 via regulation of inflammation and multiple cell death pathways after ischemic stroke in mice. FASEB J. 2019, 33, 10680–10691. [Google Scholar] [CrossRef]
- Yokomizo, T.; Izumi, T.; Chang, K.; Takuwa, Y.; Shimizu, T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 1997, 387, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Haworth, O.; Cernadas, M.; Yang, R.; Serhan, C.N.; Levy, B.D. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 2008, 9, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.F.; Dona, M.; Fredman, G.; Krishnamoorthy, S.; Irimia, D.; Serhan, C.N. Resolvin E2 formation and impact in inflammation resolution. J. Immunol. 2012, 188, 4527–4534. [Google Scholar] [CrossRef] [PubMed]
- Unno, Y.; Sato, Y.; Fukuda, H.; Ishimura, K.; Ikeda, H.; Watanabe, M.; Tansho-Nagakawa, S.; Ubagai, T.; Shuto, S.; Ono, Y. Resolvin E1, but not resolvins E2 and E3, promotes fMLF-induced ROS generation in human neutrophils. FEBS Lett. 2018, 592, 2706–2715. [Google Scholar] [CrossRef]
- Liang, M.; Lv, J.; Jiang, Z.; He, H.; Chen, C.; Xiong, Y.; Zhu, X.; Xue, Y.; Yu, Y.; Yang, S.; et al. Promotion of Myofibroblast Differentiation and Tissue Fibrosis by the Leukotriene B4 -Leukotriene B4 Receptor Axis in Systemic Sclerosis. Arthritis Rheumatol. 2020, 72, 1013–1025. [Google Scholar] [CrossRef]
- Kamata, M.; Amano, H.; Ito, Y.; Fujita, T.; Otaka, F.; Hosono, K.; Kamata, K.; Takeuchi, Y.; Yokomizo, T.; Shimizu, T.; et al. Role of the high-affinity leukotriene B4 receptor signaling in fibrosis after unilateral ureteral obstruction in mice. PLoS ONE 2019, 14, e0202842. [Google Scholar] [CrossRef]
- Lv, J.; Xiong, Y.; Li, W.; Yang, W.; Zhao, L.; He, R. BLT1 Mediates Bleomycin-Induced Lung Fibrosis Independently of Neutrophils and CD4+ T Cells. J. Immunol. 2017, 198, 1673–1684. [Google Scholar] [CrossRef]
- Flak, M.B.; Koenis, D.S.; Sobrino, A.; Smith, J.; Pistorius, K.; Palmas, F.; Dalli, J. GPR101 mediates the pro-resolving actions of RvD5n-3 DPA in arthritis and infections. J. Clin. Investig. 2020, 130, 359–373. [Google Scholar] [CrossRef]
- Zhao, Y.; Calon, F.; Julien, C.; Winkler, J.W.; Petasis, N.A.; Lukiw, W.J.; Bazan, N.G. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer’s disease models. PLoS ONE 2011, 6, e15816. [Google Scholar] [CrossRef]
- Liao, Z.; Dong, J.; Wu, W.; Yang, T.; Wang, T.; Guo, L.; Chen, L.; Xu, D.; Wen, F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARgamma/NF-kappaB pathway. Respir. Res. 2012, 13, 110. [Google Scholar] [CrossRef]
- Kokeny, G.; Calvier, L.; Legchenko, E.; Chouvarine, P.; Mozes, M.M.; Hansmann, G. PPARgamma is a gatekeeper for extracellular matrix and vascular cell homeostasis: Beneficial role in pulmonary hypertension and renal/cardiac/pulmonary fibrosis. Curr. Opin. Nephrol. Hypertens. 2020, 29, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Li, T.; Zhuang, Y.; Li, Z.; Yang, W.; Huang, Q.; Li, D.; Wu, H.; Zhang, G.; Yang, T.; et al. Involvement of lncR-30245 in Myocardial Infarction-Induced Cardiac Fibrosis Through Peroxisome Proliferator-Activated Receptor-gamma-Mediated Connective Tissue Growth Factor Signalling Pathway. Can. J. Cardiol. 2019, 35, 480–489. [Google Scholar] [CrossRef]
- Park, C.K. Maresin 1 Inhibits TRPV1 in Temporomandibular Joint-Related Trigeminal Nociceptive Neurons and TMJ Inflammation-Induced Synaptic Plasticity in the Trigeminal Nucleus. Mediat. Inflamm. 2015, 2015, 275126. [Google Scholar] [CrossRef]
- Chiang, N.; Libreros, S.; Norris, P.C.; de la Rosa, X.; Serhan, C.N. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J. Clin. Investig. 2019, 129, 5294–5311. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Jiang, M.; Huang, W.; Liu, S. Antarctic Krill Oil Attenuates Oxidative Stress via the KEAP1-NRF2 Signaling in Patients with Coronary Heart Disease. Evid. Based Complement Altern. Med. 2020, 2020, 9534137. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, Y.; Kato, A.; Sango, K.; Himeno, T.; Kondo, M.; Kato, Y.; Kamiya, H.; Nakamura, J.; Kato, K. Omega-3 polyunsaturated fatty acids exert anti-oxidant effects through the nuclear factor (erythroid-derived 2)-related factor 2 pathway in immortalized mouse Schwann cells. J. Diabetes Investig. 2019, 10, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, J.; Sekhar, K.R.; Yin, H.; Yared, N.F.; Schneider, S.N.; Sasi, S.; Dalton, T.P.; Anderson, M.E.; Chan, J.Y.; et al. Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J. Biol. Chem. 2007, 282, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, C.; Yang, L.; Yoshizaki, T.; Nakagawa, F.; Ishikado, A.; Kondo, M.; Morino, K.; Sekine, O.; Ugi, S.; Nishio, Y.; et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/HO-1 pathway in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2013, 430, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Zgorzynska, E.; Dziedzic, B.; Gorzkiewicz, A.; Stulczewski, D.; Bielawska, K.; Su, K.P.; Walczewska, A. Omega-3 polyunsaturated fatty acids improve the antioxidative defense in rat astrocytes via an Nrf2-dependent mechanism. Pharmacol. Rep. 2017, 69, 935–942. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Mao, L.; Leak, R.K.; Shi, Y.; Zhang, W.; Hu, X.; Sun, B.; Cao, G.; Gao, Y.; et al. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J. Neurosci. 2014, 34, 1903–1915. [Google Scholar] [CrossRef]
- Mildenberger, J.; Johansson, I.; Sergin, I.; Kjobli, E.; Damas, J.K.; Razani, B.; Flo, T.H.; Bjorkoy, G. N-3 PUFAs induce inflammatory tolerance by formation of KEAP1-containing SQSTM1/p62-bodies and activation of NFE2L2. Autophagy 2017, 13, 1664–1678. [Google Scholar] [CrossRef] [PubMed]
- Picou, F.; Debeissat, C.; Bourgeais, J.; Gallay, N.; Ferrie, E.; Foucault, A.; Ravalet, N.; Maciejewski, A.; Vallet, N.; Ducrocq, E.; et al. n-3 Polyunsaturated fatty acids induce acute myeloid leukemia cell death associated with mitochondrial glycolytic switch and Nrf2 pathway activation. Pharmacol. Res. 2018, 136, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kim, G.D.; Yang, H.; Son, G.W.; Park, H.R.; Cho, J.J.; Ahn, H.J.; Park, C.S.; Park, Y.S. Effects of Eicosapentaenoic Acid on the Cytoprotection Through Nrf2-Mediated Heme Oxygenase-1 in Human Endothelial Cells. J. Cardiovasc. Pharmacol. 2015, 66, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, K.; Krishnaswamy, R.; Paramasivan, P.; Chih-Yang, H.; Vishwanadha, V.P. Eicosapentaenoic acid prevents TCDD-induced oxidative stress and inflammatory response by modulating MAP kinases and redox-sensitive transcription factors. Br. J. Pharmacol. 2015, 172, 4726–4740. [Google Scholar] [CrossRef] [PubMed]
- Vara-Messler, M.; Mukdsi, J.H.; Osieki, N.I.; Benizio, E.; Repossi, G.M.; Ajayi, E.I.O.; Garcia, N.H. Eicosapentaenoic acid prevents salt sensitivity in diabetic rats and decreases oxidative stress. Nutrition 2020, 72, 110644. [Google Scholar] [CrossRef]
- Jamil, M.U.; Kim, J.; Yum, H.W.; Kim, S.H.; Kim, S.J.; Kim, D.H.; Cho, N.C.; Na, H.K.; Surh, Y.J. 17-Oxo-docosahexaenoic acid induces Nrf2-mediated expression of heme oxygenase-1 in mouse skin in vivo and in cultured murine epidermal cells. Arch. Biochem. Biophys. 2020, 679, 108156. [Google Scholar] [CrossRef]
- Tsai, C.H.; Shen, Y.C.; Chen, H.W.; Liu, K.L.; Chang, J.W.; Chen, P.Y.; Lin, C.Y.; Yao, H.T.; Li, C.C. Docosahexaenoic acid increases the expression of oxidative stress-induced growth inhibitor 1 through the PI3K/Akt/Nrf2 signaling pathway in breast cancer cells. Food Chem. Toxicol. 2017, 108, 276–288. [Google Scholar] [CrossRef]
- Johansson, I.; Monsen, V.T.; Pettersen, K.; Mildenberger, J.; Misund, K.; Kaarniranta, K.; Schonberg, S.; Bjorkoy, G. The marine n-3 PUFA DHA evokes cytoprotection against oxidative stress and protein misfolding by inducing autophagy and NFE2L2 in human retinal pigment epithelial cells. Autophagy 2015, 11, 1636–1651. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lii, C.K.; Wei, Y.L.; Li, C.C.; Lu, C.Y.; Liu, K.L.; Chen, H.W. Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-kappaB pathways. J. Nutr. Biochem. 2013, 24, 204–212. [Google Scholar] [CrossRef]
- Zhu, W.; Cui, G.; Li, T.; Chen, H.; Zhu, J.; Ding, Y.; Zhao, L. Docosahexaenoic Acid Protects Traumatic Brain Injury by Regulating NOX2 Generation via Nrf2 Signaling Pathway. Neurochem. Res. 2020, 45, 1839–1850. [Google Scholar] [CrossRef]
- Zhu, W.; Ding, Y.; Kong, W.; Li, T.; Chen, H. Docosahexaenoic Acid (DHA) Provides Neuroprotection in Traumatic Brain Injury Models via Activating Nrf2-ARE Signaling. Inflammation 2018, 41, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Takanezawa, Y.; Nakamura, R.; Hamaguchi, M.; Yamamoto, K.; Sone, Y.; Uraguchi, S.; Kiyono, M. Docosahexaenoic acid enhances methylmercury-induced endoplasmic reticulum stress and cell death and eicosapentaenoic acid potentially attenuates these effects in mouse embryonic fibroblasts. Toxicol. Lett. 2019, 306, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Dietary docosahexaenoic acid inhibits neurodegeneration and prevents stroke. J. Neurosci. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Wu, S.H.; Zhou, Y.; Tang, Y.R. Lipoxin A4-induced heme oxygenase-1 protects cardiomyocytes against hypoxia/reoxygenation injury via p38 MAPK activation and Nrf2/ARE complex. PLoS ONE 2013, 8, e67120. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zheng, Y.; Hou, X. Lipoxin A4 restores oxidative stress-induced vascular endothelial cell injury and thrombosis-related factor expression by its receptor-mediated activation of Nrf2-HO-1 axis. Cell. Signal. 2019, 60, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Yi, P.; Wu, P.; Liu, Z.; Liu, Z.; Gong, J.; Hao, H.; Cai, L.; Ye, D.; Huang, Y. Effect of lipoxin A4 on lipopolysaccharide-induced endothelial hyperpermeability. Sci. World J. 2011, 11, 148208. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, H.H.; Wu, Q.; Miao, S.; Liu, Z.J.; Wu, P.; Ye, D.Y. Lipoxin A4 Activates Nrf2 Pathway and Ameliorates Cell Damage in Cultured Cortical Astrocytes Exposed to Oxygen-Glucose Deprivation/Reperfusion Insults. J. Mol. Neurosci. 2015, 56, 848–857. [Google Scholar] [CrossRef]
- Guo, Y.; Dong, C.; Tang, J.; Deng, X.; Wang, G.; Wang, W.; Xu, H. Lipoxin A4 alleviates cerebral ischemia-reperfusion injury through up-regulating Nrf2. J. Neurosurg. Sci. 2018, 62, 225–226. [Google Scholar] [CrossRef]
- Lu, T.; Wu, X.; Wei, N.; Liu, X.; Zhou, Y.; Shang, C.; Duan, Y.; Dong, Y. Lipoxin A4 protects against spinal cord injury via regulating Akt/nuclear factor (erythroid-derived 2)-like 2/heme oxygenase-1 signaling. Biomed. Pharmacother. 2018, 97, 905–910. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Z.J.; Miao, S.; Zou, L.B.; Cai, L.; Wu, P.; Ye, D.Y.; Wu, Q.; Li, H.H. Lipoxin A4 ameliorates cerebral ischaemia/reperfusion injury through upregulation of nuclear factor erythroid 2-related factor 2. Neurol. Res. 2013, 35, 968–975. [Google Scholar] [CrossRef]
- Cheng, X.; He, S.; Yuan, J.; Miao, S.; Gao, H.; Zhang, J.; Li, Y.; Peng, W.; Wu, P. Lipoxin A4 attenuates LPS-induced mouse acute lung injury via Nrf2-mediated E-cadherin expression in airway epithelial cells. Free Radic. Biol. Med. 2016, 93, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Zheng, C.; Yu, D.; Zhang, F.; Pan, R.; Ni, X.; Shi, Z.; Zhang, Z.; Xiang, Y.; Sun, H.; et al. Lipoxin A4 Ameliorates Acute Pancreatitis-Associated Acute Lung Injury through the Antioxidative and Anti-Inflammatory Effects of the Nrf2 Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 2197017. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, J.; Ni, T.; Lin, N.; Meng, L.; Gao, F.; Luo, H.; Liu, X.; Chi, J.; Guo, H. Yellow Wine Polyphenolic Compounds prevents Doxorubicin-induced cardiotoxicity through activation of the Nrf2 signalling pathway. J. Cell. Mol. Med. 2019, 23, 6034–6047. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yao, W.; Liu, Z.; Li, H.; Zhang, Z.J.; Hei, Z.; Xia, Z. Lipoxin A4 Preconditioning Attenuates Intestinal Ischemia Reperfusion Injury through Keap1/Nrf2 Pathway in a Lipoxin A4 Receptor Independent Manner. Oxidative Med. Cell. Longev. 2016, 2016, 9303606. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Wang, M.J.; Lu, J.; Chen, X.Q. Signal transduction involved in lipoxin A4induced protection of tubular epithelial cells against hypoxia/reoxygenation injury. Mol. Med. Rep. 2017, 15, 1682–1692. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Prieto, P.; Macias, A.; Pimentel-Santillana, M.; de la Cruz, A.; Traves, P.G.; Bosca, L.; Valenzuela, C. Modulation of voltage-dependent and inward rectifier potassium channels by 15-epi-lipoxin-A4 in activated murine macrophages: Implications in innate immunity. J. Immunol. 2013, 191, 6136–6146. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Fattori, V.; Saito, P.; Pinto, I.C.; Rodrigues, C.C.A.; Melo, C.P.B.; Bussmann, A.J.C.; Staurengo-Ferrari, L.; Bezerra, J.R.; Vignoli, J.A.; et al. The Lipoxin Receptor/FPR2 Agonist BML-111 Protects Mouse Skin Against Ultraviolet B Radiation. Molecules 2020, 25, 2953. [Google Scholar] [CrossRef]
- Jaen, R.I.; Fernandez-Velasco, M.; Terron, V.; Sanchez-Garcia, S.; Zaragoza, C.; Canales-Bueno, N.; Val-Blasco, A.; Vallejo-Cremades, M.T.; Bosca, L.; Prieto, P. BML-111 treatment prevents cardiac apoptosis and oxidative stress in a mouse model of autoimmune myocarditis. FASEB J. 2020. [Google Scholar] [CrossRef]
- Wu, X.; Pan, C.; Chen, R.; Zhang, S.; Zhai, Y.; Guo, H. BML-111 attenuates high glucose-induced inflammation, oxidative stress and reduces extracellular matrix accumulation via targeting Nrf2 in rat glomerular mesangial cells. Int. Immunopharmacol. 2020, 79, 106108. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, X.; Yin, Z.; Yu, X.; Yang, Q.; Guo, Q.; Tian, D.; Xiong, X.; Xu, G.; Kuang, X. The anti-inflammatory effect of BML-111 on COPD may be mediated by regulating NLRP3 inflammasome activation and ROS production. Prostaglandins Other Lipid Mediat. 2018, 138, 23–30. [Google Scholar] [CrossRef]
- Mostafa, D.G.; Satti, H.H. Resolvin D1 Prevents the Impairment in the Retention Memory and Hippocampal Damage in Rats Fed a Corn Oil-Based High Fat Diet by Upregulation of Nrf2 and Downregulation and Inactivation of p(66)Shc. Neurochem. Res. 2020, 45, 1576–1591. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, F.; Yang, Y.; Wang, J.; Sun, S.; Xia, H.; Yao, S. Resolvin D1 attenuates ventilator-induced lung injury by reducing HMGB1 release in a HO-1-dependent pathway. Int. Immunopharmacol. 2019, 75, 105825. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Liu, Y.; Li, X.; Cao, L.; Yuan, X.; Li, W.; Cao, Q. Aspirin-Triggered Resolvin D1 Inhibits TGF-beta1-Induced EndMT through Increasing the Expression of Smad7 and Is Closely Related to Oxidative Stress. Biomol. Ther. 2016, 24, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Posso, S.V.; Quesnot, N.; Moraes, J.A.; Brito-Gitirana, L.; Kennedy-Feitosa, E.; Barroso, M.V.; Porto, L.C.; Lanzetti, M.; Valenca, S.S. AT-RVD1 repairs mouse lung after cigarette smoke-induced emphysema via downregulation of oxidative stress by NRF2/KEAP1 pathway. Int. Immunopharmacol. 2018, 56, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shen, H.; Wang, Y.; Zhang, L.; Zhao, M. Aspirin-triggered resolvin D1 alleviates paraquat-induced acute lung injury in mice. Life Sci. 2019, 218, 38–46. [Google Scholar] [CrossRef]
- Saito, P.; Melo, C.P.B.; Martinez, R.M.; Fattori, V.; Cezar, T.L.C.; Pinto, I.C.; Bussmann, A.J.C.; Vignoli, J.A.; Georgetti, S.R.; Baracat, M.M.; et al. The Lipid Mediator Resolvin D1 Reduces the Skin Inflammation and Oxidative Stress Induced by UV Irradiation in Hairless Mice. Front. Pharmacol. 2018, 9, 1242. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, X.; Qi, X.; Di, G.; Zhang, Y.; Wang, Q.; Zhou, Q. Resolvin D1 promotes corneal epithelial wound healing and restoration of mechanical sensation in diabetic mice. Mol. Vis. 2018, 24, 274–285. [Google Scholar]
- Soto, G.; Rodriguez, M.J.; Fuentealba, R.; Treuer, A.V.; Castillo, I.; Gonzalez, D.R.; Zuniga-Hernandez, J. Maresin 1, a Proresolving Lipid Mediator, Ameliorates Liver Ischemia-Reperfusion Injury and Stimulates Hepatocyte Proliferation in Sprague-Dawley Rats. Int. J. Mol. Sci. 2020, 21, 540. [Google Scholar] [CrossRef]
- Qiu, S.; Li, P.; Zhao, H.; Li, X. Maresin 1 alleviates dextran sulfate sodium-induced ulcerative colitis by regulating NRF2 and TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2020, 78, 106018. [Google Scholar] [CrossRef]
- Qiu, Y.; Wu, Y.; Zhao, H.; Sun, H.; Gao, S. Maresin 1 mitigates renal ischemia/reperfusion injury in mice via inhibition of the TLR4/MAPK/NF-kappaB pathways and activation of the Nrf2 pathway. Drug Des. Dev. Ther. 2019, 13, 739–745. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, Y.; Zhao, F.; Wang, J. Maresin 1 Ameliorates Lung Ischemia/Reperfusion Injury by Suppressing Oxidative Stress via Activation of the Nrf-2-Mediated HO-1 Signaling Pathway. Oxidative Med. Cell. Longev. 2017, 2017, 9634803. [Google Scholar] [CrossRef] [PubMed]
- Helal, M.G.; El-Kashef, D.H. Krill oil alleviates oxidative stress, iron accumulation and fibrosis in the liver and spleen of iron-overload rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 3950–3961. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, T.D.; Mason, R.P.; Budoff, M.J.; Navar, A.M.; Shearer, G.C. Mechanistic insights into cardiovascular protection for omega-3 fatty acids and their bioactive lipid metabolites. Eur. Heart J. Suppl. 2020, 22, J3–J20. [Google Scholar] [CrossRef] [PubMed]
- Shabani, P.; Ghazizadeh, Z.; Gorgani-Firuzjaee, S.; Molazem, M.; Rajabi, S.; Vahdat, S.; Azizi, Y.; Doosti, M.; Aghdami, N.; Baharvand, H. Cardioprotective effects of omega-3 fatty acids and ascorbic acid improve regenerative capacity of embryonic stem cell-derived cardiac lineage cells. Biofactors 2019, 45, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Mayyas, F.; Jaradat, R.; Alzoubi, K.H. Cardiac effects of fish oil in a rat model of streptozotocin-induced diabetes. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 592–599. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, T.D.; Block, R.C.; Huang, S.P.; Shearer, G.C. omega3-Polyunsaturated fatty acids for heart failure: Effects of dose on efficacy and novel signaling through free fatty acid receptor 4. J. Mol. Cell. Cardiol. 2017, 103, 74–92. [Google Scholar] [CrossRef]
- Eclov, J.A.; Qian, Q.; Redetzke, R.; Chen, Q.; Wu, S.C.; Healy, C.L.; Ortmeier, S.B.; Harmon, E.; Shearer, G.C.; O’Connell, T.D. EPA, not DHA, prevents fibrosis in pressure overload-induced heart failure: Potential role of free fatty acid receptor 4. J. Lipid Res. 2015, 56, 2297–2308. [Google Scholar] [CrossRef]
- Siddesha, J.M.; Valente, A.J.; Yoshida, T.; Sakamuri, S.S.; Delafontaine, P.; Iba, H.; Noda, M.; Chandrasekar, B. Docosahexaenoic acid reverses angiotensin II-induced RECK suppression and cardiac fibroblast migration. Cell Signal. 2014, 26, 933–941. [Google Scholar] [CrossRef]
- Baum, J.R.; Dolmatova, E.; Tan, A.; Duffy, H.S. Omega 3 fatty acid inhibition of inflammatory cytokine-mediated Connexin43 regulation in the heart. Front. Physiol. 2012, 3, 272. [Google Scholar] [CrossRef]
- Ramadeen, A.; Connelly, K.A.; Leong-Poi, H.; Hu, X.; Fujii, H.; Laurent, G.; Domenichiello, A.F.; Bazinet, R.P.; Dorian, P. Docosahexaenoic acid, but not eicosapentaenoic acid, supplementation reduces vulnerability to atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2012, 5, 978–983. [Google Scholar] [CrossRef]
- Takamura, M.; Kurokawa, K.; Ootsuji, H.; Inoue, O.; Okada, H.; Nomura, A.; Kaneko, S.; Usui, S. Long-Term Administration of Eicosapentaenoic Acid Improves Post-Myocardial Infarction Cardiac Remodeling in Mice by Regulating Macrophage Polarization. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Ito, S.; Sano, Y.; Nagasawa, K.; Matsuura, N.; Yamada, Y.; Uchinaka, A.; Murohara, T.; Nagata, K. Highly purified eicosapentaenoic acid ameliorates cardiac injury and adipose tissue inflammation in a rat model of metabolic syndrome. Obes. Sci. Pract. 2016, 2, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Anzai, T.; Mano, Y.; Kaneko, H.; Anzai, A.; Sugano, Y.; Maekawa, Y.; Takahashi, T.; Yoshikawa, T.; Fukuda, K. Eicosapentaenoic acid suppresses adverse effects of C-reactive protein overexpression on pressure overload-induced cardiac remodeling. Heart Vessel. 2013, 28, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Shibata, R.; Tsuji, Y.; Shimano, M.; Inden, Y.; Murohara, T. Eicosapentaenoic acid prevents atrial fibrillation associated with heart failure in a rabbit model. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1814–H1821. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Rivera, D.; Liempi, A.; Gonzalez-Herrera, F.; Fuentes-Retamal, S.; Carrillo, I.; Abarca, P.; Castillo, C.; Kemmerling, U.; Pesce, B.; Maya, J.D. Simvastatin Improves Cardiac Function through Notch 1 Activation in BALB/c Mice with Chronic Chagas Cardiomyopathy. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Boeynaems, J.M. P2Y nucleotide receptors: Promise of therapeutic applications. Drug Discov. Today 2010, 15, 570–578. [Google Scholar] [CrossRef]
- Ye, Y.; Birnbaum, G.D.; Perez-Polo, J.R.; Nanhwan, M.K.; Nylander, S.; Birnbaum, Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arter. Thromb. Vasc. Biol. 2015, 35, 1805–1814. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, G.J.; Kim, E.J.; Lee, C.H. Therapeutic Effects of Specialized Pro-Resolving Lipids Mediators on Cardiac Fibrosis via NRF2 Activation. Antioxidants 2020, 9, 1259. https://doi.org/10.3390/antiox9121259
Kang GJ, Kim EJ, Lee CH. Therapeutic Effects of Specialized Pro-Resolving Lipids Mediators on Cardiac Fibrosis via NRF2 Activation. Antioxidants. 2020; 9(12):1259. https://doi.org/10.3390/antiox9121259
Chicago/Turabian StyleKang, Gyeoung Jin, Eun Ji Kim, and Chang Hoon Lee. 2020. "Therapeutic Effects of Specialized Pro-Resolving Lipids Mediators on Cardiac Fibrosis via NRF2 Activation" Antioxidants 9, no. 12: 1259. https://doi.org/10.3390/antiox9121259
APA StyleKang, G. J., Kim, E. J., & Lee, C. H. (2020). Therapeutic Effects of Specialized Pro-Resolving Lipids Mediators on Cardiac Fibrosis via NRF2 Activation. Antioxidants, 9(12), 1259. https://doi.org/10.3390/antiox9121259




