Blunted Reducing Power Generation in Erythrocytes Contributes to Oxidative Stress in Prepubertal Obese Children with Insulin Resistance
Abstract
1. Introduction
2. Material and Methods
2.1. Subjects
2.2. Sample Collection and Preparation
2.3. Anthropometry and Biochemical Analysis of the Study Population
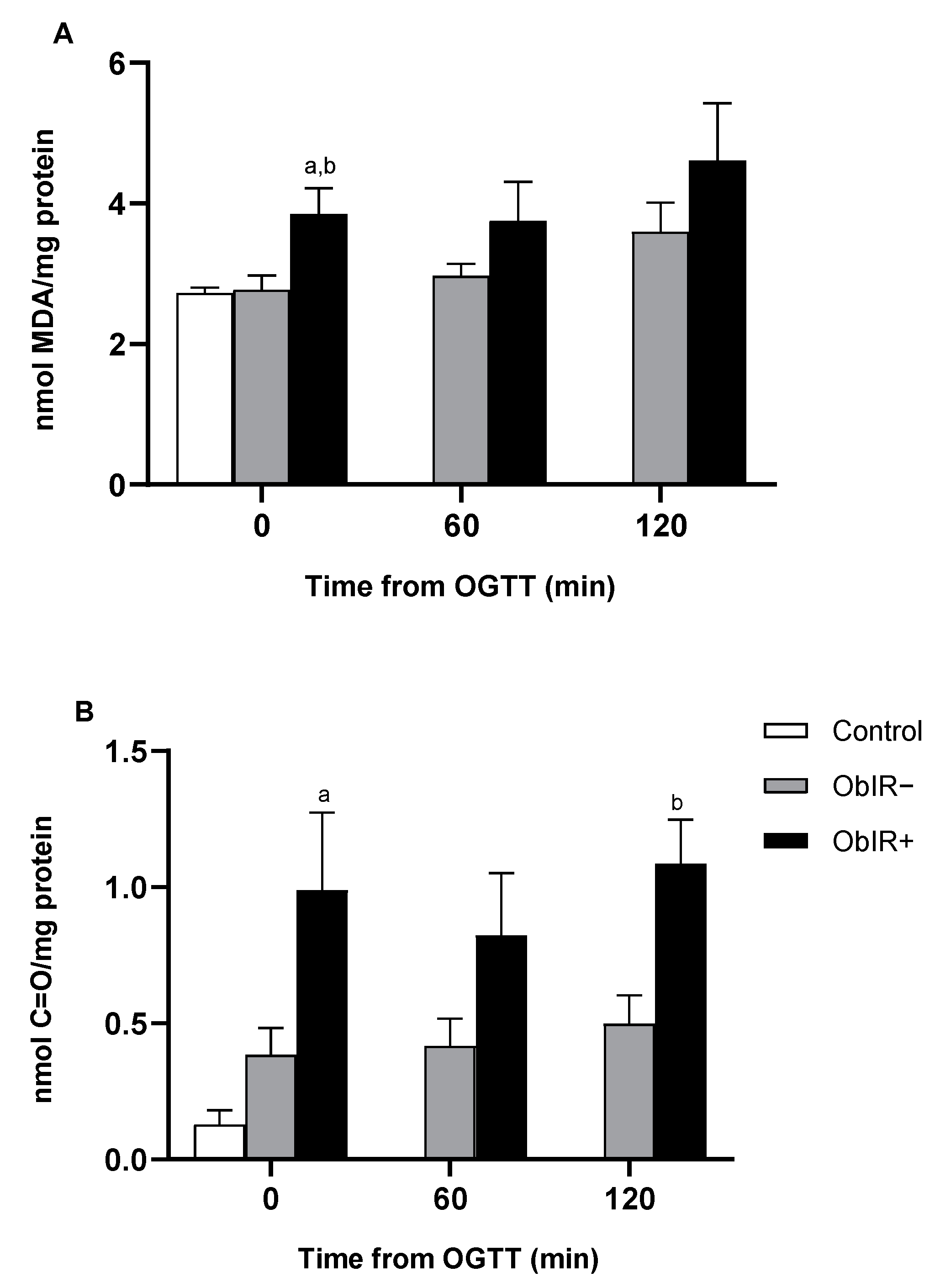
2.4. Lipid Peroxidation Quantification
2.5. Carbonyl Groups Determination
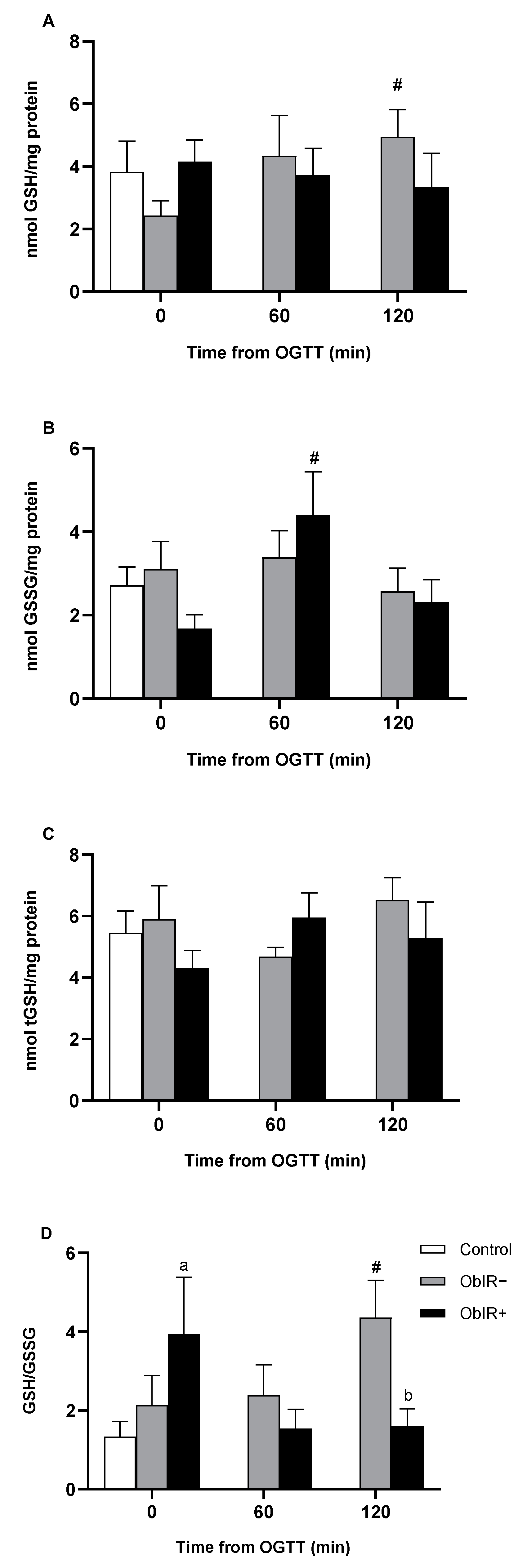
2.6. Glutathione Availability
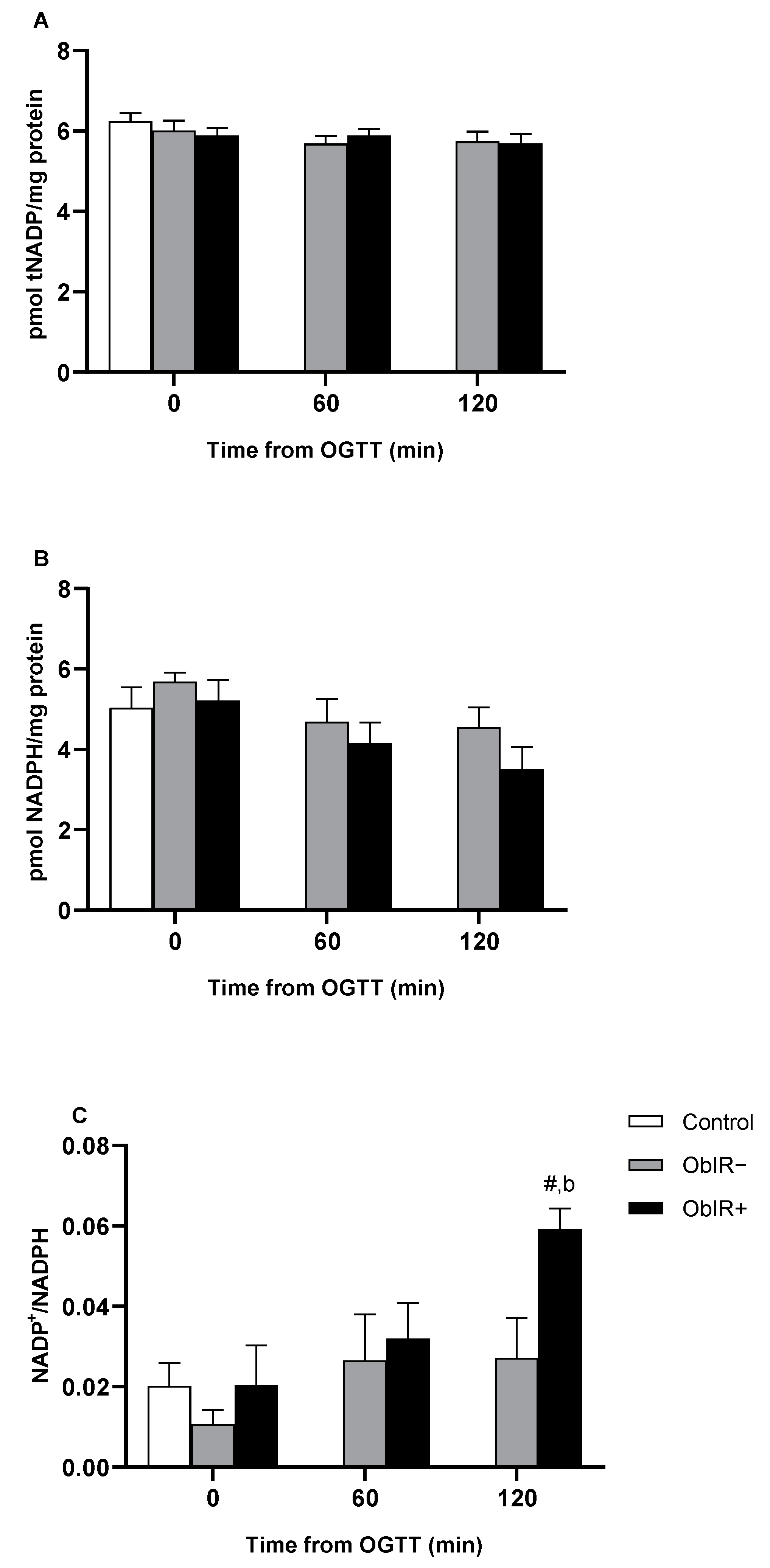
2.7. NADPH Availability
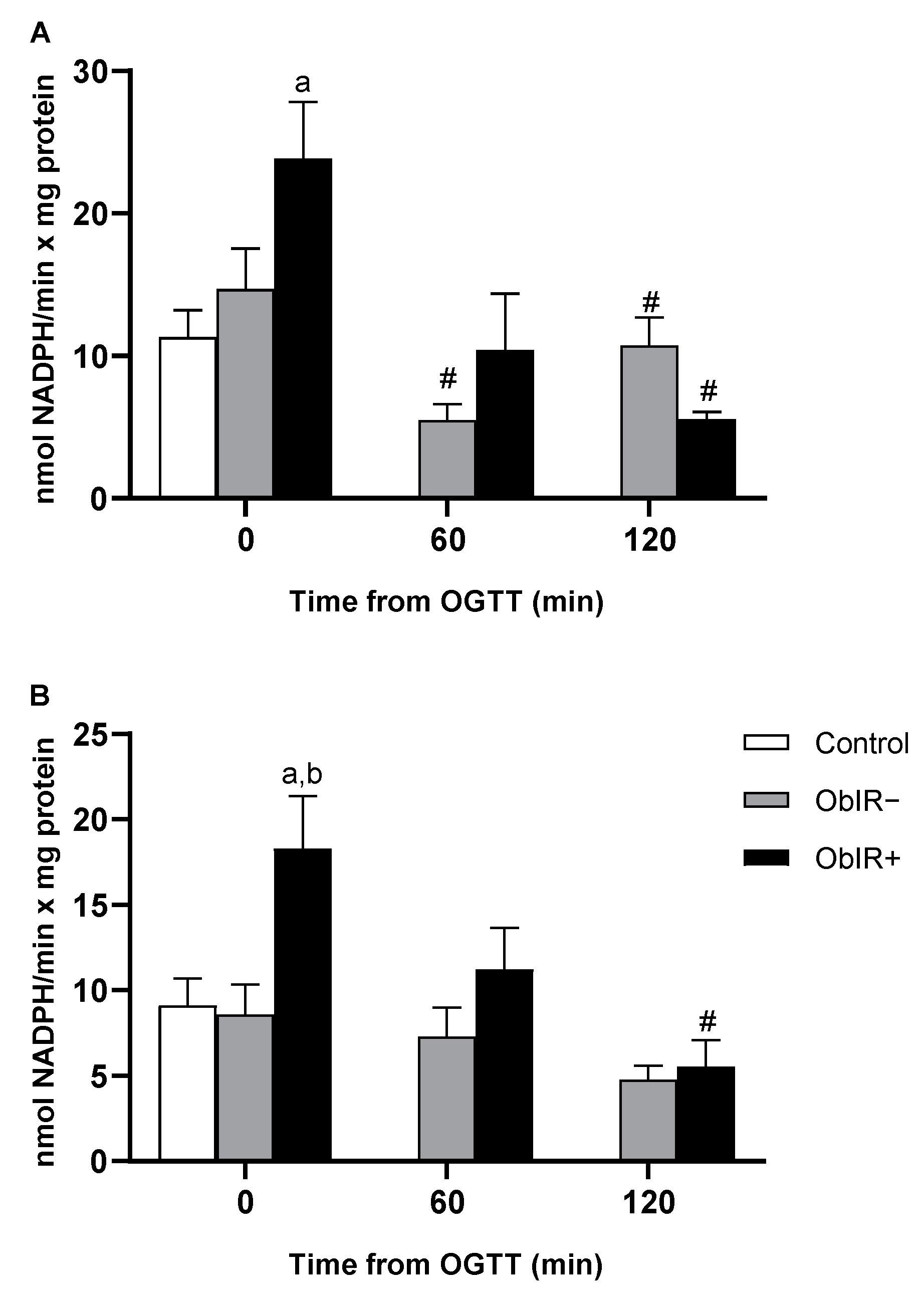
2.8. NADP-Dehydrogenase Activities
2.9. Glutathione Reductase Activity
2.10. Glutathione Peroxidase Activity
2.11. Catalase Activity
2.12. Superoxide Dismutase Activity
2.13. Statistical Analysis
3. Results
3.1. Anthropometry and Biochemical Analysis of the Study Population
3.2. Oxidative Damage
3.3. Glutathione Availability
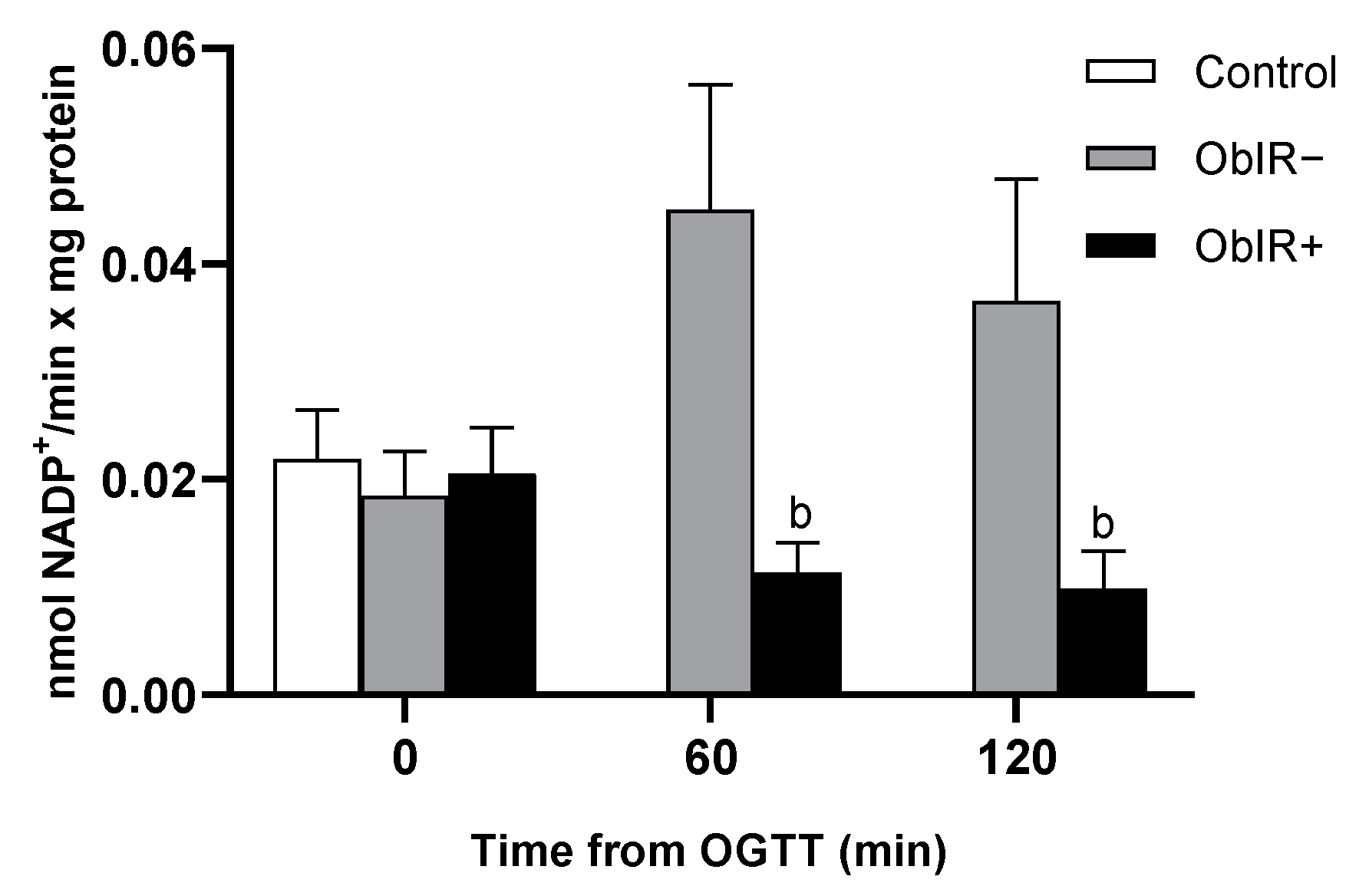
3.4. NADPH Availability
3.5. NADP-Dehydrogenases Activities
3.6. Glutathione Reductase Activity
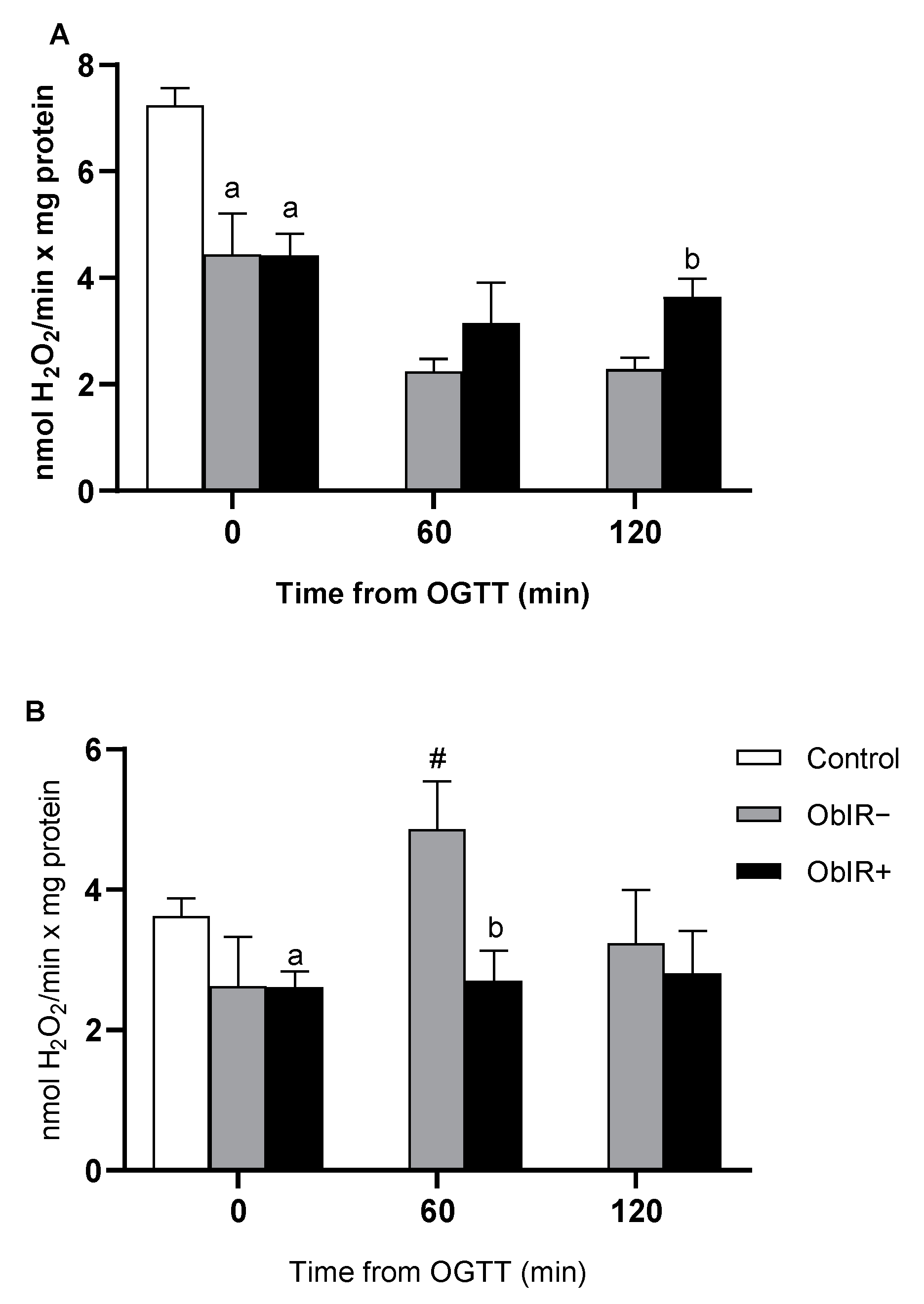
3.7. H2O2 Removing Enzymes
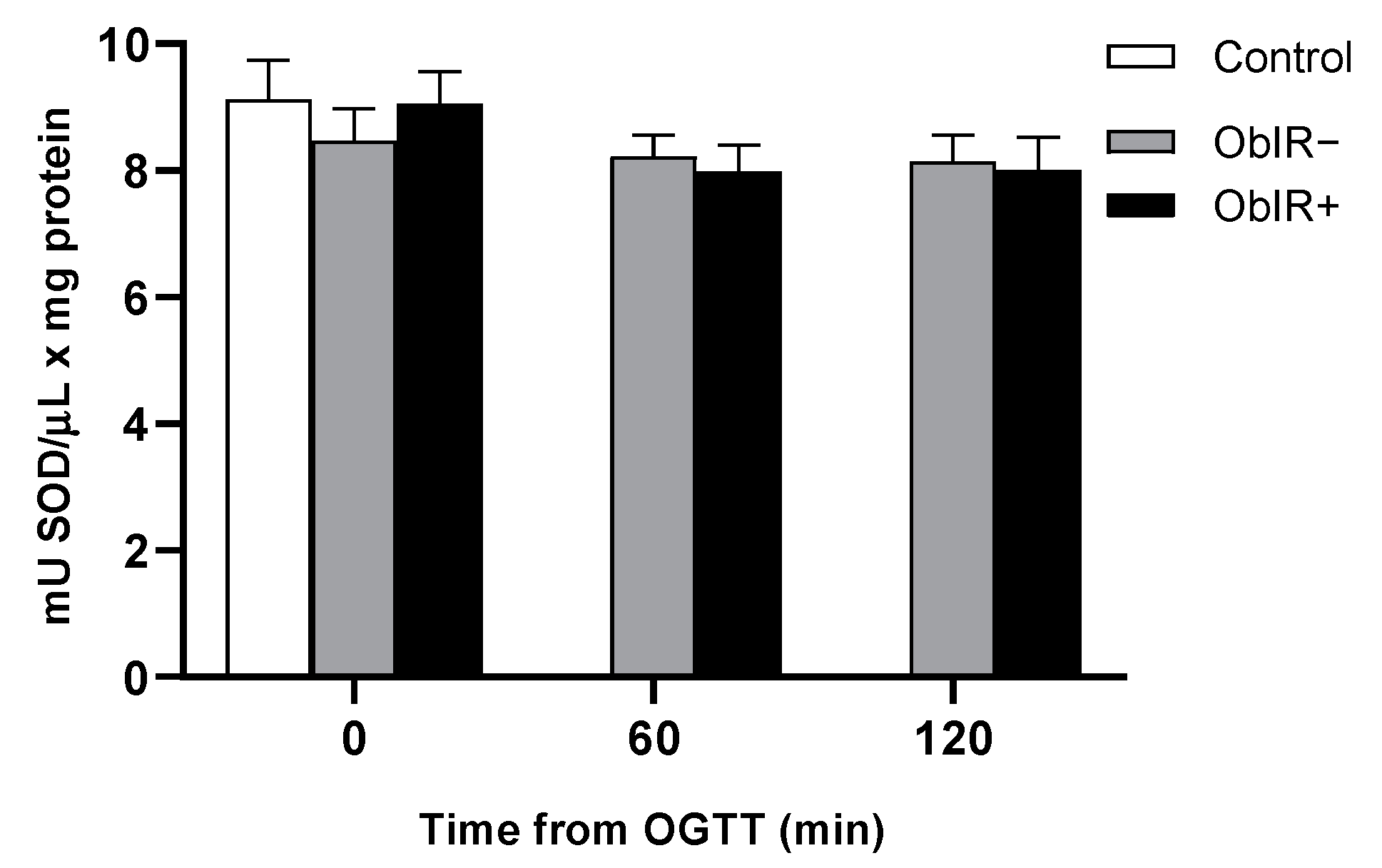
3.8. Superoxide Dismutase Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Childhood Obesity. Available online: https://www.who.int/dietphysicalactivity/childhood/en/ (accessed on 29 June 2020).
- World Health Organization. Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 29 June 2020).
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef]
- Yang, F.; Dawes, P.; Leroi, I.; Gannon, B. Measurement tools of resource use and quality of life in clinical trials for dementia or cognitive impairment interventions: A systematically conducted narrative review. Int. J. Geriatr. Psychiatry 2018, 33, e166–e176. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Oxidative stress and antioxidants: Distress or eustress? Arch. Biochem. Biophys. 2016, 595, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.; Ramteke, P.; Dholia, N.; Yadav, U.C.S. Therapeutic Interventions to Block Oxidative Stress-Associated Pathologies. In Immunity and Inflammation in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 341–362. [Google Scholar]
- Perše, M. Oxidative Stress in the Pathogenesis of Colorectal Cancer: Cause or Consequence? BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of Oxidative Stress in Neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51. [Google Scholar] [CrossRef]
- Wautier, J.L.; Wautier, M.P.; Schmidt, A.M.; Anderson, G.M.; Hori, O.; Zoukourian, C.; Capron, L.; Chappey, O.; Yan, S.D.; Brett, J.; et al. Advanced glycation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: A link between surface-associated AGEs and diabetic complications. Proc. Natl. Acad. Sci. USA 1994, 91, 7742–7746. [Google Scholar] [CrossRef]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron Metabolism in Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef] [PubMed]
- Lechuga-Sancho, A.M.; Gallego-Andujar, D.; Ruiz-Ocaña, P.; Visiedo, F.M.; Saez-Benito, A.; Schwarz, M.; Segundo, C.; Mateos, R.M. Obesity induced alterations in redox homeostasis and oxidative stress are present from an early age. PLoS ONE 2018, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, V.; Diederich, L.; Keller, T.C.S.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef] [PubMed]
- May, J.M. Ascorbate function and metabolism in the human erythrocyte. Front. Biosci. 1998, 3, A262. [Google Scholar] [CrossRef]
- Kirkman, H.N.; Rolfo, M.; Ferraris, A.M.; Gaetani, G.F. Mechanisms of Protection of Catalase by NADPH. J. Biol. Chem. 1999, 274, 13908–13914. [Google Scholar] [CrossRef]
- Dugmonits, K.N.; Ferencz, Á.; Zahorán, S.; Lázár, R.; Talapka, P.; Orvos, H.; Hermesz, E. Elevated levels of macromolecular damage are correlated with increased nitric oxide synthase expression in erythrocytes isolated from twin neonates. Br. J. Haematol. 2016, 174, 932–941. [Google Scholar] [CrossRef]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Antioxidant Defense Mechanisms in Erythrocytes and in the Central Nervous System. Antioxidants 2019, 8, 46. [Google Scholar] [CrossRef]
- Olszewska, M.; Wiatrow, J.; Bober, J.; Stachowska, E.; Gołembiewska, E.; Jakubowska, K.; Stańczyk-Dunaj, M.; Pietrzak-Nowacka, M. Oxidative stress modulates the organization of erythrocyte membrane cytoskeleton. Postepy Hig. Med. Dosw. 2012, 66, 534–542. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers. Clin. Diabetes 2019, 37, 11–34. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.M.; Bonilla-Valverde, D.; del Río, L.A.; Palma, J.M.; Corpas, F.J. NADP-dehydrogenases from pepper fruits: Effect of maturation. Physiol. Plant. 2009, 135, 130–139. [Google Scholar] [CrossRef]
- Edwards, E.A.; Rawsthorne, S.; Mullineaux, P. Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 1990, 180. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Municipal Institute of Medical Research of Barcelona Home Page. Sample Size and Power Calculator. Available online: https://www.imim.es/ofertadeserveis/software-public/granmo/ (accessed on 23 December 2020).
- Santos-Rosendo, C.; Bugatto, F.; González-Domínguez, A.; Lechuga-Sancho, A.M.; Mateos, R.M.; Visiedo, F. Placental Adaptive Changes to Protect Function and Decrease Oxidative Damage in Metabolically Healthy Maternal Obesity. Antioxidants 2020, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, V.; Ayine, P.; Fadamiro, M.; Babu, J.R.; Brown, M.; Geetha, T. Urinary Biomarkers of Inflammation and Oxidative Stress Are Elevated in Obese Children and Correlate with a Marker of Endothelial Dysfunction. Oxid. Med. Cell. Longev. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Boix-García, L.; Simó-Jordá, R.; del Castillo-Villaescusa, C.; Maset-Maldonado, J.; Valls-Bellés, V. Is obesity associated with oxidative stress in children? Int. J. Pediatr. Obes. 2010, 5, 56–63. [Google Scholar] [CrossRef]
- Karamouzis, I.; Pervanidou, P.; Berardelli, R.; Iliadis, S.; Papassotiriou, I.; Karamouzis, M.; Chrousos, G.; Kanaka-Gantenbein, C. Enhanced Oxidative Stress and Platelet Activation Combined with Reduced Antioxidant Capacity in Obese Prepubertal and Adolescent Girls with Full or Partial Metabolic Syndrome. Horm. Metab. Res. 2011, 43, 607–613. [Google Scholar] [CrossRef]
- Dei Zotti, F.; Verdoy, R.; Brusa, D.; Lobysheva, I.I.; Balligand, J.-L. Redox regulation of nitrosyl-hemoglobin in human erythrocytes. Redox Biol. 2020, 34, 101399. [Google Scholar] [CrossRef] [PubMed]
- Çimen, M.Y.B. Free radical metabolism in human erythrocytes. Clin. Chim. Acta 2008, 390, 1–11. [Google Scholar] [CrossRef]
- Guha, P.; Dey, A.; Sen, R.; Chatterjee, M.; Chattopadhyay, S.; Bandyopadhyay, S.K. Intracellular GSH Depletion Triggered Mitochondrial Bax Translocation to Accomplish Resveratrol-Induced Apoptosis in the U937 Cell Line. J. Pharmacol. Exp. Ther. 2011, 336, 206–214. [Google Scholar] [CrossRef]
- Teskey, G.; Abrahem, R.; Cao, R.; Gyurjian, K.; Islamoglu, H.; Lucero, M.; Martinez, A.; Paredes, E.; Salaiz, O.; Robinson, B.; et al. Glutathione as a Marker for Human Disease. Adv. Clin. Chem. 2018, 87, 141–159. [Google Scholar]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Bajic, V.P.; Van Neste, C.; Obradovic, M.; Zafirovic, S.; Radak, D.; Bajic, V.B.; Essack, M.; Isenovic, E.R. Glutathione “Redox Homeostasis” and Its Relation to Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Albuali, W.H. Evaluation of oxidant-antioxidant status in overweight and morbidly obese Saudi children. World J. Clin. Pediatr. 2014, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- García, J.B.; Rentero, P.Z.; Cánovas, J.M.; Jara, P.G.; Hernández, M.L.; Alemán, J.A. Marcadores bioquímicos, nutricionales y actividad antioxidante en el síndrome metabólico. Endocrinol. Nutr. 2014, 61, 302–308. [Google Scholar] [CrossRef]
- Ramezanipour, M.; Jalali, M.; Sadrzade-Yeganeh, H.; Keshavarz, S.A.; Eshraghian, M.R.; Bagheri, M.; Emami, S.S. Efeito da redução de peso sobre as enzimas antioxidantes e associação destas com a ingestão de vitaminas A, C e E. Arq. Bras. Endocrinol. Metabol. 2014, 58, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Dadan, J.; Myśliwiec, H.; Choromańska, K.; Zalewska, A.; Maciejczyk, M. A Longitudinal Study of the Antioxidant Barrier and Oxidative Stress in Morbidly Obese Patients after Bariatric Surgery. Does the Metabolic Syndrome Affect the Redox Homeostasis of Obese People? J. Clin. Med. 2020, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Vávrová, L.; Kodydková, J.; Zeman, M.; Dušejovská, M.; Macášek, J.; Stanková, B.; Tvrzická, E.; Žák, A. Altered Activities of Antioxidant Enzymes in Patients with Metabolic Syndrome. Obes. Facts 2013, 6, 39–47. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef]
- Ge, T.; Yang, J.; Zhou, S.; Wang, Y.; Li, Y.; Tong, X. The Role of the Pentose Phosphate Pathway in Diabetes and Cancer. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Park, Y.J.; Choe, S.S.; Sohn, J.H.; Kim, J.B. The role of glucose-6-phosphate dehydrogenase in adipose tissue inflammation in obesity. Adipocyte 2017, 6, 147–153. [Google Scholar] [CrossRef]
- Lee-Young, R.S.; Hoffman, N.J.; Murphy, K.T.; Henstridge, D.C.; Samocha-Bonet, D.; Siebel, A.L.; Iliades, P.; Zivanovic, B.; Hong, Y.H.; Colgan, T.D.; et al. Glucose-6-phosphate dehydrogenase contributes to the regulation of glucose uptake in skeletal muscle. Mol. Metab. 2016, 5, 1083–1091. [Google Scholar] [CrossRef]
- Zhang, Z.; Liew, C.W.; Handy, D.E.; Zhang, Y.; Leopold, J.A.; Hu, J.; Guo, L.; Kulkarni, R.N.; Loscalzo, J.; Stanton, R.C. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and β-cell apoptosis. FASEB J. 2010, 24, 1497–1505. [Google Scholar] [CrossRef]
- Heit, C.; Marshall, S.; Singh, S.; Yu, X.; Charkoftaki, G.; Zhao, H.; Orlicky, D.J.; Fritz, K.S.; Thompson, D.C.; Vasiliou, V. Catalase deletion promotes prediabetic phenotype in mice. Free Radic. Biol. Med. 2017, 103, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, A.I.; Olza, J.; Gil-Campos, M.; Leis, R.; Mesa, M.D.; Tojo, R.; Cañete, R.; Gil, A.; Aguilera, C.M. Are catalase -844A/G polymorphism and activity associated with childhood obesity? Antioxid. Redox Signal. 2013, 19, 1970–1975. [Google Scholar] [CrossRef]
- Lardinois, O.M.; Mestdagh, M.M.; Rouxhet, P.G. Reversible inhibition and irreversible inactivation of catalase in presence of hydrogen peroxide. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1996, 1295, 222–238. [Google Scholar] [CrossRef]
- Pigeolet, E.; Corbisier, P.; Houbion, A.; Lambert, D.; Michiels, C.; Raes, M.; Zachary, M.-D.; Remacle, J. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech. Ageing Dev. 1990, 51, 283–297. [Google Scholar] [CrossRef]
- Visiedo, F.; Santos-Rosendo, C.; Mateos-Bernal, R.M.; del Gil-Sánchez, M.; Bugatto, F.; Aguilar-Diosdado, M.; Segundo, C.; López-Tinoco, C. Characterization of NO-Induced Nitrosative Status in Human Placenta from Pregnant Women with Gestational Diabetes Mellitus. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.M.; Mateos, R.M.; López-Jaramillo, J.; Rodríguez-Ruiz, M.; González-Gordo, S.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant catalases as NO and H2S targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef]
- Ghosh, S.; Janocha, A.J.; Aronica, M.A.; Swaidani, S.; Comhair, S.A.A.; Xu, W.; Zheng, L.; Kaveti, S.; Kinter, M.; Hazen, S.L.; et al. Nitrotyrosine Proteome Survey in Asthma Identifies Oxidative Mechanism of Catalase Inactivation. J. Immunol. 2006, 176, 5587–5597. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Weisbach, V.; Sticht, H.; Seebahn, A.; Bussmann, J.; Zimmermann, R.; Becker, C.-M. Oxidative stress-induced posttranslational modifications of human hemoglobin in erythrocytes. Arch. Biochem. Biophys. 2013, 529, 34–44. [Google Scholar] [CrossRef]
| Control | ObIR− | ObIR+ | |
|---|---|---|---|
| Anthropometric parameters | |||
| Number of subjects (N) | 15 | 14 | 10 |
| Sex | 8♂ 7♀ | 6 ♂ 8 ♀ | 7♂ 3 ♀ |
| Age (years) | 8.33 ± 0.42 | 8.79 ± 0.48 | 8.71 ± 0.44 |
| WC (cm) | 55.76 ± 1.57 | 93.89 ± 5.00 aaaa | 92.38 ± 2.18 aaaa |
| WC (Z-Score) | −0.52 ± 0.25 | 5.20 ± 0.61 aaaa | 5.36 ± 0.70 aaaa |
| Weight (kg) | 25.17 ± 1.31 | 54.80 ± 3.88 aaaa | 59.58 ± 3.67 aaaa |
| Weight (Z-Score) | −0.65 ± 0.18 | 4.28 ± 0.34 aaaa | 5.52 ± 0.87 aaaa |
| Height (cm) | 124.25 ± 2.28 | 138.37 ± 3.15 aa | 140.38 ± 2.79 aaa |
| Height (Z-Score) | −1.07 ± 0.17 | 1.45 ± 0.31 aaaa | 3.11 ± 1.65 aaaa |
| BMI (kg/m2) | 16.11± 0.40 | 27.81 ± 1.20 aaaa | 30.16 ± 1.43 aaaa |
| BMI (Z-Score) | −0.48 ± 0.14 | 4.12 ± 0.31 aaaa | 5.02 ± 0.73 aaaa |
| Gestational age (weeks) | 39.23 ± 0.63 | 39.34 ± 0.63 | 39.78 ± 0.55 |
| Newborn weight (g) | 3199 ± 152.27 | 3418 ± 130.88 | 3155 ± 176.15 |
| Newborn weight (Z-Score) | −0.04 ± 0.22 | 0.59 ± 0.32 | −0.40 ± 0.27 b |
| Newborn height (cm) | 50.14 ± 1.07 | 51.15 ± 0.72 | 50.29 ± 1.08 |
| Newborn height (Z-Score) | 0.38 ± 0.36 | 0.80 ± 0.40 | −0.18 ± 0.54 |
| Systolic BP (mmHg) | 103.90 ± 3.60 | 118.09 ± 4.584 a | 123.50 ± 4.73 aa |
| Systolic BP (Z-Score) | 0.61 ± 0.32 | 1.49 ± 0.41 | 1.97 ± 0.41 a |
| Diastolic BP (mmHg) | 63.10 ± 4.76 | 75.27 ± 2.65 a | 77.88 ± 3.38 a |
| Diastolic BP (Z-Score) | 0.43 ± 0.41 | 1.28 ± 0.22 a | 1.38 ± 0.31 a |
| Biochemical parameters | |||
| Blood glucose curve (mg/dL) | |||
| 0 min | 85.13 ± 1.41 | 84.57 ± 1.15 | 90.70 ± 2.87 b |
| 30 min | n.d. | 118.67 ± 11.46 | 126.40 ± 15.38 |
| 60 min | n.d. | 114.55 ± 6.08 | 125.38 ± 11.45 |
| 90 min | n.d. | 110.45 ± 2.89 | 122.50 ± 7.47 |
| 120 min | n.d. | 108.55 ± 3.84 | 120.75 ± 8.16 |
| Blood insulin curve (µUI/mL) | |||
| 0 min | 4.84 ± 0.55 | 11.61 ± 1.05 aaaa | 25.29 ± 5.33 aa,b |
| 30 min | n.d. | 58.65 ± 10.52 | 208.84 ± 55.78 b |
| 60 min | n.d. | 79.98 ± 13.32 | 200.65 ± 31.98 bb |
| 90 min | n.d. | 60.80 ± 9.15 | 185.26 ± 23.99 bbbb |
| 120 min | n.d. | 47.60 ± 6.95 | 179.79 ± 31.33 bbb |
| HOMA-IR | 1.03 ± 0.13 | 2.35 ± 0.21 aaaa | 6.81 ± 1.36 aaa,bbb |
| Total Cholesterol (mg/dL) | 155.67 ± 11.06 | 163.85 ± 9.41 | 154.40 ± 9.26 |
| Triglycerides (mg/dL) | 57.60 ± 7.40 | 92.15 ± 13.80 a | 112.50 ± 19.04 aa |
| HDL-C (mg/dL) | 64.93 ± 6.18 | 45.73± 2.00 aa | 41.00 ± 2.96 aaa |
| LDL-C (mg/dL) | 93.00 ± 6.29 | 98.91 ± 8.67 | 91.00 ± 6.83 |
| Creatinine (mg/dL) | 0.5 ± 0.02 | 0.54 ± 0.02 | 0.59 ± 0.03 a |
| GOT (U/L) | 28.92 ± 1.12 | 23.09 ± 1.38 aa | 24.38 ± 2.12 |
| GPT (U/L) | 16.92 ± 1.28 | 21.67 ± 1.24 a | 27.90 ± 5.29 a |
| Ferritin (mg/mL) | 29.82 ± 3.01 | 37.72 ± 6.11 | 59.34 ± 8.24 aa,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Domínguez, Á.; Visiedo, F.; Domínguez-Riscart, J.; Ruiz-Mateos, B.; Saez-Benito, A.; Lechuga-Sancho, A.M.; Mateos, R.M. Blunted Reducing Power Generation in Erythrocytes Contributes to Oxidative Stress in Prepubertal Obese Children with Insulin Resistance. Antioxidants 2021, 10, 244. https://doi.org/10.3390/antiox10020244
González-Domínguez Á, Visiedo F, Domínguez-Riscart J, Ruiz-Mateos B, Saez-Benito A, Lechuga-Sancho AM, Mateos RM. Blunted Reducing Power Generation in Erythrocytes Contributes to Oxidative Stress in Prepubertal Obese Children with Insulin Resistance. Antioxidants. 2021; 10(2):244. https://doi.org/10.3390/antiox10020244
Chicago/Turabian StyleGonzález-Domínguez, Álvaro, Francisco Visiedo, Jesus Domínguez-Riscart, Beatriz Ruiz-Mateos, Ana Saez-Benito, Alfonso M. Lechuga-Sancho, and Rosa María Mateos. 2021. "Blunted Reducing Power Generation in Erythrocytes Contributes to Oxidative Stress in Prepubertal Obese Children with Insulin Resistance" Antioxidants 10, no. 2: 244. https://doi.org/10.3390/antiox10020244
APA StyleGonzález-Domínguez, Á., Visiedo, F., Domínguez-Riscart, J., Ruiz-Mateos, B., Saez-Benito, A., Lechuga-Sancho, A. M., & Mateos, R. M. (2021). Blunted Reducing Power Generation in Erythrocytes Contributes to Oxidative Stress in Prepubertal Obese Children with Insulin Resistance. Antioxidants, 10(2), 244. https://doi.org/10.3390/antiox10020244







