Supercritical CO2 Extraction and Microencapsulation of Lycopene-Enriched Oleoresins from Tomato Peels: Evidence on Antiproliferative and Cytocompatibility Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Tomato Fruits
2.3. Tomato Peels Supercritical CO2 Extraction Process
2.4. Evaluation of Carotenoids and Lycopene Content in Extracts and Powders
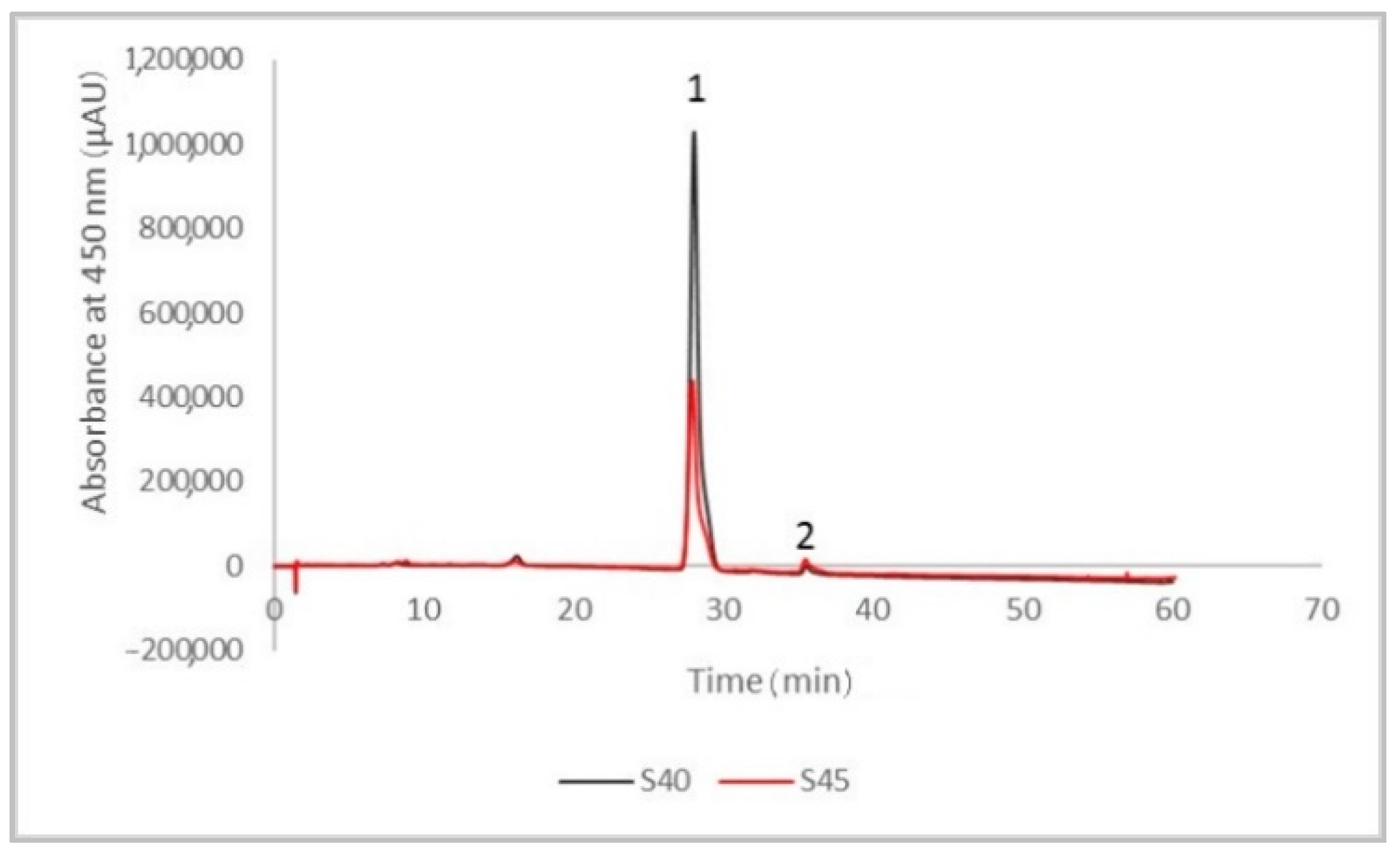
2.5. High-Performance Liquid Chromatography (HPLC) Analysis of Lycopene in the Extracts
2.6. Determination of Free Radical Scavenging Activity
2.7. Microencapsulation of the Extracts by Complex Coacervation and Freeze-Drying
2.8. Microencapsulation Efficiency
2.9. Structural and Morphological Analysis of the Powders
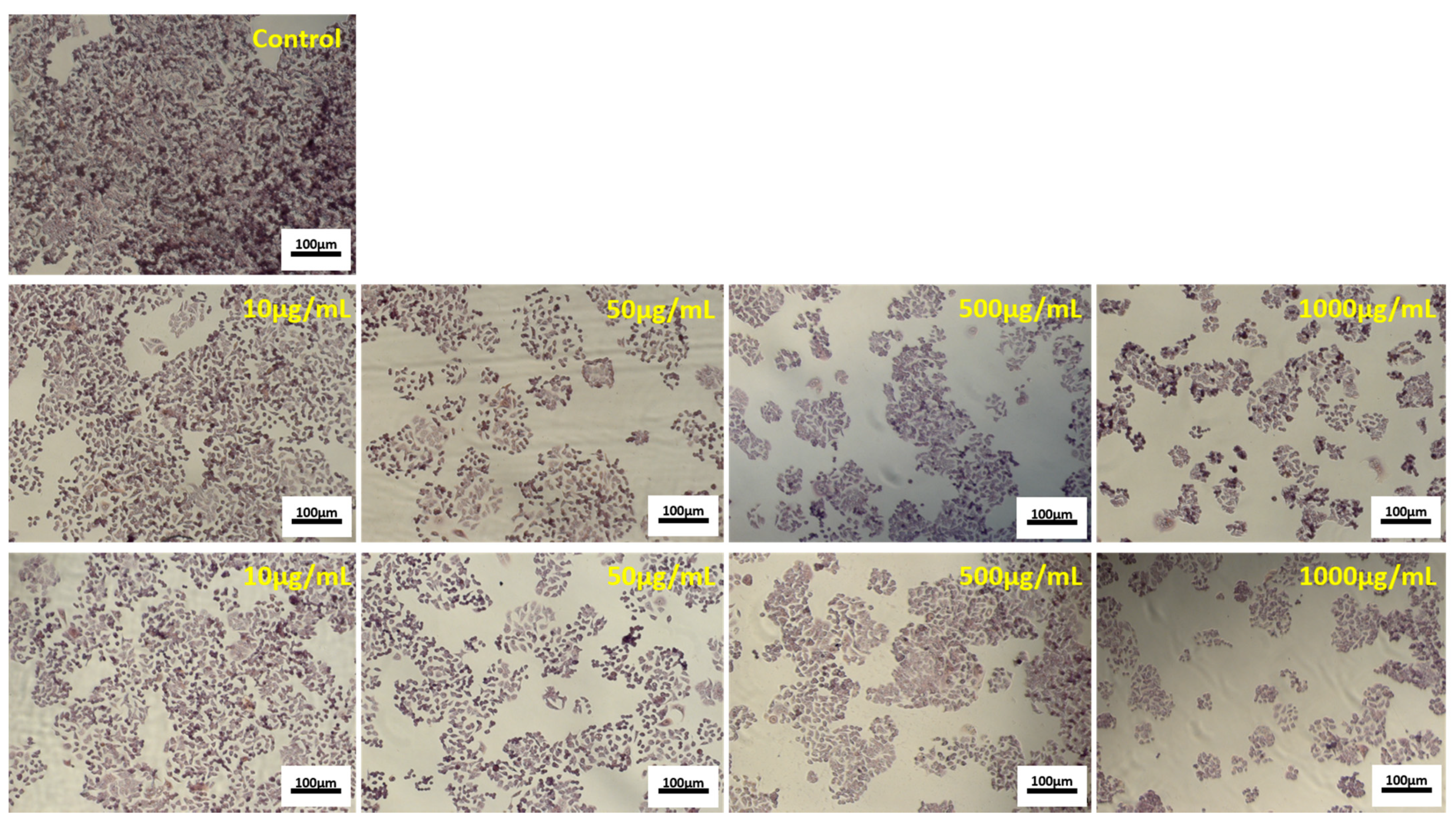
2.10. In Vitro Cytotoxicity of the Powders in HT-29 and L929 Cells by Neutral Red Assay
2.11. Light Microscopy
2.12. Stability Study
2.13. Statistical Analysis of Data
3. Results
3.1. The Phytochemical Content and Antioxidant Activity of the Tomato Peels Extract
3.2. Microencapsulation Efficiency of the Selected Extracts
3.3. Powders Phytochemical Profile and Antioxidant Activity
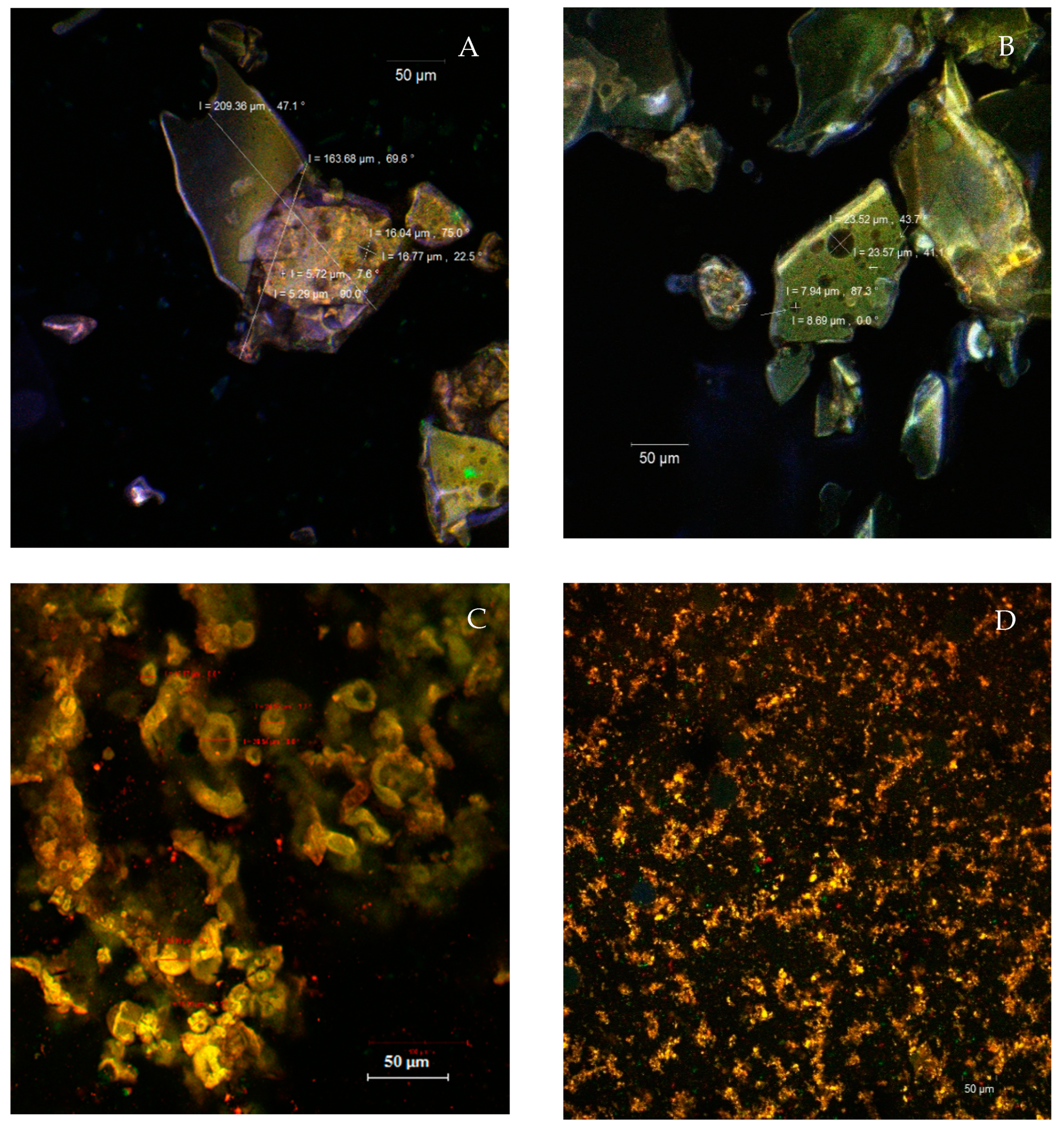
3.4. Structural and Morphological Properties of the Powders
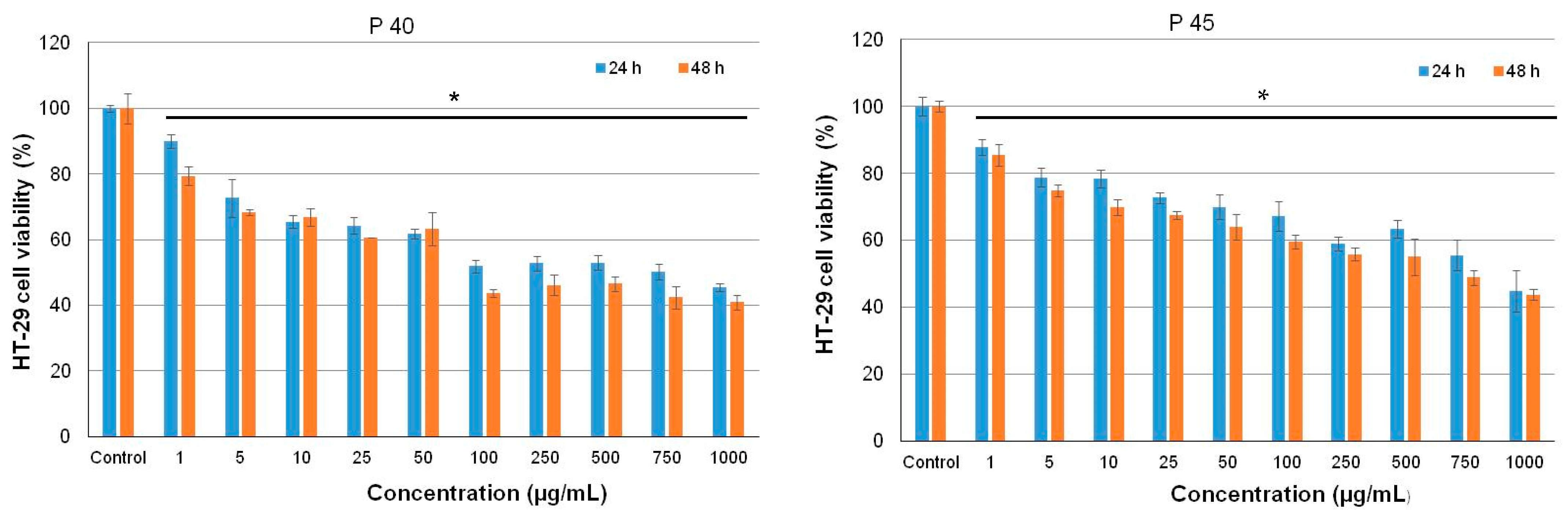
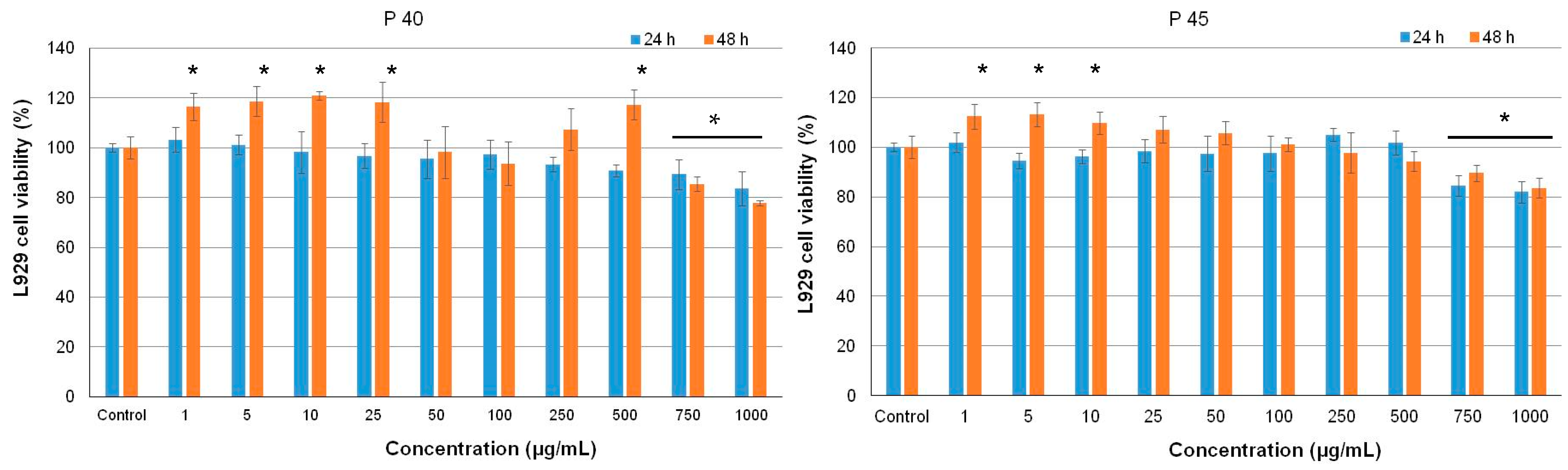
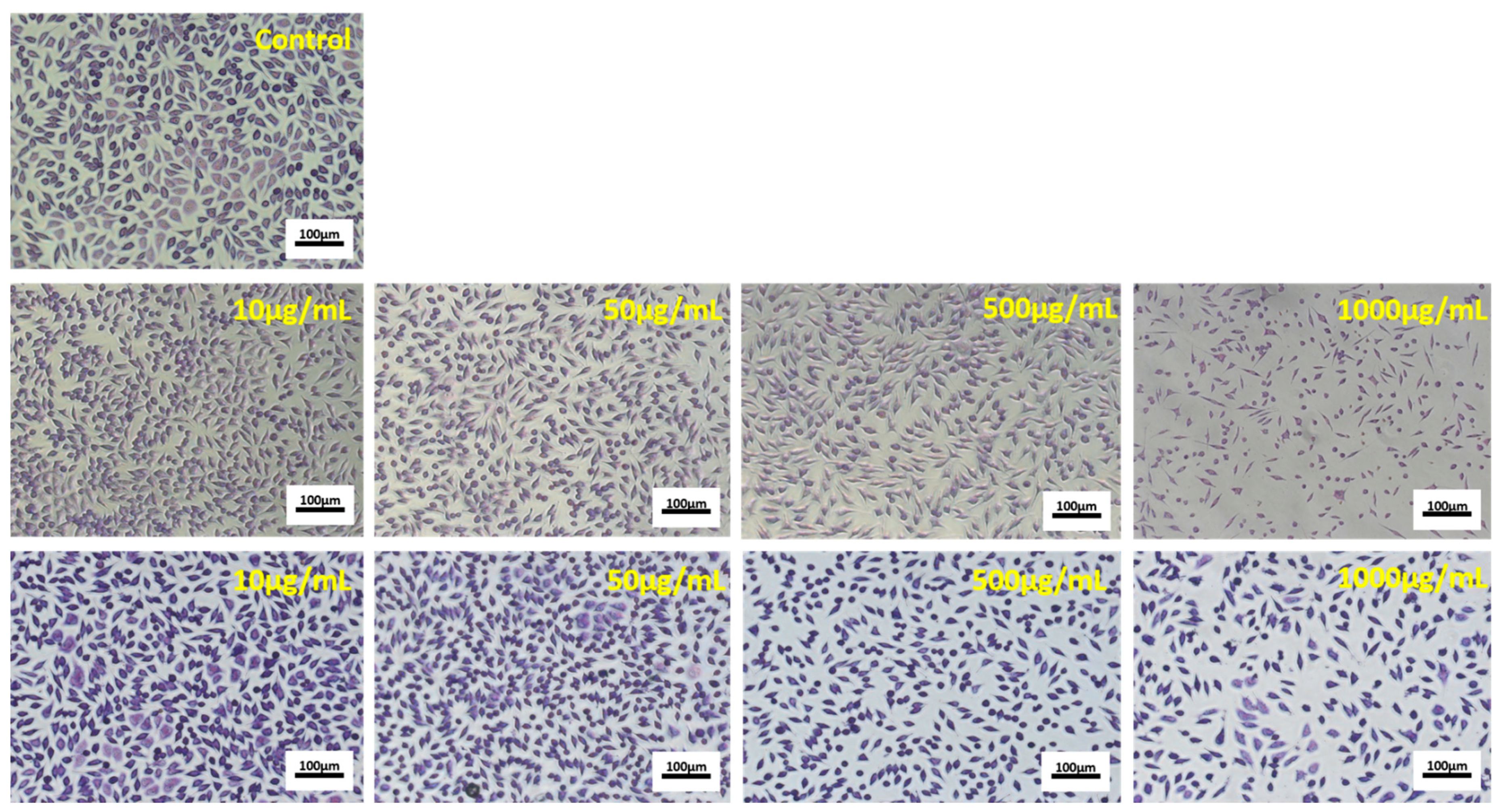
3.5. Antiproliferative Activity of the Microencapsulated Powders
3.6. Retention Rate of Lycopene at Storage Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urbonaviciene, D.; Viskelis, P. The cis-lycopene isomers composition in supercritical CO2 extracted tomato by-products. J. Food Sci. Technol. 2017, 85, 517–523. [Google Scholar]
- Colle, I.J.P.; Lemmens, L.; Tolesa, G.N.; Van Buggenhout, S.; De Vleeschouwer, K.; Van Loey, A.; Hendrickx, M. Lycopene degradation and isomerization kinetics during thermal processing of an olive oil/tomato emulsion. J. Agric. Food Chem. 2010, 58, 12784–12789. [Google Scholar] [CrossRef] [PubMed]
- Kehili, M.; Kammlott, M.; Choura, S.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Supercritical CO2 extraction and antioxidant activity of lycopene and β-carotene-enriched oleoresin from tomato (Lycopersicum esculentum L.) peels by-product of a Tunisian industry. Food Bioprod. Process. 2017, 102, 340–349. [Google Scholar] [CrossRef]
- Topal, U.; Sasaki, M.; Goto, M.; Hayakawa, K. Extraction of lycopene from tomato skin with supercritical carbon dioxide:effect of operating conditions and solubility analysis. J. Agric. Food Chem. 2006, 54, 5604–5610. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Murkovic, M. Thin-layer chromatographic analysisof carotenoids in plant and animal samples: A review. J. Planar Chromatogr. 2010, 23, 94–103. [Google Scholar] [CrossRef]
- Holzapfel, N.P.; Holzapfel, B.M.; Champ, S.; Feldthusen, J.; Clements, J.; Hutmacher, D.W. The potential role of lycopene for the prevention and therapy of prostate cancer: From molecular mechanisms to clinical evidence. Int. J. Mol. Sci. 2013, 14, 14620–14646. [Google Scholar] [CrossRef]
- Rao, A.V.; Agarwal, S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: A review. Nutr. Res. 1999, 19, 305–323. [Google Scholar] [CrossRef]
- Rafi, M.M.; Yadav, P.N.; Reyes, M. Lycopene inhibits LPS-induced proinflammatory mediator inducible nitric oxide synthase in mouse macrophage cells. J. Food Sci. 2007, 72, S69–S74. [Google Scholar] [CrossRef]
- Scaglia, B.; D’Incecco, P.; Squillace, P.; Dell’Orto, M.; De Nisi, P.; Pellegrino, L.; Botto, A.; Cavicchi, C.; Adani, F. Development of a tomato pomace biorefinery based on a CO2-supercritical extraction process for the production of a high value lycopene product, bioenergy and digestate. J. Clean. Prod. 2020, 243, 118650. [Google Scholar] [CrossRef]
- Hatami, T.M.; Meireles, A.A.; Ciftci, O.N. Supercritical carbon dioxide extraction of lycopene from tomato processing by-products: Mathematical modeling and optimization. J. Food Eng. 2019, 241, 18–25. [Google Scholar] [CrossRef]
- Barros, H.D.; Grimaldi, R.; Cabral, F.A. Lycopene-rich avocado oil obtained by simultaneous supercritical extraction from avocado pulp and tomato pomace. J. Supercrit. Fluids 2017, 120, 1–6. [Google Scholar] [CrossRef]
- Pérezmasiá, R.; Lagaron, J.M.; Lopezrubio, A. Morphology and stability of edible lycopene-containing micro- and nanocapsules produced through electrospraying and spray drying. Food Bioprocess. Technol. 2015, 8, 459–470. [Google Scholar] [CrossRef]
- Souza, A.L.R.; Hidalgo-Chávez, D.W.; Pontes, S.M.; Gomes, F.S.; Cabral, L.M.C.; Tonon, R.V. Microencapsulation by spray drying of a lycopene-rich tomato concentrate: Characterization and stability. LWT Food Sci. Technol. 2018, 91, 286–292. [Google Scholar] [CrossRef]
- Jia, C.; Cao, D.; Ji, S.; Lin, W.; Zhang, X.; Muhoza, B. Whey protein isolate conjugated with xylo-oligosaccharides via maillard reaction: Characterization, antioxidant capacity, and application for lycopene microencapsulation. LWT Food Sci. Technol. 2020, 118, 108837. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Ghoshal, G.; Kesharwani, P.; Singh, B.; Shivhare, U.S.; Katare, O.P. Lycopene loaded whey protein isolate nanoparticles: An innovative endeavor for enhanced bioavailability of lycopene and anti-cancer activity. Int. J. Pharm. 2018, 546, 97–105. [Google Scholar] [CrossRef]
- Salimi, A.; Maghsoudlou, Y.; Jafari, S.M.; Mahoonak, A.S.; Kashaninejad, M.; Ziaiifar, A.M. Preparation of lycopene emulsions by whey protein concentrate and maltodextrin and optimization by response surface methodology. J. Dispers. Sci. Technol. 2014, 36, 274–283. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control. Release 2019, 298, 38–67. [Google Scholar] [CrossRef]
- Gheonea (Dima), I.; Aprodu, I.; Cîrciumaru, A.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Microencapsulation of lycopene from tomatoes peels by complex coacervation and freeze-drying: Evidences on phytochemical profile, stability and food applications. J. Food Eng. 2021, 288, 110166. [Google Scholar] [CrossRef]
- Gaspar, A.; Crăciunescu, O.; Trif, M.; Moisei, M.; Moldovan, L. Antioxidant and anti-inflammatory properties of active compounds from Arnica montana L. Rom. Biotechnol. Lett. 2014, 19, 9353–9365. [Google Scholar]
- Machmudah, S.Z.; Winardi, S.; Sasaki, M.; Goto, M.; Kusumoto, N.; Hayakawa, K. Lycopene extraction from tomato peel by-product containing tomato seed using supercritical carbon dioxide. J. Food Eng. 2012, 108, 290–296. [Google Scholar] [CrossRef]
- Rizk, E.M.; El-Kady, A.T.; El-Bialy, A.R. Characterization of carotenoids (lycored) extracted from tomato peels and its uses as natural colorants and antioxidants of ice cream. Ann. Agric. Sci. 2014, 59, 53–61. [Google Scholar] [CrossRef]
- Ooe, E.; Ogawa, K.; Horiuchi, T.; Tada, H.; Murase, H.; Tsuruma, K.; Shimazawa, M.; Hara, H. Analysis and characterization of anthocyanins and carotenoids in Japanese blue tomato. Biosci. Biotechnol. Biochem. 2015, 80, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Etzbach, L.; Meinert, M.; Faber, T.; Klein, C.; Schieber, A.; Weber, F. Effects of carrier agents on powder properties, stability of carotenoids, and encapsulation efficiency of goldenberry (Physalis peruviana L.) powder produced by co-current spray drying. Curr. Res. Food Sci. 2020, 3, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Thakura, D.; Ghoshala, G.; Katare, O.P.; Singh, B.; Shivhare, U.S. Formation and functional attributes of electrostatic complexes involving casein and anionic polysaccharides: An approach to enhance oral absorption of lycopene in rats In Vivo. Int. J. Biol. Macromol. 2016, 93, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Neagu, C.; Mihalcea, L.; Enachi, E.; Barbu, V.; Borda, D.; Bahrim, G.E.; Stănciuc, N. Cross-Linked Microencapsulation of CO2 Supercritical Extracted Oleoresins from Sea Buckthorn: Evidence of Targeted Functionality and Stability. Molecules 2020, 25, 2442. [Google Scholar] [CrossRef] [PubMed]
- Burgess, L.C.; Rice, E.; Fischer, T.; Seekins, J.R.; Burgess, T.P.; Sticka, S.J.; Klatt, K. Lycopene has limited effect on cell proliferation in only two of seven human cell lines (both cancerous and noncancerous) in an In Vitro system with doses across the physiological range. Toxicol. In Vitro 2008, 22, 1297–1300. [Google Scholar] [CrossRef]
- Teodoro, A.J.; Oliveira, F.L.; Martins, N.B.; de Azevedo Maia, G.; Martucci, R.B.; Borojevic, R. Effect of lycopene on cell viability and cell cycle progression in human cancer cell lines. Cancer Cell Int. 2012, 12, 36. [Google Scholar] [CrossRef]
- Vasconcelos, A.G.; Valim, M.O.; Amorim, A.G.N.; do Amaral, C.P.; de Almeida, M.P.; Borges, T.K.S.; Socodato, R.; Portugal, C.C.; Brand, G.D.; Mattos, J.S.C.; et al. Cytotoxic activity of poly-ɛ-caprolactone lipid-core nanocapsules loaded with lycopene-rich extract from red guava (Psidium guajava L.) on breast cancer cells. Food Res. Int. 2020, 136, 109548. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020, 129, 110459. [Google Scholar] [CrossRef]
| Matrix | Phytochemicals | |||||
|---|---|---|---|---|---|---|
| Lycopene (mg/g DW) | β-Carotene (mg/g DW) | Antioxidant Activity (mM TEAC/g DW) | ||||
| S40 | S45 | S40 | S45 | S40 | S45 | |
| SCE extract (70 °C) | 6.06 ± 0.06 b | 9.48 ± 0.41 c | 10.88 ± 0.33 b | 18.93 ± 0.73 c | 48.52 ± 3.63 b | 24.35 ± 0.71 c |
| SCE extract (74 °C) | 5.28 ± 0.07 b | 39.11 ± 0.59 a | 12.57 ± 0.11 a | 68.24 ± 0.71 a | 77.61 ± 0.99 a | 62.74 ± 1.74 a |
| SCE extract (80 °C) | 8.11 ± 0.65 a | 30.59 ± 0.63 b | 11.85 ± 0.59 ab | 47.08 ± 1.05 b | 39.99 ± 1.02 c | 38.34 ± 2.13 b |
| Microencapsulated powder (P40) | 10.83 ± 0.09 A | 22.49 ± 1.00 A | 8.57 ± 0.74 A | |||
| Microencapsulated powder (P45) | 10.49 ± 0.23 A | 19.93 ± 0.42 B | 9.37 ± 0.48 A | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihalcea, L.; Crăciunescu, O.; Gheonea, I.; Prelipcean, A.-M.; Enachi, E.; Barbu, V.; Bahrim, G.E.; Râpeanu, G.; Oancea, A.; Stănciuc, N. Supercritical CO2 Extraction and Microencapsulation of Lycopene-Enriched Oleoresins from Tomato Peels: Evidence on Antiproliferative and Cytocompatibility Activities. Antioxidants 2021, 10, 222. https://doi.org/10.3390/antiox10020222
Mihalcea L, Crăciunescu O, Gheonea I, Prelipcean A-M, Enachi E, Barbu V, Bahrim GE, Râpeanu G, Oancea A, Stănciuc N. Supercritical CO2 Extraction and Microencapsulation of Lycopene-Enriched Oleoresins from Tomato Peels: Evidence on Antiproliferative and Cytocompatibility Activities. Antioxidants. 2021; 10(2):222. https://doi.org/10.3390/antiox10020222
Chicago/Turabian StyleMihalcea, Liliana, Oana Crăciunescu, Ionica Gheonea (Dima), Ana-Maria Prelipcean, Elena Enachi, Vasilica Barbu, Gabriela Elena Bahrim, Gabriela Râpeanu, Anca Oancea, and Nicoleta Stănciuc. 2021. "Supercritical CO2 Extraction and Microencapsulation of Lycopene-Enriched Oleoresins from Tomato Peels: Evidence on Antiproliferative and Cytocompatibility Activities" Antioxidants 10, no. 2: 222. https://doi.org/10.3390/antiox10020222
APA StyleMihalcea, L., Crăciunescu, O., Gheonea, I., Prelipcean, A.-M., Enachi, E., Barbu, V., Bahrim, G. E., Râpeanu, G., Oancea, A., & Stănciuc, N. (2021). Supercritical CO2 Extraction and Microencapsulation of Lycopene-Enriched Oleoresins from Tomato Peels: Evidence on Antiproliferative and Cytocompatibility Activities. Antioxidants, 10(2), 222. https://doi.org/10.3390/antiox10020222









