Old Plant, New Possibilities: Wild Bilberry (Vaccinium myrtillus L., Ericaceae) in Topical Skin Preparation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Wild Bilberry Isolates
2.2.1. Preparation of Wild Bilberry Leaves Extract
2.2.2. Preparation of Wild Bilberry Seeds Oil
2.3. Chemical Analysis of Wild Bilberry Isolates
2.3.1. Determination of Total Phenolics, Tannins, Flavonoids Content in Wild Bilberry Leaves Extract
2.3.2. HPLC Analysis of Wild Bilberry Leaves Extract
2.3.3. GC Analysis of Wild Bilberry Seeds Oil
2.4. Antioxidant Activity of Wild Bilberry Isolates (Leaves Extract and Seeds Oil)
2.4.1. Radical-Scavenging Activity
2.4.2. Ferric-Reducing Antioxidant Power (FRAP)
2.5. Preparation of the Creams with Wild Bilberry Isolates
2.6. Skin Study Design
Skin Study Design
2.7. Examination of Sensory Properties
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analysis of Wild Bilberry Isolates
3.1.1. Chemical Analysis of Wild Bilberry Leaves Extract
3.1.2. Chemical Analysis of Wild Bilberry Seed Oil
3.2. Antioxidant Activity of Wild Bilberry Isolates
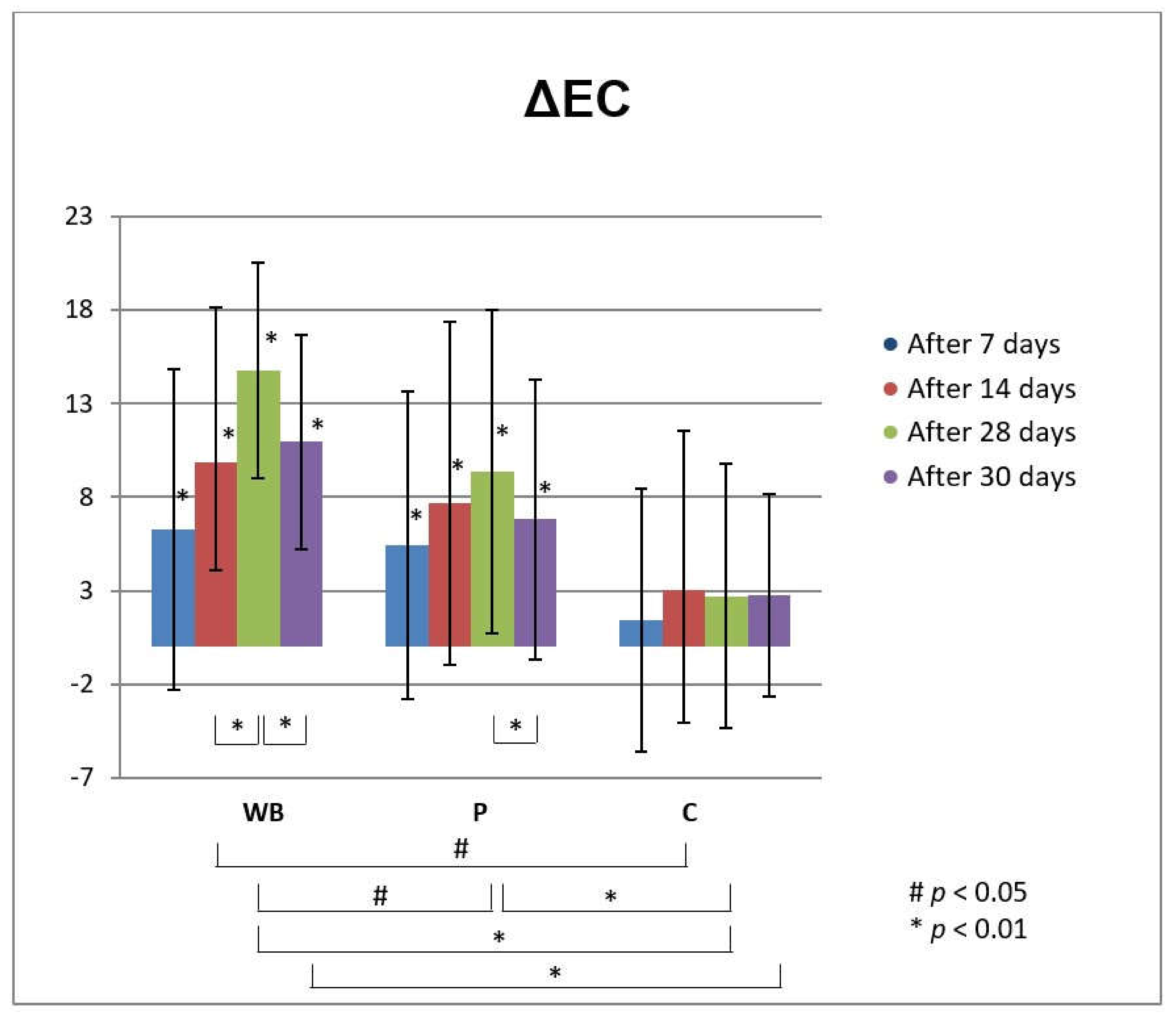
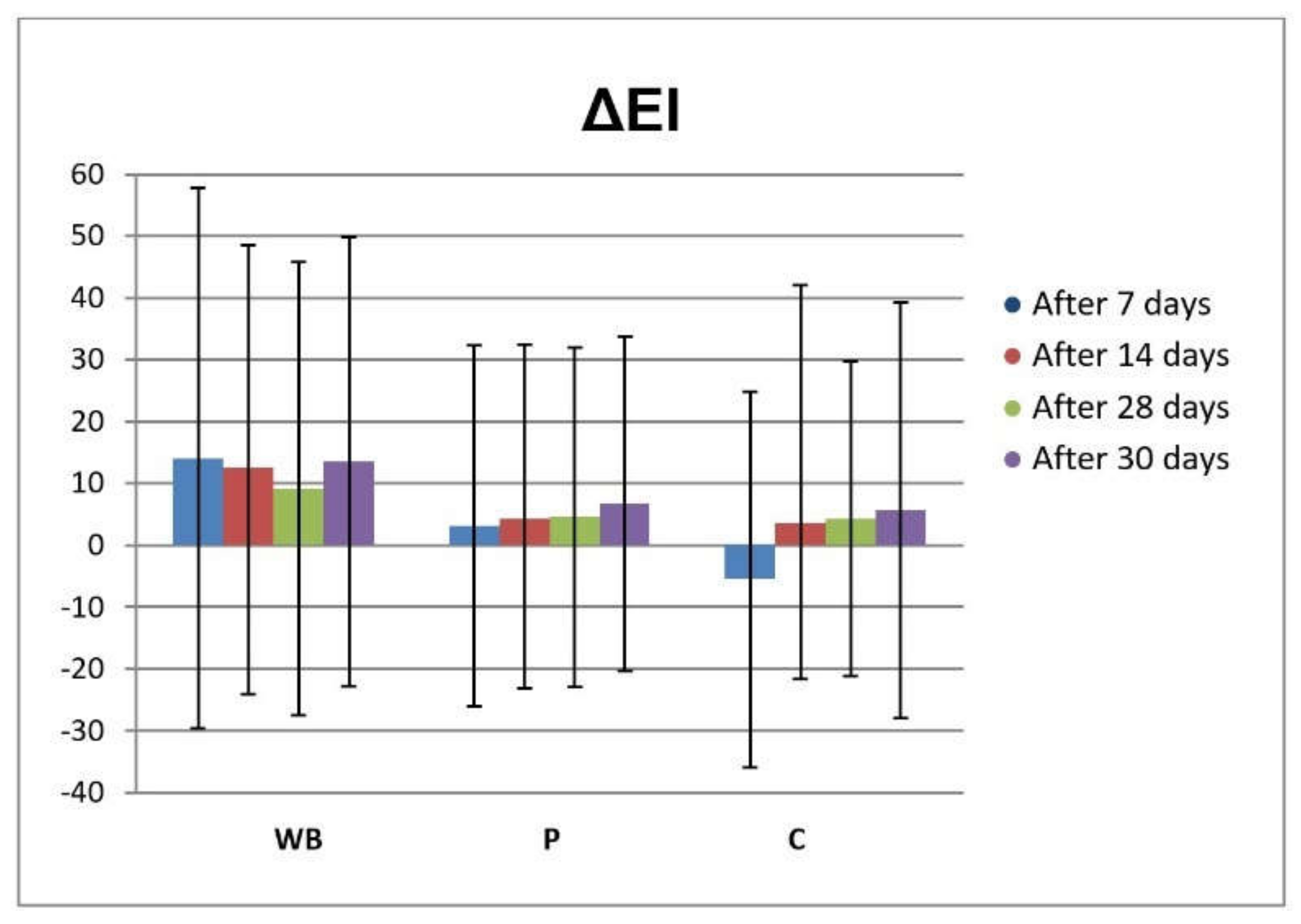
3.3. In Vivo Investigations of Cream with Wild Bilberry Isolates
Skin Effects of the Investigated Cream Assessed via Biophysical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gustinelli, G.; Eliasson, L.; Svelander, C.; Alminger, M.; Ahrnea, L. Supercritical CO2 extraction of bilberry (Vaccinium myrtillus L.) seed oil: Fatty acid composition and antioxidant activity. J. Supercrit. Fluid. 2018, 135, 91–97. [Google Scholar] [CrossRef]
- Chu, W.-K.; Cheung, S.C.M.; Lau, R.A.W.; Benzie, I.F.F. Bilberry (Vaccinium myrtillus L.). In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; Chapter 4; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Bujor, O.-C.; Tanase, C.; Popa, M.E. Phenolic antioxidants in aerial parts of wild Vaccinium species: Towards pharmaceutical and biological properties. Antioxidants 2019, 8, 649. [Google Scholar] [CrossRef] [PubMed]
- Može, S.; Polak, T.; Gašperlin, L.; Koron, D.; Vanzo, A.; Poklar Ulrih, N.; Abram, V. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Campalani, C.; Amadio, E.; Zanini, S.; Dall’Acqua, S.; Panozzo, M.; Ferrari, S.; De Nadai, G.; Francescato, S.; Selva, M.; Perosa, A. Supercritical CO2 as a green solvent for the circular economy: Extraction of fatty acids from fruit pomace. J. CO2 Util. 2020, 41, 101259. [Google Scholar] [CrossRef]
- Brasanac-Vukanovic, S.; Mutic, J.; Stankovic, D.M.; Arsic, I.; Blagojevic, N.; Vukasinovic-Pesic, V.; Tadic, V.M. Wild Bilberry (Vaccinium myrtillus L., Ericaceae), from Montenegro as a Source of Antioxidants for Use in the Production of Nutraceuticals. Molecules 2018, 23, 1864. [Google Scholar] [CrossRef]
- Lukic, M.; Jaksic, I.; Krstonosic, V.; Cekic, N.; Savic, S. A combined approach in characterization of an effective w/o hand cream: The influence of emollient on textural, sensorial and in vivo skin performance. Int. J. Cosmet. Sci. 2012, 34, 140–149. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopeia, 9th ed.; Council of Europe: Strasbourg, France, 2016. [Google Scholar]
- AOAC. No. 41.1.27 Official Method 965.49. Fatty Acids in Oils and Fats. Preparation of Methyl Esters, Final Action 1984, 16th ed.; AOAC International: Gaithersburg, MD, USA, 1998. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by GasChromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- D’Arcy, P.; Mallard, W.G. Automated Mass Spectral Deconvolution and Identification System Software (AMDIS ver. 2.1); National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2005.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Tadic, V.; Zivkovic, J.; Bigovic, D.; Zugic, A. Variation of parthenolide and phenolic compounds content in different parts of Tanacetum parthenium (L.) Schulz Bip., Asteraceae during 18 months storage. Lek. Sirovine 2019, 39, 35–39. [Google Scholar] [CrossRef]
- Vučić, D.M.; Petković, M.R.; Rodić-Grabovac, B.B.; Stefanović, O.D.; Vasić, S.M.; Čomić, L.R. Antibacterial and antioxidant activities of bilberry (Vaccinium myrtillus L.) in vitro. Afr. J. Microbiol. Res. 2013, 7, 5130–5136. [Google Scholar]
- Roslon, W.; Osinska, E.; Pioro-Jabrucka, E.; Grabowska, A. Morphological and chemical variability of wild populations of bilberry (Vaccinium myrtillus L.). Pol. J. Environ. Stud. 2011, 20, 237–243. [Google Scholar]
- Bujor, O.-C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal variations of the phenolic constituents in bilberry (Vaccinium myrtillus L.) leaves, stems and fruits, and their antioxidant activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, J.; Mattila, S.; Jaakola, L.; Pirttilä, A.M.; Tolonen, A. Identification of phenolic compounds from lingonberry (Vaccinium vitis-idaea L.), bilberry (Vaccinium myrtillus L.) and hybrid bilberry (Vaccinium x intermedium Ruthe L.) leaves. J. Agric. Food Chem. 2009, 57, 9437–9447. [Google Scholar] [CrossRef] [PubMed]
- Bljajic, K.; Petlevski, R.; Vujic, L.; Čačić, A.; Šoštarić, N.; Jablan, J.; Carvalho, I.S.; Končić, M.Z. Chemical composition, antioxidant and α-glucosidase-inhibiting activities of the aqueous and hydroethanolic extracts of Vaccinium myrtillus leaves. Molecules 2017, 22, 703. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ahotupa, M.; Määttä, P.; Kallio, H. Composition and antioxidative activities of supercritical CO2-extracted oils from seeds and soft parts of northern berries. Food Res. Int. 2011, 44, 2009–2017. [Google Scholar] [CrossRef]
- Ishak, W.M.W.; Katas, H.; Yuen, N.P.; Abdullah, M.A.; Zulfakar, M.H. Topical application of omega-3-, omega-6-, and omega-9-rich oil emulsions for cutaneous wound healing in rats. Drug Deliv. Transl. Res. 2019, 9, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Ko, G.-A.; Shrestha, S.; Cho, S.K. Sageretia thea fruit extracts rich in methyl linoleate and methyl linolenate downregulate melanogenesis via the Akt/GSK3β signaling pathway. Nutr. Res. Pract. 2018, 12, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Godin, B.; Karl, Y.; Bujanover, S.; Becker, Y. Oleic acid, a skin penetration enhancer, affects Langerhans cells and corneocytes. J. Control. Release 2002, 80, 1–7. [Google Scholar] [CrossRef]
- Stajčić, S.M.; Tepić, A.N.; Djilas, S.M.; Šumić, Z.M.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Vulić, J.J.; Tumbas, V.T. Chemical composition and antioxidant activity of berry fruits. Acta Period. Technol. 2012, 43, 93–105. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2017, 105, 940–949. [Google Scholar] [CrossRef]
- Chew, K.K.; Ng, S.Y.; Thoo, Y.Y.; Khoo, M.Z.; Wan Aida, W.M.; Ho, C.W. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Centella asiatica extracts. Int. Food Res. J. 2011, 18, 571–578. [Google Scholar]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Gerszon, J.; Rodacka, A.; Puchala, M. Antioxidant properties of resveratrol and its protective effects in neurodegenerative diseases. Adv. Cell Biol. 2015, 4, 97–117. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef]
- Gustinelli, G.; Eliasson, L.; Svelander, C.; Andlid, T.; Lundin, L.; Ahrné, L.; Alminger, M. Supercritical fluid extraction of berry seeds: Chemical composition and antioxidant activity. J. Food Qual. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Man, M.Q.; Xin, S.J.; Song, S.P.; Cho, S.Y.; Zhang, X.J.; Tu, C.X.; Feingold, K.R.; Elias, P.M. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large Chinese population. Skin Pharmacol. Physiol. 2009, 22, 190–199. [Google Scholar] [CrossRef]
- Kitagawa, S.; Yoshii, K.; Morita, S.Y.; Teraoka, R. Efficient topical delivery of chlorogenic acid by an oil-in-water microemulsion to protect skin against UV-induced damage. Chem. Pharm. Bull. 2011, 59, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Akhtar, N.; Mustafa, R. An overview of dermatological and cosmeceutical benefits of Diospyros kaki and its phytoconstituents. Rev. Bras. Farmacogn. 2017, 27, 650–662. [Google Scholar] [CrossRef]
- Bhatia, N.; Kaur, G.; Soni, V.; Kataria, J.; Dhawan, R.K. Evaluation of the wound healing potential of isoquercetin-based cream on scald burn injury in rats. Burn. Trauma 2016, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Aikens, P.A.; Friberg, S.E. Emulsifiers. In Dry Skin and Moisturizers, 1st ed.; Loden, M., Maibach, H., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 183–201. [Google Scholar]
- McCusker, M.M.; Grant-Kels, J.M. Healing fats of the skin: The structural and immunologic roles of the omega-6 and omega-3 fatty acids. Clin. Dermatol. 2010, 28, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, R.K.; Krissansen, G.W.; Black, P.N.; MacGibbon, A.K.H. Compositions of Cis-9, Trans-11 Conjugated Linoleic Acid and Vaccenic Acid and Uses Thereof. 2009. Available online: https://patentimages.storage.googleapis.com/47/1c/4b/fd33705c87c5d7/US20090048339A1.pdf (accessed on 26 January 2021).
- De Paepe, K.; Derde, M.P.; Roseeuw, D.; Rogiers, V. Claim substantiation and efficiency of hydrating body lotions and protective creams. Contact Dermat. 2000, 42, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Le, T.K.; De Mon, P.; Schalkwuk, J.; Van Der Valk, P.G. Effect of a topical corticosteroid, a retinoid and a vitamin D3 derivative on sodium dodecyl sulphate induced skin irritation. Contact Dermat. 1997, 37, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Effendy, I.; Maibach, H.I. Acute irritant contact dermatitis: Recovery time in man. Contact Dermat. 1997, 36, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, V. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol. Physiol. 2001, 14, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Lamoreux, M.R.; Sternbach, M.R.; Hsu, W.T. Erythema multiforme. Am. Fam. Phys. 2006, 74, 1883–1888. [Google Scholar]
- Munke, S.; Assmus, U.; Banowski, B.; Blaak, J.; Brock, M.; Erasmy, J.; Fitzner, A.; Kortemeier, U.; Langer, S.; Schmidt-Lewerkühne, H.; et al. The impact of cleansing products on the skin surface. Int. Fed. Soc. Cos. Chem. 2013, 3, 17–24. [Google Scholar]


| Component | WB | P | Function | |
|---|---|---|---|---|
| Trade Name | INCI Name | |||
| Oil phase | ||||
| Myritol® 318 | Caprylic/capric triglycerides | 5.50 | 5.50 | emollient |
| Sabowax® AE | Glyceryl Stearate (and) ceteareth-20 (and) ceteareth-12 (and) cetearyl alcohol (and) cetyl palmitate | 8.50 | 8.50 | O/W emulsifier, surfactant |
| Lanette 16 | Cetyl alcohol | 0.75 | 0.75 | emollient |
| Stearyl alcohol | Stearyl alcohol | 0.75 | 0.75 | emollient |
| Water phase | ||||
| Glycerin | Glycerin | 2.00 | 2.00 | humectant |
| Sodium benzoate | Sodium benzoate | 0.50 | 0.50 | preservative |
| Purified water | Water distilled to | 100.0 | 100.0 | water phase |
| Active substances of plant origin | ||||
| Wild bilberry oil | Vaccinium myrtillus L., seed oil | 6.00 | - | active component |
| Wild bilberry extract | Vaccinium myrtillus L. leaves maceration with 70% (v/v) ethanol | 6.00 | - | active component |
| Before Application | |
|---|---|
| Consistency | liquid/semi-solid |
| Gloss level | matt/pearl gloss/slightly gloss/gloss/very gloss |
| During application | |
| Spreadability | easy to spread/difficult to spread/very difficult to spread |
| Adhesion | not sticky/slightly sticky/sticky/very sticky |
| Density | rare/slightly dense/dense/very dense |
| Grease | not greasy/slightly greasy/greasy/very greasy |
| Gloss | not shiny/slightly shiny/ shiny/very shiny |
| Absorption rate | slow/moderate/fast |
| After application | |
| Residual film | no film/moderate film/expressive film |
| Stickiness | not sticky/slightly sticky/sticky/very sticky |
| Grease | not greasy/slightly greasy/greasy/very greasy |
| Gloss | not shiny/slightly shiny/shiny/very shiny |
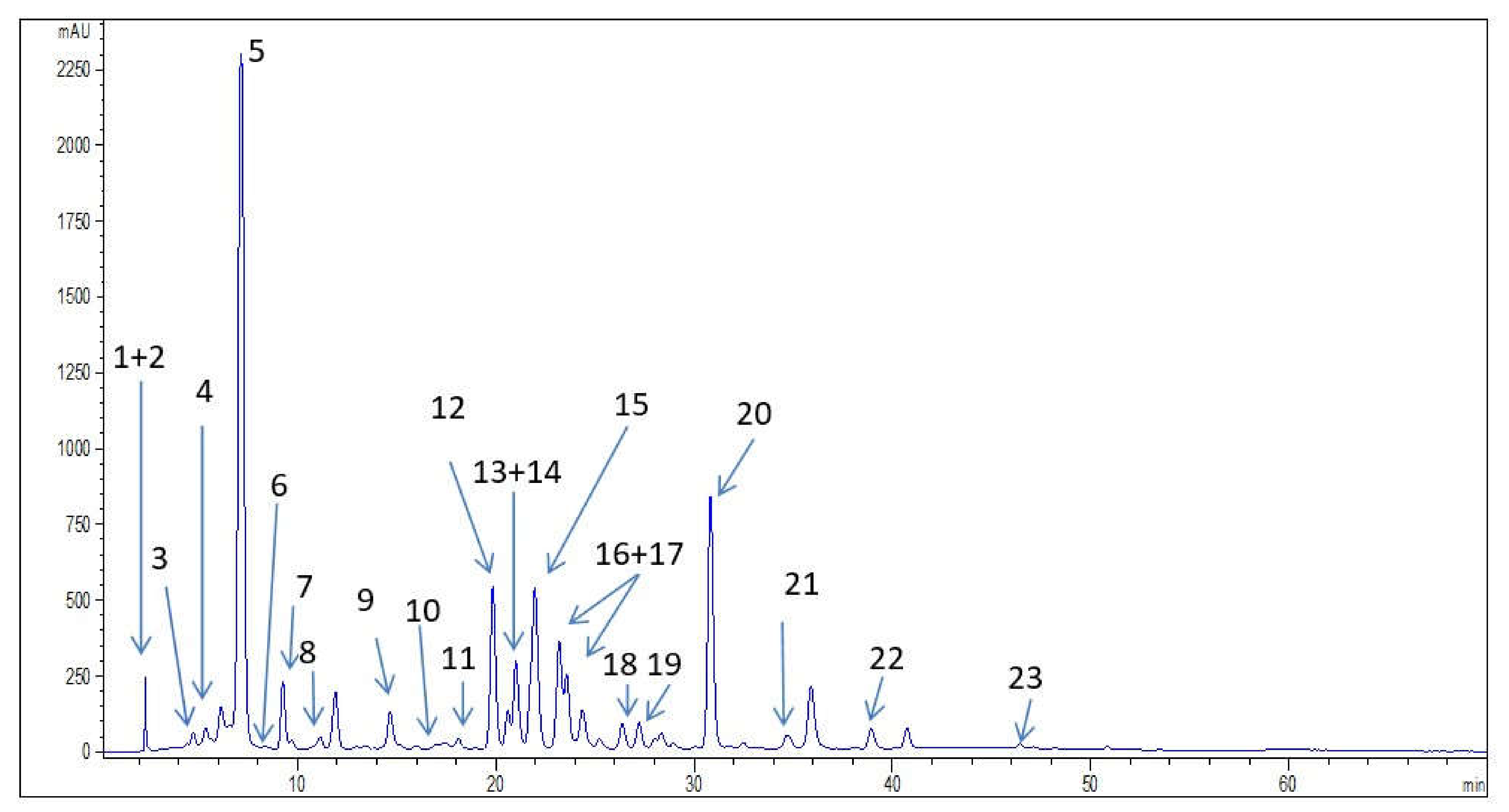
| Phenolic Compounds | Numbers in Figure 1 | Wild Bilberry Leaves Extract |
|---|---|---|
| Derivatives of hydroxycinnamic acid | ||
| Neochlorogenic acid | 3 | 0.34 |
| Chlorogenic acid | 5 | 45.51 |
| Chlorogenic acid derivative | 21 | * |
| Caffeic acid | 7 | 1.95 |
| p-coumaric acid | 9 | 1.26 |
| Sinapic acid | 10 | 0.18 |
| Ferulic acid | 11 | 0.26 |
| Derivatives of hydroxybenzoic acid | ||
| Gallic acid | 1 | 0.80 |
| Protocatechuic acid | 4 | 1.40 |
| Flavones and flavonols | ||
| Rutin | 13 | 4.73 |
| Hyperoside | 14 | 2.51 |
| Isoquercetin | 15 | 14.62 |
| Kaempferol-3-O-glucoside | 18 | 1.56 |
| Quercetin | 22 | 2.11 |
| Quercetin derivative 1 | 19 | * |
| Kaempferol | 23 | 0.10 |
| Flavanols | ||
| Procyanidin B2 | 6 | 1.29 |
| Epicatechin | 8 | 7.53 |
| Stilbenes | ||
| Resveratrol | 20 | 7.25 |
| Stilbenoid derivative 1 | 12 | * |
| Stilbenoid derivative 2 | 16 | * |
| Stilbenoid derivative 3 | 17 | * |
| Pyrogallol | 2 | 2.45 |
| Name | CAS | RI—Retention Index | % |
|---|---|---|---|
| Methyl hexadecanoate | 112-39-0 | 1921 | 4.61 |
| Methyl linoleate, ω-6 | 112-63-0 | 2095 | 38.68 |
| Methyl oleate, ω-9 | 112-62-9 | 2108 | 18.14 |
| Methyl α-linolenate, ω-3 | 301-00-8 | 2113 | 37.79 |
| Methyl stearate (methyl octadecanoate) | 112-61-8 | 2124 | 0.55 |
| 8,11,14-eicosatrienoic acid, methyl ester | 17364-32-8 | 2249 | 0.11 |
| cis-11,14-eicosadienoic acid, methyl ester | 24603-02-7 | 2305 | 0.11 |
| Samples | DPPH Assay (IC50 (mg/mL)) | FRAP Assay (mmol Fe2+/g of Extract) |
|---|---|---|
| Wild bilberry leaves extract | 2.13 | 3.6348 |
| Wild bilberry seed oil | 3.37 | 0.2045 |
| α-tocopherol | 0.47 | 16.9616 |
| BHT | 2.07 | 11.3152 |
| Before Application | ||||||
|---|---|---|---|---|---|---|
| Consistency | Gloss Level | |||||
| WB | Semisolid (100.0%) | Matt (69.2%) | ||||
| P | Semisolid (92.3%) | Slightly gloss (46.2%) | ||||
| During Application | ||||||
| Spreadability | Adhesion | Density | Grease | Gloss | Absorption Rate | |
| WB | Easy to spread (61.5%) | Not sticky (61.5%) | Slightly dense (46.2%) | Not greasy (46.2%) | Slightly shiny (69.2%) | Fast (61.5%) |
| P | Easy to spread (76.9%) | Not sticky (46.2%) | Dense (46.2%) | Not greasy (53.8%) | Slightly shiny (53.8%) | Fast (50%) |
| After Application | ||||||
| Residual Film | Stickiness | Grease | Gloss | |||
| WB | Moderate film (69.2%) | Slightly sticky (61.5%) | Not greasy (53.8%) | Slightly shiny (100%) | ||
| P | Moderate film (53.8%) | Slightly sticky (46.2%) | Not greasy (69.2%) | Slightly shiny (46.2%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadić, V.M.; Nešić, I.; Martinović, M.; Rój, E.; Brašanac-Vukanović, S.; Maksimović, S.; Žugić, A. Old Plant, New Possibilities: Wild Bilberry (Vaccinium myrtillus L., Ericaceae) in Topical Skin Preparation. Antioxidants 2021, 10, 465. https://doi.org/10.3390/antiox10030465
Tadić VM, Nešić I, Martinović M, Rój E, Brašanac-Vukanović S, Maksimović S, Žugić A. Old Plant, New Possibilities: Wild Bilberry (Vaccinium myrtillus L., Ericaceae) in Topical Skin Preparation. Antioxidants. 2021; 10(3):465. https://doi.org/10.3390/antiox10030465
Chicago/Turabian StyleTadić, Vanja M., Ivana Nešić, Milica Martinović, Edward Rój, Snežana Brašanac-Vukanović, Svetolik Maksimović, and Ana Žugić. 2021. "Old Plant, New Possibilities: Wild Bilberry (Vaccinium myrtillus L., Ericaceae) in Topical Skin Preparation" Antioxidants 10, no. 3: 465. https://doi.org/10.3390/antiox10030465
APA StyleTadić, V. M., Nešić, I., Martinović, M., Rój, E., Brašanac-Vukanović, S., Maksimović, S., & Žugić, A. (2021). Old Plant, New Possibilities: Wild Bilberry (Vaccinium myrtillus L., Ericaceae) in Topical Skin Preparation. Antioxidants, 10(3), 465. https://doi.org/10.3390/antiox10030465







