Comparative Analysis of the Polyphenols, Caffeine, and Antioxidant Activities of Green Tea, White Tea, and Flowers from Azorean Camellia sinensis Varieties Affected by Different Harvested and Processing Conditions
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Sample Origin, Withering, and Drying Methodologies
2.3. Sample Preparation for Antioxidant Assays and Extraction Methodology for Crude Catechins and CAF Content
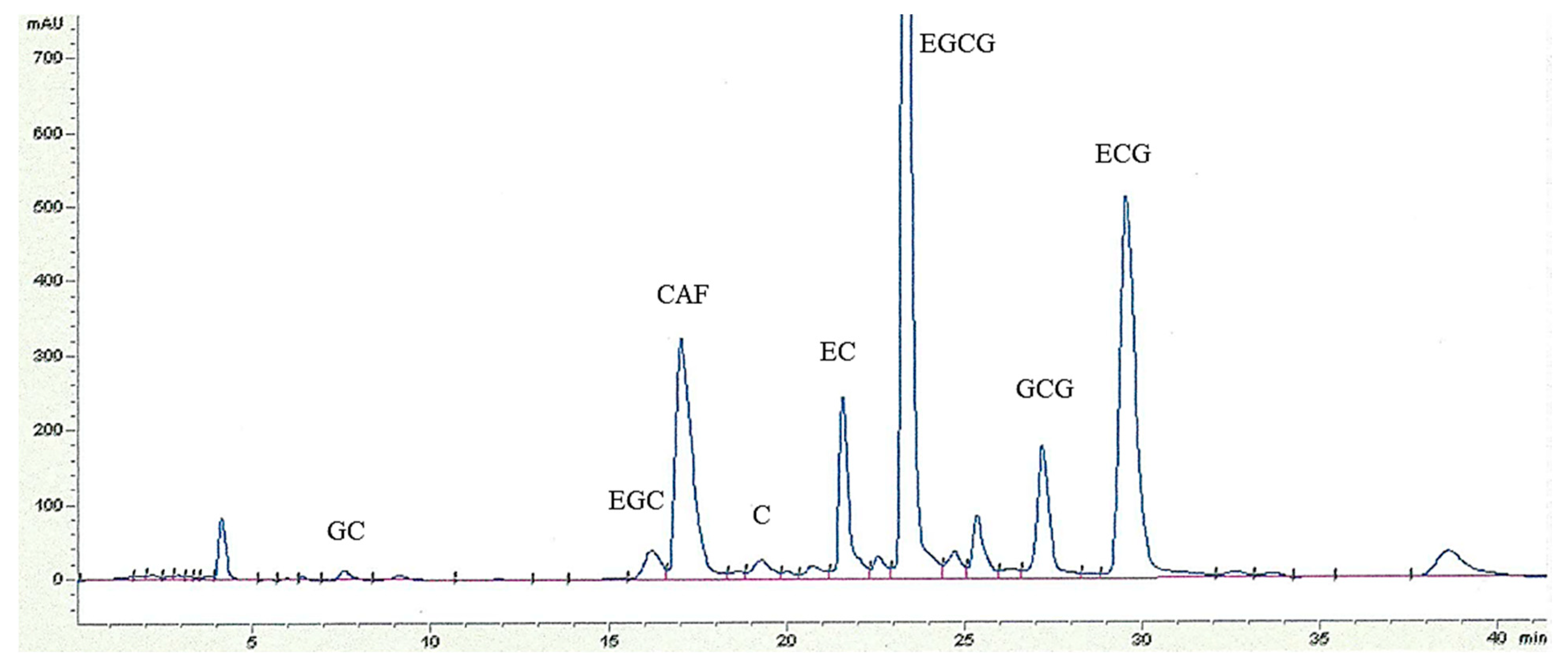
2.4. RP-HPLC Analysis of Catechins and CAF
2.5. Determination of the Total Phenolic and Total Flavonoid Content of TPs Extracts
2.6. Determination of the in Vitro Antioxidant Activity of TPs Extracts
2.6.1. Determination of DPPH-Free Radical Scavenging Activity (FRSA)
2.6.2. Determination of Ferric Reducing Antioxidant Power (FRAP)
2.6.3. Determination of Ferrous Ion-Chelating (FIC) Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Collection and Processing the Different Azorean C. sinensis Samples and Determination of their Catechin Profile and CAF Content
3.1.1. Extraction Process
3.1.2. Catechin Profiles and CAF Content of WT, Tea Flowers, and GT Samples
3.1.3. Impact of Plucking Season and Tea Processing on Catechin Profiles and CAF Content
3.2. TPC and TFC of C. sinensis Extract Samples
3.3. In Vitro Antioxidant Activity on TPs extracts
3.3.1. DPPH-FRSA Assay
3.3.2. FRAP Assay
3.3.3. FIC Activity Assay
3.4. Pearson Correlation between Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, C.-N.; Tang, G.-Y.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Liu, Q.; Mao, Q.-Q.; Shang, A.; Li, H.-B. Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R. Environmental and nutritional requirements for tea cultivation. Folia Hort. 2017, 29, 199–220. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Tounekti, T.; Joubert, E.; Hernández, I.; Munné-Bosch, S. Improving the polyphenol content of tea. Crit. Rev. Plant. Sci. 2013, 32, 192–215. [Google Scholar] [CrossRef]
- Derouiche, S. Oxidative stress associated with SARS-Cov-2 (COVID-19) increases the severity of the lung disease: A systematic review. J. Infect. Dis. Epidemiol. 2020, 6, 121. [Google Scholar]
- Zhu, Y.; Xie, D.-Y. Docking characterization and in vitro inhibitory activity of flavan-3-ols and dimeric proanthocyanidins against the main protease activity of SARS-Cov-2. Front. Plant. Sci. 2020, 11, 601316. [Google Scholar] [CrossRef]
- Too, J.C.; Kinyanjui, T.; Wanyoko, J.K.; Wachira, F.N. Effect of sunlight exposure and different withering durations on theanine levels in tea (Camellia sinensis). Food Nutr. Sci. 2015, 6, 1014–1021. [Google Scholar]
- Chen, M.-L. Tea and health—An overview. In Tea Bioactivity and Therapeutic Potential; Zhen, Y., Chen, Z., Cheng., S., Chen, M., Eds.; Taylor and Francis: London, UK, 2002; pp. 1–16. [Google Scholar]
- Collings, E.R.; Alamar, M.C.; Redfern, S.; Cools, K.; Terry, L.A. Spatial changes in leaf biochemical profile of two tea cultivars following cold storage under two different vapour pressure deficit (VPD) conditions. Food Chem. 2019, 277, 179–185. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, P.; Lin, L.; Harnly, J.M.; Yu, L.; Li, Z. Tentative identification, quantitation, and principal component analysis of green pu-erh, green, and white teas using UPLC/DAD/MS. Food Chem. 2011, 126, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Griffin, T.S.; Kraner, D.; Schaffner, M.K.; Sharma, D.; Hazel, M.; Leitch, A.R.; Orians, C.M.; Han, W.; Stepp, J.R.; et al. Environmental factors variably impact tea secondary metabolites in the context of climate change. Front. Plant. Sci. 2019, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Bacchetti, T.; Padella, L.; Tiano, L.; Carloni, P. Antioxidant activity of different white teas: Comparison of hot and cold tea infusions. J. Food Compos. Anal. 2014, 33, 59–66. [Google Scholar] [CrossRef]
- Kosińska, A.; Andlauer, W. Antioxidant capacity of tea: Effect of processing and storage. In Processing and Impact on Antioxidants in Beverages; Preedy, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 109–120. [Google Scholar]
- Hilal, Y.; Engelhardt, U. Characterisation of white tea—Comparison to green and black tea. J. Verbr. Lebensm. 2007, 2, 414–421. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A. Analysis of antioxidant activity, chlorogenic acid and rutin content of Camellia sinensis infusions using response surface methodology optimization. Food Anal. Methods 2014, 7, 2033–2041. [Google Scholar] [CrossRef]
- Unachukwu, U.J.; Ahmed, S.; Kavalier, A.; Lyles, J.T.; Kennelly, E.J. White and green teas (Camellia sinensis var. sinensis): Variation in phenolic, methylxanthine, and antioxidant profiles. J. Food Sci. 2010, 75, C541–C548. [Google Scholar]
- Zhang, L.; Ho, C.-T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis teas: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef]
- Tomlins, K.I.; Mashingaidze, A. Influence of handling on the manufacturing and quality of black teas: A review. Food Chem. 1997, 60, 573–580. [Google Scholar] [CrossRef]
- Baptista, J.; Lima, E.; Paiva, L.; Castro, A.R. Value of off-season fresh Camellia sinensis leaves. Antiradical activity, total phenolics content and catechin profiles. LWT—Food Sci. Technol. 2014, 59, 1152–1158. [Google Scholar] [CrossRef]
- Baptista, J.A.B.; Tavares, J.F.P.; Carvalho, R.C.B. Comparison of catechins and aromas among different green teas, using HPLC/SPME-GC. Food Res. Int. 1999, 31, 729–736. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons: New York, NY, USA, 2002; pp. I1.1.1–I1.1.8. [Google Scholar]
- Paiva, L.; Lima, E.; Motta, M.; Marcone, M.; Baptista, J. Variability of antioxidant properties, catechins, caffeine, L-theanine and other amino acids in different plant parts of Azorean Camellia sinensis. Curr. Res. Food Sci. 2020, 3, 227–334. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Luca, V.S.; Stan, A.-M.; Trifan, A.; Miron, A.; Aprotosoaie, A.C. Catechins profile, caffeine content and antioxidant of Camellia sinensis teas commercialized in Romania. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2016, 120, 457–463. [Google Scholar]
- Cheng, H.; Wei, K.; Wang, L. The impact of variety, environment and agricultural practices on catechins and caffeine in plucked tea leaves. In Processing and Impact on Active Components in Food; Preedy, V.R., Ed.; Academic Press: London, UK, 2015; pp. 597–603. [Google Scholar]
- Sabhapondit, S.; Karak, T.; Bhuyan, L.P.; Goswami, B.C.; Hazarika, M. Diversity of catechin in northeast Indian tea cultivars. Sci. World J. 2012, 2012, 485193. [Google Scholar] [CrossRef]
- Lin, Y.S.; Tsai, Y.J.; Tsay, J.S.; Lin, J.K. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J. Agric. Food Chem. 2003, 51, 1864–1873. [Google Scholar] [CrossRef]
- Sharma, V.; Joshi, R.; Gulati, A. Seasonal clonal variations and effects of stresses on quality chemicals and prephenate dehydratase enzyme activity in tea (Camellia sinensis). Eur. Food Res. Technol. 2011, 232, 307–317. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Zeng, L.; Dong, F.; Tu, Y.; Yang, Z. Occurrence of functional molecules in the flowers of tea (Camellia sinensis) plants: Evidence for a second resource. Molecules 2018, 23, 790. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Wu, S.-S.; Lin, J.-K. Determination of tea polyphenols and caffeine in tea flowers (Camellia sinensis) and their hydroxyl radical scavenging and nitric oxide suppressing effects. J. Agric. Food Chem. 2003, 51, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Lima, E.; Motta, M.; Baptista, J. The surplus value of Azorean Camellia sinensis flowers as an important contributor affecting the nutraceutical benefits of green tea quality. Pharm. Pharmacol. Int. J. 2019, 7, 327–332. [Google Scholar]
- Lee, L.-S.; Kim, S.-H.; Kim, Y.-B.; Kim, Y.-C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef]
- Zhao, C.; Li, C.; Liu, S.; Yang, L. The galloyl contributing to main antioxidant capacity of tea made from Camellia sinensis in China. Sci. World J. 2014, 2014, 863984. [Google Scholar] [CrossRef] [PubMed]
- Kottawa-Arachchi, J.D.; Gunasekare, M.T.K.; Ranatunga, M.A.B. Biochemical diversity of global tea [Camellia sinensis (L.) O. Kuntze] germplasm and its exploitation: A review. Genet. Resour. Crop. Evol. 2019, 66, 259–273. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Ding, Z.; Liu, F. The dynamic changes of catechins and related genes in tea (Camellia sinensis) flowers. Acta Physiol. Plant. 2019, 41, 30. [Google Scholar] [CrossRef]
- Chen, D.; Chen, G.; Sun, Y.; Zeng, X.; Ye, H. Physiological genetics, chemical composition, health benefits and toxicology of tea (Camellia sinensis L.) flowers: A review. Food Res. Int. 2020, 137, 109584. [Google Scholar] [CrossRef]
- Rohadi, R.; Lelita, D.I.; Putri, A.S. Antioxidant capacity of white tea (Camelia sinensis) extract: Compared to green, oolong and black tea. IOP Conf. Ser. Earth Environ. Sci. 2019, 292, 012018. [Google Scholar] [CrossRef]
- Ulewicz-Magulska, B.; Wesolowski, M. Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant. Foods Hum. Nutr. 2019, 74, 61–67. [Google Scholar] [CrossRef]
- Yang, Z.; Tu, Y.; Baldermann, S.; Dong, F.; Xu, Y.; Watanabe, N. Isolation and identification of compounds from the ethanolic extract of flowers of the tea (Camellia sinensis) plant and their contribution to the antioxidant capacity. Food Sci. Technol. 2009, 42, 1439–1443. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant. Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Duh, P.-D. Antioxidant activity of burdock (Arctium lappa Linné): It’s scavenging effect on free radical and active oxygen. J. Am. Oil Chem. Soc. 1998, 75, 455–461. [Google Scholar] [CrossRef]
- Xu, P.; Chen, L.; Wang, Y. Effect of storage time on antioxidant activity and inhibition on α-amylase and α-glucosidase of white tea. Food Sci. Nutr. 2019, 7, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Lipophilized epigallocatechin gallate (EGCG) derivatives as novel antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef]
- Mandel, S.; Amit, T.; Reznichenko, L.; Weinreb, O.; Youdim, M.B.H. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol. Nutr. Food Res. 2006, 50, 229–234. [Google Scholar] [CrossRef]
| Samples | Colleting Time | Withering | Drying Time 2 | Yield (%) 3 | |
|---|---|---|---|---|---|
| Time | Degree 1 | ||||
| WT1 | Apr, 2016 | 25 h 40 min | 64.05 | 119 h | 21.70 |
| WT2 | May, 2016 | 43 h 35 min | 51.34 | 27 h 15 min | 20.02 |
| WT3 | July, 2016 | 43 h 50 min | 49.25 | 51 h 40 min | 22.09 |
| WT4 | July, 2016 | 23 h | 45.41 | 71 h 30min | 22.26 |
| WT5 | Oct, 2016 | 19 h 50 min | 43.38 | 7 h 30 min | 21.29 |
| Fl | Nov, 2016 | - | - | 49 h 10 min | 14.89 |
| GTs | July–Sep, 2016 | 12 h | 46.71 | 30 min | 18.60 * |
| ECDs | Linear Range (µmol/L) | Linear Equation | R2 | LOD (µmol/L) | LOQ (µmol/L) |
|---|---|---|---|---|---|
| EGC | 0.125–2.0 | y = 115784x − 85881 | 0.9951 | 1.10 | 3.67 |
| EC | 23.4–375 | y = 555366x − 23354 | 0.9999 | 1.10 | 3.62 |
| EGCG | 0.312–5.0 | y = 963349x + 929779 | 0.9926 | 0.70 | 2.33 |
| ECG | 0.093–3.0 | y = 1 × 106x + 322547 | 0.9990 | 0.72 | 2.40 |
| Catechins and CAF | Camellia sinensis Tea and Flowers Samples | ||||||
|---|---|---|---|---|---|---|---|
| Var. Assamica | Var. Sinensis | ||||||
| WT1 | WT2 | WT3 | WT4 | WT5 | Fl | GTs | |
| GC | 4.44 ± 0.38 a | 3.17 ± 0.39 b | 3.30 ± 0.23 b | 3.49 ± 0.26 b | 2.13 ± 0.21 c | 0.81 ± 0.10 d | 0.46 ± 0.09 d |
| EGC | 0.96 ± 0.09 c,d | 1.31 ± 0.17 c | 0.96 ± 0.11 c,d | 1.15 ± 0.10 c | 2.23 ± 0.11 b | 1.07 ± 0.09 c | 5.22 ± 0.22 a |
| C | 1.01 ± 0.12 e | 1.72 ± 0.15 d,e | 2.75 ± 0.24 c | 2.09 ± 0.12 c,d | 2.95 ± 0.21 c | 8.35 ± 0.28 a | 4.45 ± 0.20 b |
| EC | 9.48 ± 0.28 e | 12.97 ± 0.47 b | 8.51 ± 0.33 f | 8.34 ± 0.41 f | 11.77 ± 0.39 c | 10.34 ± 0.41 d | 27.58 ± 0.88 a |
| EGCG | 62.68 ± 2.91 c,d | 57.92 ± 1.02 d | 133.68 ± 2.83 b | 132.37 ± 2.71 b | 131.71 ± 2.37 b | 68.94 ± 1.87 c | 198.35 ± 3.01 a |
| GCG | 1.25 ± 0.20 g | 3.83 ± 0.25 e | 4.96 ± 0.21 d | 5.84 ± 0.31 c | 2.93 ± 0.28 f | 11.26 ± 0.29 b | 24.71 ± 0.53 a |
| ECG | 40.21 ± 1.83 c | 39.08 ± 1.21 c | 50.84 ± 1.31 b | 51.72 ± 1.47 b | 51.29 ± 1.51 b | 31.73 ± 1.19 d | 89.25 ± 1.93 a |
| CAF | 16.90 ± 1.18 c | 21.48 ± 0.44 b | 21.68 ± 0.38 b | 27.73 ± 0.61 a | 21.60 ± 0.21 b | 6.15 ± 0.12 d | 15.98 ± 0.25 c |
| CAT Groups | |||||||
| ECDs | 113.33 ± 1.34 c | 111.28 ± 0.48 c | 193.99 ± 1.24 b | 193.58 ± 1.18 b | 197.00 ± 1.04 b | 112.08 ± 0.80 c | 320.40 ± 1.22 a |
| Est. CAT | 104.14 ± 1.36 c | 100.83 ± 0.51 c | 189.48 ± 1.32 b | 189.93 ± 1.20 b | 185.93 ± 1.05 b | 111.93 ± 0.79 c | 312.31 ± 1.24 a |
| Non-est. CAT | 15.89 ± 0.14 c | 19.17 ± 0.16 b | 15.52 ± 0.09 c | 15.07 ± 0.14 c | 19.08 ± 0.12 b | 20.57 ± 0.15 b | 37.71 ± 0.36 a |
| EC + ECG | 49.69 ± 0.50 c | 52.05 ± 0.52 c | 59.35 ± 0.19 b | 60.06 ± 0.67 b | 63.06 ± 0.53 b | 42.07 ± 0.55 d | 116.83 ± 0.74 a |
| EGC + EGCG | 63.64 ± 0.62 d | 59.23 ± 0.70 d | 134.64 ± 1.82 b | 133.52 ± 1.85 b | 133.94 ± 1.60 b | 70.01 ± 0.73 c | 203.57 ± 1.97 a |
| CATRAT | 0.78 b | 0.88 a | 0.44 e | 0.45 e | 0.47 e | 0.60 c | 0.57 c |
| C. sinensis Samples | TPC (mg GAE/g DE) | TFC (mg RE/g DE) |
|---|---|---|
| WT1 | 246.03 ± 4.60 d | 56.83 ± 1.04 b |
| WT2 | 208.24 ± 3.36 e | 49.50 ± 0.87 c |
| WT3 | 269.78 ± 2.39 b | 49.00 ± 1.80 c |
| WT4 | 258.45 ± 1.46 c | 41.67 ± 2.08 d |
| WT5 | 272.61 ± 1.07 b | 35.17 ± 1.61 e |
| Fl | 125.91 ± 0.83 f | 48.83 ± 0.58 c |
| GTs | 295.37± 5.13 a | 69.67 ± 1.04 a |
| C. sinensis Samples and Control | FRSA (EC50 2, µg/mL) | FRAP (EC50 3, µg/mL) | FIC (%) |
|---|---|---|---|
| WT1 | 7.7 ± 0.23 c | 7.6 ± 0.14 d | 58.55 ± 1.46 b,c |
| WT2 | 9.2 ± 0.37 d | 8.4 ± 0.11 e | 39.56 ± 2.42 e |
| WT3 | 3.6 ± 0.12 a | 6.7 ± 0.18 c | 65.33 ± 0.94 b |
| WT4 | 4.8 ± 0.14 b | 4.8 ± 0.09 a | 82.82 ± 1.47 a |
| WT5 | 4.3 ± 0.15 a,b | 5.5 ± 0.15 b | 48.27 ± 0.70 c,d |
| Fl | 17.3 ± 0.38 e | 16.5 ± 0.28 f | 47.09 ± 1.16 d,e |
| GTs | 4.1 ± 0.13 a,b | 7.0 ± 0.17 c,d | 63.25 ± 1.78 b |
| BHT * | 23.9 ± 0.32 f | 5.4 ± 0.13 b | - |
| EDTA * | - | - | 92.22 ± 0.26 a |
| Heading | ECDs | TPC | TFC | FRSA | FIC Activity |
|---|---|---|---|---|---|
| ECDs | 1 | - | - | - | - |
| TPC | 0.719 | 1 | - | - | - |
| TFC | 0.411 | 0.163 | 1 | - | - |
| FRSA | 0.629 | 0.989 | 0.083 | 1 | - |
| FIC activity | 0.456 | 0.504 | 0.062 | 0.556 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, L.; Rego, C.; Lima, E.; Marcone, M.; Baptista, J. Comparative Analysis of the Polyphenols, Caffeine, and Antioxidant Activities of Green Tea, White Tea, and Flowers from Azorean Camellia sinensis Varieties Affected by Different Harvested and Processing Conditions. Antioxidants 2021, 10, 183. https://doi.org/10.3390/antiox10020183
Paiva L, Rego C, Lima E, Marcone M, Baptista J. Comparative Analysis of the Polyphenols, Caffeine, and Antioxidant Activities of Green Tea, White Tea, and Flowers from Azorean Camellia sinensis Varieties Affected by Different Harvested and Processing Conditions. Antioxidants. 2021; 10(2):183. https://doi.org/10.3390/antiox10020183
Chicago/Turabian StylePaiva, Lisete, Clara Rego, Elisabete Lima, Massimo Marcone, and José Baptista. 2021. "Comparative Analysis of the Polyphenols, Caffeine, and Antioxidant Activities of Green Tea, White Tea, and Flowers from Azorean Camellia sinensis Varieties Affected by Different Harvested and Processing Conditions" Antioxidants 10, no. 2: 183. https://doi.org/10.3390/antiox10020183
APA StylePaiva, L., Rego, C., Lima, E., Marcone, M., & Baptista, J. (2021). Comparative Analysis of the Polyphenols, Caffeine, and Antioxidant Activities of Green Tea, White Tea, and Flowers from Azorean Camellia sinensis Varieties Affected by Different Harvested and Processing Conditions. Antioxidants, 10(2), 183. https://doi.org/10.3390/antiox10020183







