Association of Circulating Heme Oxygenase-1, Lipid Profile and Coronary Disease Phenotype in Patients with Chronic Coronary Syndrome
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Population
2.2. Clinical Definitions
2.3. HO-1 Measurements and Bio-Humoral Profile
2.4. Non-Invasive Imaging
2.4.1. Coronary CTA
2.4.2. Non-Invasive Stress Imaging Analysis
2.5. Statistical Analysis
3. Results
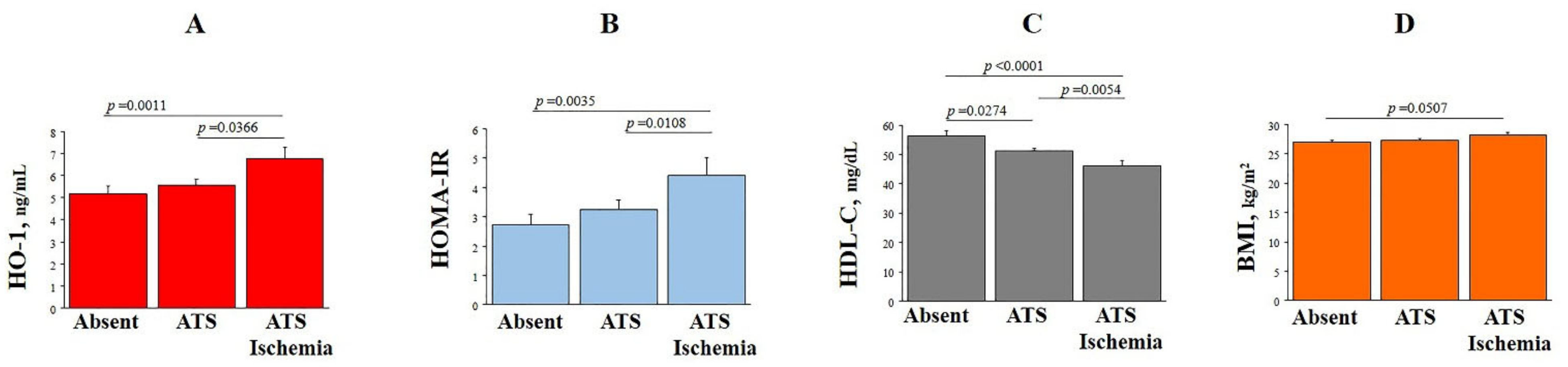
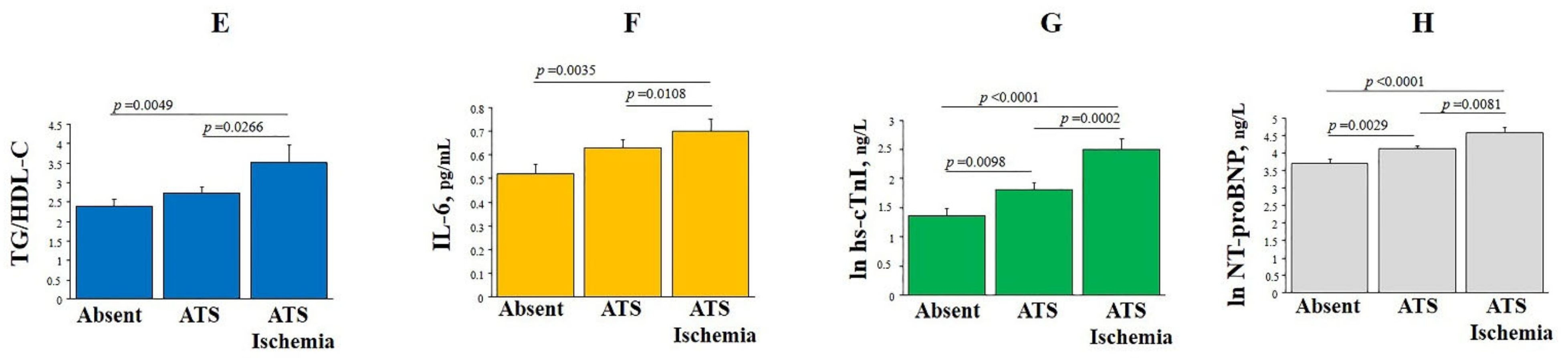
3.1. HO-1 Plasma Levels, Clinical and Bio-Humoral Profiles
3.2. Plasma HO-1, Coronary Atherosclerosis and Myocardial Ischemia
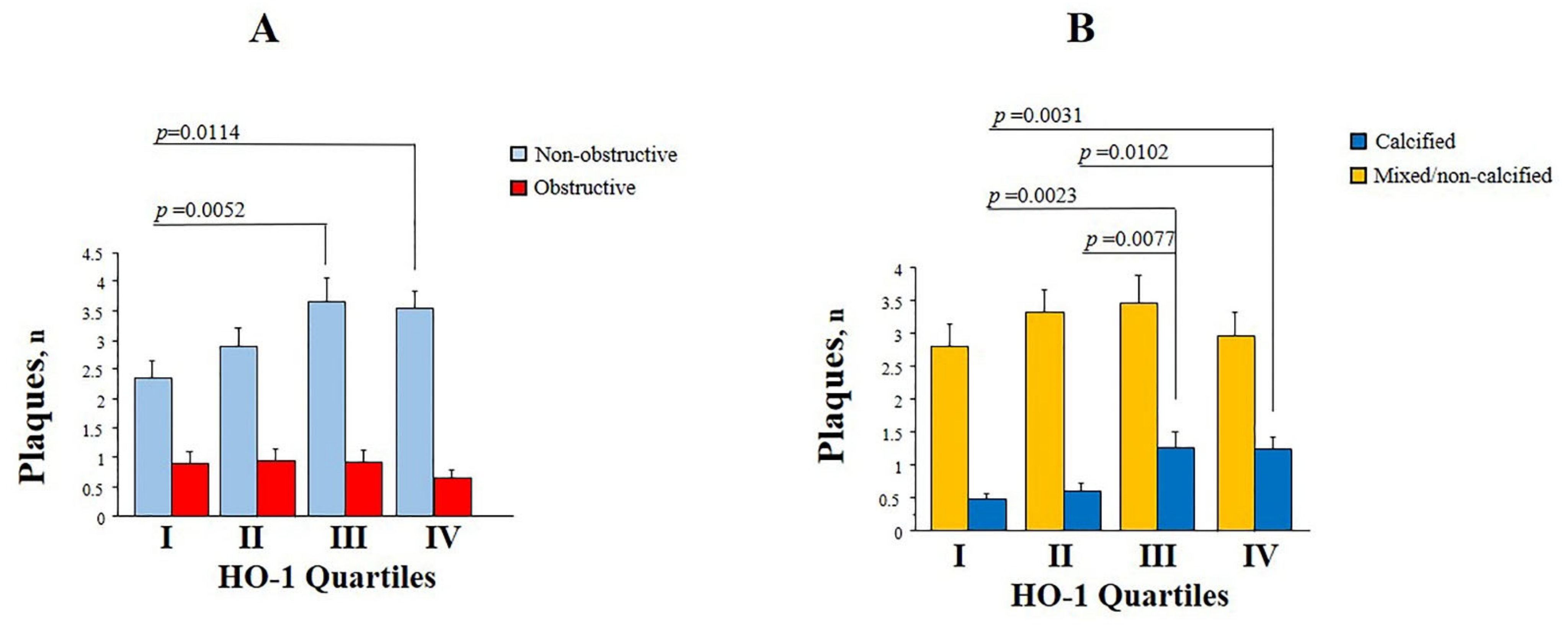
3.3. Plasma HO-1 Levels and Coronary Disease Phenotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statements
Acknowledgments
Conflicts of Interest
References
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC guidelines on the diagnosis and management of chronic coronary syndromes: The task force for diagnosis and management of chronic coronary syndromes of the European society of cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Hansson, G.K. From Focal Lipid Storage to Systemic Inflammation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 24, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Bornfeldt, K.E.; Tall, A.R. Atherosclerosis: Successes, Surprises, and Future Challenges. Circ. Res. 2016, 118, 531–534. [Google Scholar] [CrossRef]
- Wu, M.Y.; Li, C.J.; Hou, M.F.; Chu, P.Y. New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers. 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Lee, T.S.; Lee, F.Y.; Pai, R.C.; Chau, L.Y. Expression of Heme Oxygenase-1 in atherosclerotic lesions. Am. J. Pathol. 1998, 152, 711–720. [Google Scholar] [PubMed]
- Morita, T. Heme oxygenase and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1786–1795. [Google Scholar] [CrossRef]
- Fiorelli, S.; Porro, B.; Cosentino, N.; Di Minno, A.; Manega, C.M.; Fabbiocchi, F.; Niccoli, G.; Fracassi, F.; Barbieri, S.; Marenzi, G.; et al. Activation of Nrf2/HO-1 Pathway and Human Atherosclerotic Plaque Vulnerability: An In Vitro and In Vivo Study. Cells 2019, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Kondo, K.; Momiyama, Y. The Protective Role of Heme Oxygenase-1 in Atherosclerotic Diseases. Int. J. Mol. Sci. 2019, 20, 3628. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Rong, J. Therapeutic Potential of Heme Oxygenase-1/carbon Monoxide System Against Ischemia-Reperfusion Injury. Curr. Pharm. Des. 2017, 23, 3884–3898. [Google Scholar] [CrossRef]
- Perrella, M.A.; Yet, S.F. Role of Heme Oxygenase-1 in cardiovascular function. Curr. Pharm. Des. 2003, 9, 2479–2487. [Google Scholar] [CrossRef]
- Bak, I.; Papp, G.; Turoczi, T.; Varga, E.; Szendrei, L.; Vecsernyes, M.; Joo, F.; Tosaki, A. The role of heme oxygenase-related carbon monoxide and ventricular fibrillation in ischemic/reperfused hearts. Free Radic. Biol. Med. 2002, 33, 639–648. [Google Scholar] [CrossRef]
- Hangaishi, M.; Ishizaka, N.; Aizawa, T.; Kurihara, Y.; Taguchi, J.; Nagai, R.; Kimura, S.; Ohno, M. Induction of Heme Oxygenase-1 can act protectively against cardiac ischemia/reperfusion in vivo. Biochem. Biophys. Res. Commun. 2000, 279, 582–588. [Google Scholar] [CrossRef]
- Ooi, B.K.; Goh, B.H.; Yap, W.H. Oxidative Stress in Cardiovascular Diseases: Involvement of Nrf2 Antioxidant Redox Signaling in Macrophage Foam Cells Formation. Int. J. Mol. Sci. 2017, 18, 2336. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, L.; Ding, H.; Huang, S.; He, M.; Zhang, X.; Cheng, L.; Wang, D.; Hu, F.B.; Wu, T. Short (GT) (n) repeats in Heme Oxygenase-1 gene promoter are associated with lower risk of coronary heart disease in subjects with high levels of oxidative stress. Cell Stress Chaperones 2012, 17, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Endler, G.; Exner, M.; Schillinger, M.; Marculescu, R.; Sunder-Plassmann, R.; Raith, M.; Jordanova, N.; Wojta, J.; Mannhalter, C.; Wagner, O.F.; et al. A microsatellite polymorphism in the Heme Oxygenase-1 gene promoter is associated with increased bilirubin and HDL levels but not with coronary artery disease. Thromb. Haemost. 2004, 91, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Neglia, D.; Rovai, D.; Caselli, C.; Pietila, M.; Teresinska, A.; Aguadé-Bruix, S.; Pizzi, M.N.; Todiere, G.; Gimelli, A.; Schroeder, S.; et al. Detection of significant coronary artery disease by non-invasive anatomical and functional imaging. Circ. Cardiovasc. Imaging 2015, 8, e002179. [Google Scholar] [CrossRef]
- Caselli, C.; Del Turco, S.; Ragusa, R.; Lorenzoni, V.; De Graaf, M.; Basta, G.; Scholte, A.; De Caterina, R.; Neglia, D. Association of PCSK9 plasma levels with metabolic patterns and coronary atherosclerosis in patients with stable angina. Cardiovasc. Diabetol. 2019, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Caselli, C.; Rovai, D.; Lorenzoni, V.; Carpeggiani, C.; Teresinska, A.; Aguade, S.; Todiere, G.; Gimelli, A.; Schroeder, S.; Casolo, G.; et al. A New Integrated Clinical-Biohumoral Model to Predict Functionally Significant Coronary Artery Disease in Patients with Chronic Chest Pain. Can. J. Cardiol. 2015, 31, 709–716. [Google Scholar] [CrossRef]
- Caselli, C.; De Graaf, M.A.; Lorenzoni, V.; Rovai, D.; Marinelli, M.; Del Ry, S.; Giannessi, D.; Bax, J.J.; Neglia, D.; Scholte, A.J. HDL cholesterol, leptin and interleukin-6 predict high risk coronary anatomy assessed by CT angiography in patients with stable chest pain. Atherosclerosis 2015, 241, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B.; Bose, S.; McCord, J.M. Phytochemical Combination PB125 Activates the Nrf2 Pathway and Induces Cellular Protection against Oxidative Injury. Antioxidants 2019, 8, 119. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Y.; Wang, W.; Han, X.; Han, J.; Chen, M.; Zhang, J.; Wang, C.; Li, S.; Luo, J.; et al. Nuclear Factor Erythroid 2 Related Factor 2 Activator JC-5411 Inhibits Atherosclerosis Through Suppression of Inflammation and Regulation of Lipid Metabolism. Front. Pharmacol. 2020, 11, 532568. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Chang, C.C.; Zhu, Y.; Shyy, J.Y. Simvastatin induces Heme Oxygenase-1: A novel mechanism of vessel protection. Circulation 2004, 110, 1296–1302. [Google Scholar] [CrossRef]
- Heeba, G.; Moselhy, M.E.; Hassan, M.; Khalifa, M.; Gryglewski, R.; Malinski, T. Anti-atherogenic effect of statins: Role of nitric oxide, peroxynitrite and haem oxygenase-1. Br. J. Pharmacol. 2009, 156, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Sung, K.C.; Wild, S.H.; Byrne, C.D. Controlling for apolipoprotein A-I concentrations changes the inverse direction of the relationship between high HDL-C concentration and a measure of pre-clinical atherosclerosis. Atherosclerosis 2013, 231, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.C.; Ryu, S.; Wild, S.H.; Byrne, C.D. An increased high-density lipoprotein cholesterol/apolipoprotein A-I ratio is associated with increased cardiovascular and all-cause mortality. Heart 2015, 101, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.J.; Byrne, C.D.; Sung, K.C. The HDL cholesterol/apolipoprotein A-I ratio: An indicator of cardiovascular disease. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Sun, W.; Tan, Y.; Liu, Y.; Zheng, Y.; Liu, Q.; Cai, L.; Sun, J. Sulforaphane attenuation of type 2 diabetes-induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxid. Med. Cell Longev. 2014, 2014, 123963. [Google Scholar] [CrossRef]
- Choi, S.H.; Park, S.; Oh, C.J.; Leem, J.; Park, K.G.; Lee, I.K. Dipeptidyl peptidase-4 inhibition by gemigliptin prevents abnormal vascular remodeling via NF-E2-related factor 2 activation. Vascul. Pharmacol. 2015, 73, 11–19. [Google Scholar] [CrossRef]
- Nakazato, R.; Gransar, H.; Berman, D.S.; Cheng, V.Y.; Lin, F.Y.; Achenbach, S.; Al-Mallah, M.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q.; et al. Statins use and coronary artery plaque composition: Results from the International Multicenter CONFIRM Registry. Atherosclerosis 2012, 225, 148–153. [Google Scholar] [CrossRef]
- Lee, S.E.; Chang, H.J.; Sung, J.M.; Park, H.B.; Heo, R.; Rizvi, A.; Lin, F.Y.; Kumar, A.; Hadamitzky, M.; Kim, Y.J.; et al. Effects of Statins on Coronary Atherosclerotic Plaques: The PARADIGM Study. JACC Cardiovasc. Imaging 2018, 11, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Smit, J.M.; van Rosendael, A.R.; El Mahdiui, M.; Neglia, D.; Knuuti, J.; Saraste, A.; Buechel, R.R.; Teresinska, A.; Pizzi, M.N.; Roque, A.; et al. Impact of Clinical Characteristics and Statins on Coronary Plaque Progression by Serial Computed Tomography Angiography. Circ. Cardiovasc. Imaging 2020, 13, e009750. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Hou, Y.; Chen, Y.; Yang, B.; Fu, J.; Zheng, H.; Yarborough, K.; Woods, C.G.; Liu, D.; Yamamoto, M.; et al. Adipose deficiency of Nrf2 in ob/ob mice results in severe metabolic syndrome. Diabetes 2013, 62, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, L.V.; Savova, M.S.; Amirova, K.M.; Dinkova-Kostova, A.T.; Georgiev, M.I. Obesity and NRF2-mediated cytoprotection: Where is the missing link? Pharmacol. Res. 2020, 156, 104760. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Leung, L.; Xue, P.; Wang, W.; Hou, Y.; Liu, D.; Yehuda-Shnaidman, E.; Lee, C.; Lau, J.; Kurtz, T.W.; et al. Deficiency in the nuclear factor E2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. J. Biol. Chem. 2010, 19, 9292–9300. [Google Scholar] [CrossRef]
- Hou, Y.; Xue, P.; Bai, Y.; Liu, D.; Woods, C.G.; Yarborough, K.; Fu, J.; Zhang, Q.; Sun, G.; Collins, S.; et al. Nuclear factor erythroid-derived factor 2-related factor 2 regulates transcription of CCAAT/enhancer-binding protein β during adipogenesis. Free Radic. Biol. Med. 2012, 52, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Son, Y.; Kim, N.H.; Jeong, H.J.; Chang, K.C.; Chung, H.T. Role of heme oxygenase in preserving vascular bioactive NO. Nitric Oxide 2010, 23, 251–257. [Google Scholar] [CrossRef]
- Cao, J.; Vecoli, C.; Neglia, D.; Tavazzi, B.; Lazzarino, G.; Novelli, M.; Masiello, P.; Wang, Y.T.; Puri, N.; Paolocci, N.; et al. Cobalt-Protoporphyrin Improves Heart Function by Blunting Oxidative Stress and Restoring NO Synthase Equilibrium in an Animal Model of Experimental Diabetes. Front. Physiol. 2012, 3, 160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vecoli, C.; Cao, J.; Neglia, D.; Inoue, K.; Sodhi, K.; Vanella, L.; Gabrielson, K.K.; Bedja, D.; Paolocci, N.; L’abbate, A.; et al. Apolipoprotein A-I mimetic peptide L-4F prevents myocardial and coronary dysfunction in diabetic mice. J. Cell. Biochem. 2011, 112, 2616–2626. [Google Scholar] [CrossRef]
- Vecoli, C.; Novelli, M.; Pippa, A.; Giacopelli, D.; Beffy, P.; Masiello, P.; L’Abbate, A.; Neglia, D. Partial deletion of eNOS gene causes hyperinsulinemic state, unbalance of cardiac insulin signaling pathways and coronary dysfunction independently of high fat diet. PLoS ONE 2014, 9, e104156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caselli, C.; Prontera, C.; Liga, R.; De Graaf, M.A.; Gaemperli, O.; Lorenzoni, V.; Ragusa, R.; Marinelli, M.; Del Ry, S.; Rovai, D.; et al. Effect of Coronary Atherosclerosis and Myocardial Ischemia on Plasma Levels of High-Sensitivity Troponin T and NT-proBNP in Patients With Stable Angina. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 757–764. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, M.; Ma, Y.; Wang, R.; Liu, W.; Xia, W.; Guan, A.; Xing, C.; Lu, F.; Ji, X. Fenofibrate Increases Heme Oxygenase 1 Expression and Astrocyte Proliferation While Limits Neuronal Injury During Intracerebral Hemorrhage. Curr. Neurovasc. Res. 2017, 14, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Li Volti, G.; Sacerdoti, D.; Di Giacomo, C.; Barcellona, M.L.; Scacco, A.; Murabito, P.; Biondi, A.; Basile, F.; Gazzolo, D.; Abella, R.; et al. Natural Heme Oxygenase-1 inducers in hepatobiliary function. World J. Gastroenterol. 2008, 14, 6122–6132. [Google Scholar] [CrossRef] [PubMed]
- Pittala, V.; Vanella, L.; Salerno, L.; Romeo, G.; Marrazzo, A.; Di Giacomo, C.; Sorrenti, V. Effects of Polyphenolic Derivatives on Heme Oxygenase-System in Metabolic Dysfunctions. Curr. Med. Chem. 2018, 25, 1577–1595. [Google Scholar] [CrossRef] [PubMed]

| Clinical Population n = 526 | Low HO-1 n = 263 | High HO-1 n = 263 | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 60 ± 9 | 61 ± 8 | 60 ± 9 | ns |
| Males | 318 (60) | 145 (55) | 173 (66) | 0.0125 |
| Clinical characteristics | ||||
| Typical angina | 139 (26) | 76 (29) | 63 (24) | ns |
| LVEF% | 60 ± 8 | 61 ± 8 | 59 ± 8 | 0.0110 |
| CAD probability | 48 ± 19 | 48 ± 20 | 49 ± 18 | ns |
| Cardiovascular risk factors | ||||
| Family history of CAD | 186 (35) | 94 (36) | 92 (37) | ns |
| Diabetes | 177 (34) | 83 (32) | 94 (36) | ns |
| Hypercholesterolemia | 316 (60) | 157 (60) | 159 (60) | ns |
| Hypertension | 349 (66) | 166 (63) | 163 (62) | ns |
| Smoking | 129 (24) | 64 (24) | 65 (25) | ns |
| BMI, kg/m2 | 27.7 ± 4.3 | 27.2 ± 4 | 28.2 ± 4.6 | 0.0076 |
| Metabolic syndrome | 181 (34) | 80 (30) | 101 (38) | 0.0539 |
| Pharmacological therapies | ||||
| Beta-blockers | 212 (40) | 100 (38) | 112 (43) | ns |
| Calcium channel blockers | 72 (14) | 30 (11) | 42 (16) | ns |
| ACE Inhibitors | 157 (30) | 86 (33) | 71 (27) | ns |
| ARBs | 89 (17) | 41 (16) | 48 (18) | ns |
| Diuretics | 88 (17) | 44 (17) | 44 (17) | ns |
| Anti-diabetic | 109 (21) | 45 (17) | 51 (19) | ns |
| Statins | 274 (52) | 128 (49) | 146 (56) | ns |
| Aspirin | 309 (59) | 155 (59) | 154 (59) | ns |
| Nitrates | 58 (11) | 23 (9) | 35 (13) | ns |
| Anti-coagulants | 11 (2) | 4 (1) | 7 (3) | ns |
| Clinical Population n = 526 | Low HO-1 n = 263 | High HO-1 n = 263 | p Value | |
|---|---|---|---|---|
| Oxidative stress | ||||
| HO-1, ng/mL | 5.65 ± 4.19 | 2.33 ± 1.43 | 8.97 ± 3.13 | <0.0001 |
| GGT, IU/L | 40 ± 30 | 38 ± 27 | 42 ± 32 | 0.0074 |
| Metabolic (glucose) | ||||
| FPG, mg/dL | 112 ± 36 | 112 ± 34 | 113 ± 38 | ns |
| Insulin, μUI/mL | 11.6 ± 11.0 | 12.7 ± 10.7 | 11.5 ± 11 | ns |
| HOMA-IR index | 3.5 ± 4.2 | 3.4 ± 4.1 | 3.5 ± 4.1 | ns |
| Metabolic (lipid) | ||||
| Total-C, mg/dL | 183 ± 49 | 188 ± 50 | 178 ± 49 | 0.0166 |
| LDL-C, mg/dL | 106 ± 40 | 110 ± 40 | 102 ± 40 | 0.0130 |
| HDL-C, mg/dL | 52 ± 17 | 54 ± 16 | 51 ± 18 | 0.0054 |
| Remnant-C, mg/dL | 24 ± 15 | 23 ± 14 | 25 ± 15 | ns |
| Non-HDL-C, mg/dL | 130 ± 43 | 134 ± 43 | 126 ± 42 | 0.0528 |
| Apo A1, mg/dL | 143 ± 32 | 145 ± 33 | 142 ± 32 | ns |
| HDL-C/Apo A1 | 0.37 ± 0.12 | 0.38 ± 0.13 | 0.36 ± 0.1 | 0.0258 |
| Apo B, mg/dL | 87 ± 28 | 90 ± 28 | 84 ± 28 | 0.0079 |
| Apo A1/Apo B | 1.80 ± 0.85 | 1.71 ± 0.52 | 1.89 ± 1.07 | 0.0167 |
| Lp (a) | 20 ± 22 | 23.6 ± 24.1 | 18.6 ± 21.8 | 0.0076 |
| Triglycerides, mg/dL | 124 ± 81 | 120 ± 75 | 128 ± 86 | ns |
| TG/HDL-C | 2.74 ± 2.45 | 2.55 ± 2.33 | 2.94 ± 2.55 | ns |
| PCSK9, ng/mL | 213 ± 105 | 227 ± 110 | 199 ± 98 | 0.0024 |
| Adipose tissue | ||||
| Adiponectin, μg/mL | 9.8 ± 6.9 | 10.2 ± 6.4 | 9.2 ± 6.9 | 0.0030 |
| Leptin, ng/mL | 9.9 ± 10.7 | 8.8 ± 8.3 | 11.2 ± 12.7 | ns |
| Hepatic | ||||
| AST, IU/L | 24 ± 10 | 24 ± 9 | 25 ± 11 | ns |
| ALT, IU/L | 21 ± 13 | 20 ± 13 | 22 ± 14 | ns |
| Remodeling | ||||
| MMP-2, ng/mL | 159 ± 61 | 157 ± 63 | 161 ± 58 | ns |
| MMP-9, ng/mL | 145 ± 206 | 162 ± 221 | 127 ± 187 | 0.0511 |
| ALP, IU/L | 51 ± 18 | 53 ± 18 | 50 ± 18 | 0.0433 |
| Inflammatory | ||||
| hs-CRP, mg/dL | 0.40 ± 1.09 | 0.34 ± 0.56 | 0.47 ± 1.45 | ns |
| IL-6, ng/L | 1.35 ± 2.35 | 1.12 ± 1.33 | 1.47 ± 2.83 | 0.0575 |
| Cardiac | ||||
| hs-cTnT, ng/L | 910 ± 21 | 10 ± 19 | 10 ± 22 | ns |
| hs-cTnI, ng/L | 54 ± 240 | 41 ± 215 | 66 ± 262 | 0.0006 |
| NT-proBNP, ng/L | 139 ± 291 | 119 ± 173 | 158 ± 374 | ns |
| Renal | ||||
| Creatinine, mg/dL | 0.96 ± 0.23 | 0.85 ± 0.23 | 0.91 ± 0.22 | 0.0014 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Coefficient | SE | p Value | Coefficient | SE | p Value | |
| Age | −0.010 | 0.004 | 0.0218 | −0.011 | 0.004 | 0.0128 |
| Males | 0.243 | 0.079 | 0.0022 | |||
| LVEF% | −0.010 | 0.005 | 0.0286 | |||
| BMI | 0.025 | 0.009 | 0.0068 | |||
| Beta-blockers | 0.141 | 0.079 | 0.0748 | |||
| Statins | 0.198 | 0.078 | 0.0108 | |||
| Nitrates | 0.249 | 0.124 | 0.0452 | 0.267 | 0.124 | 0.0317 |
| GGT | 0.173 | 0.072 | 0.0159 | |||
| Total-C | −0.466 | 0.143 | 0.0012 | |||
| LDL-C | −0.289 | 0.095 | 0.0026 | |||
| HDL-C | −0.394 | 0.122 | 0.0013 | |||
| Non-HDL-C | −0.002 | 0.001 | 0.0065 | |||
| HDL-C/Apo A1 | −1.214 | 0.332 | 0.0003 | −1.281 | 0.343 | 0.0002 |
| Apo B | −0.369 | 0.117 | 0.0145 | −0.390 | 0.126 | 0.0017 |
| Apo A1/Apo B | 0.111 | 0.046 | 0.0163 | |||
| Lp (a) | −0.116 | 0.038 | 0.0024 | −0.101 | 0.038 | 0.0087 |
| PCSK9 | −0.306 | 0.080 | 0.0002 | |||
| Adiponectin | −0.186 | 0.061 | 0.0024 | |||
| MMP-9 | −0.063 | 0.037 | 0.0876 | |||
| ALP | −0.284 | 0.108 | 0.0088 | |||
| IL-6 | 0.244 | 0.081 | 0.0027 | 0.180 | 0.081 | 0.0274 |
| hs-cTnI | 0.093 | 0.027 | 0.0005 | 0.064 | 0.027 | 0.0189 |
| Imaging Population n = 347 | Low HO-1 n = 174 | High HO-1 n = 173 | p Value | |
|---|---|---|---|---|
| Coronary Anatomy | ||||
| Normals | 95 (27) | 52 (30) | 42 (24) | ns |
| Patients with non-obstructive | 131 (38) | 59 (34) | 72 (42) | |
| Patients with obstructive | 121 (35) | 62 (36) | 59 (34) | |
| Coronary Plaques | ||||
| Total No. of plaques | 4 ± 3.8 | 3.6 ± 3.5 | 4.5 ± 4 | 0.0265 |
| No. of non-obstructive plaques | 3.1 ± 3 | 2.6 ± 2.6 | 3.6 ± 3.3 | 0.0018 |
| No. of obstructive plaques | 0.9 ± 14.7 | 0.9 ± 1.8 | 0.8 ± 1.5 | ns |
| No. of calcified plaques | 0.9 ± 1.7 | 0.5 ± 1 | 1.2 ± 2.1 | 0.0002 |
| No. of non-calcified plaques | 0.5 ± 0.9 | 0.4 ± 0.9 | 0.5 ± 0.9 | ns |
| No. of mixed plaques | 2.7 ± 3.2 | 2.6 ± 3.1 | 2.83 ± 3.4 | ns |
| Risk Scores | ||||
| CTA risk score | 11.9 ± 11 | 11 ± 10.6 | 12.8 ± 11.3 | ns |
| CAC score (n = 286) | 292 ± 604 | 222 ± 414 | 361 ± 739 | 0.0497 |
| Myocardial Ischemia | ||||
| Patients with myocardial ischemia | 83 (24) | 34 (20) | 49 (28) | 0.0477 |
| SDS at MPI (n = 274) | 3.41 ± 7.71 | 2.19 ± 5.12 | 4.14 ± 8.78 | 0.0272 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caselli, C.; De Caterina, R.; Ragusa, R.; Liga, R.; Gimelli, A.; Scholte, A.J.H.A.; Clerico, A.; Knuuti, J.; Neglia, D. Association of Circulating Heme Oxygenase-1, Lipid Profile and Coronary Disease Phenotype in Patients with Chronic Coronary Syndrome. Antioxidants 2021, 10, 2002. https://doi.org/10.3390/antiox10122002
Caselli C, De Caterina R, Ragusa R, Liga R, Gimelli A, Scholte AJHA, Clerico A, Knuuti J, Neglia D. Association of Circulating Heme Oxygenase-1, Lipid Profile and Coronary Disease Phenotype in Patients with Chronic Coronary Syndrome. Antioxidants. 2021; 10(12):2002. https://doi.org/10.3390/antiox10122002
Chicago/Turabian StyleCaselli, Chiara, Raffaele De Caterina, Rosetta Ragusa, Riccardo Liga, Alessia Gimelli, Arthur J. H. A. Scholte, Aldo Clerico, Juhani Knuuti, and Danilo Neglia. 2021. "Association of Circulating Heme Oxygenase-1, Lipid Profile and Coronary Disease Phenotype in Patients with Chronic Coronary Syndrome" Antioxidants 10, no. 12: 2002. https://doi.org/10.3390/antiox10122002
APA StyleCaselli, C., De Caterina, R., Ragusa, R., Liga, R., Gimelli, A., Scholte, A. J. H. A., Clerico, A., Knuuti, J., & Neglia, D. (2021). Association of Circulating Heme Oxygenase-1, Lipid Profile and Coronary Disease Phenotype in Patients with Chronic Coronary Syndrome. Antioxidants, 10(12), 2002. https://doi.org/10.3390/antiox10122002







